Abstract
BAALC and ERG expression levels are prognostic markers in younger (< 60 years) cytogenetically normal acute myeloid leukemia (CN-AML) adults; their prognostic impact in older (≥ 60 years) patients requires further investigation. We evaluated pretreatment expression of BAALC and ERG in 158 de novo patients treated on cytarabine/daunorubicin-based protocols. The patients were also characterized for other established molecular prognosticators. Low BAALC and ERG expression levels were associated with better outcome in univariable and multivariable analyses. Expression levels of both BAALC and ERG were the only factors significantly associated with overall survival upon multivariable analysis. To gain biological insights, we derived gene expression signatures associated with BAALC and ERG expression in older CN-AML patients. Furthermore, we derived the first microRNA expression signatures associated with the expression of these 2 genes. In low BAALC expressers, genes associated with undifferentiated hematopoietic precursors and unfavorable outcome predictors were down-regulated, whereas HOX genes and HOX-gene–embedded microRNAs were up-regulated. Low ERG expressers presented with down-regulation of genes involved in the DNA-methylation machinery, and up-regulation of miR-148a, which targets DNMT3B. We conclude that in older CN-AML patients, low BAALC and ERG expression associates with better outcome and distinct gene and microRNA expression signatures that could aid in identifying new targets and novel therapeutic strategies for older patients.
Introduction
Acute myeloid leukemia (AML) is a cytogenetically and molecularly heterogeneous disease characterized by clonal proliferation of myeloid precursors and maturation arrest. Despite progress in our understanding of the biology of this disease and investigation of therapies targeting distinct clinical, cytogenetic, and/or molecular subsets, outcome remains poor for the majority of patients. This is especially true for patients aged 60 years or more, of whom only approximately 7%-15% achieve long-term survival.1,2 The reasons for the poor outcome of this older patient population may not only relate to higher frequencies of secondary disease (ie, AML after antecedent hematologic disorders and/or therapy-related disease), high-risk cytogenetics, clinical comorbid conditions, and poor performance status, but also to the presence of specific molecular genetic alterations, including gene mutations and changes in gene expression.3
To date, the prognostic significance of molecular genetic alterations has been studied most extensively in younger (< 60 years) patients and found its maximum applicability in cytogenetically normal AML (CN-AML), which constitutes the largest AML subset.4 In this cytogenetic subset, mutations in the NPM15 and CEBPA genes,6,7 and lower expression levels of the BAALC8,9 and ERG10,11 genes have been associated with favorable outcome, whereas internal tandem duplication of the FLT3 gene (FLT3-ITD)12 and WT1 mutations13 have been shown to confer adverse prognosis. Furthermore, because these molecular alterations are not mutually exclusive, combinations of 2 or more of them have been used to refine prognostication of CN-AML patients and are recommended by best practice guidelines for cytogenetic/molecular risk stratification of AML patients.14
CN-AML is also the largest cytogenetic subset among patients aged 60 years or older.1,2 But, despite the relatively large number of patients presenting with this feature, few studies have investigated the prognostic significance of molecular markers in this age group. Recently, we reported a study demonstrating that NPM1 mutations are associated with a more favorable outcome in older CN-AML patients.15 However, to our knowledge, studies testing the prognostic impact of BAALC and ERG gene expression levels in relatively large cohorts of older CN-AML patients have not been reported.
The BAALC (brain and acute leukemia, cytoplasmic) gene, located at chromosome band 8q22.3, was cloned in the course of research aimed at the identification of genes associated with a trisomy of chromosome 8 in AML.16 High levels of BAALC expression were, indeed, found in AML patients with trisomy 8, but also in a subset of CN-AML patients.8,16 The ETS-related gene, ERG, located at chromosome band 21q22.3, was first shown to be involved in leukemogenesis as a fusion partner with the FUS gene in the rare, but recurrent in AML, t(16;21)(p11;q22).17 Moreover, ERG overexpression was demonstrated in AML patients with complex karyotypes with cryptic amplification of chromosome 21,18 which was first discovered using spectral karyotyping,19 and it was also found in a fraction of patients with CN-AML.10 Our group was the first to report that high expression levels of the BAALC and ERG genes contribute to poor prognosis in younger CN-AML patients,8,10 and these results have been corroborated by others.20-24
Herein, we sought to determine the prognostic impact of the expression of BAALC and ERG in older de novo CN-AML patients. We show, for the first time, that low levels of expression of these 2 genes are significantly associated with improved outcome in older CN-AML patients, even after adjustment for other prognostic clinical and molecular variables, including NPM1 mutations. In addition, using genome-wide microarray profiling, we reveal changes in the expression of specific genes and microRNAs in low BAALC and ERG expressers that may contribute to the less aggressive disease in these patients. These biological features may potentially be exploited and lead to new treatment strategies.
Methods
Patients and treatment
A total of 158 patients aged 60 years or older with de novo CN-AML, who had pretreatment blood available, were analyzed for BAALC and ERG expression. The patients were treated with intensive cytarabine/daunorubicin-based regimens on Cancer and Leukemia Group B (CALGB) front-line clinical protocols 8525, 8923, 9420, 9720, or 10201 (see supplemental Methods for details, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Patients with antecedent hematologic disorders or therapy-related AML, and those transplanted in first complete remission (CR), were excluded. The Ohio State University Institutional Review Board–approved, written informed consent for participation in these studies was obtained from all patients in accordance with the Declaration of Helsinki.
Cytogenetics and additional mutation markers
Pretreatment cytogenetic analyses of bone marrow (BM) were performed by CALGB-approved institutional cytogenetic laboratories as part of CALGB 8461, a prospective cytogenetic companion study, and the results were reviewed centrally.25 To be considered cytogenetically normal, at least 20 metaphase cells had to be analyzed and the karyotype found to be normal.25
RNA extraction and real-time RT-PCR to measure BAALC and ERG expression levels
Preparation of pretreatment blood samples and analysis of BAALC and ERG expression were performed as previously described.8-11 Briefly, total RNA was extracted using the Trizol method and complementary DNA was synthesized from total RNA. Quantitative real-time reverse-transcription–polymerase chain reaction (RT-PCR) amplification of BAALC, ERG, and ABL1 was performed using standard curves. BAALC and ERG expression levels are reported as copy numbers normalized to ABL1 copy numbers.
Gene and microRNA expression profiling
For gene and microRNA expression profiling, total RNA was extracted from pretreatment BM or blood mononuclear cells. The gene and micro RNA expression profiling was performed using the Affymetrix U133 plus 2.0 array (Affymetrix) and The Ohio State University custom microRNA array (OSU_CCC Version 4.0), respectively, as previously reported15,28 and detailed in supplemental Methods. The microarray data are available on ArrayExpress under accession numbers E-TABM-1071 and E-TABM-1072.
Definition of clinical endpoints and statistical analysis
The main objective of this study was to evaluate the prognostic impact of BAALC and ERG expression on clinical outcome in older CN-AML patients. The median BAALC/ABL1 or ERG/ABL1 copy number values, respectively, were used to define low and high BAALC or ERG expressers. This cutoff was based on the trends for overall survival (OS) of patients divided into quartiles by expression values, where patients in quartile 1 had better outcome than patients in quartile 2, followed by patients in quartiles 3 and 4 for BAALC and ERG expression (P < .001 and P < .001, test for trend29 ).
Definitions of clinical endpoints (eg, CR, disease-free survival [DFS] and OS) and details of statistical analyses, including variable selection for statistical modeling, are provided in the supplemental material. Associations between patients with low or high expression of BAALC or ERG for baseline demographic, clinical, and molecular features were compared using the Fisher exact and Wilcoxon rank-sum tests for categorical and continuous variables, respectively. Estimated probabilities of DFS and OS were calculated using the Kaplan-Meier method, and the log-rank test evaluated differences between survival distributions. Multivariable logistic regression models were constructed to analyze factors related to the probability of achieving CR using a limited backward selection procedure. Multivariable proportional hazards models were constructed for DFS and OS to evaluate the impact of BAALC and ERG expression (low/high) by adjusting for other variables using a limited backward selection procedure. For achievement of CR, estimated odds ratios, and for survival endpoints, hazard ratios with their corresponding 95% confidence intervals were obtained for each significant prognostic factor.
For the gene and microRNA expression profiling, summary measures of gene and microRNA expression were computed, normalized, and filtered (see supplemental material). The profiles were derived by comparing gene and microRNA expression between low and high BAALC expressers and between low and high ERG expressers. Univariable significance levels of .001 for gene and .005 for microRNA expression profiling were used to determine, respectively, the probe sets and microRNA probes that comprised the signatures.
All analyses were performed by the CALGB Statistical Center.
Results
Associations of BAALC and ERG expression with clinical and molecular characteristics
At diagnosis, low BAALC expression was associated with mutated NPM1 (P < .001), wild-type CEBPA (P = .02), and low ERG expression levels (P < .001; Table 1).
Clinical and molecular characteristics at diagnosis according to BAALC and ERG expression status in older CN-AML patients
| Characteristic . | Low BAALC (n = 79) . | High BAALC (n = 79) . | P . | Low ERG (n = 79) . | High ERG (n = 79) . | P . |
|---|---|---|---|---|---|---|
| Age, y | .17 | .95 | ||||
| Median | 67 | 69 | 68 | 69 | ||
| Range | 60-79 | 60-83 | 60-81 | 60-83 | ||
| Sex, no. (%) | .52 | .11 | ||||
| Male | 46 (58) | 41 (52) | 38 (48) | 49 (62) | ||
| Female | 33 (42) | 38 (48) | 41 (52) | 30 (38) | ||
| Race, no. (%) | .40 | 1.0 | ||||
| White | 73 (94) | 68 (89) | 70 (92) | 71 (91) | ||
| Nonwhite | 5 (6) | 8 (11) | 6 (8) | 7 (9) | ||
| Hemoglobin, g/dL | .43 | .76 | ||||
| Median | 9.6 | 9.5 | 9.5 | 9.5 | ||
| Range | 5.4-15.0 | 6.8-14.5 | 5.4-13.6 | 6.0-15.0 | ||
| Platelet count, ×109/L | .31 | .51 | ||||
| Median | 55 | 67 | 69 | 55 | ||
| Range | 5-481 | 4-850 | 4-271 | 11-850 | ||
| WBC count, ×109/L | .78 | .005 | ||||
| Median | 27.8 | 33.5 | 20.6 | 38.0 | ||
| Range | 1.4-450.0 | 1.0-173.1 | 1.1-234.5 | 1.0-450.0 | ||
| Blood blasts, % | .36 | < .001 | ||||
| Median | 52 | 59 | 34 | 68 | ||
| Range | 0–97 | 0–99 | 0–93 | 0–99 | ||
| BM blasts, % | .80 | .001 | ||||
| Median | 70 | 67 | 59 | 75 | ||
| Range | 11-97 | 7-97 | 7-97 | 23-97 | ||
| Extramedullary involvement, no. (%) | 16 (21) | 19 (25) | .70 | 16 (21) | 19 (25) | .56 |
| NPM1, no. (%) | < .001 | .33 | ||||
| Mutated | 64 (81) | 33 (42) | 45 (57) | 52 (66) | ||
| Wild-type | 15 (19) | 46 (58) | 34 (43) | 27 (34) | ||
| FLT3-ITD, no. (%) | .14 | < .001 | ||||
| Present | 24 (30) | 34 (43) | 12 (15) | 46 (58) | ||
| Absent | 55 (70) | 45 (57) | 67 (85) | 33 (42) | ||
| FLT3-TKD, no. (%) | .61 | .61 | ||||
| Present | 10 (13) | 7 (9) | 10 (13) | 7 (9) | ||
| Absent | 69 (87) | 72 (91) | 69 (87) | 72 (91) | ||
| WT1, no. (%) | .21 | .21 | ||||
| Mutated | 3 (4) | 8 (10) | 3 (4) | 8 (10) | ||
| Wild-type | 76 (96) | 71 (90) | 76 (96) | 71 (90) | ||
| CEBPA, no. (%) | .02 | .65 | ||||
| Mutated | 6 (8) | 17 (22) | 10 (13) | 13 (16) | ||
| Wild-type | 73 (92) | 62 (78) | 69 (87) | 66 (84) | ||
| IDH1, no. (%) | .09 | .40 | ||||
| Mutated | 4 (6) | 10 (17) | 5 (8) | 9 (15) | ||
| Wild-type | 59 (94) | 50 (83) | 59 (92) | 53 (85) | ||
| IDH2*, no. (%) | .30 | .10 | ||||
| R140 IDH2 | 18 (29) | 7 (12) | 17 (28) | 8 (13) | ||
| R172 IDH2 | 0 (0) | 5 (8) | 2 (3) | 3 (5) | ||
| Wild-type | 45 (71) | 48 (80) | 42 (69) | 51 (82) | ||
| MLL-PTD, no. (%) | .68 | .42 | ||||
| Present | 2 (4) | 4 (7) | 4 (7) | 2 (3) | ||
| Absent | 55 (96) | 55 (93) | 51 (93) | 59 (97) | ||
| ERG expression†, no. (%) | < .001 | |||||
| Low | 51 (65) | 28 (35) | ||||
| High | 28 (35) | 51 (65) | ||||
| BAALC expression†, no. (%) | < .001 | |||||
| Low | 51 (65) | 28 (35) | ||||
| High | 28 (35) | 51 (65) |
| Characteristic . | Low BAALC (n = 79) . | High BAALC (n = 79) . | P . | Low ERG (n = 79) . | High ERG (n = 79) . | P . |
|---|---|---|---|---|---|---|
| Age, y | .17 | .95 | ||||
| Median | 67 | 69 | 68 | 69 | ||
| Range | 60-79 | 60-83 | 60-81 | 60-83 | ||
| Sex, no. (%) | .52 | .11 | ||||
| Male | 46 (58) | 41 (52) | 38 (48) | 49 (62) | ||
| Female | 33 (42) | 38 (48) | 41 (52) | 30 (38) | ||
| Race, no. (%) | .40 | 1.0 | ||||
| White | 73 (94) | 68 (89) | 70 (92) | 71 (91) | ||
| Nonwhite | 5 (6) | 8 (11) | 6 (8) | 7 (9) | ||
| Hemoglobin, g/dL | .43 | .76 | ||||
| Median | 9.6 | 9.5 | 9.5 | 9.5 | ||
| Range | 5.4-15.0 | 6.8-14.5 | 5.4-13.6 | 6.0-15.0 | ||
| Platelet count, ×109/L | .31 | .51 | ||||
| Median | 55 | 67 | 69 | 55 | ||
| Range | 5-481 | 4-850 | 4-271 | 11-850 | ||
| WBC count, ×109/L | .78 | .005 | ||||
| Median | 27.8 | 33.5 | 20.6 | 38.0 | ||
| Range | 1.4-450.0 | 1.0-173.1 | 1.1-234.5 | 1.0-450.0 | ||
| Blood blasts, % | .36 | < .001 | ||||
| Median | 52 | 59 | 34 | 68 | ||
| Range | 0–97 | 0–99 | 0–93 | 0–99 | ||
| BM blasts, % | .80 | .001 | ||||
| Median | 70 | 67 | 59 | 75 | ||
| Range | 11-97 | 7-97 | 7-97 | 23-97 | ||
| Extramedullary involvement, no. (%) | 16 (21) | 19 (25) | .70 | 16 (21) | 19 (25) | .56 |
| NPM1, no. (%) | < .001 | .33 | ||||
| Mutated | 64 (81) | 33 (42) | 45 (57) | 52 (66) | ||
| Wild-type | 15 (19) | 46 (58) | 34 (43) | 27 (34) | ||
| FLT3-ITD, no. (%) | .14 | < .001 | ||||
| Present | 24 (30) | 34 (43) | 12 (15) | 46 (58) | ||
| Absent | 55 (70) | 45 (57) | 67 (85) | 33 (42) | ||
| FLT3-TKD, no. (%) | .61 | .61 | ||||
| Present | 10 (13) | 7 (9) | 10 (13) | 7 (9) | ||
| Absent | 69 (87) | 72 (91) | 69 (87) | 72 (91) | ||
| WT1, no. (%) | .21 | .21 | ||||
| Mutated | 3 (4) | 8 (10) | 3 (4) | 8 (10) | ||
| Wild-type | 76 (96) | 71 (90) | 76 (96) | 71 (90) | ||
| CEBPA, no. (%) | .02 | .65 | ||||
| Mutated | 6 (8) | 17 (22) | 10 (13) | 13 (16) | ||
| Wild-type | 73 (92) | 62 (78) | 69 (87) | 66 (84) | ||
| IDH1, no. (%) | .09 | .40 | ||||
| Mutated | 4 (6) | 10 (17) | 5 (8) | 9 (15) | ||
| Wild-type | 59 (94) | 50 (83) | 59 (92) | 53 (85) | ||
| IDH2*, no. (%) | .30 | .10 | ||||
| R140 IDH2 | 18 (29) | 7 (12) | 17 (28) | 8 (13) | ||
| R172 IDH2 | 0 (0) | 5 (8) | 2 (3) | 3 (5) | ||
| Wild-type | 45 (71) | 48 (80) | 42 (69) | 51 (82) | ||
| MLL-PTD, no. (%) | .68 | .42 | ||||
| Present | 2 (4) | 4 (7) | 4 (7) | 2 (3) | ||
| Absent | 55 (96) | 55 (93) | 51 (93) | 59 (97) | ||
| ERG expression†, no. (%) | < .001 | |||||
| Low | 51 (65) | 28 (35) | ||||
| High | 28 (35) | 51 (65) | ||||
| BAALC expression†, no. (%) | < .001 | |||||
| Low | 51 (65) | 28 (35) | ||||
| High | 28 (35) | 51 (65) |
BM, bone marrow; FLT3-ITD, internal tandem duplication of the FLT3 gene; FLT3-TKD, tyrosine kinase domain mutation of the FLT3 gene; MLL-PTD, partial tandem duplication of the MLL gene; WBC, white blood count.
P value is for the comparison of IDH2 mutated (R140 or R172) versus IDH2 wild-type.
The median expression value was used as a cut point.
Low ERG expression was associated with lower white blood count (WBC; P = .005), lower percentages of blood (P < .001) and BM (P = .001) blasts, the absence of FLT3-ITD (P < .001), and low BAALC expression (P < .001; Table 1).
Prognostic value of BAALC and ERG expression
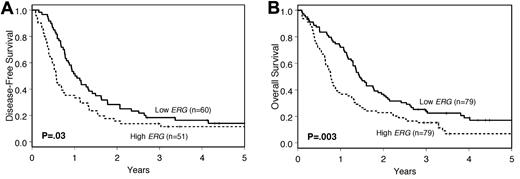
Low BAALC expressers had a higher CR rate (86% vs. 54%; P < .001) and longer DFS (P = .01; Figure 1A) and OS (P < .001; Figure 1B) than high expressers (Table 2). Similarly, patients with low ERG expression had a trend for better CR rates (76% vs. 65%; P = .12) and longer DFS (P = .03; Figure 2A) and OS (P = .003; Figure 2B; Table 2). The median follow-up for patients alive was 3.8 years (range: 2.8-11.6 years).
Outcome of cytogenetically normal older AML patients according to BAALC expression levels. (A) DFS. (B) OS.
Outcome of cytogenetically normal older AML patients according to BAALC expression levels. (A) DFS. (B) OS.
Outcomes according to BAALC and ERG expression in older CN-AML patients
| Outcome . | Low BAALC (n = 79) . | High BAALC (n = 79) . | P . | Low ERG (n = 79) . | High ERG (n = 79) . | P . |
|---|---|---|---|---|---|---|
| Complete remission rate, no. (%) | 68 (86) | 43 (54) | < .001 | 60 (76) | 51 (65) | .12 |
| Disease-free survival | .01 | .03 | ||||
| Median, y | 1.0 | 0.6 | 1.0 | 0.6 | ||
| Disease-free at 3 y, % (95% CI) | 19 (11-29) | 12 (4-23) | 18 (10-29) | 14 (6-25) | ||
| Overall survival | < .001 | .003 | ||||
| Median, y | 1.5 | 0.8 | 1.4 | 0.8 | ||
| Alive at 3 y, % (95% CI) | 29 (19-39) | 10 (5-18) | 24 (15-34) | 15 (8-24) |
| Outcome . | Low BAALC (n = 79) . | High BAALC (n = 79) . | P . | Low ERG (n = 79) . | High ERG (n = 79) . | P . |
|---|---|---|---|---|---|---|
| Complete remission rate, no. (%) | 68 (86) | 43 (54) | < .001 | 60 (76) | 51 (65) | .12 |
| Disease-free survival | .01 | .03 | ||||
| Median, y | 1.0 | 0.6 | 1.0 | 0.6 | ||
| Disease-free at 3 y, % (95% CI) | 19 (11-29) | 12 (4-23) | 18 (10-29) | 14 (6-25) | ||
| Overall survival | < .001 | .003 | ||||
| Median, y | 1.5 | 0.8 | 1.4 | 0.8 | ||
| Alive at 3 y, % (95% CI) | 29 (19-39) | 10 (5-18) | 24 (15-34) | 15 (8-24) |
CI indicates confidence interval.
Outcome of cytogenetically normal older AML patients according to ERG expression levels. (A) DFS. (B) OS.
Outcome of cytogenetically normal older AML patients according to ERG expression levels. (A) DFS. (B) OS.
In a multivariable model for CR attainment (see supplemental material for full model building description), BAALC expression remained a significant prognosticator (P < .001) after adjustment for NPM1 mutation status (P = .04) and WBC (P = .02; Table 3). The odds for achieving a CR were more than 4 times higher for the low BAALC-expressing group than for high BAALC expressers. BAALC expression, but not ERG expression, was also a significant factor for DFS (P = .03), after adjustment for the FLT3-ITD (P < .001) and age (P = .05; Table 3). The risk of relapse or death was reduced by 36% for low BAALC expressers. Furthermore, expression levels of both BAALC and ERG were the only factors significantly associated with OS upon multivariable analysis (P < .001 and P = .03, respectively; Table 3). The risk of death was reduced by approximately one-half in the low BAALC-expressing group and by approximately one-third in the low ERG-expressing group.
Multivariable logistic regression analysis for achievement of CR, DFS, and OS in older CN-AML patients
| Group . | CR* . | DFS† . | OS‡ . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| BAALC, low vs high | 4.32 | 1.83-10.20 | < .001 | 0.64 | 0.42-0.97 | .03 | 0.49‖ | 0.34-0.71 | <.001 |
| ERG, low vs high | 0.69 | 0.48-0.97 | .03 | ||||||
| NPM1, mutated vs wild-type | 2.34 | 1.03-5.35 | .04 | ||||||
| FLT3-ITD, present vs absent | 1.79§ | 1.11-2.87 | < .001 | ||||||
| WBC, each 50-unit increase | 0.68 | 0.49-0.93 | .02 | ||||||
| Age, each 10-year increase | 0.65 | 0.42-1.00 | .05 | ||||||
| Group . | CR* . | DFS† . | OS‡ . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| OR . | 95% CI . | P . | HR . | 95% CI . | P . | HR . | 95% CI . | P . | |
| BAALC, low vs high | 4.32 | 1.83-10.20 | < .001 | 0.64 | 0.42-0.97 | .03 | 0.49‖ | 0.34-0.71 | <.001 |
| ERG, low vs high | 0.69 | 0.48-0.97 | .03 | ||||||
| NPM1, mutated vs wild-type | 2.34 | 1.03-5.35 | .04 | ||||||
| FLT3-ITD, present vs absent | 1.79§ | 1.11-2.87 | < .001 | ||||||
| WBC, each 50-unit increase | 0.68 | 0.49-0.93 | .02 | ||||||
| Age, each 10-year increase | 0.65 | 0.42-1.00 | .05 | ||||||
Odds ratios greater than (less than) 1.0 mean higher (lower) CR rate for the higher values of the continuous variables and the first category listed for the categorical variables. Hazard ratios greater than (less than) 1.0 indicate higher (lower) risk for relapse or death (DFS) or death (OS) for the higher values of the continuous variables and the first category listed for the categorical variables.
Variables considered in the model based on univariable analyses were BAALC expression (low vs high; median cut), ERG expression (low vs high; median cut), NPM1 (mutated vs wild-type), WBC (in 50-unit increments), and age (in 10-year increments).
Variables considered in the model based on univariable analyses were BAALC expression (low vs high; median cut), ERG expression (low vs high; median cut), FLT3-ITD (positive vs negative), FLT3-TKD (positive vs negative), and age (in 10-year increments).
Variables considered in the model based on univariable analyses were BAALC expression (low vs high; median cut), ERG expression (low vs high; median cut), NPM1 (mutated vs wild-type), FLT3-ITD (positive vs negative), and platelet count.
Does not meet the proportional hazards assumption. Low BAALC was associated with better OS until approximately 2 years, and after that, did not seem to impact OS. The hazard ratio for BAALC, low vs high, is reported at 1 year.
Does not meet the proportional hazards assumption. FLT3-ITD was associated with worse DFS until approximately 1 year, and after that, its adverse impact for DFS declined. The hazard ratio for FLT3-ITD is reported at 9 months.
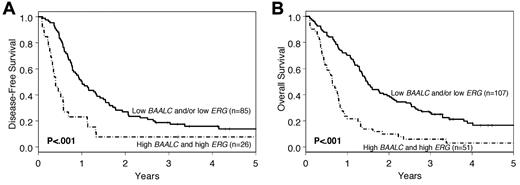
Because both expression markers were significantly associated with OS, we investigated the combination of the 2 markers. Patients who had lower expression of BAALC or ERG, or both, had a significantly longer DFS and OS (P < .001 for both DFS and OS; Figure 3) than those expressing both markers at high levels. At 3 years, 26% of the patients with low expression of BAALC or ERG, or both, were alive, compared with only 6% of patients who expressed BAALC and ERG at high levels. However, there were no significant differences in outcome between patients with low expression of both BAALC and ERG and those who had low expression of only 1 of these 2 genes.
Outcome of cytogenetically normal older AML patients according to a combination of BAALC and ERG expression levels. Patients with low expression of BAALC or ERG, or both, were compared with those having high expression of both BAALC and ERG. (A) DFS. (B) OS.
Outcome of cytogenetically normal older AML patients according to a combination of BAALC and ERG expression levels. Patients with low expression of BAALC or ERG, or both, were compared with those having high expression of both BAALC and ERG. (A) DFS. (B) OS.
We recently reported a strong prognostic impact of NPM1 mutations on CR rates, DFS, and OS in older CN-AML patients.15 However, in the set of patients investigated here, NPM1 mutation status was retained only in our model for CR. Therefore, we compared different multivariable models for CR attainment, DFS, and OS using the Akaike information criterion (AIC; supplemental Table 1A-C). We found that the multivariable model for DFS that included BAALC expresser status, FLT3-ITD mutation status, and age, excluding NPM1 mutation status, appeared to be better than other evaluated models that comprised NPM1 mutation status (supplemental Table 1B). For OS, we found that a model including only the BAALC and ERG expresser status was better than 2 other evaluated models that comprised NPM1 mutation status (supplemental Table 1C).
We have also recently reported a trend toward a lower CR rate and shorter OS in older CN-AML patients harboring WT1 mutations.13 This report suggested that the impact of WT1 mutation status might be obscured by the generally poor outcome of older CN-AML patients. In the current study, we only had 11 patients who harbored WT1 mutations, and we did not see a significant impact of WT1 mutation status on outcome upon univariable analyses. Furthermore, only 3 of the 11 WT1-mutated cases were low BAALC or low ERG expressers. Therefore, we were not able to develop multivariable regression models for outcome that would have evaluated WT1 mutations in the context of low BAALC or ERG expression status.
Genome-wide gene expression profiling
To gain insights into the biology of older CN-AML patients, we derived gene expression signatures associated with BAALC and ERG expresser status.
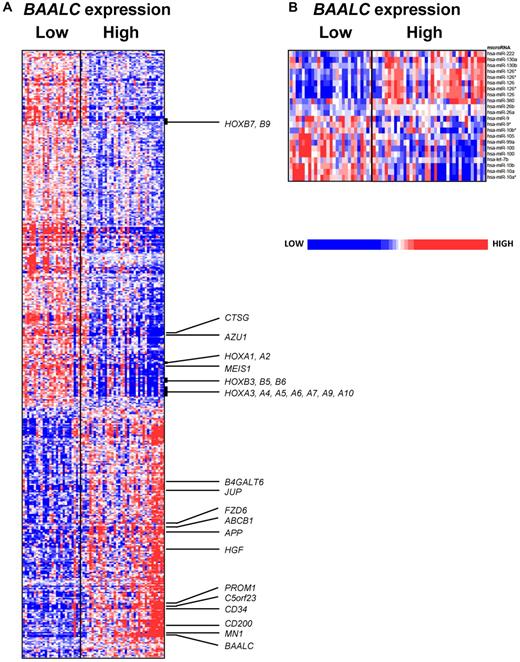
We observed that BAALC expression was associated with the differential expression of 482 probe sets, representing 292 annotated genes. Of these, 283 probe sets, which represented 165 annotated genes, were up-regulated, and 199 probe sets, representing 127 annotated genes, were down-regulated in low BAALC expressers (Figure 4A; supplemental Table 2). Probe sets representing BAALC were among the most down-regulated probe sets in low BAALC expressers, confirming our real-time RT-PCR results. The gene expression signature of BAALC derived in older CN-AML patients was very similar to that we reported in patients younger than 60 years.9 Low BAALC expression was associated with up-regulation of genes of the HOXA and HOXB clusters, as well as the HOX cofactor, MEIS1, that are important for developmental processes and hematopoietic stem cell function.30 We also observed a higher expression of CTSG and AZU1—genes that are expressed in mature neutrophils.31 As in younger patients and consistent with the more favorable prognosis associated with low BAALC expression, we found that low BAALC expressers had down-regulation of genes previously associated with worse outcome in CN-AML, including the transcription coregulator, MN1,32 the surface marker, CD200,33 and the growth factor, HGF34 (Figure 4A). Furthermore, we observed a down-regulation of genes found expressed in less differentiated precursors, such as PROM1, CD34, JUP, C5orf23, FZD6, B4GALT6, and APP (Figure 4A),18,35,36 and ABCB1 (MDR1), a gene encoding the multidrug resistance protein, whose high expression has been associated with worse outcome in older AML patients.37
Heat maps of the BAALC signatures. Derived gene expression (A) and microRNA expression signatures (B) associated with BAALC expression in the group of CN-AML patients ≥ 60 years. Patients are ordered from left to right by increasing BAALC expression. Expression values of the gene probe sets (microRNA probes) are represented by color, with blue indicating expression less than and red indicating expression greater than the median value for the given gene probe set (microRNA probe). For the gene expression heat map, up- and down-regulated genes mentioned in the text are indicated.
Heat maps of the BAALC signatures. Derived gene expression (A) and microRNA expression signatures (B) associated with BAALC expression in the group of CN-AML patients ≥ 60 years. Patients are ordered from left to right by increasing BAALC expression. Expression values of the gene probe sets (microRNA probes) are represented by color, with blue indicating expression less than and red indicating expression greater than the median value for the given gene probe set (microRNA probe). For the gene expression heat map, up- and down-regulated genes mentioned in the text are indicated.
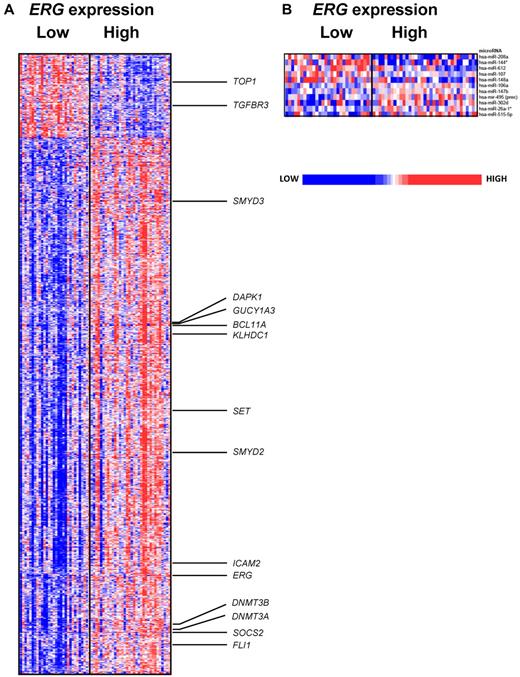
The gene expression signature associated with ERG expression comprised 1554 differentially expressed probe sets, representing 1089 genes, of which 208 probe sets, representing 127 genes, were up-regulated, and 1346 probe sets, representing 962 genes, were down-regulated in low ERG expressers (Figure 5A; supplemental Table 3). Probe sets representing ERG were among the most down-regulated in low ERG expressers, again confirming our real-time RT-PCR results. We found previously identified putative ERG targets and binding partners, such as ICAM2, SOCS2, and FLI1,38,39 down-regulated in low ERG expressers. Also down-regulated were DNMT3A and DNMT3B, genes associated with aberrant DNA hypermethylation in AML40 ; SET, which inhibits active DNA demethylation via chromatin modification and inhibits the activation of the tumor suppressor, PP2A phosphatase41,42 ; and the histone methyltransferases, SMYD2 and SMYD3, that have a role in cell proliferation in cancer.43,44 Among the overexpressed genes associated with low ERG levels were TGFBR3, which was shown to suppress breast cancer progression,45 and TOP1, which has been associated with enhanced sensitivity to chemotherapy.46 Furthermore, when we compared the gene expression signature of ERG derived in older CN-AML patients to the signature reported in patients younger than 60 years, we found them to be very similar.10 We found that genes such as BCL11A, DAPK1, GUCY1A3, and KLHDC1,10 were also down-regulated in low ERG expressers in the current study.
Heat maps of the ERG signatures. Derived gene expression (A) and microRNA expression signatures (B) associated with ERG expression in the group of CN-AML patients ≥ 60 years. Patients are ordered from left to right by increasing ERG expression. Expression values of the gene probe sets (microRNA probes) are represented by color, with blue indicating expression less than and red indicating expression greater than the median value for the given gene probe set (microRNA probe). For the gene expression heat map, up- and down-regulated genes mentioned in the text are indicated.
Heat maps of the ERG signatures. Derived gene expression (A) and microRNA expression signatures (B) associated with ERG expression in the group of CN-AML patients ≥ 60 years. Patients are ordered from left to right by increasing ERG expression. Expression values of the gene probe sets (microRNA probes) are represented by color, with blue indicating expression less than and red indicating expression greater than the median value for the given gene probe set (microRNA probe). For the gene expression heat map, up- and down-regulated genes mentioned in the text are indicated.
Genome-wide microRNA expression profiling
To further investigate the biology related to low BAALC and ERG expression, we derived microRNA expression signatures associated with BAALC and ERG expresser status. For the first time, we were able to derive a microRNA expression signature associated with BAALC expression (Figure 4B). We found 22 differentially expressed probes, representing 18 microRNAs, with 10 up-regulated and 8 down-regulated in low BAALC-expressing patients. Consistent with the higher expression of HOX genes in low BAALC expressers, we observed up-regulation of microRNAs embedded in the HOX cluster, namely, miR-10a, miR-10b, and miR-9. Underexpressed was miR-126, which we previously found correlated with MN1 expression in younger patients,32 as well as miR-222, which is known to target KIT and has been linked to hematologic lineage differentiation.47
We also report here the first microRNA expression signature associated with ERG expression in CN-AML. We observed 11 differentially expressed probes, representing 11 microRNAs, associated with ERG expression (Figure 5B), with 5 up-regulated and 6 down-regulated in low ERG expressers. Among the up-regulated microRNAs in low ERG-expressing patients was miR-107, known to target NFIX, a gene involved in a regulatory feedback loop involving miR-223 and CEBPA during granulocytic differentiation.48 Interestingly, also up-regulated was miR-148a, which is shown to target DNMT3B,49 and miR-208, which is predicted in silico to target ERG itself. Another noteworthy down-regulated microRNA was miR-302d that has been associated with early developmental stages and “stemness.”50
Discussion
Although some studies have shown an association of mutation markers and outcome in older CN-AML patients,13,15,26 relatively little is known with regard to the prognostic value of gene expression markers in older patients with CN-AML. The aim of this study was to elucidate the prognostic impact of BAALC and ERG expression, in the context of other well-established molecular markers, in older de novo CN-AML patients similarly treated with intensive chemotherapy.
First, we demonstrate that low expression levels of both BAALC and ERG are associated with better outcome in older patients with CN-AML. As in younger CN-AML patients,9 low BAALC expression was associated with a higher CR rate and longer DFS and OS in older CN-AML patients investigated here. Low ERG expression was also associated with longer DFS and OS and a trend toward a higher CR rate in older patients, which is similar to our findings in younger CN-AML patients, where low ERG expressers had higher CR rates and longer event-free survival (EFS) than high ERG expressers.11 In multivariable analyses, BAALC, but not ERG, expression levels were significant factors for CR attainment and DFS. Expression levels of both BAALC and ERG were the only factors associated with OS in our cohort of older CN-AML patients.
To our knowledge, only 3 studies that analyzed the prognostic significance of BAALC expression and one study analyzing ERG expression in CN-AML included patients age 60 years or older.20,21,24 Two of these studies that comprised, respectively, 67 and 98 adults with CN-AML, including an unspecified number of patients age 60 years or older, showed that low BAALC expression was associated with better outcome, but did not evaluate older patients separately. The third study20 also included older patients and showed that low BAALC expression was associated with higher probability of CR achievement and longer EFS and OS. In their subgroup of 101 patients age 60 years or older, low BAALC expression showed a trend toward a higher CR rate (66% vs. 50%; P = .1) and a longer EFS (P = .03; Klaus H. Metzeler, Ludwig-Maximilians-Universität München, e-mail, April 26, 2010). On the other hand, low ERG expression seemed to be a stronger factor for outcome in the study of Metzeler et al,20 where it was significantly associated with a higher CR rate and longer EFS and OS in all patients analyzed. Subset analyses revealed a significant impact of low ERG expression on OS in both the younger patients and those aged 60 years or older, but neither of these age groups was evaluated separately in multivariable models considering other molecular markers.
Second, because we recently reported a strong prognostic impact of NPM1 mutations on CR rates, DFS, and OS in older CN-AML patients,15 it was of particular interest to us to investigate the relationship of NPM1 mutation status with BAALC and ERG expresser status. In multivariable models, NPM1 mutation status remained a strong, favorable prognostic marker for achievement of CR, but did not remain in the models for DFS and OS. Interestingly, in our previously reported gene expression signature associated with NPM1 mutations in older patients with CN-AML, expression of both the BAALC and ERG genes was low.15 We believe that measuring BAALC and ERG expression at diagnosis seems to provide more prognostic information with regard to survival than NPM1 mutational status alone. Indeed, patients with high BAALC expression had worse outcome irrespective of their NPM1 mutational status. This is supported by the comparison of different multivariable models in our dataset. Thus, once reliable methods to measure the pretreatment expression levels of BAALC and ERG in individual patients are available, these markers should be included in diagnostic testing for a more accurate risk stratification of older CN-AML patients.
Finally, to gain insights into the biology of the disease, we derived gene and, for the first time, microRNA expression signatures associated with BAALC and ERG expression. Many genes in the BAALC signature for older patients reported here have also been found in the gene expression signature in younger patients.9 This indicates that low BAALC expression defines a distinct subset of biologically similar patients, regardless of age. The characteristic features of this signature included overexpression of the HOXA and HOXB gene clusters, underexpression of known adverse outcome predictors, and down-regulation of genes associated with an undifferentiated state. Furthermore, in the first microRNA expression signature associated with BAALC expression, we found down-regulation of microRNAs embedded in the HOX cluster and up-regulation of miR-222, which is known to target the tyrosine kinase, KIT. The lower expression of ABCB1 (MDR1) observed in older CN-AML patients with low BAALC expression may account, at least in part, for the observed higher CR rate and better survival.
Interestingly, patient subgroups characterized by mutated NPM1 or low BAALC expression shared some common features in their gene and microRNA expression profiles. The second most down-regulated gene in NPM1 mutated patients was BAALC.15 Both the gene and microRNA expression profiles associated with mutated NPM115 and those associated with low BAALC expression show down-regulation of HOX genes and cofactors such as MEIS1 and up-regulation of CTSG, as well as overexpression of miR-9, miR-10a, miR-10b, and miR-100, and underexpression of miR-126 and miR-130a. Thus, both low BAALC expression and mutated NPM1 appear to share some biological features. To our knowledge, no molecular link between the 2 markers has been hitherto found. Further studies of possible biological links between those 2 markers are needed.
As with BAALC, the gene expression signature associated with ERG was similar to the signature we previously derived in younger patients.10 Not surprisingly, we found lower expression of previously identified putative ERG targets and binding partners associated with low ERG expression. Up-regulation of topoisomerase 1 might, in part, explain outcome differences caused by enhanced sensitivity to chemotherapy of low ERG expressers. Furthermore, low ERG expressers also had down-regulation of DNA methyltransferases, histone methyltransferases, and an up-regulation of miR-148a, which targets DNMT3B.49 We also observed a down-regulation of SET, which inhibits active DNA demethylation. This may indicate a higher activity of the DNA methylation machinery in high ERG expressers, which could contribute to their worse outcome. This should be further investigated and, if confirmed, might potentially lead to new treatment strategies in this subset of patients.
The similarity of the respective BAALC and ERG gene expression signatures between younger and older CN-AML patients, and the fact that these expression markers affect outcomes of both younger and older CN-AML patients, suggest that 60 years might not be an optimal age cutoff to separate younger and older patients' eligibility for treatment trials. Older patients with favorable molecular risk factors, such as low BAALC and/or ERG expression, if treated more intensively, might have outcomes comparable with those of younger patients with corresponding molecular features.
In conclusion, we show that low BAALC expression is an important factor for CR achievement and longer DFS; low BAALC and ERG expresser status are both significant factors associated with improved survival in older CN-AML patients. However, before the pretreatment expression of the 2 genes can be used for risk-stratification of older CN-AML patients, future studies should establish a standardized method of BAALC and ERG expression quantification and define absolute cutoff points. Once this obstacle is overcome, these important markers should be included in future risk-classification schemas. Furthermore, the derived gene and microRNA expression signatures shed light on the biology of this complex disease and identified new targets that might help in developing new therapeutic strategies for older CN-AML patients.
The online version of this article contains a data supplement.
Presented, in part, at the 51st annual meeting of the American Society of Hematology, December 7, 2009, New Orleans, LA, and published in abstract form.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Donna Bucci of the CALGB Leukemia Tissue Bank at The Ohio State University Comprehensive Cancer Center, Columbus, OH, for sample processing and storage services; Lisa J. Sterling and Colin G. Edwards for data management; and The Ohio State University Comprehensive Cancer Center's Nucleic Acid and Microarray Shared Resources for technical support.
This work was supported, in part, by National Cancer Institute grants CA101140, CA114725, CA140158, CA31946, CA33601, CA16058, CA77658, and CA129657, The Coleman Leukemia Research Foundation, and the Deutsche Krebshilfe–Dr Mildred Scheel Cancer Foundation (to H.B.).
National Institutes of Health
Authorship
Contribution: S.S., G.M., K. Maharry, M.D.R., K. Mrózek, and C.D.B. contributed to the design and analysis of this study and the writing of the manuscript, and all authors agreed on the final version; S.S., H.B., S.P.W., Y.-Z.W., and K.H.M. carried out laboratory-based research; K. Maharry, M.D.R., K.B.H., and D.M. performed statistical analyses; and G.M., B.L.P., J.E.K., T.H.C., J.O.M., M.R.B., M.A.C., R.A.L., and C.D.B. were involved directly or indirectly in the care of patients and/or sample procurement.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
For a complete list of participating Cancer and Leukemia Group B institutions, principal investigators, and cytogeneticists, please see the supplemental Appendix.
Correspondence: Clara D. Bloomfield, The Ohio State University, James Cancer Hospital and Solove Research Institute, Rm 1216, 300 West 10th Ave, Columbus, OH 43210; e-mail: clara.bloomfield@osumc.edu; or Guido Marcucci, MD, The Ohio State University, Comprehensive Cancer Center, Biomedical Research Tower, 460 W 12th Ave, Columbus, OH 43210; e-mail: guido.marcucci@osumc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal