Abstract
New treatment options are required for primary systemic amyloid light chain (AL) amyloidosis. This phase 1/2 dose-escalation study aimed to determine the maximum tolerated dose (MTD) of lenalidomide in combination with melphalan and dexamethasone (M-dex), and assess the efficacy and tolerability of this therapy for patients with de novo AL amyloidosis. Twenty-six patients were enrolled across 4 cohorts: M-dex + lenalidomide 5, 10, 15, and 20 mg once daily on days 1 to 21 in a 28-day cycle. No dose limiting toxicity (DLT) was observed in cohorts 1, 2, and 3. 4. Seven patients in cohort 4, M-dex + lenalidomide 20 mg/day, experienced DLT. MTD was defined as 15 mg of lenalidomide. A complete hematologic response was achieved in 42% at the dose of 15 mg of lenalidomide per day. After a median follow-up of 19 months, estimated 2-year overall survival (OS) and event-free survival (EFS) were 80.8% and 53.8%, respectively. Hematologic and organ responses were both associated with superior EFS rates (P = .0001). A higher EFS was also observed in patients whose free light chains decreased by more than 50% during therapy (P = .019). Lenalidomide 15 mg/d + M-dex is a new effective combination therapy in patients with newly diagnosed AL amyloidosis. This study is registered at www.clinicaltrials.gov as NCT00621400.
Introduction
Systemic amyloid light chain (AL) amyloidosis is a protein conformational disease consecutive to a marrow clonal plasma cell proliferation. AL amyloidosis results from tissue deposition of amyloid protein, mainly consisting of monoclonal light chain fragments produced by the clone.1 In most patients, the amyloidogenic monoclonal immunoglobulin can be detected in serum and/or urine, either by immunofixation or by the use of free light chain assay (FLC). Multiple organ dysfunction comes from extracellular amyloid deposits and the natural history of AL amyloidosis is that of a progressive and fatal disease. AL amyloidosis therapy aims at rapidly reducing the supply of amyloid-forming monoclonal free light chains by suppression of underlying plasma cell dyscrasia. The regimens used to treat subjects with systemic AL amyloidosis are based on those used in multiple myeloma therapy. Hematologic response usually translates into clinically improved organ function and combines with a substantial survival advantage and improved quality of life. Strategies for the effective management of subjects with systemic AL amyloidosis have been reported.2,3 First-line therapy includes treatments such as high-dose melphalan followed by autologous stem cell transplantation (ASCT) in patients eligible for high-dose therapy, or oral melphalan + dexamethasone (M-dex). In a recent prospective randomized trial performed in patients with newly diagnosed AL amyloidosis comparing oral M-dex given monthly with a high-dose therapeutic approach, the median overall survival (OS) for patients receiving M-Dex was 56 months, indicating that this combination could be considered as an interesting therapeutic option in patients with newly diagnosed AL amyloidosis.4 Lenalidomide has shown significant activity in patients with multiple myeloma alone and in combination with dexamethasone, in relapse or in newly diagnosed patients.5,6 Lenalidomide has also been associated in patients with de novo multiple myeloma with melphalan and prednisone. This oral melphalan and prednisone regimen was well tolerated with a significant response rate of approximately 80%.7 The recommended dose in the melphalan and prednisone regimen was 0.18 mg/kg melphalan given for 4 consecutive days every 4 to 6 weeks, and 10 mg/d lenalidomide for 21 consecutive days every 4 weeks. Based on these encouraging results in myeloma patients, lenalidomide + dexamethasone was tested in patients with relapsing AL amyloidosis. Sanchorawala et al reported that the initial dose of lenalidomide used (25 mg/d) was poorly tolerated in such patients, but a reduced dose of 15 mg/d was well tolerated and effective, with an overall hematologic response rate of 67% in combination with dexamethasone.8 Dispenzieri et al confirmed that the combination of lenalidomide + dexamethasone achieved a 75% hematologic response rate, with a 42% organ response rate, with a median follow-up of 17 months in a series mainly including relapsed patients.9 These authors also recommended a lower dose of 15 mg/d. Both groups concluded that lenalidomide + dexamethasone was an interesting option in patients with AL amyloidosis.
To improve the hematologic and organ response rates, it might be interesting to combine M-dex, which many investigators consider as the standard of care at the time of diagnosis, and lenalidomide. Until now, no trial of this specific combination has been performed in patients with newly diagnosed AL-amyloidosis patients, and the toxicity of this regimen is unknown. We therefore have initiated a multicenter single-arm, open-label phase 1/2 dose-escalation study of lenalidomide administered in combination with M-dex.
Methods
Patients
Patients from 18 to 70 years with a diagnosis of de novo AL amyloidosis, including measurable hematologic and amyloid-related end-organ involvement, were eligible. The diagnosis of AL-amyloidosis required a histologic tissue diagnosis of amyloid deposition proven by Congo red staining of a tissue biopsy, plus proof of plasma cell dyscrasia (measurable M-protein in serum or urine by immunofixation or electrophoresis, and/or elevated free light chain [FLC] higher than 100 mg/L and an abnormal FLC ratio). Adequate organ function defined as absolute neutrophil count > 1.0 × 109/L, platelet count > 100 × 109/L, aspartate transaminase (AST) and alanine transaminase (ALT) < 2× upper normal limit, total bilirubin < 1.5 mg/dL, creatinin serum level < 150μM, and ECOG performance status of < 2 at study entry were required. For females of childbearing potential a negative serum or urine pregnancy test at screening visit, as well as within 24 hours of starting lenalidomide, was mandatory. All patients were counseled at a minimum of every 28 days about pregnancy precautions and risks of fetal exposure due to lenalidomide therapy. Patients had to be disease free from any prior malignancies for > 5 years with exception of currently treated basal cell, squamous cell carcinoma of the skin, or carcinoma in situ of the cervix or breast.
Patients were excluded if they had symptomatic multiple myeloma, if they had received prior treatment with lenalidomide or any other experimental drug within 28 days of baseline, or any prior treatment for amyloidosis, if they were known positive for HIV or infectious hepatitis, type A, B, or C. Pregnant or breast feeding females were excluded. There was no eligibility restriction for any degree of cardiac involvement.
All patients provided written, informed consent in accordance with the Declaration of Helsinki.
Study design
Patients were enrolled in this phase 1/2, multicenter, single-arm, open-label study of dose escalation of lenalidomide administered in combination with M-dex (www.clinicaltrials.gov NCT00621400, Eudract 2007-004739-43) on 11 sites in France, between March 2008 and February 2009. The primary end point of this study was to determine the incidence of dose-limiting toxicities (DLT) during the first cycle of lenalidomide at a given dose level in combination with M-dex to define the maximal tolerated dose (MTD) in a dose-escalation study design. Secondary objectives were to determine hematologic response rate of the combination, organ response rate, and safety (type, frequency, severity, and relationship of adverse events [AEs] to study treatment), and to determine the prognostic value of FLC reduction during therapy. The study was carried out in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki, and was approved by a single Institutional Review Board/Independent Ethics Committee (Tours, France) for all the centers involved according to French law.
A standard 3 + 3 dose-escalation design was used to determine the MTD. For each dose level, 3 subjects were exposed with up to 6 subjects depending on DLT. If no DLT was observed among the first cohort of 3 subjects at a given dose level, the next set of 3 subjects was entered on the following higher dose. If 2 or more DLTs were observed, the previous dose level was identified as MTD. If 1 DLT was observed on the initial 3 subjects, an additional group of 3 subjects was entered. If none of the additional 3 subjects experienced any DLT, the dose was escalated; otherwise the lower dose was identified as MTD. After the MTD had been defined, 9 additional subjects were enrolled at MTD to define the safety profile further and to estimate the hematologic and organ response rate.
Using National Cancer Institute common toxicity criteria during the first 4 weeks of treatment (1 cycle), DLT was defined as at least grade 2 cardiac arrhythmia, at least grade 3 nonhematologic toxicity, grade 4 neutropenia lasting > 7 days, or any grade 4 other hematologic toxicity or treatment delay due to toxicity occurring during the first cycle.
Treatment schedule was as follows: in addition to melphalan 0.18 mg/kg/d on days 1-4 in each 28-day cycle and dexamethasone 40 mg/d on days 1-4 in each 28-day cycle, subjects received lenalidomide. Three successive cohorts of subjects were exposed to escalating doses of lenalidomide (5, 10, and 15 mg once daily on days 1-21 in a 28-day cycle). The dosage of lenalidomide in cohort 3 (ie, 15 mg/d on days 1-21) had been anticipated as the highest tolerable, according to the reports of previous publications showing that this dosage was the optimal dosage in the relapse setting, using lenalidomide as single-agent or with dexamethasone, and without combining with melphalan. However, in November 2008, no DLT had been observed at the lenalidomide dose of 15 mg/d in combination with M-dex, so the protocol was amended to open a fourth cohort at 20 mg/d. Patients could receive up to 9 cycles of treatment according to the protocol. A prophylactic anti-thrombotic treatment consisting of aspirin, low-molecular-weight heparin (LMWH) or warfarin at the physician's discretion was recommended. In spite of aspirin prophylaxis, patient 103 experienced one thrombosis event during cycle 2. The protocol was amended and daily LMWH administration was mandatory during the first 4 cycles of the protocol. In absence of thrombosis during these first 4 months of therapy, aspirin could be introduced, replacing LMWH infusion, for the further treatment cycles. Granulocyte colony-stimulating factor was not permitted during the first cycle of therapy.
Assessment
Baseline assessments and procedures included a complete physical examination, assessment of performans status, amyloid-related organ involvement, and laboratory assessments for hematology and clinical chemistry, serum/urine M-protein analysis, FLC analysis, bone marrow aspirate, and biopsy. Patients were regularly seen during therapy for a follow-up visit including physical examination and laboratory assessments, every 4 weeks or more often, if clinically required.
Organ involvement and response to therapy were assessed according to the international consensus guidelines defined during the 10th International Symposium on Amyloidosis,10 except that bone marrow studies were not required to define a complete response.4 A clinical response was defined as improvement of an organ known to be involved with amyloid. A complete hematologic response was defined as the complete disappearance of the monoclonal immunoglobulin or light chain in a serum or urine specimen; a partial hematologic response was defined as more than a 50% reduction in these proteins. Responses were assessed during the rest period of each treatment cycle, at the end-of-treatment visit, and then every 4 to 6 weeks until progression. Safety was assessed by the incidence of AE or any clinically laboratory changes. Adverse events were recorded throughout the study and until 30 days after the last dose of lenalidomide. Any patient receiving one dose of treatment with lenalidomide was assessable for safety.
Statistical analysis
Kaplan-Meier curves for event-free survival (EFS, defined by the time between diagnosis and the occurrence of progression, relapse, or death) and overall survival (OS) were plotted and compared by the use of the log-rank test.
Results
Patient characteristics
A total of 27 patients with newly diagnosed AL amyloidosis were enrolled in the 4 cohorts of this study. One patient (313) withdrew consent during the first month of the trial and was lost to follow-up. Twenty-six patients were thus assessable for efficacy and toxicity. Baseline demographics and disease characteristics are shown in Table 1. Overall median age was 57 years (range, 27-70 years); 10 patients (38%) had 1 organ involved and 16 patients (62%) 2 organs or more. A total of 10 patients (38%) were considered at high risk.4,11 The plasma cell dyscrasia was λ in 20, κ in 5, and biclonal in 1.
Patient characteristics
| Characteristics . | All patients (n = 26) . | |
|---|---|---|
| Number . | Percentage . | |
| Age, y | ||
| Median | 57 | |
| Range | 27-70 | |
| Sex | ||
| Male | 16 | 62 |
| Female | 10 | 38 |
| No. of involved organs | ||
| Median | 2 | |
| Range | 1-6 | |
| Heart | 15 | 58 |
| Kidney | 15 | 58 |
| Liver | 7 | 27 |
| Peripheral nerve | 3 | 12 |
| Patients with high-risk disease | 10 | 38 |
| Involved serum FLC, mg/L | ||
| Median | 187 | |
| Range | 18-9650 | |
| NT-pro BNP, ng/L | ||
| Median | 1140 | |
| Range | 194-21 091 | |
| Missing | 11 | |
| > 332 ng/L12 | 12/15 | 80 |
| Characteristics . | All patients (n = 26) . | |
|---|---|---|
| Number . | Percentage . | |
| Age, y | ||
| Median | 57 | |
| Range | 27-70 | |
| Sex | ||
| Male | 16 | 62 |
| Female | 10 | 38 |
| No. of involved organs | ||
| Median | 2 | |
| Range | 1-6 | |
| Heart | 15 | 58 |
| Kidney | 15 | 58 |
| Liver | 7 | 27 |
| Peripheral nerve | 3 | 12 |
| Patients with high-risk disease | 10 | 38 |
| Involved serum FLC, mg/L | ||
| Median | 187 | |
| Range | 18-9650 | |
| NT-pro BNP, ng/L | ||
| Median | 1140 | |
| Range | 194-21 091 | |
| Missing | 11 | |
| > 332 ng/L12 | 12/15 | 80 |
NT-pro BNP indicates N-terminal pro brain natriuretic peptide.
DLT and definition of MTD (Table 2)
Three patients were enrolled in cohort 1, M-dex + 5 mg of lenalidomide, and no DLT was observed. Four patients (1 patient, 204, died from progressive disease during the first month of therapy and was not assessable for DLT) were enrolled in cohort 2, M-dex + 10 mg of lenalidomide, and no DLT was observed. Three patients were enrolled in cohort 3, M-dex + 15 mg of lenalidomide, no DLT was observed. Nine additional patients were treated to further define safety at this dose level, which had been anticipated to be the highest. No DLT was observed on these 9 patients. The protocol was thus subsequently amended to open cohort 4, M-dex + 20 mg of lenalidomide. Among the first 3 patients treated in cohort 4, 1 experienced DLT: grade 4 anemia. Further 4 patients received M-dex + 20 mg of lenalidomide and 3 of them experienced DLT: 1 grade 4 neutropenia lasting 10 days, 1 grade 3 muscle pain, and 1 grade 3 cerebrovascular ischemia. Overall, on the 7 patients treated in cohort 4, 4 DLTs were reported, and 15 mg of lenalidomide was defined as MTD, in combination with M-dex.
DLT, responses, and outcome
| Patient no. . | Dose of Len, mg/d . | DLT . | Hematologic response . | OR* . | No. of cycles . | Relapse progression . | Death . |
|---|---|---|---|---|---|---|---|
| 101 | 5 | − | PR | + (H,L,K) | 9 | − | − |
| 102 | 5 | − | Stable | − | 7 | − | − |
| 103 | 5 | − | PR | − | 9 | + | − |
| 204 | 10 | − | No | − | 1 | + | + |
| 205 | 10 | − | PR | + (K) | 6 | − | + |
| 206 | 10 | − | CR | + (K) | 9 | − | − |
| 207 | 10 | − | Stable | − | 6 | + | − |
| 308 | 15 | − | CR | + (K) | 9 | − | − |
| 309 | 15 | − | PR | + (H) | 9 | − | − |
| 310 | 15 | − | No | − | 2 | + | + |
| 311 | 15 | − | Stable | − | 2 | + | − |
| 312 | 15 | − | PR | − | 4 | + | − |
| 314 | 15 | − | PR | + (H,K) | 8 | − | − |
| 315 | 15 | − | No | − | 2 | + | + |
| 316 | 15 | − | Stable | − | 3 | + | − |
| 317 | 15 | − | CR | + (K) | 9 | − | − |
| 318 | 15 | − | CR | + (K,L) | 9 | − | − |
| 319 | 15 | − | CR | + (H,K) | 9 | − | − |
| 320 | 15 | − | CR | + (L) | 9 | − | − |
| 421 | 20 | − | No | − | 2 | + | + |
| 422 | 20 | − | PR | + (H,K) | 9 | − | − |
| 423 | 20 | Grade 4 anemia | No | − | 2 | + | − |
| 424 | 20 | Grade 4 neutropenia, 10 days | PR | + (K) | 8 | − | − |
| 425 | 20 | Grade 3 cerebrovascular ischemia | Stable | − | 1 | − | − |
| 426 | 20 | Grade 3 muscle pain | No | − | 4 | + | − |
| 427 | 20 | − | PR | + (H,K) | 9 | − | − |
| Patient no. . | Dose of Len, mg/d . | DLT . | Hematologic response . | OR* . | No. of cycles . | Relapse progression . | Death . |
|---|---|---|---|---|---|---|---|
| 101 | 5 | − | PR | + (H,L,K) | 9 | − | − |
| 102 | 5 | − | Stable | − | 7 | − | − |
| 103 | 5 | − | PR | − | 9 | + | − |
| 204 | 10 | − | No | − | 1 | + | + |
| 205 | 10 | − | PR | + (K) | 6 | − | + |
| 206 | 10 | − | CR | + (K) | 9 | − | − |
| 207 | 10 | − | Stable | − | 6 | + | − |
| 308 | 15 | − | CR | + (K) | 9 | − | − |
| 309 | 15 | − | PR | + (H) | 9 | − | − |
| 310 | 15 | − | No | − | 2 | + | + |
| 311 | 15 | − | Stable | − | 2 | + | − |
| 312 | 15 | − | PR | − | 4 | + | − |
| 314 | 15 | − | PR | + (H,K) | 8 | − | − |
| 315 | 15 | − | No | − | 2 | + | + |
| 316 | 15 | − | Stable | − | 3 | + | − |
| 317 | 15 | − | CR | + (K) | 9 | − | − |
| 318 | 15 | − | CR | + (K,L) | 9 | − | − |
| 319 | 15 | − | CR | + (H,K) | 9 | − | − |
| 320 | 15 | − | CR | + (L) | 9 | − | − |
| 421 | 20 | − | No | − | 2 | + | + |
| 422 | 20 | − | PR | + (H,K) | 9 | − | − |
| 423 | 20 | Grade 4 anemia | No | − | 2 | + | − |
| 424 | 20 | Grade 4 neutropenia, 10 days | PR | + (K) | 8 | − | − |
| 425 | 20 | Grade 3 cerebrovascular ischemia | Stable | − | 1 | − | − |
| 426 | 20 | Grade 3 muscle pain | No | − | 4 | + | − |
| 427 | 20 | − | PR | + (H,K) | 9 | − | − |
Len indicates lenalidomide.
Type of organ response: PR, partial response; H, heart; L, liver; and K, kidney.
Hematologic response (Table 2)
Overall, disregarding the dose of lenalidomide given, on an intent-to-treat basis, a hematologic response was achieved in 15 of 26 cases (58%), complete in 6 patients and partial in 9 patients. Responses were observed at each dose level, but the higher complete response (CR) rate, 42% (5 of 12 cases), was achieved at the dose of 15 mg of lenalidomide per day, defined as MTD. Five patients had stable disease, and 6 did not respond to therapy.
Organ response (Table 2)
Organ response was achieved in 13 of 26 cases (50%), and responses were observed at each dose level. Organ response and hematologic response were strongly correlated: hematologic reponse was observed in all 13 patients experiencing organ response, as compared with 2 cases of hematologic response among the remaining 13 patients (P < .001, Fisher exact test).
Survival and progression
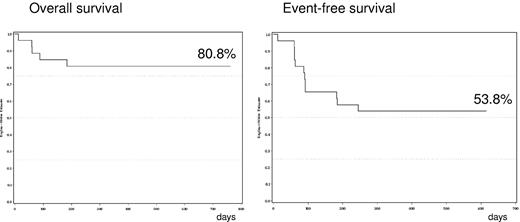
At the cutoff date of March 1, 2010, median follow-up time from diagnosis for surviving patients was 19 months. Twelve patients had progressed or died (Table 2). Five deaths were observed (median time from diagnosis 2 months, 1-7), 4 due to progressive disease (cardiac, liver and gastrointestinal involvement in 1 case, cardiac in 2 cases, and renal involvement in 1 case), and 1 due to cholangiocarcinoma occurring 7 months after starting treatment, with AL amyloidosis responding to therapy. For the 7 patients alive with disease progression: 2 never responded to M-dex-lenalidomide (1 was rescued with bortezomib + dexamethasone), 2 progressed after an initial response (1 was rescued with bortezomib-dexamethasone), and 3 progressed after an initial phase of stable hematologic disease. Estimated 2-year OS and EFS were 80.8% and 53.8%, respectively (Figure 1). Hematologic and organ responses were both associated with superior EFS rates (estimated 2-year EFS of 81.8% and 84.6% in patients with hematologic (CR plus partial response) and organ responses, versus 20 [P = .0001] and 7.7% [P < .0001], respectively (Figure 2). Similarly, a higher EFS was observed in patients whose FLCs decreased by more than 50% during therapy (70% versus 28.6% at 2 years, P = .019, Figure 2).12
Event-free survival according to response. Event-free survival according to: (A) Hematologic response (complete response plus partial response vs stable disease or no response, P = .0001); (B) Organ response (response vs no response, P < .0001); (C) FLCs decreased by more than 50% during therapy or not, P = .019.
Event-free survival according to response. Event-free survival according to: (A) Hematologic response (complete response plus partial response vs stable disease or no response, P = .0001); (B) Organ response (response vs no response, P < .0001); (C) FLCs decreased by more than 50% during therapy or not, P = .019.
Toxicity, treatment exposure
A median of 7 cycles were given (1 to 9) and 11 patients received the planned 9 cycles of combination therapy (Table 2). All 26 patients are assessable for safety. The most commonly reported adverse events (AEs) of any grade by organ class were hematologic events (31% of 302 AEs overall), gastrointestinal events (16% of all AEs consisting mainly of nausea), general disorders such as fatigue and asthenia (12% of all AEs) and infections (19 episodes, 6% of all AEs; Table 3). No case of neuropathy related to therapy was reported. Twenty-one grade 3 or 4 AEs were observed, leading to discontinuations or dose-reductions of therapy in 10 cases (Table 4).
Treatment-emergent AEs of any grade (all cycles)
| AEs, total N = 302, 26 patients . | Number . | Percentage . |
|---|---|---|
| Any | 100 | |
| Hematologic | 95 | 31 |
| Neutropenia | 29 | |
| Anemia | 30 | |
| Thrombocytopenia | 21 | |
| Lymphopenia | 14 | |
| Eosinophilia | 1 | |
| Gastrointestinal | 49 | 16 |
| Nausea | 39 | |
| Constipation | 5 | |
| Diarrhea | 5 | |
| Dry mouth | 3 | |
| Abdominal distension | 3 | |
| General disorders | 37 | 12 |
| Fatigue | 15 | |
| Anorexia | 4 | |
| Pyrexia | 4 | |
| Asthenia | 14 | |
| Dermatologic | 29 | 10 |
| Skin rash | 11 | |
| Pruritis | 3 | |
| Skin atrophy | 5 | |
| Dry skin | 1 | |
| Hyperpigmentation | 9 | |
| Cardiovascular | 22 | 7 |
| Left ventricular systolic dysfunction | 6 | |
| Peripheral edema | 6 | |
| Hypotension | 5 | |
| Atrial fibrillation | 1 | |
| Thrombosis | 2 | |
| Cardiac ischemia | 2 | |
| Infectious | 19 | 6 |
| Others | 51 | 17 |
| AEs, total N = 302, 26 patients . | Number . | Percentage . |
|---|---|---|
| Any | 100 | |
| Hematologic | 95 | 31 |
| Neutropenia | 29 | |
| Anemia | 30 | |
| Thrombocytopenia | 21 | |
| Lymphopenia | 14 | |
| Eosinophilia | 1 | |
| Gastrointestinal | 49 | 16 |
| Nausea | 39 | |
| Constipation | 5 | |
| Diarrhea | 5 | |
| Dry mouth | 3 | |
| Abdominal distension | 3 | |
| General disorders | 37 | 12 |
| Fatigue | 15 | |
| Anorexia | 4 | |
| Pyrexia | 4 | |
| Asthenia | 14 | |
| Dermatologic | 29 | 10 |
| Skin rash | 11 | |
| Pruritis | 3 | |
| Skin atrophy | 5 | |
| Dry skin | 1 | |
| Hyperpigmentation | 9 | |
| Cardiovascular | 22 | 7 |
| Left ventricular systolic dysfunction | 6 | |
| Peripheral edema | 6 | |
| Hypotension | 5 | |
| Atrial fibrillation | 1 | |
| Thrombosis | 2 | |
| Cardiac ischemia | 2 | |
| Infectious | 19 | 6 |
| Others | 51 | 17 |
Grade 3 or 4 AEs (all cycles)
| Any grade 3 or 4 AE . | Total N = 21, in 26 patients . |
|---|---|
| Cohort 5 mg | |
| Thombosis | 1 |
| Eosinophilia | 1 |
| Cohort 10 mg | |
| Congestive heart failure | 2 |
| Neutropenia | 2 |
| Gastrointestinal hemorrhage | 1 |
| Atrial fibrillation | 1 |
| Cholangiocarcinoma | 1 |
| Urinary retention | 1 |
| Cohort 15 mg | |
| Cardiac ischemia | 1 |
| Congestive heart failure | 1 |
| Delirium | 1 |
| Hypoxia | 1 |
| Liver dysfunction | 1 |
| Cohort 20 mg | |
| Anemia | 1 |
| Neutropenia | 1 |
| Cerebrovascular ischemia | 1 |
| Muscle pain | 1 |
| Congestive heart failure | 1 |
| Renal failure | 1 |
| Any grade 3 or 4 AE . | Total N = 21, in 26 patients . |
|---|---|
| Cohort 5 mg | |
| Thombosis | 1 |
| Eosinophilia | 1 |
| Cohort 10 mg | |
| Congestive heart failure | 2 |
| Neutropenia | 2 |
| Gastrointestinal hemorrhage | 1 |
| Atrial fibrillation | 1 |
| Cholangiocarcinoma | 1 |
| Urinary retention | 1 |
| Cohort 15 mg | |
| Cardiac ischemia | 1 |
| Congestive heart failure | 1 |
| Delirium | 1 |
| Hypoxia | 1 |
| Liver dysfunction | 1 |
| Cohort 20 mg | |
| Anemia | 1 |
| Neutropenia | 1 |
| Cerebrovascular ischemia | 1 |
| Muscle pain | 1 |
| Congestive heart failure | 1 |
| Renal failure | 1 |
Discussion
This study is the first reporting results of the combination of M-dex + lenalidomide in patients with newly diagnosed AL-amyloidosis. We were able to define the MTD of lenalidomide as follows: 15 mg once daily on days 1 to 21 of a 28-day cycle. This dose was recommended in relapse when patients received lenalidomide alone or in combination with dexamethasone, without addition of melphalan.8,9 In the 2 series already reported of lenalidomide in relapsed AL amyloidosis, most patients were previously treated with alkylating agents including high-dose melphalan in some cases, that could preclude the administration of higher doses of lenalidomide. In first-line treatment, 15 mg of lenalidomide + melphalan and dexamethasone could be given without significant toxicity.
The goal of the combination is to increase both the hematologic and organ response rates, which are associated with longer survival. Note that our dose-escalation schedule tends to underestimate response rates. In our series, on an intent-to-treat basis, hematologic response rates was 58% and organ response rates was 50%. It is sometimes difficult to compare the results of clinical studies in AL amyloidosis due to the wide variability of patient cohorts from trial to trial. For example, the median age of patients in our series is 57 years, which is approximately 10 years younger than the age of patients in the community. Nevertheless, although the number of patients was small, the 42% CR rate achieved at MTD compares favorably with the CR rate achieved with melphalan and dexamethasone without lenalidomide. In the first experience of M-dex reported by the Italian group, the CR rate was 33%.13 Recently updated results on a larger number of patients (159 cases) showed a CR rate achieved with M-dex alone of 25%.14 The 2-year OS estimated to 80% also compared favorably with the OS achieved with M-dex alone. In our recent prospective trial comparing ASCT with M-dex, the 2-year survival in the M-dex arm was 65% in 50 patients.4
Lenalidomide is not the only novel agent effective in AL amyloidosis. Thalidomide was the first novel agent tested in this disease with significant activity, mostly in combination with cyclophosphamide and dexamethasone.15 Bortezomib has been increasingly used with promising results, both in relapse and frontline treatment.16-18 A recent phase 2 trial of M-dex + bortezomib yielded high response rates, including a 45% CR rate.19 It is important to note that M-dex + lenalidomide can be delivered safely in patients with neuropathy, as opposed to combination using bortezomib or thalidomide. Another difference with bortezomib-based therapy is oral availability. Recently pomalidomide has also shown effectiveness in relapse setting but has only been tested in combination with dexamethasone.20
In fit and good-risk patients with adequate organ function, low NT-proBNP and troponin T, high-dose therapy with ASCT may be considered as front-line treatment.2,11,21-23 Nevertheless, this group of patients only represents 15% to 20% of the total AL-amyloidosis population.3 Thus, for most patients and given the poor prognosis of this disease, determining a new effective oral combination therapy, such as M-dex lenalidomide without neurotoxicity as defined in this trial, is an important step forward.
This work has been presented previously at the 51st American Society of Hematology annual meeting, New Orleans, December 7, 2009, oral presentation, abstract 427; and the XIIth International Symposium on Amyloidosis, Rome, Italy, April 21, 2010, oral presentation, abstract 86.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.M., A.J., and J.P.F. designed the research; P.M., A.J., L.B., B.R., X.L., F.B., G.S., V.L., M.R., O.H., J.L.H., and J.P.F. performed the research; P.M. collected data; P.M., A.J., and J.P.F. analyzed and interpreted data; L.P. performed statistical analysis; P.M., A.J., and J.P.F. wrote the paper; and all authors reviewed and approved the paper.
Conflict-of-interest disclosure: P.M., A.J., X.L., G.S., M.R., J.L.H., and J.P.F. have received honoraria from Celgene. M.A. is employee of Celgene. The remaining authors declare no competing financial interests.
Correspondence: Philippe Moreau, Hematology Department, Centre Hospitalier Universitaire Hôtel-Dieu, Place A.Ricordeau, 44093, Nantes, France; e-mail: philippe.moreau@chu-nantes.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal