Abstract
Inherited hematologic defects that lack an in vivo selective advantage following gene correction may benefit from effective yet minimally toxic cytoreduction of endogenous hematopoietic stem cells (HSCs) prior to transplantation of gene-modified HSCs. We studied the efficacy of administering a novel sequential treatment of parenteral ACK2, an antibody that blocks KIT, followed by low-dose irradiation (LD-IR) for conditioning of wild-type and X-linked chronic granulomatous disease (X-CGD) mice. In wild-type mice, combining ACK2 and LD-IR profoundly decreased endogenous competitive long-term HSC repopulating activity, and permitted efficient and durable donor-derived HSC engraftment after congenic transplantation. ACK2 alone was ineffective. The combination of ACK2 and LD-IR was also effective conditioning in X-CGD mice for engraftment of X-CGD donor HSCs transduced ex vivo with a lentiviral vector. We conclude that combining ACK2 with LD-IR is a promising approach to effectively deplete endogenous HSCs and facilitate engraftment of transplanted donor HSCs.
Introduction
Chronic granulomatous disease (CGD) is an inherited hematological disease resulting from absence or dysfunction of the leukocyte superoxide-generating nicotinamide adenine dinucleotide phosphate (NADPH) oxidase that plays an important role in microbial killing.1-3 Gene defects in any one of 5 NADPH oxidase subunits can cause CGD, with two-thirds of cases due to mutations in an X-linked gene encoding the gp91phox (NOX2) subunit of the oxidase flavocytochrome b.1,4 Current management includes antibiotic prophylaxis and aggressive treatment of acute infections, and HSC transplantation has been used for patients failing standard therapies.1,2,5 CGD is also a candidate for gene therapy using autologous hematopoietic stem cells (HSCs).1,5 Because CGD affects neutrophil function and no selective advantage exists for gene correction, cytoreduction of uncorrected endogenous marrow cells is required in order to achieve a sufficient level of long-term engraftment of gene-modified HSCs, ideally with regimens that minimize exposure to genotoxic agents.
KIT and its ligand stem cell factor (SCF) contribute to the survival and proliferation of hematopoietic stem and progenitor cells (HSPCs).6 The monoclonal antibody ACK2 blocks binding and activation of murine KIT by SCF.7 Administration of ACK2 transiently reduces murine HSPCs, permitting substantial repopulation with donor HSCs in immunodeficient Rag2−/−γc−/− mice, but not immunocompetent mice.8 Here, we used congenic models to examine the effectiveness of combining ACK2 with low-dose irradiation (LD-IR) as conditioning for transplantation of immunocompetent wild-type mice and for HSC gene therapy of X-linked CGD (X-CGD) mice.
Methods
Mice used in this study include wild-type C57Bl/6J (C57; CD45.2+), B6.SJL-PtrcaPep3b/BoyJ (BoyJ; CD45.1+), and F1 progeny of C57 and BoyJ mice. X-CGD mice9 in C57, BoyJ, and CD45.1 × CD45.2 F1 backgrounds were also used. Mice were treated with 2 mg of the KIT-blocking monoclonal antibody ACK27 (injected intraperitoneally), 300 cGy, or both, followed by analysis of marrow HSPCs or transplantation of congenic marrow, either freshly isolated or transduced with the lentiviral vector CL20-gp91-OPT10 for expression of gp91phox, performed as described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). All animal protocols were approved by the Institutional Animal Care and Use Committee of the Indiana University School of Medicine.
Results and discussion
Effect of ACK2 and/or LD-IR on HSCs and HSPCs in immunocompetent mice
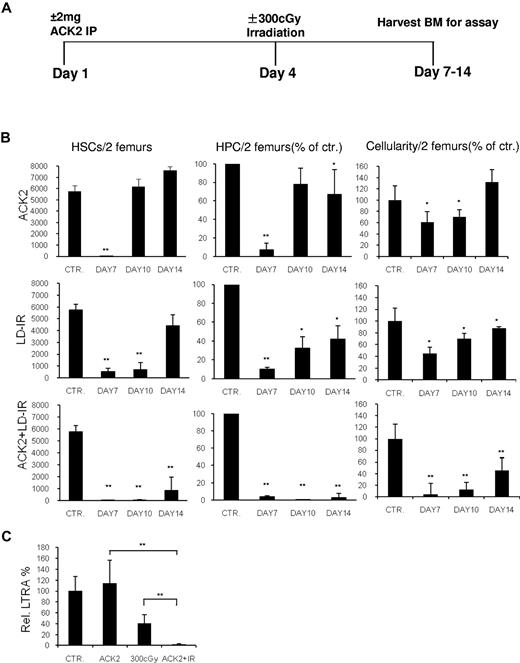
To determine whether ACK2 + LD-IR at 300 cGy could deplete HSCs in immunocompetent mice, marrow was harvested from wild-type mice treated with ACK2, LD-IR, or both (Figure 1A), and the frequency of HSCs (lin− Sca-1+ KIT+ CD135−CD150+) and HPCs (lin− Sca-1−KIT+ CD34lowFcγRlow or colony forming unit-granulocyte, monocyte) was determined. Mice in all 3 treatment arms showed HSC and HPC depletion on day 7; whereas recovery occurred in mice treated with either agent alone, these remained profoundly depressed in mice treated with ACK2 + LD-IR through day 14 (Figure 1B). Bone marrow (BM) cellularity followed a similar pattern (Figure 1B). Peripheral blood (PB) counts obtained on day 10 also showed more substantial effects of the combination treatment compared with ACK2 or LD-IR alone (supplemental Table 1).
Effect of ACK2 with low-dose irradiation treatment on marrow HSPC. (A) Schematic of treatment schedule using 2 mg ACK2 (IP) ± LD-IR at 300 cGy. Control mice received 0.5 cc saline on day 1. (B) Marrow HSCs (lin− Sca-1+ KIT+ CD135−CD150+), HPCs (common myeloid progenitor lin− Sca-1−KIT+ CD34lowFcγRlow or, for the ACK2 treatment group at day 7, colony forming unit-granulocyte, monocyte) and cellularity at different times following treatment of F1 C57/BoyJ mice with ACK2, LD-IR, or ACK + LD-IR compared with control mice treated with saline. Data are either the number per 2 femurs or the percentage of the saline-treated cohort on the day of study; mean ± standard deviation (n = 3 to 4). *P < .05; **P < .01 versus control. (C) Relative LTRA was determined by a competitive repopulation assay. Marrow obtained at day 7 (Figure 1A) was studied from either control mice or mice conditioned with ACK2 (n = 4), LD-IR (n = 4), or ACK2 + LD-IR (n = 8). **P < .01.
Effect of ACK2 with low-dose irradiation treatment on marrow HSPC. (A) Schematic of treatment schedule using 2 mg ACK2 (IP) ± LD-IR at 300 cGy. Control mice received 0.5 cc saline on day 1. (B) Marrow HSCs (lin− Sca-1+ KIT+ CD135−CD150+), HPCs (common myeloid progenitor lin− Sca-1−KIT+ CD34lowFcγRlow or, for the ACK2 treatment group at day 7, colony forming unit-granulocyte, monocyte) and cellularity at different times following treatment of F1 C57/BoyJ mice with ACK2, LD-IR, or ACK + LD-IR compared with control mice treated with saline. Data are either the number per 2 femurs or the percentage of the saline-treated cohort on the day of study; mean ± standard deviation (n = 3 to 4). *P < .05; **P < .01 versus control. (C) Relative LTRA was determined by a competitive repopulation assay. Marrow obtained at day 7 (Figure 1A) was studied from either control mice or mice conditioned with ACK2 (n = 4), LD-IR (n = 4), or ACK2 + LD-IR (n = 8). **P < .01.
HSC function of these 3 cohorts of mice was assessed with a competitive long-term repopulating assay.11-13 7 days after ACK2 administration, marrow long-term repopulating activity (LTRA) was normal (Figure 1C), indicating that HSC functional capacity was intact, despite the marked reduction in marrow lin− Sca-1+ KIT+ CD135−CD150+ cells (Figure 1B). This is consistent with the full recovery in the number of marrow lin− Sca-1+ KIT+ CD135−CD150+ cells 10 days after ACK2 (Figure 1B). As reported,12,13 LTRA was reduced to approximately 30% of normal 3 days following LD-IR alone. However, the combination of ACK2 + LD-IR almost entirely eliminated marrow LTRA (2.5% ± 0.8% of normal; Figure 1C).
We examined the clearance of ACK2 antibody in serum using a serial dilution assay (supplemental Methods). ACK2 concentration was estimated at 2-5 μg/mL serum at day 7 and was undetectable by day 10 (supplemental Figure 1). These results suggest that serum ACK2 levels were reduced by at least 200-fold 7 days after administration compared with the predicted level of 1 mg/mL on the day of ACK2 administration.
ACK2 followed by LD-IR allows stable engraftment of freshly isolated and lentivirus-transduced HSCs in wild-type and X-CGD mice
To determine whether marrow depletion of HSCs using ACK2 + LD-IR could allow engraftment of donor HSC in immunocompetent mice, 1 × 106 marrow cells from CD45.2+ C57 mice were transplanted into CD45.1+CD45.2+ (F1) recipients on day 7 after ACK2 ± LD-IR (see Figure 1A). In mice preconditioned with ACK2 + LD-IR, donor chimerism in PB leukocytes reached 79.3% ± 8.3%, while LD-IR alone permitted only 15.3% ± 4.6% engraftment 24 weeks posttransplantation (Figure 2A). In a second experiment, we transplanted 20 × 106 marrow cells into mice treated with ACK2 alone to exclude the possibility that a low number of donor HSCs may be insufficient to engraft in the host niche in this setting, while 1 × 106 cells were transplanted into mice conditioned with LD-IR or ACK2 + LD-IR. Donor chimerism in ACK2 + LD-IR–treated mice (76.5% ± 5.5%) was significantly higher than in the single-treatment groups: LD-IR group, 7.3% ± 2.6%; ACK2 group, 5.0% ± 2.5% (Figure 2B). The enhancement of PB donor chimerism in mice conditioned with ACK2 + LD-IR was multilineage (supplemental Figure 2A). Long-term donor chimerism in marrow lin− Sca-1+ KIT+ CD135−CD150+ HSC from primary recipients was similar to that in PB (supplemental Figure 2B), and donor chimerism following secondary transplantation into lethally irradiated recipients was similar to that seen in primary recipients (Figure 2C), further establishing that HSC depletion with ACK2 + LD-IR permits engraftment of multilineage, long-term repopulating HSCs.
ACK2 treatment plus LD-IR allows long-term engraftment of syngeneic wild-type bone marrow cells and gene-modified donor HSCs. (A) Posttransplantation donor chimerism of F1 C57/BoyJ mice conditioned with LD-IR (300 cGy) or ACK2 + LD-IR prior to transplantation with 1 × 106 freshly isolated C57 marrow cells. Recipients received 2 mg ACK2 or saline on day 1 and LD-IR on day 4, followed by transplantation on day 7. Peripheral blood was sampled at different times posttransplantation. Solid squares, ACK2 + LD-IR cohort; gray diamonds, LD-IR cohort. Donor cell chimerism at 24 weeks in ACK2 + LD-IR cohort was 79.3 ± 8.3% versus 15.3 ± 4.6% in LD-IR cohort (**P < .01, n = 4). (B) Posttransplant donor chimerism of F1 C57/BoyJ mice conditioned with saline, ACK2 alone, LD-IR (300 cGy), or ACK2 + LD-IR prior to transplantation with 20 × 106 (saline or ACK2) or 1 × 106 (LD-IR or ACK2 + IR) freshly isolated C57 marrow cells. PB leukocyte donor chimerism was determined 24 weeks posttransplantation. Bars represent the mean donor cell CD45.2 chimerism. **P < .01 (n = 4 in each group). (C) Secondarily transplanted donor HSCs from ACK2-treated mice give rise to long-term engraftment in secondary recipients. Marrow from 2 primary recipients in each cohort shown in Figure 2A was harvested at 24 weeks posttransplantation and for each primary recipient, 1 × 106 cells were transplanted into each of 4 lethally irradiated F1 C57/BoyJ mice. Donor chimerism in primary recipients was 3.4% and 5.2% for the 300 cGy mice and 71.2% and 69.3% for the ACK2 + LD-IR mice. Secondary recipients were analyzed for PB leukocyte donor chimerism 18 weeks after transplantation (mean ± standard deviation is shown). **P < .01 compared with 300 cGy cohort. One of 2 independent experiments is shown. (D) Conditioning with ACK2 + LD-IR prior to transplantation of lentivirus-tranduced cells in murine X-CGD. Lineage-negative BM cells from C57 X-CGD mice were purified using a MACS Separation System, then transduced in vitro with vesicular stomatitis virus–glycoprotein–pseudotyped CL20i4r-EF1a-gp91-OPT lentiviral vector overnight in the presence of interleukin-6 and SCF. Recipient F1C57/BoyJ X-CGD mice were treated with LD-IR (300 cGy) alone or ACK2 + LD-IR as described in panel A prior to transplantation with 5 × 105 transduced X-CGD lin- BM cells. n = 4 in each group. PB leukocyte donor chimerism and neutrophil NADPH oxidase activity was assayed at various times after transplantation. Different colored symbols represent individual mice. *P < .02 compared with LD-IR cohort. One of 2 independent experiments is shown.
ACK2 treatment plus LD-IR allows long-term engraftment of syngeneic wild-type bone marrow cells and gene-modified donor HSCs. (A) Posttransplantation donor chimerism of F1 C57/BoyJ mice conditioned with LD-IR (300 cGy) or ACK2 + LD-IR prior to transplantation with 1 × 106 freshly isolated C57 marrow cells. Recipients received 2 mg ACK2 or saline on day 1 and LD-IR on day 4, followed by transplantation on day 7. Peripheral blood was sampled at different times posttransplantation. Solid squares, ACK2 + LD-IR cohort; gray diamonds, LD-IR cohort. Donor cell chimerism at 24 weeks in ACK2 + LD-IR cohort was 79.3 ± 8.3% versus 15.3 ± 4.6% in LD-IR cohort (**P < .01, n = 4). (B) Posttransplant donor chimerism of F1 C57/BoyJ mice conditioned with saline, ACK2 alone, LD-IR (300 cGy), or ACK2 + LD-IR prior to transplantation with 20 × 106 (saline or ACK2) or 1 × 106 (LD-IR or ACK2 + IR) freshly isolated C57 marrow cells. PB leukocyte donor chimerism was determined 24 weeks posttransplantation. Bars represent the mean donor cell CD45.2 chimerism. **P < .01 (n = 4 in each group). (C) Secondarily transplanted donor HSCs from ACK2-treated mice give rise to long-term engraftment in secondary recipients. Marrow from 2 primary recipients in each cohort shown in Figure 2A was harvested at 24 weeks posttransplantation and for each primary recipient, 1 × 106 cells were transplanted into each of 4 lethally irradiated F1 C57/BoyJ mice. Donor chimerism in primary recipients was 3.4% and 5.2% for the 300 cGy mice and 71.2% and 69.3% for the ACK2 + LD-IR mice. Secondary recipients were analyzed for PB leukocyte donor chimerism 18 weeks after transplantation (mean ± standard deviation is shown). **P < .01 compared with 300 cGy cohort. One of 2 independent experiments is shown. (D) Conditioning with ACK2 + LD-IR prior to transplantation of lentivirus-tranduced cells in murine X-CGD. Lineage-negative BM cells from C57 X-CGD mice were purified using a MACS Separation System, then transduced in vitro with vesicular stomatitis virus–glycoprotein–pseudotyped CL20i4r-EF1a-gp91-OPT lentiviral vector overnight in the presence of interleukin-6 and SCF. Recipient F1C57/BoyJ X-CGD mice were treated with LD-IR (300 cGy) alone or ACK2 + LD-IR as described in panel A prior to transplantation with 5 × 105 transduced X-CGD lin- BM cells. n = 4 in each group. PB leukocyte donor chimerism and neutrophil NADPH oxidase activity was assayed at various times after transplantation. Different colored symbols represent individual mice. *P < .02 compared with LD-IR cohort. One of 2 independent experiments is shown.
We then sought to implement ACK2 + LD-IR as conditioning for transplantation of X-CGD mice with HSC transduced with a lentiviral vector for gp91phox expression. In mice transplanted with of 5 × 105 CL20-gp91-OPT10 –transduced lin− cells, donor chimerism at 20 weeks posttransplantation was only approximately 1.0% in recipients conditioned with LD-IR but ranged from 8.0% to 76.0% (45.5% ± 32.1% mean ± standard deviation) in mice conditioned with ACK2 + LD-IR (Figure 2D). The percentage of DHR-positive neutrophils in this latter cohort ranged from 9.1% to 42.0% at 20 weeks (Figure 2D), indicating engraftment of CL20-gp91-OPT–transduced HSC and expression of gp91phox in neutrophils to reconstitute NADPH oxidase activity. This fraction of functional phagocytes is expected to provide significant activity against pathogenic microbes.1,5,14
In summary, we show for the first time that combination treatment with the KIT-blocking antibody ACK2 and LD-IR nearly ablates marrow HSCs in immunocompetent mice, as measured by a HSC competitive repopulation assay. This combination enables substantial and durable engraftment after transplantation of modest numbers of congenic donor BM. ACK2 given as a single agent transiently reduced HSPCs in immunocompetent mice, as in a previous report8 ; however, HSC recovery was rapid and LTRA was unaffected on day 7 after administration. These findings likely explain why there is little engraftment of congenic donor marrow in immunocompetent mice conditioned with ACK2 alone, even with transplantation of high cell doses. Our data further demonstrate that conditioning with ACK2 and LD-IR in X-CGD mice enables efficient engraftment of HSC cultured ex vivo for lentivirus-mediated gene transfer in a murine model of autologous transplantation for gene therapy. We conclude that ACK2 combined with LD-IR is a promising approach for reducing exposure to genotoxic agents while still achieving substantial cytoreduction of endogenous marrow to facilitate engraftment of transplanted HSCs. This conditioning strategy may be useful for gene therapy of CGD and other inherited blood cell disorders for which no selective advantage exists for gene correction, and may also be applicable for depletion of recipient HSCs in allogeneic transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Catherine Matthews for help with preparing the manuscript and Karl Staser and Wade Clapp for bone marrow–derived mast cells as controls for ACK2 staining. We also thank Harry Malech, Uimook Choi, Suk See De Ravin, and Elizabeth Kang (Laboratory for Host Defenses, Genetic Immunotherapy Section, National Institute of Allergy and Infectious Diseases) for the CL20-gp91-OPT lentiviral vector.
This work was supported by National Institutes of Health grants P01 HL53586 (M.C.D., M.C.Y., and E.F.S.) and the Riley Children's Foundation (M.C.D. and M.C.Y.). The Indiana University Simon Cancer Center (P30 CA082709) provided partial support for flow cytometry and mouse cores.
National Institutes of Health
Authorship
Contribution: X.X., N.K.P., W.C.S., M.C.Y., and M.C.D. designed, performed, and analyzed experiments; X.X., M.C.Y., and M.C.D. drafted the manuscript and figures; E.F.S. helped with experimental design, analysis, and editing of the manuscript; and M.C.D. oversaw the project including experimental design, analysis, interpretation of the data, and preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.C.D. is Department of Pediatrics, Washington University School of Medicine, St Louis, MO.
Correspondence: Mary Dinauer, Washington University School of Medicine, 660 South Euclid Ave, Box 8208, St Louis, MO 63100; e-mail: dinauer_m@kids.wustl.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal