Abstract
Understanding molecular mechanisms in the dominant inheritance of von Willebrand disease would improve our knowledge of pathophysiologic processes underlying its prevalence. Cellular models of severe type 2 von Willebrand disease, caused by a heterozygous deletion in the von Willebrand factor (VWF) gene, were produced to investigate the altered biosynthesis. Coexpression of the wild-type and in-frame deleted (p.P1127_C1948delinsR) VWF forms impaired protein secretion, high molecular weight multimer formation and function (VWF collagen-binding 1.9% ± 0.5% of wild-type), which mimicked the patient's phenotype. mRNA, protein, and cellular studies delineated the highly efficient dominant-negative mechanism, based on the key role of heterodimers as multimer terminators. The altered VWF, synthesized in large amounts with the correctly encoded “cysteine knot” domain, formed heterodimers and heterotetramers with wild-type VWF, in addition to deleted homodimers. Impaired multimerization was associated with reduced amounts of VWF in late endosomes. Correction of the dominant-negative effect was explored by siRNAs targeting the mRNA breakpoint, which selectively inhibited the in-frame deleted VWF expression. Although the small amount of the deleted protein synthesized after inhibition still exerted dominant, even though weakened, negative effects, the siRNA treatment restored secretion of large multimers with improved function (VWF collagen-binding 28.0% ± 3.3% of wild-type).
Introduction
Dominant inheritance characterizes von Willebrand disease (VWD), the most prevalent1 disorder of hemostasis, and particularly type 1 VWD, a quantitative deficiency, and type 2 VWD, which includes a large and still increasing group of von Willebrand factor (VWF) qualitative deficiencies.2 Differently, the rare and severe type 3 VWD is recessively inherited. Based on functional and structural defects impairing platelet adhesion or factor VIII binding, type 2 has been classified into 4 subtypes: A, B, M, and N.2 Type 2A refers to qualitative defects characterized by a decreased proportion of large multimers3 and by numerous mutations interfering with the multiple biosynthetic steps of the protein maturation, subgrouped in IIA, IIC, IID, and IIE.2,4,5
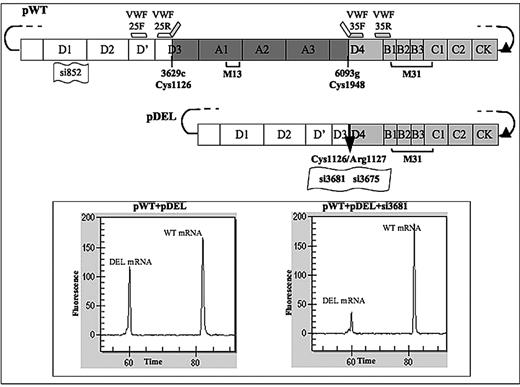
We have previously described a large, de novo, and heterozygous deletion in VWF gene associated with severe type 2 VWD,6 characterized by reduced VWF antigen levels, impaired function, and virtual absence of high molecular weight multimers (HMWMs). The altered VWF mRNA7 (Figure 1) was supposed to encode an in-frame deleted protein, the characterization of which would contribute to define its dominant-negative features.
VWF dimerization and multimerization provide a rationale for the dominant inheritance, caused by the interaction of wild-type and mutant monomers during polymerization. The strength of the dominant-negative effects produced by VWF variants8-12 would be associated with the ample variation of residual VWF level and function, and would participate in the phenotypic severity and penetrance of VWD type 1 and type 2.13 As a contribution to answer these open questions in VWF biology and VWD molecular base definition, cellular models14 expressing the in-frame deleted variant were constructed, and the biosynthetic steps of VWF maturation and secretion investigated.
Methods
Expression vectors
The full-length VWF cDNA expression vector pSVHVWF1 was a generous gift from Prof J.E. Sadler (Washington University, St Louis, MO).14 Site-directed mutagenesis (QuickChange XL Stratagene; primers SacF and SacR; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) was used to abolish the Sac I restriction site at position 8816 (124 bases downstream of the stop codon, Figure 1), thus producing the pSVHVWF vector (defined as pWT). pWT expression resulted in secreted VWF antigen (VWF:Ag) and collagen-binding activity (VWF:CB) levels indistinguishable from that of the pSVHVWF1 (data not shown).
An overlapping polymerase chain reaction (PCR) strategy,15,16 exploiting 2 pairs of primers (25F-25R and 35F-35R, Figure 1; supplemental Table 1), was used to create the P1127_C1948delinsR17,18 deletion in pWT vector. The purified amplicons were then annealed to generate, through PCR amplification with external primers 25F and 35R, the deleted fragment. This was subsequently cloned into the pWT vector in the SacI restriction sites at positions 3335 and 6926 upstream and downstream of the breakpoint, respectively. The correct sequence of vectors was confirmed by sequencing.
Expression of recombinant VWF in eukaryotic cells
COS-1 cells14 at 60% to 70% confluence in 6-well plates were transiently transfected by Lipofectamine2000 (Invitrogen) with single plasmids or a combination of them (the relative molar proportion is reported in the figures), in the presence of serum-free medium. The total amount of DNA in each transfection was set to 4.5 μg by adding, when needed, a gutted pUC18 plasmid. Seventy-two hours after transfection, conditioned media were collected and cells lysed19 for intracellular VWF investigation and for total RNA isolation (NucleoSpin RNAII isolation Kit, Macherey-Nagel).
Silencing
To specifically silence the wild-type VWF expression, the siRNA 852 and the siNC, as negative control, were designed by the Invitrogen software BLOCK-iT RNAi Designer (https://rnaidesigner.invitrogen.com/rnaiexpress; Figure 1; supplemental Table 2). None of the current computer programs for RNA interference (RNAi) design was able to predict siRNAs targeting the breakpoint region, which led us to evaluate the mRNA conformation and accessibility at this site by the Mfold, Version 3.220 software (http://mfold.bioinfo.rpi.edu/). We designed the si3681 and si3675 in the respect of the siRNA design rules. siRNAs were purchased form Invitrogen and transfected (10-80nM) with VWF expression vectors as mentioned.
Wild-type and deleted mRNA before and after siRNA treatment was evaluated by capillary electrophoresis (Experion automated electrophoresis station,21 Bio-Rad Laboratories) of reverse-transcriptase PCR products (F1, R1, and R2 primers, supplemental Table 1).
Antibodies
Polyclonal rabbit anti–human VWF (A0082), polyclonal rabbit anti–human VWF/horseradish peroxidase (P0226), polyclonal swine anti–rabbit immunoglobulin/tetramethylrhodamine isothiocyanate (R0156), and polyclonal goat anti–mouse immunoglobulin G/horseradish peroxidase antibodies were purchased from Dako Denmark. The mouse monoclonal (2G11) antibody to mannose 6–phosphate receptor (late endosome marker; ab2733) was purchased from Abcam. The fluorescein isothiocyanate–conjugated AffiniPure goat anti–mouse immunoglobulin G (H + L; 115-095-146) was from Jackson ImmunoResearch Laboratories. Monoclonal anti-VWF M31 and M13 were obtained as described.22,23
Analysis of recombinant VWF
VWF:Ag in conditioned media and cell lysates was determined by enzyme-linked immunosorbent assay, essentially as described by Federici et al.24 VWF:CB was measured in conditioned media using the CollagenBinding Assay kit (Life Therapeutics).
The multimeric pattern of VWF was detected by sodium dodecyl sulfate (0.1%) agarose gel (1.5%, 1.8%, 3%) electrophoresis25,26 followed by Western blotting and revealed by immunoenzymatic stain (polyclonal goat antimouse immunoglobulin G/horseradish peroxidase antibodies, Dako Denmark).
Western blots of VWF in media and lysates were performed in reducing condition with monoclonal M31 or M13 anti-VWF antibodies.
Western blot films were scanned by the GS 700 Imaging Densitometer and analyzed by the Quantity One software, Version 4.4.1 (Bio-Rad).
Confocal immunofluorescence microscopy
For immunofluorescence labeling, cells were cultured on coverslips at subconfluence in 6- or 12-well plates and transfected. At 24 hours after transfection, cells were fixed in methanol and saturated with 4% bovine serum albumin/phosphate-buffered saline. Primary antibodies (1:100 in 4% bovine serum albumin/phosphate-buffered saline) and secondary antibodies (1:50) were incubated 1 hour at room temperature. Confocal images were acquired as previously described27 with a Zeiss LSM 510 microscope (Carl Zeiss).
Results
The experimental approach was designed to produce cellular models of the severe type 2 VWD, characterized by absence in plasma of HMWMs and by antigen levels reduced to one-fourth (supplemental Figure 3).6 To investigate the molecular mechanism through which the heterozygous deletion p.1127_1948delinsR produced the dominant-negative effect, we constructed the pDEL vector (Figure 1) containing the VWF cDNA breakpoint previously characterized in the patient mRNA.7 The in-frame junction between codon C1126 and mutated codon 1948 (C1127R) was inserted in the vector.
Expression plasmids for VWF variants and breakpoint-specific siRNAs. Schematic representation of the expression vectors for the wild-type (pWT) and in-frame deleted (pDEL) VWF variants. The amino, carboxyl-terminus, and deleted (r.3629-6093del, p.1127_C1948delinsR) VWF domains are reported as white, light gray, and dark gray rectangles, respectively. Accordingly, PCR primers (VWF-25F, VWF-25R. VWF-35F, and VWF-35R) used to create the deletion are indicated by short white or light gray bars. The black arrow in the pDEL vector indicates the breakpoint. Position and numbering of sequences targeted by siRNA molecules are indicated by small flags. Square brackets indicate the localization of VWF epitopes recognized by antibody pools (M13, M31) used for Western blotting. The filled triangle represents the position (134 bases downstream of the stop codon) of the suppressed SacI restriction site. (Inset) Capillary electrophoresis of wild-type and deleted mRNA reverse-transcribed PCR products from cells transfected with equimolar amounts of the indicated vectors, and on treatment with 40nM si3681 (right).
Expression plasmids for VWF variants and breakpoint-specific siRNAs. Schematic representation of the expression vectors for the wild-type (pWT) and in-frame deleted (pDEL) VWF variants. The amino, carboxyl-terminus, and deleted (r.3629-6093del, p.1127_C1948delinsR) VWF domains are reported as white, light gray, and dark gray rectangles, respectively. Accordingly, PCR primers (VWF-25F, VWF-25R. VWF-35F, and VWF-35R) used to create the deletion are indicated by short white or light gray bars. The black arrow in the pDEL vector indicates the breakpoint. Position and numbering of sequences targeted by siRNA molecules are indicated by small flags. Square brackets indicate the localization of VWF epitopes recognized by antibody pools (M13, M31) used for Western blotting. The filled triangle represents the position (134 bases downstream of the stop codon) of the suppressed SacI restriction site. (Inset) Capillary electrophoresis of wild-type and deleted mRNA reverse-transcribed PCR products from cells transfected with equimolar amounts of the indicated vectors, and on treatment with 40nM si3681 (right).
Expression of the in-frame deleted VWF
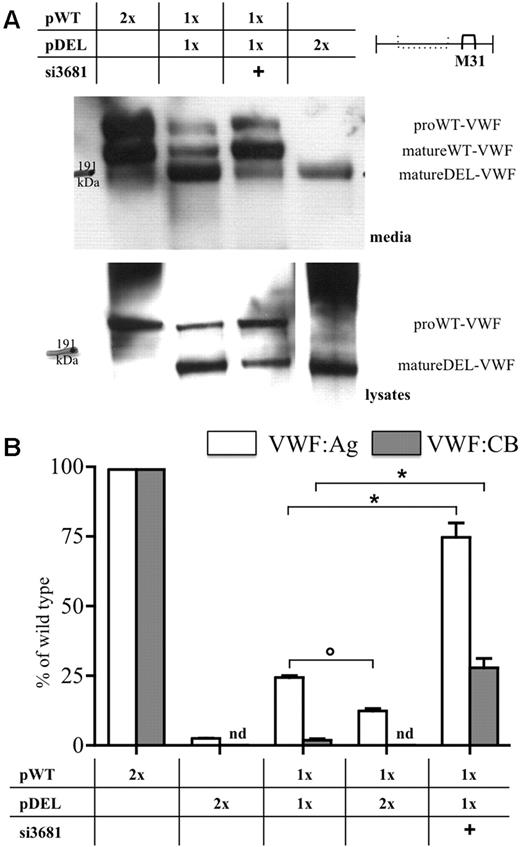
The expression of the pDEL was studied both in media and cell lysates (Figure 2A; supplemental Figure 4). Western blots were obtained with the M31 (Figure 2A; supplemental Figure 4B) or M13 (supplemental Figure 4A) antibodies,22,23 recognizing VWF domains downstream of the breakpoint junction and within the deleted region, respectively. The biosynthesis of the in-frame deleted molecule was supported by the band detected by the M31 but not by the M13 antibody, with electrophoretic migration compatible with protein size (∼ 160 kDa) inferred from the extent of the deleted domains (821 amino acids). Although only the mature form of the deleted VWF was virtually detected (supplemental Figure 4B), pro-wild-type VWF was abundant both in media and lysates, in accordance with published data.14,28,29
Wild-type and mutant VWF expression in cellular models. (A) Western blot analysis of wild-type and mutant VWF in conditioned media (top panel) and in cell lysates (bottom panels) with the monoclonal antibody pool M31,22,23 recognizing domains downstream of the breakpoint junction. Migration of the mature wild-type (matureWT-VWF) and deleted (matureDEL-VWF) proteins, and of forms containing the propeptide (proWT-VWF), is indicated. For comparison, see the Western blots in supplemental Figure 4. (B) VWF:Ag (white bars) and VWF:CB (gray bars) levels in conditioned media. The relative molar amount of pWT, pDEL, and vectors and the addition of siRNAs (40nM) are indicated in the table. 100% VWF:Ag corresponds to 445.1 ± 54.5 ng/mL and 100% of VWF:CB to 108.0% ± 14.7% of normal standard. Results from at least 3 independent experiments are reported as mean ± SEM. Statistical significance was evaluated by one-way analysis of variance with Bonferroni posttest: *P < .001; ○P < .01. nd indicates not detectable.
Wild-type and mutant VWF expression in cellular models. (A) Western blot analysis of wild-type and mutant VWF in conditioned media (top panel) and in cell lysates (bottom panels) with the monoclonal antibody pool M31,22,23 recognizing domains downstream of the breakpoint junction. Migration of the mature wild-type (matureWT-VWF) and deleted (matureDEL-VWF) proteins, and of forms containing the propeptide (proWT-VWF), is indicated. For comparison, see the Western blots in supplemental Figure 4. (B) VWF:Ag (white bars) and VWF:CB (gray bars) levels in conditioned media. The relative molar amount of pWT, pDEL, and vectors and the addition of siRNAs (40nM) are indicated in the table. 100% VWF:Ag corresponds to 445.1 ± 54.5 ng/mL and 100% of VWF:CB to 108.0% ± 14.7% of normal standard. Results from at least 3 independent experiments are reported as mean ± SEM. Statistical significance was evaluated by one-way analysis of variance with Bonferroni posttest: *P < .001; ○P < .01. nd indicates not detectable.
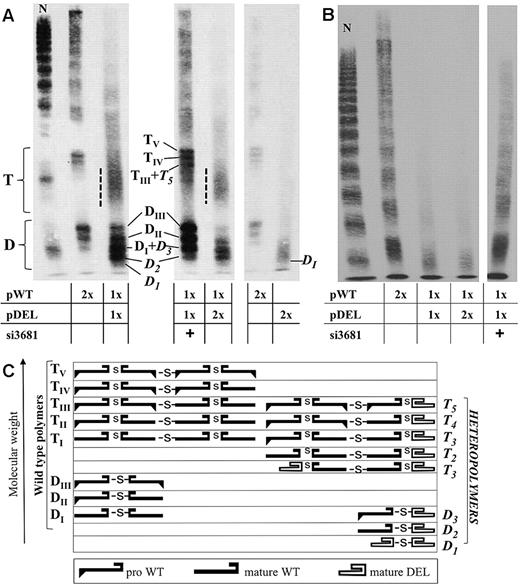
Antigen and collagen-binding activity levels in media from cells expressing the pDEL vector (Figure 2B) were very low and undetectable, respectively. The multimer analysis in high resolution gels (Figure 3A right) showed the presence of a broad band with increased mobility compared with wild-type and did not reveal the presence of tetramers.
Multimer analysis of VWF in conditioned media. (A) High-resolution multimer analysis (3% agarose gels) and (C) schematic representation of multimer composition. Dimers (D) and tetramers (T) are indicated together with sub-band composition in accordance with the schematic representation.30,31 DI-III and TI-V (subscript Roman numerals) indicate polymers containing combinations of wild-type VWF (pro- or mature VWF). D1-3 and T1-5 (Italics, subscript Arabic numerals) indicate polymers containing deleted VWF (mature). A dotted line flanks the fast-migrating and smeared bands containing heterotetramers. The similar extent of the deleted and propeptide region causes comigration of bands with different subunit composition. Comigrating proteins are indicated in the same line (scheme), and comigrating bands (DI + D3, TIII + T5) are indicated by the sum of specific dimers or tetramers. A total of 1 ng (left and center panels) and 0.25 ng (right panel) of protein were loaded in each lane, based on VWF:Ag concentration in media. Accordingly, the gel in the right panel was overexposed. (B) Multimer analysis in 1.8% agarose gels. Equal volumes of media (15 μL) were loaded in each lane to highlight both quantitative and qualitative differences among samples. The relative molar amounts of each vector and siRNA are reported in the tables. N (normal plasma from an healthy person with normal values of VWF) was diluted 1/200.
Multimer analysis of VWF in conditioned media. (A) High-resolution multimer analysis (3% agarose gels) and (C) schematic representation of multimer composition. Dimers (D) and tetramers (T) are indicated together with sub-band composition in accordance with the schematic representation.30,31 DI-III and TI-V (subscript Roman numerals) indicate polymers containing combinations of wild-type VWF (pro- or mature VWF). D1-3 and T1-5 (Italics, subscript Arabic numerals) indicate polymers containing deleted VWF (mature). A dotted line flanks the fast-migrating and smeared bands containing heterotetramers. The similar extent of the deleted and propeptide region causes comigration of bands with different subunit composition. Comigrating proteins are indicated in the same line (scheme), and comigrating bands (DI + D3, TIII + T5) are indicated by the sum of specific dimers or tetramers. A total of 1 ng (left and center panels) and 0.25 ng (right panel) of protein were loaded in each lane, based on VWF:Ag concentration in media. Accordingly, the gel in the right panel was overexposed. (B) Multimer analysis in 1.8% agarose gels. Equal volumes of media (15 μL) were loaded in each lane to highlight both quantitative and qualitative differences among samples. The relative molar amounts of each vector and siRNA are reported in the tables. N (normal plasma from an healthy person with normal values of VWF) was diluted 1/200.
Dominant-negative effect of the VWF deletion
To mimic the heterozygous condition in COS-1 cells, the pWT vector was coexpressed with increasing amounts of the pDEL, which progressively decreased wild-type VWF expression (Figure 2B). Equimolar amounts of both plasmids produced 24% (Figure 2B white bars) of wild-type antigen levels and 2% of collagen-binding activity (gray bars). A double proportion of the deleted vector decreased the VWF:Ag levels to 12% of wild-type and abolished collagen-binding activity.
These values were remarkably similar to those reported for patient plasma (supplemental Figure 3),6 both for protein amount and function (VWF:Ag and ristocetin cofactor activity, 24% and 3% of normal plasma, respectively).
The proportion between the normal and deleted VWF, which might modulate the dominant-negative effects, was evaluated by comparing the mature and propeptide forms of wild-type with the mature deleted VWF (Figure 2A). On densitometric analysis of Western blot, the ratios (deleted/wild-type) were 1.8 and 3.5, in cell media and lysates, respectively, which indicates higher expression of the altered VWF. Moreover, the amount of the deleted protein was higher in the cotransfection model than in cells transfected with the pDEL plasmid alone (Figure 2A).
In media from cotransfected cells (Figure 3), the pDEL expression impaired in a dose-dependent manner the secretion of oligomers, and particularly of HMWMs, virtually absent in patient plasma (supplemental Figure 3).6 Patient's multimer pattern also displayed an abnormal morphology of triplets and a fast-migrating component, potentially containing the deleted VWF. In high-resolution gels from conditioned media (Figure 3A), wild-type and deleted dimers (heterodimers) were clearly detectable. Subunit composition of dimers (D) and tetramers (T), reported in the scheme, would produce comigrating bands because of the similar extent of the deleted (821 amino acids) and propeptide regions (741 amino acids). Despite this complication, the heterodimer band D2, faster than DI (wild-type) and slower than D1 (deleted homodimer), is clearly distinguishable. In addition, the fast-migrating and smeared bands (dotted line), caused by the high number of different tetramers, supported formation of heterotetramers.
Immunofluorescence staining was used to investigate the biosynthesis and intracellular localization of normal and deleted proteins. Transfected cells were stained with anti-VWF antibodies and with endoplasmic reticulum and late endosome32 markers. In the first compartment, we did not detect differences in the fluorescence patterns between the wild-type and in-frame deleted VWF-expressing cells (not shown). Differently, the analysis of late endosomes (Figure 4) revealed that the degree of overlapping between the VWF (red)- and mannose 6-phosphate receptor (green)-specific fluorescence was (1) high in cells expressing the pWT vector (panels A-B), (2) barely detectable in cells expressing the pDEL (Figure 4C-D), and (3) intermediate in cells expressing both vectors (Figure 4E-F). Although inefficiently transported to the late endosome compartment, the deleted protein was efficiently synthesized as indicated by the strong red fluorescence in pDEL-expressing cells (Figure 4C-D), and by Western blot analysis (Figure 2A).
Intracellular distribution of recombinant VWF in late endosomal compartment. Cells transfected with vectors and siRNA indicated above the images (A-H) were coimmunostained for VWF (tetramethylrhodamine isothiocyanate, red) and mannose 6-phosphate receptor (fluorescein isothiocyanate, green) as late endosome marker. Images were taken with Carl Zeiss LSM 510 equipped with a Fluar 40×/1.3 oil immersion objective at room temperature on fixed cells mounting in glycerol/1,4 diazabicyclo[2.2.2]octane/4,6-diamidino-2-phenylindole medium. Briefly, 488- and 543-nm excitation wavelengths were provided, respectively, by Argon/2 and HeNE laser sources at a 5% intensity. Superimposition of red and green fluorescence, not specifically caused by colocalization, was excluded by applying to the green channel a beam path admitting the acquisition of fluorescence composed among 505 and 550 nm and to the red channel a light path excluding all fluorescence less than 560 nm. Pinhole values ranged from 40 to 80 μm. Images in the top panels were digitally zoomed in twice the original size with LSM examiner software Version 3.0 (Carl Zeiss).
Intracellular distribution of recombinant VWF in late endosomal compartment. Cells transfected with vectors and siRNA indicated above the images (A-H) were coimmunostained for VWF (tetramethylrhodamine isothiocyanate, red) and mannose 6-phosphate receptor (fluorescein isothiocyanate, green) as late endosome marker. Images were taken with Carl Zeiss LSM 510 equipped with a Fluar 40×/1.3 oil immersion objective at room temperature on fixed cells mounting in glycerol/1,4 diazabicyclo[2.2.2]octane/4,6-diamidino-2-phenylindole medium. Briefly, 488- and 543-nm excitation wavelengths were provided, respectively, by Argon/2 and HeNE laser sources at a 5% intensity. Superimposition of red and green fluorescence, not specifically caused by colocalization, was excluded by applying to the green channel a beam path admitting the acquisition of fluorescence composed among 505 and 550 nm and to the red channel a light path excluding all fluorescence less than 560 nm. Pinhole values ranged from 40 to 80 μm. Images in the top panels were digitally zoomed in twice the original size with LSM examiner software Version 3.0 (Carl Zeiss).
The analysis did not suggest accumulation of the deleted protein inside cotransfected cells, an observation confirmed by Western blots (Figure 2A) and VWF antigen levels in cell lysates (supplemental Figure 5).
Taken together, these experiments demonstrated the ability of the in-frame deleted VWF to interact with wild-type protein in the biosynthetic process, thus causing quantitative and qualitative alteration of secreted VWF and impairment of HMWM formation.
Interfering with dimerization of the in-frame deleted protein by RNA silencing
We investigated whether siRNAs (Figure 1), specifically directed to the VWF mRNA breakpoint in the pDEL, were able to rescue wild-type VWF biosynthesis and secretion. The positive effect of the si3681 treatment was indicated by the lower amounts of the deleted mRNA (Figure 1 inset) and by Western blotting results (Figure 2A), demonstrating that the silencing treatment substantially increased the proportion between wild-type and deleted molecules. This occurred both in media (Figure 2A top panel) and in the intracellular compartments (Figure 2A bottom panel). The densitometric analysis indicates that the ratios between the altered and normal VWF decreased from 3.5 to 0.5 in lysates and from 1.8 to 0.3 in media.
The si3681 treatment also produced a 3-fold increase in antigen levels (Figure 2B; supplemental Figure 6A white bars). The collagen-binding activity, undetectable before treatment, improved to 28.0% plus or minus 3.3% of wild-type (Figures 2B; supplemental Figure 6A gray bars); accordingly, the HMWMs (Figure 3) were better represented. Moreover, the bands containing wild-type homodimers (DII-III) and homotetramers (TIV-V) were increased in intensity (Figure 3A). Finally, the fluorescence pattern of treated cells appeared similar to that of wild-type VWF-expressing cells (Figure 4G-H vs Figure 4A-B).
Taken together, these data support both quantitative and qualitative improvement induced by the siRNA treatment.
Discussion
The design and expression of recombinant vectors to create cellular models of the in-frame deletion enabled us to evaluate the main biosynthetic steps of the deleted VWF and its interaction with wild-type protein, thus contributing to interpret the disease at the macromolecular level.
The “heterozygous” cellular model provided us with intermediate phenotypes, which paralleled patient plasma findings6 (supplemental Figure 3) for qualitative and quantitative alteration of VWF and supported the occurrence of a particularly efficient dominant-negative mechanism causing the severe VWD.
In cells transfected with the deleted construct, the altered mRNA was efficiently translated, and large amounts of the VWF variant were detected in cell lysates. Furthermore, inside cotransfected cells, the deleted protein was much more represented than the wild-type form, which would potentiate the dominant-negative effect of the altered molecules. By comparison, dominant-negative missense mutations8-12 would probably permit biosynthesis of equimolar amounts of mutant and wild-type proteins, having indistinguishable size.
For the first time, the presence and proportion of heteropolymers, which should play a key role in the dominant-negative behavior, are not only hypothesized but also visualized through high-resolution multimer analysis of VWF in conditioned media. A properly folded “cysteine knot” domain, localized at the carboxyl-terminus of the protein33,34 and correctly encoded in the in-frame deleted VWF, enables it to participate in the dimerization process in the absence of all A domains,35 in accordance with features and evolution of A domain-containing proteins.36 Whereas deleted homodimers cannot sustain multimerization, because of the absence of the Cys 1142 involved in interdimer disulfide bond formation,37-40 heterodimers permit an asymmetric multimerization. By acting as terminators16,41 at one side of the growing polymer, heterodimers produce detectable heterotetramers. At variance with known dimerization mutants,41 the remarkable amounts of terminated LMWMs do not contain unpaired bands because of the efficient dimerization of altered molecules.
Cell immunostaining provided information about the pDEL-induced modification of intracellular distribution of VWF. Late endosomes displayed an intermediate amount of VWF in the cotransfected cells compared with the wild-type and deleted models. Neither in transfection nor in cotransfection experiments, we obtained evidence for sites of intracellular accumulation, which would contribute to the decreased amount of secreted molecules. In comparison, the extensively studied dominant-negative C1149R mutation resulted in intracellular retention of mutant molecules,8,9,11 suggesting that intracellular removal would be more efficient for the deleted than for the missense VWF variant.
The dominant-negative effect was further investigated and counteracted by RNAi. The allele-specific RNAi strategy formally validated the presence of the dominant-negative mechanism through reduced availability of the deleted mRNA. Consequently, the low amounts of the deleted protein and decreased rate of heterodimer formation resulted in improved collagen-binding activity and secretion of HMWMs in media of treated cells. However, the small amount of the deleted protein synthesized after siRNA treatment still exerts a dominant, even though weakened, negative effect on multimerization. Accordingly, the quantitative improvement (VWF antigen levels) was larger than the qualitative rescue. Although allele-specific RNA silencing has been previously used for a few disease models,42-44 we think that our experiments provide the first attempt aimed at counteracting the negative effects of a dominant VWF variant at the cellular level.
Overall data provide evidence for a series of coherent features, which pertain to the molecular mechanism producing the dominant-negative effects:
The amount of the deleted protein in cell lysate was higher than that of wild-type VWF.
The deleted protein, efficiently matured, forms abundant heterodimers with wild-type VWF.
The heterodimers act as terminators16,41 of the multimerization process in the Golgi compartment.
VWF, present in low amounts in late endosomes, is secreted as LMWMs.
The allele-specific RNAi strategy efficiently rescues VWF secretion and function but incompletely restores VWF multimerization.
These original observations support the notion that the dominant-negative character originates from molecular mechanisms with an ample gradient of quantitative and qualitative efficiency, and help to interpret the plasma phenotype and clinical severity45 in dominant VWD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work is in memory of the late Giorgio Ballerini and Severino Guerra, who triggered this study.
The authors thank Prof J. E. Sadler (Departments of Medicine, Biochemistry, and Molecular Biophysics, Washington University School of Medicine, St Louis, MO) for providing the pSVHVWF1 expression vector, Patrizia Sabatelli (IGM-CNR Unit of Bologna, Bologna, Italy) for advice on immunofluorescence staining technique, Donato Gemmati (Department of Biomedical Sciences and Advanced Therapies, Section of Hematology, Center for the Study of Hemostasis and Thrombosis, University of Ferrara, Ferrara, Italy) for assistance with the VWF antigen assay, and Alessandro Canella (Department of Biochemistry and Molecular Biology, University of Ferrara) for technical assistance.
This work was supported by University of Ferrara (C.C., M.P., F.B.), Telethon-Italy (GGP09183; C.C., M.P.), Fondazione CARIFE (M.P.), and Ministero dell'Università e della Ricerca-Progetti di Ricerca di Interesse Nazionale (C.C., M.P., S.L., R.D.C).
Authorship
Contribution: C.C. performed experiments, analyzed results, made the figures, and wrote the paper; M.P. designed the experimental strategies and wrote the paper; S.L. performed multimer analysis experiments; E.A. performed confocal microscopy and analyzed images; R.D.C. and A.C. analyzed data and revised the paper; and F.B. designed research, analyzed results, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Bernardi, Department of Biochemistry and Molecular Biology, University of Ferrara, via Fossato di Mortara 74, 44121 Ferrara, Italy; e-mail: ber@unife.it.

![Figure 4. Intracellular distribution of recombinant VWF in late endosomal compartment. Cells transfected with vectors and siRNA indicated above the images (A-H) were coimmunostained for VWF (tetramethylrhodamine isothiocyanate, red) and mannose 6-phosphate receptor (fluorescein isothiocyanate, green) as late endosome marker. Images were taken with Carl Zeiss LSM 510 equipped with a Fluar 40×/1.3 oil immersion objective at room temperature on fixed cells mounting in glycerol/1,4 diazabicyclo[2.2.2]octane/4,6-diamidino-2-phenylindole medium. Briefly, 488- and 543-nm excitation wavelengths were provided, respectively, by Argon/2 and HeNE laser sources at a 5% intensity. Superimposition of red and green fluorescence, not specifically caused by colocalization, was excluded by applying to the green channel a beam path admitting the acquisition of fluorescence composed among 505 and 550 nm and to the red channel a light path excluding all fluorescence less than 560 nm. Pinhole values ranged from 40 to 80 μm. Images in the top panels were digitally zoomed in twice the original size with LSM examiner software Version 3.0 (Carl Zeiss).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/24/10.1182_blood-2010-02-268920/4/m_zh89991061610004.jpeg?Expires=1769083007&Signature=R9ZRMUgHxw1Oxy4MMoUL5lhxxgXMGwj39gnbUmkpM4Umliz36rdAM-tTMll1voBJtPb9zdGoH1I5DapB1WhDGYI43Y4pvxG8Up8vG3Xz4rZ6KrZgb7XQVpXrGD-KKIJ8MN0kAypNO2GZyNdqUErbCsK1aNwVdrX3MfwqzfHLz97bkiFioWLzCIU8YVZtvhRUpmwkaTWy0Wrx6ODk62Xgm9Koa1ZcZSG6bhgIYMB5j9MEb7ZpjHgu6IC3Fy3~dbaWMc3XOAgXeHP3WTohnfTCjr3-8~vAJXpRf7zTfQ9m1LfhESO-P4ATIML2vlsJ~bCw48odA5eWLdpm8ljf7UdeRg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal