Abstract
Progressive bone marrow failure is a major cause of morbidity and mortality in human Fanconi Anemia patients. In an effort to develop a Fanconi Anemia murine model to study bone marrow failure, we found that Fancd2−/− mice have readily measurable hematopoietic defects. Fancd2 deficiency was associated with a significant decline in the size of the c-Kit+Sca-1+Lineage− (KSL) pool and reduced stem cell repopulation and spleen colony-forming capacity. Fancd2−/− KSL cells showed an abnormal cell cycle status and loss of quiescence. In addition, the supportive function of the marrow microenvironment was compromised in Fancd2−/− mice. Treatment with Sirt1-mimetic and the antioxidant drug, resveratrol, maintained Fancd2−/− KSL cells in quiescence, improved the marrow microenvironment, partially corrected the abnormal cell cycle status, and significantly improved the spleen colony-forming capacity of Fancd2−/− bone marrow cells. We conclude that Fancd2−/− mice have readily quantifiable hematopoietic defects, and that this model is well suited for pharmacologic screening studies.
Introduction
Fanconi anemia (FA) is a rare, autosomal, recessive genetic disorder associated with severe birth defects, cancer predisposition, and bone marrow failure. Thirteen causative genes (FANCA, FANCB, FANCC, FANCD1/BRCA2, FANCD2, FANCE, FANCF, FANCG/XRCC9, FANCI, FANCL/PHF9/Pog, FANCJ/BRIP1/BACH1, FANCM/Hef, and FANCN/PALB2) have been identified and cloned to date, and the encoded proteins are believed to work together in a common DNA damage-response pathway to maintain genomic integrity and protect the genome from DNA damage induced by cross-linking agents.1,2 Although deficiency in DNA cross-link repair renders all FA cells susceptible to cross-linking agents, bone marrow is the most affected organ system. Mutations in any of the different FA genes almost universally lead to bone marrow failure, which is the primary cause of mortality in FA.3
The pathogenesis of bone marrow failure in FA remains elusive. Mutations in several genes involved in DNA damage repair, including Atr, XPD, and Ercc1, caused either hematopoietic stem cell (HSC) loss or impaired HSC function under conditions of stress.4-6 These studies suggest that the maintenance of genome integrity is critical for HSC survival and function. However, the extent to which genotoxicity, resulting from impaired DNA damage repair, contributes to bone marrow failure in FA is unclear.7 Other pathways associated with hematopoietic failure, such as altered cytokine signaling, may also contribute to FA pathogenesis.8,9 For example, levels of proapoptotic cytokines tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ) are elevated in FA lymphocytes, bone marrow cells, and FA patient serum samples.10-12 FA bone marrow cells (at least of the C complementation group) are also hypersensitive to these cytokines and undergo apoptosis when exposed to even low levels of them.13-15
To better understand FA, multiple murine knockout models, including Fanca−/−, Fancc−/−, Fancg−/−, Fancd2−/−, Fanca−/−-Fancc−/− double, and Fancl−/− mice, have been developed.16 Notably, in contrast to humans with mutations of these genes, anemia has not been reported in any of these mice, although the bone marrow cells from the well-characterized Fancc−/− mice have shown reduced repopulating ability in transplantation experiments.17-20
Similar to human FA patients, Fancd2−/− mice have a higher incidence of tumors, a phenotype that occurs rarely in Fancc−/− or other models with a deficient FA core-complex gene.16,21 However, the hematopoietic properties of Fancd2−/− mice have not been fully characterized previously. FANCD2 is thought to play a central role in the FA/BRCA pathway and is well conserved among different multicellular eukaryotic species, whereas the majority of FA core complex genes do not exist in many lower eukaryotes.22,23 Interestingly, there is some evidence that patients with FANCD2 mutations have earlier onset and more rapid progression of hematologic manifestations.24 It is also noteworthy that all the FANCD2 mutations identified in human patients are hypomorphic, whereas Fancd2−/− mice have null mutations.21,24 We therefore hypothesized that Fancd2−/− mice might have more severe hematopoietic defects. In this study, we characterized hematopoietic properties of Fancd2−/− mice in detail. Our results show that Fancd2−/− mice had multiple hematopoietic defects, including HSC and progenitor loss in early development, abnormal cell-cycle status and loss of quiescence in hematopoietic stem and progenitor cells, and compromised functional capacity of HSCs. These readily quantifiable hematopoietic defects make Fancd2−/− mice well suited for pharmacologic studies for bone marrow failure. Indeed, we conducted studies designed to evaluate the effect of resveratrol using this murine model.
Resveratrol is a polyphenol found in grapes and red wine. Although the full spectrum of bioactivity of resveratrol is not known, it has antioxidant properties and is a known activator of SirT1 deacetylase. SirT1, the mammalian ortholog of Sir2, is a member of the Sirtuins family of nicotine adenine dinucleotide–dependent protein deacetylases involved in the regulation of many nuclear processes, including genome stability maintenance, cellular senescence, apoptosis, aging, and longevity.25,26 It has been reported that resveratrol extends yeast lifespan and suppresses tumorigenesis in mice.27,28 More recently, SirT1 has been shown to be directly involved in DNA double-strand break repair after oxidative damage and to protect against genome instability.29,30 Therefore, resveratrol treatment could activate SirT1 and potentially benefit FA patients in their battle against oxidative genotoxicity and genome instability. Since resveratrol has the potential to benefit FA patients, has been previously used in rodents, and is nontoxic,31 we administered resveratrol to Fancd2−/− mice, and found that this agent partially corrected hematopoietic defects in these mice.
Methods
Mice
All Fancd2 or Fancc mutant mice and ROSA26 transgenic mice were maintained on the 129S4 background. Heterozygotes were inbred to generate mutant mice and littermate controls. The resveratrol diet was made by mixing powdered resveratrol (3,5,4′-trihydroxystilbene; Orchid Chemicals and Pharmaceuticals, Ltd) with common rodent diet (Bio-Serv) and given to the mice at 250 mg/kg body weight/d. All animals were treated in accordance with the guidelines of the Institutional Animals Care and Use Committee. Unless specified otherwise, the mice used were between 3 and 8 months of age.
Flow cytometry
Bone marrow cells were isolated from femura of either Fancd2 mutant mice or wild-type controls and treated with 1× red blood cell Lysis Buffer (eBioscience) to lyse red blood cells. To count total nucleated cells, a small aliquot of cell suspension was diluted to 1/100 in 3% acetic acid and counted using a hemocytometer. All the antibodies were obtained from eBioscience unless otherwise indicated.
For c-Kit+Sca-1+Lineage− (KSL) cells, cells were stained with a KSL staining cocktail of phycoerythrin (PE)-conjugated anti-mouse lineage markers (CD3e, CD4, CD5, CD8a, B220, Ter119, NK1.1, Mac1, and Gr1), allophycocyanin (APC)–conjugated anti-mouse CD117 (c-Kit), and PE-Cy7–conjugated anti-Ly-6A/E (Sca-1) in phosphate-buffered saline (PBS), supplemented with 1% bovine serum albumin (BSA). Samples were examined on a Cytopeia Influx cell sorter, and cytometric data were analyzed using FlowJo software Version 6.4.7 (TreeStar).
Myeloid progenitors and common lymphoid progenitors were analyzed on an LSR II flow cytometer (BD Biosciences) as previously described.32 For the analysis of myeloid progenitors, bone marrow cells were labeled with a PE-conjugated lineage mixture (CD3e, CD4, CD8a, B220, Ter119, Gr1, CD19, and immunoglobulin M) and anti-interleukin-7 receptor-alpha (IL-7R-α)–PE, anti-Sca-1–PE, anti-c-Kit–APC, anti-CD34–fluorescein isothiocyanate (FITC), and anti-Fc-γRII/III–PE-Cy7 antibodies. Common myeloid progenitors (CMPs) were defined as IL-7R-α−Lin−Sca-1−c-Kit+CD34+Fc-γRII/IIIlow, and granulocyte-macrophage progenitors (GMPs) were defined as IL-7R-α−Lin−Sca-1−c-Kit+CD34+Fc-γRII/IIIhigh. For common lymphoid progenitors (CLPs), cells were stained with a PE-conjugated lineage mixture (CD3e, CD4, CD8a, B220, Ter119, Gr1, and Mac1) and anti-IL-7R-α–FITC, anti-Sca-1–PE-Cy7, and anti-c-Kit–APC antibodies. CLPs were defined as IL-7R-α+Lin−Sca-1lowc-Kitlow.
Cell cycle analysis was done as previously described by Wilson et al.33 Briefly, mouse bone marrow cells were stained with a KSL staining cocktail, fixed in PBS with 2% paraformaldehyde, and permeabilized in PBS/3% bovine calf serum (BCS) with 0.5% saponin. Cells were then stained with anti-Ki-67–FITC (BD Biosciences) in PBS/3% BCS, supplemented with 10 μg/mL Hoeschst 33342 (Sigma-Aldrich). Mouse IgG1-FITC (BD Biosciences) staining was performed in parallel to serve as an isotype control. For cell cycle analysis on CD34−KSL cells, anti-CD34–PE was added to the KSL staining cocktail.
For the measurement of reactive oxygen species (ROS) in KSL cells, 7000 double-sorted KSL cells were stained with 10μM 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA; Invitrogen) for 30 minutes, followed by flow cytometric analysis.
Cobblestone area–forming cell (CAFC) assay
Murine stromal layers were established on 96-well plates by culturing freshly isolated bone marrow cells at 2 × 106 cells/mL in MyeloCult medium (StemCell Technologies), supplemented with 10−6M freshly prepared hydrocortisone. A stromal layer was established after 2 weeks of culture at 33°C, with weekly half-medium changes. Hematopoietic progenitor cells within the stromal layer were then inactivated by irradiation (1500 rad) from a 137Cs γ–irradiation source. Seven days later, whole marrow cells were cultured on irradiated stromal layers at 3 different densities (3 × 104, 1.5 × 104, and 7.5 × 103 cells per well), with 16 wells for each density. For the assessment of cobblestone colonies, all wells were examined under a microscope for the presence of 6 or more closely associated cells underneath the stromal layer. Each well was scored as positive (≥ 1 colonies) or negative (no colonies). Colony frequency was calculated based on Poisson distribution using L-Calc software (StemCell Technologies). In the case of resveratrol treatment, resveratrol was added into the freshly prepared medium at a final concentration of 10 or 25μM.
In vivo competitive repopulation assay
Competitive repopulation assays were done as previously described.20 All the mice used were 8-10 weeks old. Briefly, donor bone marrow cells were isolated from FA mice (Fancc−/− or Fancd2−/−) and ROSA26Tg/O mice. In the case of Fancd2−/− donors, Fancd2−/− bone marrow cells were mixed with ROSA26Tg/O bone marrow cells at a 1:1 ratio and then transplanted into lethally irradiated Fancc−/− recipient mice. In the case of Fancc−/− donors, Fancc−/− bone marrow cells were mixed with ROSA26Tg/O bone marrow cells at a 1:1 ratio and then transplanted into lethally irradiated Fancd2−/− recipient mice. All recipient mice were lethally irradiated at a split dose of 1200 rad (600 rad each, 4 hours apart) 1 day before transplantation. Nucleated whole bone marrow cells were counted, and 2 million mixed FA donor cells and ROSA26Tg/O donor cells were transplanted into each recipient FA mouse. DNA was isolated from peripheral blood 7-9 months posttransplantation, and quantitative real-time polymerase chain reaction (qPCR) analysis was performed to analyze the contribution of FA donor cells or ROSA26Tg/O donor cells to nucleated mature blood cells in recipient mice. The following primers were used for the qPCR amplification:
ROSA26 transgenic allele:
MG1717, 5′ CATCAGCCGCTACAGTCAACAG3′;
MG1718, 5′ CAGCCATGTGCCTTCTTCCGC3′.
Fancc mutant allele:
MG1711, 5′ GAGCTGCCTGATACGGATGCTG 3′;
MG1791, 5′ GGGCTGCTAAAGCGCATGCTC 3′.
Fancd2 mutant allele:
MG2279, 5′ TGGAAGAGATGAAGGTTACGATTG 3′;
MG2280, 5′ GGTTCTATACTGTTGACCCAATGC 3′.
Colony-forming unit-spleen (CFU-S) assay
Recipient mice (wild-type, 8-12 weeks old) were irradiated with a split dose of 1100 rad (550 rad each, 4 hours apart) 1 day before transplantation. Forty thousand bone marrow cells from each donor mouse were transplanted into each recipient mouse. Twelve days posttransplantation, spleens were harvested and fixed with Bouin fixative solution.
ELISA
TNF-α levels were measured with enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) as previously described.34 Fancc shRNA-treated or nontargeted control shRNA-treated THP1 cells were stably integrated with the shRNA-expressing cassette. R848 (imidazoquinoline resiquimod; AXXORA) was used to induce TNF-α production. Cells were incubated with R848 for 24 hours before resveratrol was added. Resveratrol treatment lasted 12 hours, and then TNF-α levels were measured.
Statistical analysis
Unless specified otherwise, P values were calculated by the 2-tailed, unpaired Student t test using Prism 4.0 software (GraphPad Software Inc). A P value less than .05 was considered significant.
Results
Fancd2 deficiency caused loss of hematopoietic stem and progenitor cells
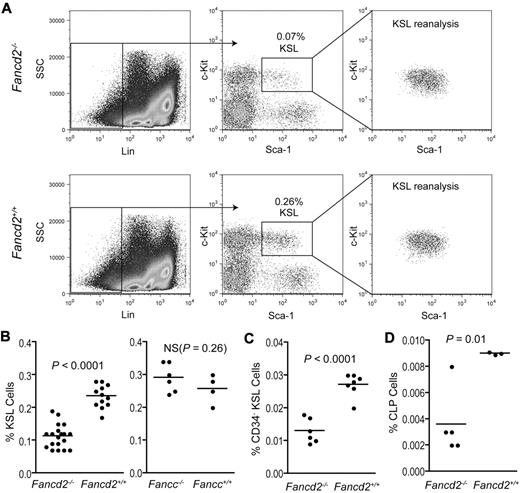
To determine whether Fancd2−/− mice have hematopoietic defects, we first performed a complete blood count (CBC) on blood samples from 5-6-month-old Fancd2−/− or Fancd2+/+ mice. Despite a significant drop in platelet numbers in the blood of Fancd2−/− mice (P < .05), all the other hematologic parameters measured for both genotypes were within the normal range (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), indicating the absence of significant anemia in the peripheral blood. We next characterized hematopoietic properties of Fancd2−/− bone marrow cells. Both Fancd2−/− and wild-type mice had equivalent numbers of total nucleated bone marrow cells (data not shown). Compared with wild-type controls, bone marrow cells of 3- to 8-month-old Fancd2−/− mice had a 50% reduction in the frequency of c-Kit+Sca-1+Lineage− (KSL) cells (P < .0001), a population enriched for short- and long-term HSCs (Figure 1A-B). In contrast, Fancc−/− mice had a KSL compartment comparable with their wild-type littermates (P > .05; Figure 1B). CD34 staining of KSL cells also showed a significant reduction in long-term HSC-enriched CD34−KSL cells in Fancd2−/− bone marrow (P < .0001; Figure 1C). A thorough comparison between young and aged mice found that even 3-week-old Fancd2−/− mice had a smaller KSL pool than wild-type controls (supplemental Figure 1), suggesting that this defect might be generated in early development. KSL frequencies in Fancd2−/− mice appeared to stabilize after 1 month of age and did not decline further even in aged mice as old as 12 months.
Fancd2−/− mice have a smaller KSL stem and progenitor pool. (A) FACS profiles after KSL staining of the bone marrow cells from Fancd2 mutant mice and wild-type littermate controls. The percentage of the KSL gate is referring to the proportion of KSL cells in the whole nucleated bone marrow. To confirm the purity of double-sorted KSL cells, 3000 cells were analyzed for each genotype. (B) Quantification of hematopoietic KSL stem and progenitor frequencies in the bone marrow of FA mice (Fancd2−/− or Fancc−/−) and wild-type controls. All the mice used were between 3 and 8 months of age. In the Fancd2−/− group, n = 19 for Fancd2−/− mice and n = 12 for Fancd2+/+ littermate controls; in the Fancc−/− group, n = 6 for Fancc−/− mice and n = 4 for Fancc+/+ littermate controls. NS denotes not significant. (C) Quantification of CD34−KSL hematopoietic stem-cell frequencies in the bone marrow of Fancd2−/− mice or wild-type controls. N = 6 for Fancd2−/− mice and n = 7 for Fancd2+/+ mice. (D) Quantification of CLP frequencies in the bone marrow of Fancd2−/− mice or wild-type controls. N = 5 for Fancd2−/− mice and n = 3 for Fancd2+/+ mice.
Fancd2−/− mice have a smaller KSL stem and progenitor pool. (A) FACS profiles after KSL staining of the bone marrow cells from Fancd2 mutant mice and wild-type littermate controls. The percentage of the KSL gate is referring to the proportion of KSL cells in the whole nucleated bone marrow. To confirm the purity of double-sorted KSL cells, 3000 cells were analyzed for each genotype. (B) Quantification of hematopoietic KSL stem and progenitor frequencies in the bone marrow of FA mice (Fancd2−/− or Fancc−/−) and wild-type controls. All the mice used were between 3 and 8 months of age. In the Fancd2−/− group, n = 19 for Fancd2−/− mice and n = 12 for Fancd2+/+ littermate controls; in the Fancc−/− group, n = 6 for Fancc−/− mice and n = 4 for Fancc+/+ littermate controls. NS denotes not significant. (C) Quantification of CD34−KSL hematopoietic stem-cell frequencies in the bone marrow of Fancd2−/− mice or wild-type controls. N = 6 for Fancd2−/− mice and n = 7 for Fancd2+/+ mice. (D) Quantification of CLP frequencies in the bone marrow of Fancd2−/− mice or wild-type controls. N = 5 for Fancd2−/− mice and n = 3 for Fancd2+/+ mice.
To evaluate the effects of Fancd2 deficiency on defined hematopoietic progenitor populations, we first examined the common myeloid progenitors and granulocyte-macrophage progenitors in Fancd2−/− mice (supplemental Figure 2). The average frequency of each population in Fancd2−/− mice was slightly, but nonsignificantly, lower than their counterpart in wild-type mice. However, we did notice that 20% of Fancd2−/− mice displayed a marked reduction in the frequency of CMPs or GMPs. Further analysis found that CLPs are more than 50% fewer in Fancd2−/− mice than those in the wild-type controls (P = .01; Figure 1D). Thus, loss of Fancd2 resulted in a smaller HSC pool and reduced lymphoid progenitor frequencies. Taking into account that progenitor cell numbers can be reduced in FA patients before the onset of overt bone marrow failure,9,35 the fact that Fancd2−/− mice show hematopoietic defects, even in the absence of clinical anemia, is congruent with the human condition.
Fancd2−/− bone marrow suffered functional loss of hematopoietic stem and progenitor cells and had a compromised marrow environment
To assess the functional properties of Fancd2−/− HSCs, we used a long-term in vivo competitive repopulation assay and transplanted bone marrow cells from either Fancd2−/− or Fancc−/− mice into lethally irradiated FA mice (Fancd2−/− donor cells into Fancc−/− recipients or vice versa), along with an equal number of cells from ROSA26Tg/O mice, which are hemizygous for the ROSA26 transgenic strain and have an intact FA pathway. Like wild-type mice, ROSA26Tg/O mice do not have any overt phenotypes and the transgene is used simply as a genetic marker. Each different strain (Fancd2−/−, Fancc−/−, or ROSA26Tg/O) has a unique genotype that can be distinguished from one another by qPCR. Analysis of peripheral blood 7-9 months posttransplantation revealed a dramatic reduction in donor-derived FA cells. Starting from an initial input ratio of 1:1 (FA: ROSA26Tg/O), the ratio of donor-derived FA cells vs. donor-derived ROSA26Tg/O cells in the peripheral blood dropped to 1:20 in the case of Fancd2−/− donor and 1:7 in the case of Fancc−/− donor cells (Figure 2A). Together, these data show compromised repopulating capacity by both Fancd2−/− and Fancc−/− bone marrow cells.
Hematopoietic defects in Fancd2−/− mice. (A) In vivo competitive repopulation of mixed FA (Fancc−/− or Fancd2−/−) and ROSA26Tg/O bone marrow cells. Quantitative real-time PCR (qPCR) analyses were performed to evaluate donor contribution to the peripheral blood cells from each donor (7 or 9 months posttransplantation for Fancd2−/− or Fancc−/− donors, respectively). Three independent qPCR analyses were performed for each sample, and results from 5 animals were pooled together for each experimental group. P values were calculated by the 2-tailed, paired Student t test. Error bars represent SEM. (B) Representative picture for CAFC assay. The arrow indicates cobblestone colony. The image was acquired on an Axiovert 200 inverted microscope with an AxioCam MRc color camera at room temperature using AxioVision Release 4.8 software (Carl Zeiss MicroImaging). Original magnification ×100 (with 10× objective lens). (C) Quantification of CAFC results. P values were calculated by 2-tailed, paired Student t test.
Hematopoietic defects in Fancd2−/− mice. (A) In vivo competitive repopulation of mixed FA (Fancc−/− or Fancd2−/−) and ROSA26Tg/O bone marrow cells. Quantitative real-time PCR (qPCR) analyses were performed to evaluate donor contribution to the peripheral blood cells from each donor (7 or 9 months posttransplantation for Fancd2−/− or Fancc−/− donors, respectively). Three independent qPCR analyses were performed for each sample, and results from 5 animals were pooled together for each experimental group. P values were calculated by the 2-tailed, paired Student t test. Error bars represent SEM. (B) Representative picture for CAFC assay. The arrow indicates cobblestone colony. The image was acquired on an Axiovert 200 inverted microscope with an AxioCam MRc color camera at room temperature using AxioVision Release 4.8 software (Carl Zeiss MicroImaging). Original magnification ×100 (with 10× objective lens). (C) Quantification of CAFC results. P values were calculated by 2-tailed, paired Student t test.
Next, we measured the frequency of Fancd2−/− primitive progenitors in vitro using the CAFC assay. In this assay, freshly isolated bone marrow cells were used to establish an adherent stromal/feeder layer, which comprised endothelial cells, fibroblasts, and adipocytes. The stromal layer provides a microenvironment that supports proliferation and differentiation of HSCs. When plated on wild-type bone marrow–derived feeder/stromal layers, Fancd2−/− bone marrow cells formed fewer than half the number of colonies generated by Fancd2+/+ controls, with an average CAFC frequency of only 1/34 000 cells, compared with 1/16 000 cells in the wild type (P < .005; Figure 2B-C). The reduction of CAFC frequency in Fancd2−/− bone marrow cells reflects the change in hematopoietic stem and progenitor numbers in these animals, consistent with the observed reduction of KSL cells. Along the same line, a CFU-S assay also confirmed that Fancd2−/− bone marrow cells formed 65% fewer macroscopic splenic colonies than wild-type controls (supplemental Figure 3), suggesting a reduced number and function of progenitors in Fancd2−/− bone marrow.
To our surprise, we also found that Fancd2−/− bone marrow–derived feeder layer cells were less supportive of progenitor growth than wild-type controls in the CAFC assay. Both wild-type and mutant marrow cells formed significantly fewer cobblestone colonies when plated onto mutant feeders. In a direct comparison, the CAFC frequency of wild-type marrow was only 1/27 000 on Fancd2−/− feeders, compared with 1/16 000 on Fancd2+/+ feeder cells (P < .05; Figure 2C). Similarly, Fancd2−/− bone marrow had the lowest colony-forming frequency (1/74 000) when plated on Fancd2−/− feeders. Collectively, these results suggest that Fancd2 deficiency affects not only the stem cells directly, but also causes a defective marrow microenvironment.
Fancd2−/− KSL cells showed an abnormal cell-cycle status
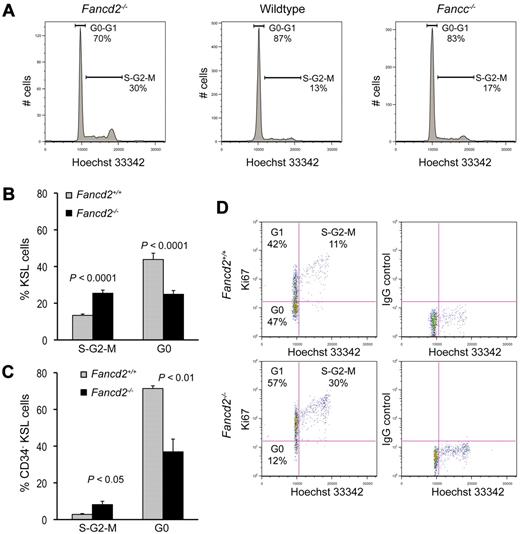
We next examined the cell-cycle status of Fancd2−/− KSL cells using DNA-binding dye Hoechst 33342. The proportion of Fancd2−/− KSL cells in the S-G2-M phases of the cell cycle was markedly higher than that in wild-type KSL cells (Figure 3A-B). Nearly twice as many cells had G2 DNA content, indicating a spontaneous delay in S/G2/M. Importantly, this late S-phase/G2-M delay is a hallmark of FA cells in culture and has been used diagnostically.36 Similar trends were also seen in Fancc−/− KSL cells, although the difference between Fancc−/− KSL cells and wild-type controls was less than in the Fancd2−/− strain (Figure 3A).
Fancd2−/− KSL cells have abnormal cell cycle status. (A) Representative cell-cycle profiles of KSL cells from a Fancd2−/− mouse and its wild-type littermate control. DNA content was measured with Hoechst 33342. Data were analyzed with FlowJo (TreeStar) using the Dean-Jett-Fox model for the quantification of each cell-cycle phase. (B) Pooled results from cell cycle analysis on KSL cells. N = 9 for each group. (C) Quantification of the cell cycle analysis on CD34−KSL cells. N = 3 for each group. (D) Representative picture after costaining for DNA content (Hoechst 33342) and Ki67 expression in KSL cells from a Fancd2−/− mouse and its wild-type littermate control. Percentages for each gate were denoted.
Fancd2−/− KSL cells have abnormal cell cycle status. (A) Representative cell-cycle profiles of KSL cells from a Fancd2−/− mouse and its wild-type littermate control. DNA content was measured with Hoechst 33342. Data were analyzed with FlowJo (TreeStar) using the Dean-Jett-Fox model for the quantification of each cell-cycle phase. (B) Pooled results from cell cycle analysis on KSL cells. N = 9 for each group. (C) Quantification of the cell cycle analysis on CD34−KSL cells. N = 3 for each group. (D) Representative picture after costaining for DNA content (Hoechst 33342) and Ki67 expression in KSL cells from a Fancd2−/− mouse and its wild-type littermate control. Percentages for each gate were denoted.
To confirm that Fancd2−/− stem cells were less quiescent, we combined the above cell-cycle analysis approach with antibody staining for Ki-67, which is not expressed by quiescent cells. Because cells in both G0 and G1 phases of the cell cycle have 2c DNA content by Hoechst 33342 staining, the use of Ki-67 antibody allowed us to distinguish G0 (negative Ki-67 staining) from G1 cells (positive Ki-67 staining). We found that the average G0 proportion of Fancd2−/− KSL cells was 25%, significantly lower than the 45% observed in the wild-type counterparts (P < .0001; Figure 3B). A similar result was seen in CD34−KSL cells. Fancd2−/− CD34−KSL cells showed a 2-fold higher frequency of S/G2/M cells (P < .05) and a significantly lower frequency of G0 cells than Fancd2+/+ CD34−KSL cells (P < .01; Figure 3C). In some extreme cases, such as that shown in Figure 3D, as few as 12% of Fancd2−/− KSL cells were in G0, compared with 47% G0 frequency in KSL cells from a littermate wild-type control. These results demonstrate that Fancd2 deficiency led to increased cell-cycle entry and the loss of quiescence in HSCs.
Resveratrol treatment partially corrected the hematopoietic defects in Fancd2−/− mice
The abnormal quantitative phenotypes described in this Fancd2−/− murine model provide an opportunity to directly ascertain the therapeutic effects of drugs on HSCs.
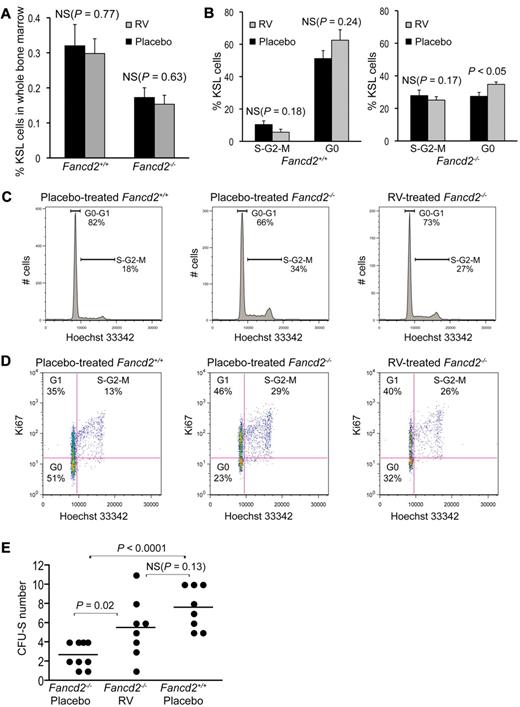
In light of the bioactivities of resveratrol,27,28,31 we hypothesized that this compound could enhance hematopoiesis in Fancd2−/− mice. Fancd2−/− mice and their wild-type littermates were treated with either control diet or diet supplemented with resveratrol. Treatment began at 1 month of age and continued for up to 9 months. Experimental mice showed no signs of weight loss or drug toxicity. Flow cytometric analysis showed no difference in KSL frequencies between resveratrol-treated and placebo-treated mice (Figure 4A). However, cell cycle analysis revealed a 27% increase in the G0 proportion of KSL cells in resveratrol-treated Fancd2−/− mice (Figure 4B-D). Importantly, CFU-S assays with whole bone marrow demonstrated that the frequency of primitive spleen colony-forming cells in resveratrol-treated Fancd2−/− mice was significantly improved, compared with placebo-treated controls (P = .02; Figure 4E). The numbers of CFU-S doubled after resveratrol treatment, and many were in the range obtained from normal bone marrow. These data suggest that resveratrol can partially correct the hematopoietic defects of Fancd2−/− mice.
Resveratrol partially corrects hematopoietic defects in Fancd2−/− mice. (A) KSL frequency was not significantly changed in either Fancd2−/− or Fancd2+/+ mice after resveratrol (RV) treatment. (B) Statistical quantification of the cell-cycle analysis on KSL cells in resveratrol- versus placebo-treated mice. N = 3 for each group. (C) Representative cell cycle profiles of a placebo-treated Fancd2−/− mouse, its resveratrol-treated Fancd2−/− littermate control, and placebo-treated wild-type control. Cells are stained for KSL and DNA content (Hoechst 33342). Data were analyzed with FlowJo using Dean-Jett-Fox model for the quantification of each cell cycle phase. (D) Representative picture after a costaining for DNA content (Hoechst 33342) and Ki67 expression in KSL subsets of the bone marrow cells from placebo- or resveratrol-treated Fancd2−/− mice and a placebo-treated wild-type littermate control. Percentages for each gate were denoted. (E) Resveratrol treatment significantly improved the CFU-S–forming capacity of Fancd2−/− bone marrow cells. Data represent 3 donors for each group with 3 (or 2 in 1 case) recipients for each donor.
Resveratrol partially corrects hematopoietic defects in Fancd2−/− mice. (A) KSL frequency was not significantly changed in either Fancd2−/− or Fancd2+/+ mice after resveratrol (RV) treatment. (B) Statistical quantification of the cell-cycle analysis on KSL cells in resveratrol- versus placebo-treated mice. N = 3 for each group. (C) Representative cell cycle profiles of a placebo-treated Fancd2−/− mouse, its resveratrol-treated Fancd2−/− littermate control, and placebo-treated wild-type control. Cells are stained for KSL and DNA content (Hoechst 33342). Data were analyzed with FlowJo using Dean-Jett-Fox model for the quantification of each cell cycle phase. (D) Representative picture after a costaining for DNA content (Hoechst 33342) and Ki67 expression in KSL subsets of the bone marrow cells from placebo- or resveratrol-treated Fancd2−/− mice and a placebo-treated wild-type littermate control. Percentages for each gate were denoted. (E) Resveratrol treatment significantly improved the CFU-S–forming capacity of Fancd2−/− bone marrow cells. Data represent 3 donors for each group with 3 (or 2 in 1 case) recipients for each donor.
We next tested whether resveratrol could affect the function of the bone marrow stromal cells in CAFC assays. Fancd2−/− bone marrow–derived feeder/stromal layers were established in the absence or presence of resveratrol. When wild-type bone marrow cells were plated on top of the different feeders, CAFC frequencies on resveratrol-treated Fancd2−/− feeder layers were 42% higher than those on placebo-treated Fancd2−/− feeder layers (P < .05, 2-tailed paired Student t test; n = 3), indicating that resveratrol treatment alleviates the defects of Fancd2−/− stromal cells in supporting hematopoiesis. There was no difference in CAFC frequencies between resveratrol-treated wild-type bone marrow–derived stromal layers and placebo-treated wild-type controls (data not shown), suggesting that the beneficial effect of resveratrol on Fancd2−/− feeder function is not due to a general improvement of colony formation on all the feeder layers.
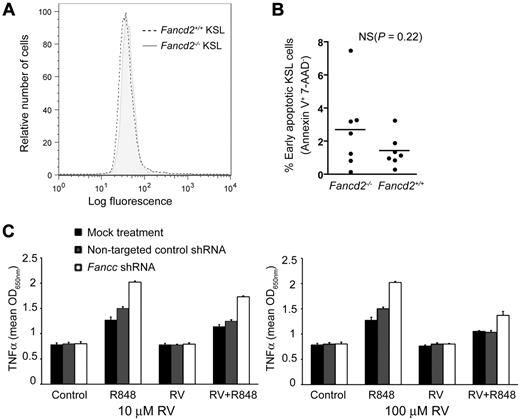
Given that FA cells are hypersensitive to reactive oxygen species (ROS),19,37 we therefore tested the hypothesis that ROS levels are increased in KSL cells from Fancd2−/− mice. Double-sorted KSL cells were stained with carboxy-H2DCFDA to measure the intracellular concentrations of ROS. Under our experimental conditions, we failed to detect any difference in ROS levels between Fancd2−/− KSL cells and wild-type controls (Figure 5A).
ROS levels, apoptosis, and cytokine response in Fancd2−/− and wild-type bone marrow cells. (A) Fluorescence intensity in KSL subsets of Fancd2−/− and wild-type bone marrow after carboxy-H2DCFDA staining for intracellular ROS. Oxidation of carboxy-H2DCFDA by ROS generated fluorescence detectable by flow cytometry. (B) Quantification of early apoptotic KSL cells after 7-AAD, annexin V, and KSL staining. Data represent 7 mice for each group. NS denotes not significant. (C) Resveratrol suppressed R848-induced TNF-α overproduction in Fancc-shRNA–treated THP1 human leukemia cells. The experiments were done in triplicates on a 96-well plate.
ROS levels, apoptosis, and cytokine response in Fancd2−/− and wild-type bone marrow cells. (A) Fluorescence intensity in KSL subsets of Fancd2−/− and wild-type bone marrow after carboxy-H2DCFDA staining for intracellular ROS. Oxidation of carboxy-H2DCFDA by ROS generated fluorescence detectable by flow cytometry. (B) Quantification of early apoptotic KSL cells after 7-AAD, annexin V, and KSL staining. Data represent 7 mice for each group. NS denotes not significant. (C) Resveratrol suppressed R848-induced TNF-α overproduction in Fancc-shRNA–treated THP1 human leukemia cells. The experiments were done in triplicates on a 96-well plate.
Because resveratrol may affect production of and/or hypersensitivity to proapoptotic cytokine TNF-α in the hematopoietic microenvironment,14,38,39 we measured the levels of apoptosis in Fancd2−/− and wild-type bone marrow cells. Annexin V and 7-aminoactinomycin D (7-AAD) staining revealed no significant gross increase in early apoptotic cells, defined as annexin V positive and 7-AAD negative, in Fancd2−/− whole bone marrow cells, compared with wild-type controls. Further analysis found that Fancd2−/− KSL cells have a higher, but nonsignificant, proportion of cells undergoing apoptosis than wild-type controls (Figure 5B). It is possible that the increase of apoptosis level is insufficient to be detectable under these experimental conditions, or that the lack of statistical significance was a function of sample size.
To directly test whether resveratrol could influence cytokine response in FA cells, we used a recently developed system in which the overproduction of TNF-α by human FANCC-deficient and murine Fancc-deficient mononuclear phagocytes could be quantitatively measured by ELISA.34 This system was suitable for small-molecule screening and permitted us to test the capacity of resveratrol to influence that phenotype in FANCC-deficient cells. In the absence of resveratrol, imidazoquinoline resiquimod R848 induced the overexpression of TNF-α in FANCC-deficient THP1 cells, as quantified by TNF-α ELISA. Treatment of these cells with 10μM or 100μM resveratrol suppressed TNF-α production in these cells (Figure 5C). These results demonstrate that resveratrol can influence a quantitative hematopoietic defect in cells harboring a disrupted FA pathway. Although this effect appeared not to be specific for FA cells, as shown by Figure 5C, the benefit from resveratrol treatment could be more significant for FA cells, since these cells are more sensitive to TNF-α.
Discussion
In this work, we characterized hematopoietic phenotypes in Fancd2−/− mice and found multiple defects, including stem-cell and progenitor loss in early development, compromised functional capacity of HSCs, and a less supportive marrow environment. This work highlights the critical role of Fancd2 in stem-cell survival and function.
It should be noted that others have reported Fancc-mutant mice to display progenitor defects when on a C57/B6 background.18 Nonetheless, in a direct comparison of Fancd2−/− and Fancc−/− mice on the same 129S4 mouse strain, we show here that Fancd2−/− mice have markedly lower numbers of KSL stem and progenitor cells, a phenotype that might be formed in early development and not shared by Fancc−/− mice. Consistent with our findings, developmental abnormalities have also been observed in Fancd2-knockdown zebrafish.40 These data support the notion that Fancd2 might have an especially critical function in some early developmental processes, including hematopoiesis.2 In humans, FANCD2 could be essential for embryonic survival, considering that all the FANCD2 mutations identified in human patients are hypomorphic.24 The fact that Fancc−/− mice have a normal-sized KSL stem and progenitor pool could imply that nonubiquitinated Fancd2 has indispensable roles in these developmental processes, as some have recently speculated.2
An important finding of this study is that Fancd2−/− mice have a compromised marrow environment that is less supportive for stem cell population. Similarly, it has recently been reported that Fancg−/− mesenchymal stem/progenitor cells have defects in their ability to support the proliferation and differentiation of hematopoietic stem/progenitor cells.41 Considering the crucial function of marrow environment in balancing stem cell self-renewal and differentiation, we propose this phenotype is very likely to contribute to the pathogenesis of FA.
Despite the marked reduction in HSC and progenitor numbers and the reduced capacity for stem-cell function, Fancd2−/− mice did not show spontaneous anemia in their peripheral blood and maintained relatively normal blood counts. How do Fancd2−/− mice maintain the homeostasis in their blood system? We have observed in this work that HSCs and progenitors in Fancd2−/− mice failed to maintain their quiescence and, hence, enter the cell cycle more frequently. We speculate that this could compensate for consequences from the loss in HSC number and function. However, in doing so, the animals could face a long-term risk of stem-cell exhaustion that could eventually lead to bone marrow failure, given the compelling evidence that enhanced cell cycle entry causes stem cell exhaustion and leads to long-term loss of stem cell function.42-44
The loss of quiescence and reduction in the KSL pool along with a dysfunctional marrow stroma converge to create several quantitative phenotypes ideally suited for pharmacological studies. Not surprisingly, the developmental defect of reduced KSL numbers could not be corrected by postnatal treatment with resveratrol. Encouragingly, however, this Sirt1-mimetic compound showed measurable benefit in functional hematopoietic assays both in vivo (CFU-S, cell cycle profile) and in vitro (CAFC). By improving the function of HSCs and progenitors, resveratrol could relieve the pressure of enhanced cell cycle entry faced by those cells and has the potential to slow down the path toward stem cell exhaustion.
There are several possible mechanisms by which resveratrol could improve hematopoietic function in Fancd2−/− mice. Because SirT1 is expressed at high levels in HSCs,45 resveratrol could stimulate SirT1 activity and directly enhance SirT1-mediated DNA repair.29,30 However, the function of SirT1 in HSC remains elusive. Further work needs to be done to determine, among the myriad of SirT1 target proteins, which protein is specifically influenced by resveratrol treatment to enhance hematopoiesis. Alternatively, resveratrol might alter HSC activity through SirT1-independent mechanisms.46 We did find that resveratrol treatment could suppress TNF-α production in FA-deficient cells. It remains to be determined whether resveratrol could have a similar effect on TNF-α production in FA cells in vivo. It is also important to emphasize that our data do not address whether the effects of resveratrol are specific to only FA or whether hematopoietic stem cells from wild-type mice also respond to this compound. Given the fact that resveratrol has shown beneficial effects in many non-FA phenotypes, it appears unlikely that it acts in an FA-specific way.47,48 Nonetheless, it partially normalized important hematopoietic parameters in our Fancd2−/− mouse model and hence should be further pursued therapeutically.
While the hematopoietic correction from resveratrol was incomplete, more powerful Sirt1-stimulating compounds have been recently developed and may prove more potent in achieving hematopoietic enhancement.49 In the meantime, resveratrol itself may be suitable for clinical trials in FA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Pamela Canady, Mandy Boyd, and Dorian LaTocha at the Oregon Health & Science University flow cytometry core for fluorescence activated cell sorting.
This work was supported by grants 1P01HL48546, HL077818, HL069133, and 1R01CA138237 and by the Veterans Affairs Merit Review.
National Institutes of Health
Authorship
Contributions: Q.-S.Z. designed the study, performed research, analyzed and interpreted data, and wrote the manuscript; L.M.-L., L.E., and K.W.-S. performed research; A.W.D. and D.C.G. designed the study and wrote the manuscript; P.A. and R.K.R. performed the research; W.H.F. interpreted data and wrote the manuscript; G.C.B. designed TNF-α assays, interpreted data, and wrote the manuscript; and M.G. designed the study, analyzed and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qing-Shuo Zhang, Oregon Stem Cell Center, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd, Portland, OR 97239; e-mail: zhangqi@ohsu.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal