Abstract
It has been recognized for nearly 80 years that insoluble aluminum salts are good immunologic adjuvants and that they form long-lived nodules in vivo. Nodule formation has long been presumed to be central for adjuvant activity by providing an antigen depot, but the composition and function of these nodules is poorly understood. We show here that aluminum salt nodules formed within hours of injection and contained the clotting protein fibrinogen. Fibrinogen was critical for nodule formation and required processing to insoluble fibrin by thrombin. DNase treatment partially disrupted the nodules, and the nodules contained histone H3 and citrullinated H3, features consistent with extracellular traps. Although neutrophils were not essential for nodule formation, CD11b+ cells were implicated. Vaccination of fibrinogen-deficient mice resulted in normal CD4 T-cell and antibody responses and enhanced CD8 T-cell responses, indicating that nodules are not required for aluminum's adjuvant effect. Moreover, the ability of aluminum salts to retain antigen in the body, the well-known depot effect, was unaffected by the absence of nodules. We conclude that aluminum adjuvants form fibrin-dependent nodules in vivo, that these nodules have properties of extracellular traps, and the nodules are not required for aluminum salts to act as adjuvants.
Introduction
In 1926, Alexander Glenny and colleagues1 reported that toxoid precipitated with aluminum potassium sulfate, referred to as alum, induced better antibody production when injected into guinea pigs than soluble toxoid alone. It was later reported that nodules formed at the injection site.2 The presence of antigenic toxoid within the nodules for days2 or weeks3 was proven when the nodules were ground up and injected into naive guinea pigs, thereby inducing antitoxoid antibodies. These observations are the basis of the “depot theory,” which states that aluminum salts are effective adjuvants because antigen is slowly released over time, providing both a priming effect and a boosting effect with one vaccination.
Decades later, the first histologic characterization of “the alum granuloma” was reported.4 It was found that 1 day after subcutaneous injection, a mass appears that contains aluminum adjuvant, neutrophils, and macrophages. By day 14, the mass was surrounded by organized layers of macrophages, then blasting B cells, and then collagen and fibroblasts.
The formation of these nodules is widely known among researchers that use aluminum salts either for vaccination or for induction of experimental asthma. However, these nodules have been poorly studied and the kinetics of nodule formation, their protein composition, and mode of production have never been reported. We demonstrate here that aluminum salt nodules form within 2-4 hours of injection into mice. Nodule formation requires the presence of fibrinogen, which must be processed to its active form, fibrin, by thrombin. Contrary to expectations, these nodules are not required for insoluble aluminum salts either to act as adjuvants, or to act as depots for antigen. Aluminum nodules have features consistent with those of extracellular traps (ETs), and aluminum adjuvants may prove useful for studying ETs in vivo.
Methods
Mouse strains
C57BL/6 mice, gp91/phox knockout (KO) mice, RAG-1 KO mice, IL-5 KO mice, W/Wv mice, CD11c-DTR mice, and OT-I T-cell receptor transgenic (TCR Tg) mice were purchased from The Jackson Laboratory. Fibrinogen-α−/− mice,5 fibrinogen-γ 390-396A mice,6 PHIL mice,7 and 508 TCR Tg mice8 have been previously described. Fibrinogen-α−/− mice and fibrinogen-γ 390-396A mice have been backcrossed onto the C57BL/6 background for 6 generations. Fibrinogen-α−/− mice have a high rate of neonatal mortality that worsens with increased backcrossing to C57BL/6 mice. All mice were housed and/or bred at the Biological Resource Center at National Jewish Health. All studies were approved by the Institutional Animal Care and Use Committee. Except where indicated, 3-5 mice were used per group.
Aluminum adjuvants and nodule collection
Alhydrogel brand aluminum hydroxide (Accurate Chemical), Adju-Phos brand aluminum phosphate (Accurate Chemical), aluminum potassium sulfate, also known as alum (Sigma-Aldrich), and Imject Alum Adjuvant (Thermo Fisher Scientific) were purchased commercially. Aluminum potassium sulfate was precipitated by neutralization to pH 7.0 with 1M KOH. All adjuvants were washed and stored in phosphate-buffered saline (PBS). Except where noted, 4 mg of Alhydrogel was injected intraperitoneally in 0.2 mL of PBS. Aluminum nodules were collected with forceps into preweighed microcentrifuge tubes containing PBS. To determine nodule wet weight, PBS was removed, tubes were centrifuged at 10 000g for 2 minutes, and residual PBS was removed. Unless otherwise noted, nodules were collected 4-6 hours after injection.
Photographs
Photos of aluminum nodules were taken with a Nikon CoolPix 5100 digital camera. Note that regions of glare were dimmed by the use of Adobe Photoshop to enhance nodule visibility.
Protein gels and Western blot analysis
Proteins were extracted from nodules by boiling in 2% sodium dodecyl sulfate (SDS). An estimate of protein content was made by determining the A280, assuming 1 mg/mL protein = 1 OD. For total protein analysis, proteins were analyzed with Coomassie blue stain. For Western blot analysis, proteins were separated by SDS–polyacrylamide gel electrophoresis (PAGE), transferred to a PVDF membrane (Millipore), and then blocked with 5% nonfat milk. Antibodies to histone H3 (H3; Abcam Y173), citrullinated H3 (citH3; Abcam Ab5103), and mouse immunoglobulin M heavy chain (Jackson ImmunoResearch Laboratories) were purchased. Rabbit anti–mouse fibrinogen has been previously described.5 Detection was performed with the use of appropriate horseradish peroxidase– or fluorophore-conjugated secondaries. Data were collected with a VersaDoc imager (Bio-Rad) and analyzed with Quantity One software Version 4.6.5 (Bio-Rad).
Mass spectrometry
Forty milligrams of alum were injected intraperitoneally into male C57BL/6 mice. Two hours later, alum aggregates were removed by dissection, boiled in 2% SDS, then run onto an SDS-PAGE gel for 7 minutes to eliminate particulate material. The gel was cut into pieces, digested with trypsin, and analyzed by mass spectrometry (details are provided in supplemental Methods, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Resulting peptides were sequenced and matched to proteins via a search of the Swissprot protein database by the use of the database search program Spectrum Mill (Agilent Technologies).
Fibrinogen reconstitution
Human fibrinogen (Calbiochem) was used for reconstitution experiments because it is cleavable by mouse thrombin9 and available much more economically than mouse fibrinogen. Human fibrinogen was dissolved in PBS by warming it to 37°C and by gentle pipetting. Mice were injected intraperitoneally with 1 mg of human fibrinogen in PBS 5-10 minutes before injection of aluminum adjuvant. We estimated that a 25-g mouse has approximately 2.5 mL of blood, approximately one-half of which is plasma. Mouse plasma has approximately 2.2 mg/mL fibrinogen, or a total of approximately 2.75 mg of plasma fibrinogen. There may be a similar level of nonplasma fibrinogen in other extracellular spaces, or approximately 5.5 mg of total fibrinogen in a mouse. Thus, local reconstitution with 1 mg of fibrinogen would be < 20% of normal total body fibrinogen.
Peritoneal lavage
Mice were killed by CO2 inhalation. Peritoneal lavage fluid was collected by the use of 5 mL of ice-cold PBS.
Warfarin, lepirudin, and uricase treatment
Warfarin (Sigma-Aldrich) was administered in drinking water at 2.5 μg/mL for 7 days before adjuvant injection. Fifty μg of lepirudin (Refludan; Bayer) was injected intraperitoneally in 0.1 mL of PBS, 10 minutes before adjuvant injection. Fifty U of uricase (Sigma-Aldrich; U0880) was injected intraperitoneally in 0.1 mL of PBS, 1 day and also 10 minutes before adjuvant injection, as previously described (Kool et al10 and B. Lambrecht, University of Liege, written communication, April 28, 2008). Peritoneal lavage fluid from each mouse was centrifuged to remove cells, and then the uric acid concentration of the supernatant was tested with a commercial kit (Molecular Probes; A22181). The uric acid concentration was multiplied by the volume of the lavage fluid to determine the amount of uric acid per mouse peritoneal cavity.
Aluminum nodule digestion
Mice were injected with 4 mg of Alhydrogel. Four hours later, nodules from 3 mice were collected, pooled, divided into separate tubes, and weighed. Buffer containing 20mM Tris-HCl, pH 8.4, with 5mM MgCl2 was added to each tube, and 100 μg/mL DNase I, Grade 2 (Roche) or buffer control was added to each sample as indicated and incubated at 37°C for 30 minutes. Finally, 10 μg/mL recombinant proteinase K, PCR grade, (Roche) or buffer control was added to each sample as indicated and incubated at 37°C for an additional 30 minutes. Samples were pelleted at 13 000g for 1 minute then supernatants were transferred to new tubes. Supernatant A280 values were normalized to the starting nodule weights.
Microscopy
Alhydrogel nodules, collected from mice injected 4 hours previously, were embedded in OCT compound, permeabilized with 0.5% Triton X-100, blocked, then transferred to poly-L-lysine coverslips. Myeloperoxidase was stained with mouse anti–mouse myeloperoxidase and anti–mouse secondary. Hoechst stain was used to detect DNA. Wright-Giemsa stain was performed with the Hema 3 System (Fisher) then visualized with an Olympus BX40 microscope with 100×/1.30 oil objective with a Q-Color 3 camera (Olympus) and QCapture software Version 2.68.2 (Q Imaging Corporation).
Cell depletions and antibody blockade
Neutrophils were depleted by intravenous injection of 200 μg of anti–Gr-1 antibody (RB6-8C5) 1 day before adjuvant injection. Depletion was confirmed by Wright-Giemsa staining of peritoneal lavage cells. Peritoneal macrophages were depleted by intraperitoneal injection of clodronate liposomes in 200 μL of PBS 1 day before adjuvant injection and confirmed by F4/80 fluorescence-activated cell sorting staining of peritoneal lavage cells.
Antigens and vaccinations
3K peptide (FEAQKAKANKAVDGGGC) was synthesized by Genemed. 3K peptide was covalently attached to ovalbumin (OVA) by use of the Imject maleimide-activated OVA kit (Pierce). Conjugation was verified by testing interleukin-2 production by the 3K-specific hybridoma B3K0508. Mice were vaccinated intraperitoneally with 10 μg of 3K-OVA conjugate precipitated with 4 mg of aluminum potassium sulfate (Sigma-Aldrich) in 200 μL of PBS.
In vivo antigen presentation assay
Recipient mice were vaccinated as described previously. To test for in vivo antigen presentation, cells from 508 TCR Tg mice, specific for I-Ab-3K, and from OT-I TCR Tg mice, specific for Kb-SIINFEKL, were used. Spleen and lymph node cells were combined then labeled with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes). Cells were adoptively transferred by intraperitoneal injection because an intravenous injection would likely cause uncontrolled bleeding in FibA KO mice. Forty hours later, spleen and inguinal lymph node cells from recipient mice were analyzed on a CYAN flow cytometer (Dako Cytomation). Cells that were CD4+CD8−B220−Va2+Vb14+CFSE+ were considered to be 508 TCR Tg CD4 T cells. Cells that were CD4−CD8+B220-Va2+Vb5+CFSE+ were considered to be OT-I TCR Tg T cells.
CD4 T-cell, CD8 T-cell, and antibody responses
I-Ab-3K tetramers were labeled with phycoerythrin and have been previously described.11 Kb-SIINFEKL tetramers were labeled with antigen-presenting cells (APCs) and have been previously described.12 Single-cell suspensions of splenocytes were treated with ammonium chloride to lyse red blood cells, counted with a Coulter Counter, then stained with tetramers at 37°C for 2 hours. Antibodies to surface markers were incubated with cells for a further 20 minutes at 4°C. B220, F4/80, and MHC II were used as dump markers. Three million events were collected per sample. Cells that were CD4+CD8−CD44hi and I-Ab-3K+ or cells that were CD8+CD4−CD44hi and Kb-SIINFEKL+ were considered to be specific for 3K or SIINFEKL, respectively. Serum anti-OVA antibodies were detected by enzyme-linked immunosorbent assay (ELISA) with OVA-coated plates by the use of AP-conjugated detection antibodies specific for IgG1 (BD clone X56) and IgG2c (BD clone R19-15) read with an ELx808 Ultra Microplate Reader (Bio-Tek Instruments).
Results
Nodules form rapidly after the injection of insoluble aluminum salts
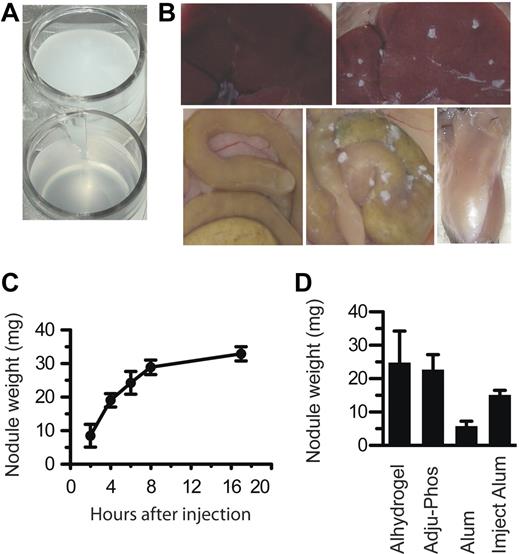
Before injection, all aluminum adjuvants are insoluble suspensions of microscopic particles that have a milky appearance (Figure 1A). After injection into animals, aluminum adjuvants formed nodules that could be seen by the naked eye, regardless of whether the adjuvant was injected intraperitoneally or intramuscularly (Figure 1B). Nodules were first visible within approximately 2 hours, and their accumulation was nearly complete within 8 hours (Figure 1C).
Aluminum adjuvants rapidly form into nodules in vivo. (A) A suspension of Alhydrogel in PBS. (B) Top left, a normal mouse liver; top right, liver of a mouse injected with Alhydrogel; bottom left, intestines of an uninjected mouse; bottom middle, intestines of a mouse injected with Alhydrogel; bottom right, leg muscle of a mouse injected with Alhydrogel. Photos taken 4 hours after injection. (C) At various times after intraperitoneal injection of 4 mg of Alhydrogel, nodules were collected and weighed. (D) At 4 hours after injection of 4 mg of Alhydrogel, Adju-Phos, aluminum potassium sulfate (Alum), or Imject Alum, nodules all form nodules within 4 hours in vivo. All experiments included at least 3 mice per group. Panel B was repeated more than 5 times. Panels C and D were repeated twice each.
Aluminum adjuvants rapidly form into nodules in vivo. (A) A suspension of Alhydrogel in PBS. (B) Top left, a normal mouse liver; top right, liver of a mouse injected with Alhydrogel; bottom left, intestines of an uninjected mouse; bottom middle, intestines of a mouse injected with Alhydrogel; bottom right, leg muscle of a mouse injected with Alhydrogel. Photos taken 4 hours after injection. (C) At various times after intraperitoneal injection of 4 mg of Alhydrogel, nodules were collected and weighed. (D) At 4 hours after injection of 4 mg of Alhydrogel, Adju-Phos, aluminum potassium sulfate (Alum), or Imject Alum, nodules all form nodules within 4 hours in vivo. All experiments included at least 3 mice per group. Panel B was repeated more than 5 times. Panels C and D were repeated twice each.
Three different insoluble aluminum salts are currently used as adjuvants in human vaccines, aluminum hydroxide (Alhydrogel), aluminum phosphate (ie, Adju-Phos), and aluminum potassium sulfate (ie, alum). Imject Alum, an adjuvant used only in animals, is a mixture of aluminum hydroxide and magnesium hydroxide, similar to Maalox brand antacid. All 4 of these substances formed nodules when injected into mice (Figure 1D).
Fibrinogen processing to fibrin is required for nodule formation
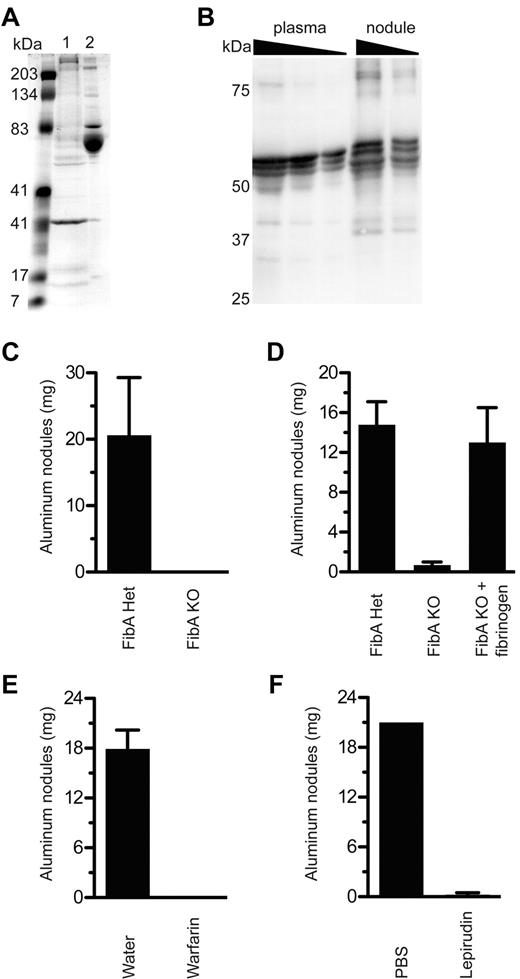
To determine how nodules are formed after injection of insoluble aluminum salts, we injected mice intraperitoneally with alum. Four hours later we isolated the nodules, boiled them in SDS-PAGE running buffer, and analyzed their contents by SDS-PAGE followed by Coomassie blue staining. The major protein in normal peritoneal fluid has a molecular weight of approximately 70 kDa and is probably mouse serum albumin (Figure 2A). Because insoluble aluminum salts adsorb a wide variety of proteins fairly nonspecifically (but to some extent interactions depend on the relative charge of the salts and the protein), we expected that the proteins in the nodules would be representative of those in peritoneal fluid. Instead, we found that albumin was extremely underrepresented within aluminum nodules (Figure 2A). Conversely, a 31-kDa protein and a protein larger than 200 kDa were overrepresented, as were several other proteins of various sizes.
Fibrinogen and thrombin are required for aluminum nodule formation. (A) At 4 hours after Alhydrogel injection, nodules were collected and protein was eluted by boiling in SDS, separation by SDS-PAGE, and analysis with Coomassie staining. Lane 1, protein eluted from aluminum nodules; lane 2, normal mouse serum. (B) Plasma (0.1, 0.05, 0.025 μL), and proteins eluted from 4-hour Alhydrogel nodules (4 μg, 2 μg), were analyzed by the use of Western blot for fibrinogen under reducing conditions. Note that the fibrinogen bands in the plasma samples are probably compressed by albumin, which is very abundant but not visualized. (C) FibA KO mice were tested for the ability to form aluminum nodules in vivo compared with littermate controls. (D) FibA KO mice were injected with 1 mg of human fibrinogen 10 minutes before an injection of Alhydrogel. At 5 hours later, mice were evaluated for nodule formation. (E) Mice given 2.5 μg/mL warfarin in their drinking water for 7 days were injected with Alhydrogel and evaluated 5 hours later for the presence of aluminum nodules. (F) Mice were injected intraperitoneally with lepirudin 10 minutes before injection with Alhydrogel, then evaluated 5 hours later for the presence of aluminum nodules. All experiments included at least 3 mice per group and were performed at least 3 times, except panel D, which was performed twice.
Fibrinogen and thrombin are required for aluminum nodule formation. (A) At 4 hours after Alhydrogel injection, nodules were collected and protein was eluted by boiling in SDS, separation by SDS-PAGE, and analysis with Coomassie staining. Lane 1, protein eluted from aluminum nodules; lane 2, normal mouse serum. (B) Plasma (0.1, 0.05, 0.025 μL), and proteins eluted from 4-hour Alhydrogel nodules (4 μg, 2 μg), were analyzed by the use of Western blot for fibrinogen under reducing conditions. Note that the fibrinogen bands in the plasma samples are probably compressed by albumin, which is very abundant but not visualized. (C) FibA KO mice were tested for the ability to form aluminum nodules in vivo compared with littermate controls. (D) FibA KO mice were injected with 1 mg of human fibrinogen 10 minutes before an injection of Alhydrogel. At 5 hours later, mice were evaluated for nodule formation. (E) Mice given 2.5 μg/mL warfarin in their drinking water for 7 days were injected with Alhydrogel and evaluated 5 hours later for the presence of aluminum nodules. (F) Mice were injected intraperitoneally with lepirudin 10 minutes before injection with Alhydrogel, then evaluated 5 hours later for the presence of aluminum nodules. All experiments included at least 3 mice per group and were performed at least 3 times, except panel D, which was performed twice.
To identify the aluminum adjuvant-adsorbed proteins, we used SDS to elute adjuvant-adsorbed proteins and then performed mass spectrometry to determine their identity. Mass spectrometry of proteins from nodules isolated only 2 hours after injection revealed high levels of the α, β, and γ chains of the clotting protein, fibrinogen, as well as histones H1, H2A, H2B, H3, and H4 (supplemental Table 1). Altogether, 35 proteins were identified by mass spectrometry.
The presence of fibrinogen was intriguing, given its role in diverse inflammatory conditions including foreign body reactions13 and experimental autoimmune arthritis.14 We confirmed by Western blot analysis the presence of fibrinogen-α, -β, and -γ chains in aluminum salt nodules (Figure 2B). Bands detected at higher and lower molecular weights indicated that covalent cross-linking by factor XIIIa and some degradation by plasmin may have occurred.
Of importance, we found that fibrinogen-α–deficient mice (FibA KO), which completely lack fibrinogen,5 did not form aluminum adjuvant nodules (Figure 2C). The defect was not simply a delayed response in FibA KO mice because nodules were also absent 1 or 9 days after adjuvant administration (data not shown). The defect was specific because reconstitution of FibA KO mice with purified fibrinogen before Alhydrogel injection restored nodule formation (Figure 2D).
We wondered whether adsorption of soluble fibrinogen to aluminum adjuvant was sufficient for nodule formation or whether fibrinogen cleavage to active fibrin by thrombin was required. Treatment of mice with warfarin, which prevents hepatic synthesis of thrombin and other components of the coagulation cascade, abolished nodule formation (Figure 2E). To test the role of thrombin specifically, mice were treated with the recombinant hirudin lepirudin, a highly specific direct thrombin inhibitor derived from the blood-sucking leech Hirudo medicinalis. Lepirudin treatment also prevented nodule formation (Figure 2F).
We concluded from these data that fibrinogen is essential for nodule formation. Furthermore, nodule formation requires cleavage of fibrinogen to its active form, fibrin, by thrombin.
Aluminum nodules have features consistent with ETs
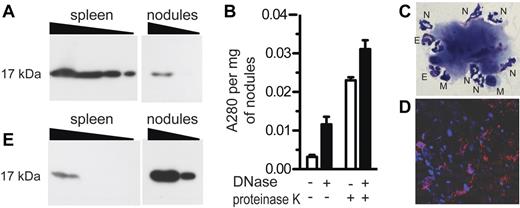
Mass spectrometry also identified the presence of histones within aluminum nodules (supplemental Table 1). To verify this finding, we analyzed aluminum nodules by Western blot and confirmed the presence of histone H3 (Figure 3A). The presence of histones indicated either that living cells were present on or within the nodules, or that extracellular chromatin was present. To distinguish between these possibilities, we treated nodules with DNase, which degrades extracellular DNA but not intracellular DNA, then measured absorbance of the supernatant at 280 nm. Absorbance was significantly increased by DNase treatment, indicating release of DNA from the nodules (Figure 3B left). Digestion with DNase also significantly enhanced disruption of the nodules with proteinase K, a relatively nonspecific endopeptidase (Figure 3B right). We concluded from these data that histones are present in aluminum adjuvant nodules, and that much of the chromatin is extracellular.
Aluminum nodules have features consistent with ETs. (A) Alhydrogel nodules (10 μg, 3 μg) were analyzed by Western blot for the presence of histone H3, compared with spleen cells (1.0, 0.3, 0.1, 0.03 × 106 cell equivalents). (B) Alhydrogel nodules were digested in the presence of DNase alone, proteinase K alone, or both. Absorbance of released material was measured at 280 nm. (C) Aluminum nodules were analyzed by Wright-Giemsa staining at ×1000 magnification. N indicates neutrophil; E, eosinophil; M, mononuclear cell. (D) Aluminum nodules were analyzed by immunofluorescence staining for DNA with DAPI (blue) and myeloperoxidase (red). (E) A gel identical to that shown in panel A (and run in parallel) was analyzed by Western blot for the presence of citH3. All experiments used Alhydrogel nodules collected 4-5 hours after injection and were performed at least 3 times.
Aluminum nodules have features consistent with ETs. (A) Alhydrogel nodules (10 μg, 3 μg) were analyzed by Western blot for the presence of histone H3, compared with spleen cells (1.0, 0.3, 0.1, 0.03 × 106 cell equivalents). (B) Alhydrogel nodules were digested in the presence of DNase alone, proteinase K alone, or both. Absorbance of released material was measured at 280 nm. (C) Aluminum nodules were analyzed by Wright-Giemsa staining at ×1000 magnification. N indicates neutrophil; E, eosinophil; M, mononuclear cell. (D) Aluminum nodules were analyzed by immunofluorescence staining for DNA with DAPI (blue) and myeloperoxidase (red). (E) A gel identical to that shown in panel A (and run in parallel) was analyzed by Western blot for the presence of citH3. All experiments used Alhydrogel nodules collected 4-5 hours after injection and were performed at least 3 times.
The presence of extracellular chromatin and histones was reminiscent of neutrophil extracellular traps (NETs), networks of chromatin extruded from neutrophils that trap and kill bacteria.15 We examined the nodules by Wright-Giemsa staining and observed that neutrophils were indeed interacting with the nodules, as were smaller numbers of eosinophils and mononuclear cells (Figure 3C). In addition, the nodules were analyzed by fluorescence microscopy for the presence of myeloperoxidase, a neutrophil-specific protease. The nodules did indeed contain myeloperoxidase (Figure 3D), most of which appeared to be extracellular.
In vitro stimulation of neutrophils to release ETs results in deimination of arginine to citrulline on histone 3 (H3).16 Peptidyl arginine deiminase 4, the enzyme that converts H3 to citrullinated H3 (citH3), is also required for chromatin decondensation and ET release from neutrophils,17 suggesting that neutrophil H3 citrullination and ET formation are functionally linked. We therefore tested aluminum nodules for the presence of citH3 by Western blot (Figure 3E). The spleen cell control had a low ratio of citH3 to total H3 (compare Figure 3E with Figure 3A). Of importance, the ratio of citH3 to total H3 in aluminum nodules was very high.
The presence of histones, extracellular chromatin, neutrophils, myeloperoxidase, and citH3 are all consistent with the idea that aluminum nodules are a type of ETs. Because these ETs are dependent on fibrin, we refer to them as fibrin-dependent extracellular traps (fibrin ETs).
Fibrinogen interacts with CD11b+ cells
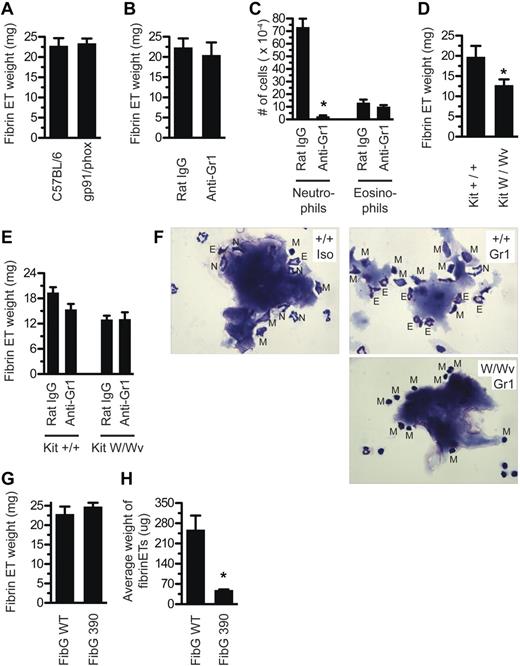
Previous investigators, such as Fuchs et al,18 have described a critical role for nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in the release of NETs from neutrophils stimulated with PMA. However, when gp91-deficient mice, which lack an essential subunit of the phagosomal oxidase NADPH oxidase, were injected with aluminum adjuvant, they still produced aluminum fibrin ETs (Figure 4A). Surprisingly, neutrophil depletion also did not alter the formation of aluminum fibrin ETs (Figure 4B), although depletion was 97% effective (Figure 4C). Depletion of macrophages, the other major phagocytic cell type present in the peritoneum, in conjunction with neutrophils, was also dispensable for aluminum fibrin ET formation (supplemental Figure 1A).
Fibrinogen interacts with CD11b+ cells but there are redundant ET-releasing cell types. (A) Mice deficient for gp91/phox were injected with Alhydrogel and evaluated for fibrin ET formation 5 hours later. (B) Mice were depleted of neutrophils with 200 μg of anti–Gr-1 intravenously at day −1, or injected with isotype control Ab, then tested for fibrin ET formation 5 hours after Alhydrogel injection. (C) Peritoneal lavage was collected from mice in Figure 4B. Cells were stained by Wright-Giemsa and neutrophils and eosinophils were counted. *P < .001 compared with Rat IgG. (D) KitW/Wv mice, or littermate controls, were injected with Alhydrogel and evaluated for fibrin ET formation 5 hours later. Note that fibrin ET formation was decreased by 35% (P = .03). (E) KitW/Wv mice, or littermate controls, were depleted of neutrophils with 200 μg of anti–Gr-1 intravenously at day −1 or given isotype control Ab. On day 0, mice were injected with Alhydrogel and evaluated for fibrin ET formation 5 hours later. (F) Nodules from the peritoneal lavage of mice in part E were stained by Wright-Giemsa. Top left, Kit+/+ mice receiving Rat IgG. Top right, Kit+/+ mice receiving anti–Gr-1. Bottom right, KitW/Wv mice receiving anti–Gr-1. N indicates neutrophil; E, eosinophil; M, mononuclear cell. (G) FibG 390-396A mice were injected with Alhydrogel and evaluated for nodule formation 5 hours later. (H) Nodules from FibG 390-396A mice or controls were counted and the average weight determined (*P = .02). All experiments included at least 3 mice per group and were performed at least 3 times, except panel E, which was performed twice with 4 mice per group and shows the average from all 8 mice.
Fibrinogen interacts with CD11b+ cells but there are redundant ET-releasing cell types. (A) Mice deficient for gp91/phox were injected with Alhydrogel and evaluated for fibrin ET formation 5 hours later. (B) Mice were depleted of neutrophils with 200 μg of anti–Gr-1 intravenously at day −1, or injected with isotype control Ab, then tested for fibrin ET formation 5 hours after Alhydrogel injection. (C) Peritoneal lavage was collected from mice in Figure 4B. Cells were stained by Wright-Giemsa and neutrophils and eosinophils were counted. *P < .001 compared with Rat IgG. (D) KitW/Wv mice, or littermate controls, were injected with Alhydrogel and evaluated for fibrin ET formation 5 hours later. Note that fibrin ET formation was decreased by 35% (P = .03). (E) KitW/Wv mice, or littermate controls, were depleted of neutrophils with 200 μg of anti–Gr-1 intravenously at day −1 or given isotype control Ab. On day 0, mice were injected with Alhydrogel and evaluated for fibrin ET formation 5 hours later. (F) Nodules from the peritoneal lavage of mice in part E were stained by Wright-Giemsa. Top left, Kit+/+ mice receiving Rat IgG. Top right, Kit+/+ mice receiving anti–Gr-1. Bottom right, KitW/Wv mice receiving anti–Gr-1. N indicates neutrophil; E, eosinophil; M, mononuclear cell. (G) FibG 390-396A mice were injected with Alhydrogel and evaluated for nodule formation 5 hours later. (H) Nodules from FibG 390-396A mice or controls were counted and the average weight determined (*P = .02). All experiments included at least 3 mice per group and were performed at least 3 times, except panel E, which was performed twice with 4 mice per group and shows the average from all 8 mice.
Cells other than neutrophils, including mast cells and eosinophils, have been reported to also release ETs when stimulated in vitro with PMA.19 We previously described several cell types present in the peritoneal cavity in unmanipulated mice or cells recruited after aluminum adjuvant injection,19 including mast cells, eosinophils, and lymphocytes, and thought these might contribute to aluminum fibrin ET formation.
We did not find any requirement for T cells (supplemental Figure 1B) or eosinophils (supplemental Figure 1C-D) in fibrin ET formation. We did detect immunoglobulin M in aluminum fibrin ETs (data not shown), possibly adsorbed from serum or else derived from B cells, but normal fibrin ET formation in RAG-deficient mice (supplemental Figure 1B) ruled out a key role for this interaction.
We found that mast cell-deficient KitW/Wv mice had significantly less aluminum fibrin ETs than control mice (Figure 4D). Thus, mast cells might contribute to, but are not absolutely required for, fibrin ET formation. When we depleted neutrophils from KitW/Wv mice, this did not result in further reduction of fibrin ET formation (Figure 4E).
We performed Wright-Giemsa staining on these samples to see which cells were interacting with the fibrin ETs. As expected, in control mice, neutrophils were the primary cell type interacting with the aluminum fibrin ETs (Figure 4F, top left). In Kit+/+ mice depleted of neutrophils, there were few neutrophils but more interactions with eosinophils and mononuclear cells (Figure 4F, top right). KitW/Wv mice injected with aluminum adjuvant recruit few eosinophils to the peritoneum,20 and in KitW/Wv mice depleted of neutrophils, we saw that virtually all cells interacting with aluminum fibrin ETs were mononuclear cells (Figure 4F bottom right).
Because it is not practical to deplete all peritoneal cell populations at the same time, we sought a different approach. Fibrinogen is able to interact with integrin αMβ2 (Mac-1 or CD11b/CD18) expressed on the surface of leukocytes. Knock-in mice containing alanine substitutions within fibrinogen-γ residues 390-396 (FibG390-396A mice) have been described. These mice have normal clotting function and platelet aggregation, but fibrinogen purified from FibG390-396A mice is unable to support αMβ2-dependent adhesion in vitro.6 When we injected FibG390-396A mice with aluminum adjuvant, the total mass of aluminum fibrin ETs was unaltered (Figure 4G), but the average size of each fibrin ET recovered was much smaller (Figure 4H).
This finding suggests that after thrombin cleaves aluminum-adsorbed fibrinogen into fibrin, CD11b+ cells are recruited to interact with, and enhance aggregation of, the nodules. Consistent with this result, other investigators, such as Neeli et al,21 have found that antibody blockade of CD11b reduced the ability of neutrophils to make NETs. Notably, we did not consistently observe smaller aluminum fibrin ETs in mice lacking neutrophils, macrophages, mast cells, T cells, B cells, or eosinophils.
Mast cell–deficient mice made fewer fibrin ETs. We concluded that mast cells may contribute directly to fibrin ETs, supported by the presence of mast cell granules in the Wright-Giemsa stains (Figures 3C and 4F). Alternatively, mast cells may enhance vascular leakage of fibrinogen into the peritoneal cavity and/or increase recruitment of other leukocytes. Furthermore, fibrinogen interaction with αMβ2 integrin is important for full aggregation of aluminum adjuvant fibrin ETs. However, the fact that no single cell type was essential for their appearance suggested that several kinds of cell might contribute in a redundant fashion to the formation of aluminum salt fibrin ETs.
Uric acid and caspase-1 are not required for fibrin ETs
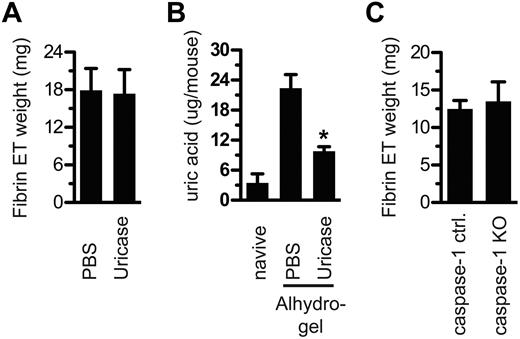
It has been reported that aluminum adjuvants induce the release of uric acid from dying cells in vivo and that uric acid is required for the aluminum adjuvants to induce CD4 T-cell proliferation.10 We tested the role of uric acid in fibrin ET formation by treating mice with uricase before adjuvant injection. Uricase did not prevent fibrin ET formation (Figure 5A), despite evidence that uricase significantly reduced uric acid levels (Figure 5B).
Uric acid and caspase-1 are dispensable for fibrin ET formation. (A) Mice were treated with uricase or PBS on day −1 and also 10 minutes before Alhydrogel injection. After 5 hours, fibrin ETs were weighed. (B) Peritoneal lavage fluid from each mouse in panel A was centrifuged to remove cells, and then the supernatant was tested for uric acid. Naive indicates mice that were never injected with PBS, uricase, or Alhydrogel. *P < .05 compared with PBS alone. (C) At 5 hours after Alhydrogel injection, caspase-1 KO mice and littermate controls were tested for fibrin ET formation. All experiments included at least 3 mice per group and were performed at least 3 times.
Uric acid and caspase-1 are dispensable for fibrin ET formation. (A) Mice were treated with uricase or PBS on day −1 and also 10 minutes before Alhydrogel injection. After 5 hours, fibrin ETs were weighed. (B) Peritoneal lavage fluid from each mouse in panel A was centrifuged to remove cells, and then the supernatant was tested for uric acid. Naive indicates mice that were never injected with PBS, uricase, or Alhydrogel. *P < .05 compared with PBS alone. (C) At 5 hours after Alhydrogel injection, caspase-1 KO mice and littermate controls were tested for fibrin ET formation. All experiments included at least 3 mice per group and were performed at least 3 times.
It has also been reported that when a mixture of aluminum hydroxide and magnesium hydroxide is used as an adjuvant, activation of the NLRP3 inflammasome and caspase-1 are essential for antibody responses,22,23 although other groups have been unable to confirm these findings.20,24-26 In any event, caspase-1–deficient mice injected with aluminum adjuvant made fibrin ETs to the same degree as wild type mice (Figure 5C).
An antigen depot forms even in the absence of aluminum salt fibrin ETs
As stated previously, it has been known for many decades that aluminum nodules contain antigen for days or weeks. It was hypothesized in 1931 that the nodules, presumed to slowly release this antigen in vivo, would thus provide both a priming effect and a continuous boosting effect. This depot theory was generally accepted for many decades. But in 1977 it was shown that aluminum adjuvant induces eosinophilia,27 an indication that innate immunity is activated. Many recent papers have confirmed and extended this finding, demonstrating that aluminum adjuvants induce rapid innate inflammation.10,20,28-30
Because aluminum fibrin ETs are completely absent in FibA KO mice, this provided an ideal system to determine what role aluminum fibrin ETs play in the depot effect, and how much contribution they make to adaptive immune responses.
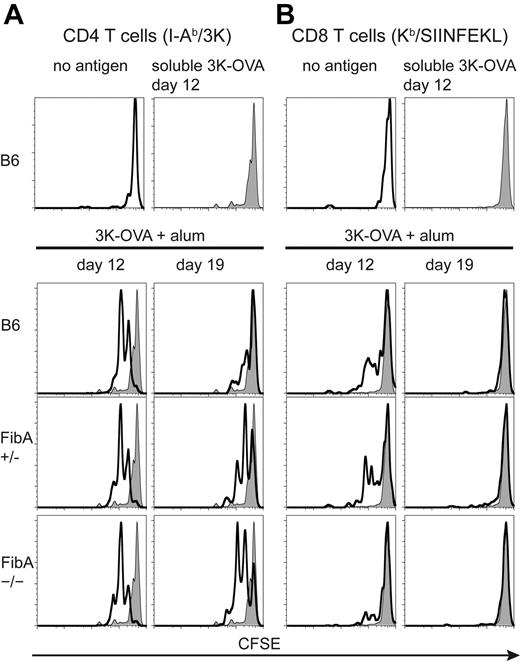
To study the depot effect, we used ovalbumin protein covalently coupled to the 3K peptide (3K-OVA) as the antigen. We immunized FibA KO mice and control mice with antigen, with or without alum. To determine whether antigen was detectable by T cells in these animals, we used CD4 T cells from 508 TCR transgenic (Tg) mice, which are specific for IAb/3K. These cells were labeled with CFSE and transferred into recipient mice at various times after the immunization. Two days after the cell transfer, the 508 Tg T cells were analyzed for dilution of CFSE as an indicator of in vivo activation. Soluble 3K-OVA was presented to 508 CD4 T cells for less than 12 days after immunization (Figure 6A, top). In contrast, 508 T cells could detect antigen at both 12 and 19 days after immunization with 3K-OVA plus alum in B6 mice or FibA Het mice. Surprisingly, 508 T cells also proliferated at days 12 and 19 in FibA KO mice (Figure 6A), which did not make fibrin ETs.
Aluminum adjuvant fibrin ETs are not required to maintain an antigen depot. C57BL/6 mice, and FibA Het (+/−), or FibA KO (−/−) recipient mice were vaccinated with 3K-OVA plus alum. Spleen and lymph node cells from 508 mice and OT-I mice, containing TCR Tg T cells specific for I-Ab-3K, and Kb-SIINFEKL, respectively, were isolated from TCR Tg mice, pooled, labeled with CFSE, and adoptively transferred into recipients. Two days later, mice were killed and splenic TCR Tg T cells were analyzed by fluorescence-activated cell sorting for proliferation of Tg CD4 T cells (A) and Tg CD8 T cells (B). The genotype of the recipient mouse is indicated in each histogram. The gray histogram indicates staining of T cells transferred to control mice that were vaccinated 12 days previously with soluble 3K-OVA. Similar results were obtained from the inguinal lymph node (not shown). There were 3 mice per group and the mouse with median CFSE dilution is shown from each group. Transfers were done on 3 days, 2 of which are shown (day 12 and day 19).
Aluminum adjuvant fibrin ETs are not required to maintain an antigen depot. C57BL/6 mice, and FibA Het (+/−), or FibA KO (−/−) recipient mice were vaccinated with 3K-OVA plus alum. Spleen and lymph node cells from 508 mice and OT-I mice, containing TCR Tg T cells specific for I-Ab-3K, and Kb-SIINFEKL, respectively, were isolated from TCR Tg mice, pooled, labeled with CFSE, and adoptively transferred into recipients. Two days later, mice were killed and splenic TCR Tg T cells were analyzed by fluorescence-activated cell sorting for proliferation of Tg CD4 T cells (A) and Tg CD8 T cells (B). The genotype of the recipient mouse is indicated in each histogram. The gray histogram indicates staining of T cells transferred to control mice that were vaccinated 12 days previously with soluble 3K-OVA. Similar results were obtained from the inguinal lymph node (not shown). There were 3 mice per group and the mouse with median CFSE dilution is shown from each group. Transfers were done on 3 days, 2 of which are shown (day 12 and day 19).
CFSE-labeled OT-I TCR Tg T cells, specific for Kb/SIINFEKL, were cotransferred with 508 TCR Tg T cells and also analyzed for in vivo proliferation. As seen with CD4 T cells, soluble 3K-OVA was undetectable by day 12 (Figure 6B, top). OT-I T cells also proliferated at day 12 in FibA KO mice, although somewhat less than in controls (Figure 6B). By day 19, presentation of Kb-SIINFEKL had ceased in all mice.
These results confirmed that antigen presentation to T cells is substantially lengthened by the presence of aluminum adjuvant. However, contrary to the assumption by us and others,2 aluminum salt fibrin ETs are not required for the depot effect.
Aluminum salt fibrin ETs are not required for adaptive immune responses
We next determined what role fibrinogen and aluminum adjuvant nodules play in adaptive immunity. We considered using adoptive transfer of TCR Tg T cells to measure CD4 and CD8 T-cell responses. However, to avoid potential artifacts introduced by the use of high T-cell precursor frequencies, we measured endogenous CD4 and CD8 T-cell responses.
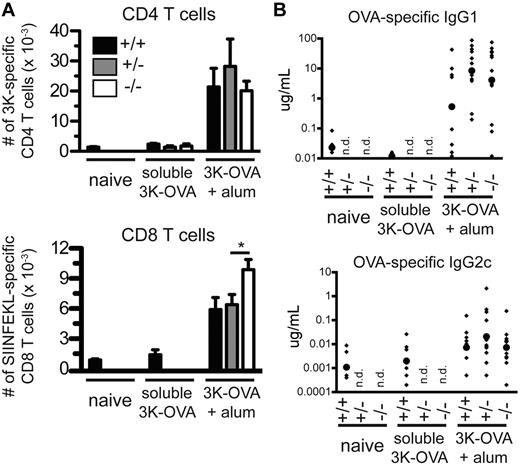
Fibrinogen-deficient mice were vaccinated with soluble 3K-OVA or 3K-OVA plus aluminum adjuvant. Nine days later, endogenous 3K-specific CD4 T cells, OVA-specific CD8 T cells, and OVA-specific antibodies were measured. The CD4 T-cell response was found to be similar in control and fibrinogen-deficient mice, whereas the CD8 T-cell response was actually increased approximately 1.5-fold (Figure 7A).
Aluminum adjuvant fibrin ETs are not required for adaptive immunity. C57BL/6, FibA Het, or FibA KO mice were vaccinated with 3K-OVA plus aluminum adjuvant. (A) Nine days later, the number of 3K-specific CD4 T cells and SIINFEKL-specific CD8 T cells per spleen were determined with IAb-3K and Kb-SIINFEKL tetramers, respectively (*P = .03). (B) Also at day 9, serum OVA-specific IgG1 and IgG2c antibodies were measured by ELISA. Small diamonds indicate individual mice and large circles indicate the geometric mean. Data are pooled from 3 experiments, n = 6-10 mice per nonadjuvant group and 8-13 mice per adjuvant group, n.d. indicates not determined.
Aluminum adjuvant fibrin ETs are not required for adaptive immunity. C57BL/6, FibA Het, or FibA KO mice were vaccinated with 3K-OVA plus aluminum adjuvant. (A) Nine days later, the number of 3K-specific CD4 T cells and SIINFEKL-specific CD8 T cells per spleen were determined with IAb-3K and Kb-SIINFEKL tetramers, respectively (*P = .03). (B) Also at day 9, serum OVA-specific IgG1 and IgG2c antibodies were measured by ELISA. Small diamonds indicate individual mice and large circles indicate the geometric mean. Data are pooled from 3 experiments, n = 6-10 mice per nonadjuvant group and 8-13 mice per adjuvant group, n.d. indicates not determined.
As expected, aluminum adjuvant induced high levels of the Th2-associated antibody isotype IgG1 but low levels of the Th1-associated antibody isotype IgG2c (Figure 7B, note difference in scales). FibA KO mice had a small decrease in OVA-specific antibodies of the IgG1 (2-fold) and IgG2c (3-fold) isotypes, compared with FibA Het mice, but the differences were not significant. Thus, we found no evidence that aluminum salt fibrin ETs are required for any aspect of adaptive immunity, although their presence appears to partially inhibit CD8 T-cell responses.
Discussion
The ability of vaccines containing aluminum adjuvants to induce the formation of nodules was observed soon after the discovery of aluminum adjuvants more than 80 years ago. However, there have been few attempts to evaluate the composition of these nodules or their contribution to adaptive immune responses. In this study we identified fibrinogen as a major component of these nodules and determined that thrombin cleavage of soluble fibrinogen to insoluble fibrin is essential for nodule formation. We also found that these aluminum nodules contain extracellular chromatin, citrullinated histones, neutrophils, and extracellular myeloperoxidase. Because these nodules require fibrin and also have features consistent with ETs, we refer to them as fibrin ETs. It has been presumed by many since the first observation and study of aluminum nodules that these nodules form an antigen depot that is central for aluminum salts to act as adjuvants. Contrary to these expectations, we show that aluminum salt fibrin ETs are dispensable for aluminum salts' depot effect and have no obvious role in inducing adaptive immunity.
The data presented here suggest a model for the formation of aluminum salt fibrin ETs in vivo (supplemental Figure 2). Step 1: fibrinogen from extracellular fluids rapidly adsorbs to the insoluble salts. Step 2: thrombin converts the adsorbed fibrinogen to fibrin, exposing fibrin's “A” and “B” knobs. These knobs interact with “a” and “b” holes on nearby fibrin molecules to recruit additional fibrin.31 These fibrin-fibrin interactions between separate aluminum salt particles results in larger aggregates. This effect is enhanced by mast cells. The mast cells may interact directly with the aluminum adjuvant, which is consistent with the appearance of granules on many nodules (Figures 3C and 4F). Alternatively or in addition, mast cells may act indirectly. For example, it is known that aluminum adjuvants induce mast cells to release histamine,20 and this is likely to promote vascular leakage of fibrinogen and to increase leukocyte migration to the injection site. Step 3: the CD11b-interacting motif of fibrinogen, absent in fibrinogen-γ 390-396A mice, then recruits and activates CD11b+ cells to release citrullinated histones onto the fibrin matrix, cross-linking the aggregates and forming fibrin ETs.
DNA and proteins both appear to contribute to the structural integrity of aluminum fibrin ETs (Figure 4). However, it is not clear how much mass of fibrin ETs is contributed by DNA or fibrinogen compared with other proteins. Optical density readings of the material liberated by DNase and proteinase treatment had stronger readings at 280 nm than at 260 nm, suggesting the fibrin ETs are primarily proteinaceous, but it is also possible this occurred because proteinase K has higher enzymatic activity than DNase I.
When aluminum adjuvants are cultured with cells in vitro, we have never observed the formation of aluminum nodules, or even the aggregation of aluminum particles. The central role of fibrinogen in this process suggests that the use of serum, which lacks fibrinogen, is a key reason that this phenomenon is only observed in vivo. These data also suggest that it will not be possible to study aluminum fibrin ETs in vitro. Whole blood collected without anticoagulants clots rapidly, consuming fibrinogen. But blood or plasma collected in the presence of anticoagulants, such as EDTA, heparin or citrate, lacks thrombin activity, another key requirement for aluminum fibrin ET formation (Figure 4).
The body's response to aluminum salts has significant parallels to foreign-body reactions, which occur when a foreign object or material, such as a splinter or medical device, is present within tissue.13 For example, medical biomaterials acquire a protein layer within minutes of implantation, and phagocytes are recruited to the implant site and begin to interact with the biomaterial within hours. Furthermore, phagocyte accumulation on the surface of biomaterials, like the full formation of aluminum fibrin ETs, depends on fibrinogen and its interaction with Mac-1 (CD11b/CD18).13
Foreign body reactions were likely selected for during evolution to deal with microbial infections, and to deal with the introduction of foreign bodies such as splinters. Presumably, when aluminum adjuvants or medical devices are inserted into the body, the result is simply a programmed response to microbial infection or splinters that also occurs when other nonphysiologic substances appear within the body. In the case of medical devices, this response is unwanted and detrimental. For aluminum adjuvants, it seems that this response is neither particularly helpful nor detrimental to the purpose of the vaccine, which is to induce antigen-specific T cell and antibody responses.
NETs and other types of ETs have been studied most extensively in vitro. One likely reason for this is that some bacterial DNases degrade NETs in vivo.32,33 Although the aluminum salt fibrin ETs described here are distinct from previously studied NETs, which require NADPH oxidase,18 aluminum fibrin ETs may be an advantageous model system for studying ETs in vivo because aluminum salts do not subvert the immune system.
Fibrinogen-deficient mice, which did not form fibrin ETs after vaccination, were found here to have normal CD4 T-cell and antibody responses, and a somewhat enhanced CD8 T-cell response. This finding directly contradicts Glenny et al's theory that aluminum salt nodules are central to the adjuvant properties of aluminum salts.
It was not surprising that naive TCR Tg CD4 T cells transferred to a mouse vaccinated as many as 5 weeks previously became activated (Figure 6 and data not shown). This finding is in line with previous studies that showed antigen persists in nodules for days2 or weeks.3 Thus, we assumed that prolonged antigen presentation occurs because newly generated APCs continuously nibble off small bites of the aluminum fibrin ETs, then traffic to lymphoid organs where they present the antigen to T cells. However, we were surprised that antigen presentation to CD4 T cells occurred for at least 19 days in fibrinogen-deficient mice (Figure 6), which did not form any discernable nodules.
To explain this finding, we propose that when aluminum adjuvants are injected, a portion of the adjuvant is retained at the site of injection and becomes encapsulated within fibrin ETs, but another portion is taken up by phagocytic APCs. Because APCs are thought to have a half-life of much less than 19 days, it is unlikely that a given APC could present the antigen for the entire 19 days. It is more likely that the first wave of APCs to phagocytose antigen and adjuvant die within a few days. These dying APCs, and the antigen and adjuvant, would then be taken up by other APCs, which present antigen until their demise. This relay of antigen presentation continues for weeks, but probably depends on small particles of antigen and adjuvant, not the large nodules that are visible to the naked eye. With this model in mind, we note that Glenny et al1 demonstrated that aluminum nodules contain antigen but did not demonstrate that antigen is released from aluminum nodules in a physiologic setting. Instead it was demonstrated that aluminum nodules, when ground up and injected into a recipient animal, contain antigen.
What is the physiologic function of the aluminum salt fibrin ETs? It appears that encasement of aluminum salt particles occurs as a biologic response to an unnatural stimulus. This response shares some similarities with a foreign-body response, which occur when a foreign object or material (eg, a medical devise), is present within tissue.13 The phenomenon of ETs has been studies primarily in vitro, and the use of aluminum adjuvants to induce fibrin ETs may provide a useful model for their study in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Volker Brinkmann, Arturo Zychlinsky, and Peter Henson for helpful discussions; Volker Brinkmann for technical assistance; and Julia Rhiannon for assistance obtaining lepirudan.
This work was supported by AI-22295, AI-18758, DOD grant USAMRMC: W81XWH-07-1-550, and National Institutes of Health grant S10RR023703 from the National Center For Research Resources.
National Institutes of Health
Authorship
Contribution: M.W.M., A.S.M., M.K.M., J.L.D., N.A.R., J.W.K., and P.M. designed the experiments; M.W.M., A.S.M., M.K.M., and R.L.P. performed the experiments; M.W.M. analyzed the data, generated the figures, and wrote the manuscript; and M.W.M., A.S.M., M.K.M., and P.M. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Philippa Marrack, Department of Immunology, Howard Hughes Medical Institute, 1400 Jackson St, K512, Denver, CO 80206; e-mail: marrackp@njhealth.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal