Abstract
To explore the effect(s) of growth hormone signaling on thrombosis, we studied signal transduction and transcription factor 5 (STAT5)–deficient mice and found markedly reduced survival in an in vivo thrombosis model. These findings were not explained by a compensatory increase in growth hormone secretion. There was a modest increase in the activity of several procoagulant factors, but there was no difference in the rate or magnitude of thrombin generation in STAT5-deficient mice relative to control. However, thrombin-triggered clot times were markedly shorter, and fibrin polymerization occurred more rapidly in plasma from STAT5-deficient mice. Fibrinogen depletion and mixing studies indicated that the effect on fibrin polymerization was not due to intrinsic changes in fibrinogen, but resulted from changes in the concentration of a circulating plasma inhibitor. While thrombin-triggered clot times were significantly shorter in STAT5-deficient animals, reptilase-triggered clot times were unchanged. Accordingly, while the rate of thrombin-catalyzed release of fibrinopeptide A was similar, the release of fibrinopeptide B was accelerated in STAT5-deficient plasma versus control. Taken together, these studies demonstrated that the loss of STAT5 resulted in a decrease in the concentration of a plasma inhibitor affecting thrombin-triggered cleavage of fibrinopeptide B. This ultimately resulted in accelerated fibrin polymerization and greater thrombosis susceptibility in STAT5-deficient animals.
Introduction
Thrombosis-related diseases such as myocardial infarction, stroke, and venous thromboembolism account for substantial morbidity and mortality worldwide. We recently demonstrated that thrombosis susceptibility is mediated, in part, by sex-specific patterns of growth hormone (GH) secretion and subsequent effects on expression of coagulation-related genes in the liver.1
GH is a pleiotropic hormone that is synthesized and secreted by the anterior pituitary gland and has diverse effects on its target tissues.2,3 Hepatic GH signaling occurs via the type I cytokine receptor, growth hormone receptor, and activation of its primary downstream effectors, the Janus kinase 2 (JAK2) and signal transduction and transcription factor 5 (STAT5). Mice deficient in both STAT5A and STAT5B are largely insensitive to GH and consequently, have an impaired insulin-like growth factor 1-mediated feedback inhibition and dysregulated GH secretion.4-8 STAT5 is also an integral component of interleukin, erythropoietin, and prolactin signaling pathways. Furthermore, STAT5 can be activated independently of JAK2 by receptor tyrosine kinases and other mechanisms.9,10 STAT5 has thus been shown to be a central component of GH signaling, as well as other important cytokine signaling pathways.
Previously, we demonstrated that GH-deficient little mice are protected from thrombosis in vivo.1 Considering the important role of STAT5 in GH signaling, we aimed to study the role of STAT5 in thrombosis. In this study, we characterized 2 mouse models of STAT5 deficiency. We predicted that mice deficient in STAT5 would have a decrease in thrombosis susceptibility, as seen in GH-deficient little mice. Surprisingly, we found that the genetic loss of STAT5 resulted in increased susceptibility to thrombosis, in vivo, and shortened clotting times, in vitro. These studies also suggested a regulatory role for STAT5 in the conversion of fibrinogen to fibrin, a process that is integral to clot formation. Overall, these findings link an important mediator of cytokine/growth factor signaling with alterations in a critical hemostatic process and suggest that specific alterations in the kinetics of fibrinopeptide B (FpB) release are important in thrombosis, in vivo.
Methods
Mice
Mouse care and use for these studies was approved by the University of California San Francisco (UCSF) Institutional Animal Care and Use Committee. All mice were assessed between 6 and 8 weeks of age, beyond the age of sexual maturity. All experiments were conducted blind to genotype. The details of strain background of each line and how they were maintained are described in detail in the supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Littermate controls were used in all experiments.
Pulmonary embolism model
This model was performed on 8-week-old mice as previously described in detail.11
In vitro coagulation assays
Whole blood and plasma were collected and tested on a KC4 δ coagulation analyzer (Trinity Biotech) as described previously and in detail in the supplemental Methods.1
Gene expression
Real-time polymerase chain reaction (RT-PCR) was performed using TaqMan primer/probe sets (5′FAM/3′BHQ; Biosearch Technologies) as previously described.1 Specific primer and probe sequences used are included in supplemental Table 1.
FXIII activity
FXIII activity was determined according to the methods of Ariëns and colleagues.12 Briefly, 100 μL 40 μg/mL human fibrinogen (Haematologic Technologies) was used to coat Nunc Immuno Maxisorp microtiter plates. Plasma (10 μl) was added to each well, and coagulation was initiated with 90 μL reaction mix containing 0.56mM dithiothreitol (Fluka), 0.11M calcium chloride (Sigma-Aldrich), 1.11M EZ-Link pentylamine-biotin (Pierce), and 1.11 U/mL human α-thrombin. Cross-links were detected via addition of 100 μL 10 μg/mL streptavidin-alkaline phosphatase (Sigma-Aldrich), 100 μL p-nitrophenyl phosphate solution (Sigma-Aldrich), and 100 μL sodium hydroxide. Absorbance was measured at 405 nm.
Thrombin generation assay
Thrombin generation was measured using a fluorogenic thrombin substrate on a multiwell automated fluorescent plate reader (ThrombinoSCOPE) according to the manufacturer's protocol. Briefly, 80 μL mouse plasma (diluted 1:8 in saline) was placed in a 96-well plate and combined with 20 μL PPP-Reagent LOW (ThrombinoSCOPE) to give a final tissue factor concentration of 1ρM. Clotting was triggered with the addition of calcium chloride buffer and a fluorogenic thrombin substrate. The amount of thrombin generated in the reaction was measured over time.
Thrombin-antithrombin complexes
Thromboelastometry
Fibrin clot formation and firmness were measured using a thromboelastometer (ROTEM). Briefly, 7 μL 0.2M calcium chloride and 7 μL 20.1 U/mL human α-thrombin were placed in a mini-cup (ROTEM). Clotting was initiated with the addition of 105 μL whole blood. For mixing experiments with human plasma, 20 μL 0.2M calcium chloride and 20 μL 20.1 U/mL human α-thrombin were placed in a large reaction cup. Clotting was initiated with the addition of 300 μL 1:1 mix of pooled defibrinated plasma and Normal Hemostasis Reference Plasma (American Diagnostica). Clot firmness was measured and recorded for 60 minutes at 37°C. At the conclusion, measurements of clot time and maximal clot firmness were generated using the included ROTEM software analysis package.
Fibrinogen concentration and defibrination
The concentration of fibrinogen in plasma was determined using an enzyme-linked immunosorbent assay specific for mouse fibrinogen (Immunology Consultants Laboratory) according to the manufacturer's protocol. Mouse plasma was defibrinated using a protocol adapted from Sheng and colleagues.14 Thawed plasma was centrifuged at 3000 relative centrifugal force (rcf) for 20 minutes and subsequently placed in a 0.22-μm spin-filter (Corning). The flow-through was incubated in a 53°C water bath for 20 minutes and then centrifuged at 10 000 rcf for 10 minutes. The supernatant was carefully isolated for analysis. Equal amounts of plasma and defibrinated plasma were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (Invitrogen), and proteins were transferred to Fluorotrans-blotting membranes (Sigma-Aldrich). Plasma fibrinogen was detected using rabbit anti–mouse fibrinogen polyclonal antibody (Abcam) in conjunction with AlexaFluor 555 goat anti–rabbit immunoglobulin G highly cross-adsorbed secondary antibody (Invitrogen). Images were acquired and quantified using VersaDoc Molecular Imaging System (Bio-Rad Laboratories). Defibrinated mouse plasma was diluted 1:4 in saline and supplemented with human fibrinogen to achieve a final concentration of 3.0 mg/mL.
Fibrin polymerization
Dynamic fibrin formation was visualized by supplementing plasma with 80 μg/mL human fibrinogen conjugated to Alexa Fluor 488 (Invitrogen). Thrombin-triggered clotting was carried out with the addition of 100 μL 1.05 U/mLl human α-thrombin (diluted in 15mM calcium chloride solution) to 50 μL supplemented plasma in the center of a 35-mm glass-bottomed dish (MatTek). A glass coverslip was placed over the solution, and polymerization was recorded at 37°C using a Nikon Ti inverted microscope (Nikon Instruments), 100×/1.49 Apo TIRF objective, Yokogowa CSU-X1 spinning disk confocal unit with 486 nm DPSS laser source, and a Cascade II 512 camera (Photometrics). z-Stacks (5-μm), with 10 slices per stack, were acquired every 20 seconds for a period of 10 minutes.
Fibrinopeptide release
Fibrinopeptide release was performed essentially as described.15 Briefly, 1.0 mg/mL human fibrinogen (in 20mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.15M NaCl, pH 7.4 [HBS], 1mM CaCl2) and 50mM ϵ-aminocaproic acid were added to a 1:4 dilution of defibrinated control and STAT5-deficient plasma. This reaction mixture was aliquoted, and the reactions were initiated by the addition of human α-thrombin at a final concentration of 1.0 U/mL. Total fibrinopeptide in each sample was determined by an infinity time point with 20 U/mL thrombin and a 60-minute incubation. All reactions were carried out at room temperature and stopped at the indicated times by incubating at 100°C for 15 minutes. The samples were cooled to room temperature and mixed with 4 times their volume of high-performance liquid chromatography (HPLC) buffer B (25mM NaH2PO4/Na2HPO4, pH 6.0, with 50% acetonitrile), incubated 15 minutes, and centrifuged at 13 000 rcf for 60 minutes at 4°C. The supernatants were passed through 0.2-μm syringe filters before HPLC.
Fibrinopeptide release was monitored by reverse-phase HPLC as described.15 Briefly, samples were loaded onto an ISCO System HPLC with a C18 column (Vydac) equilibrated with buffer A (25mM NaH2PO4/Na2HPO4, pH 6.0). Fibrinopeptides were eluted with a linear gradient from 0% buffer B to 40% buffer B and monitored by the absorbance at 206 nm. Fibrinopeptide peak area was determined using ISCO software (version 2.4; ChemResearch). Individual fibrinopeptide A (FpA) raw data curves were fit with a simple first-order reaction to derive FpA max values. Each curve was then normalized to its calculated FpA max (100%). Normalized data were fit with a simple first-order reaction to determine rate constants for release of FpA (k1), as described.16 Each FpB dataset was normalized to its calculated FpA max. Normalized data were plotted together, and while a fit was attempted, no acceptable fit was made. Each experiment was performed 4 times.
Statistical analysis
Results from the pulmonary embolism model were analyzed via Kaplan-Meier survival plots and the log-rank test to determine the effect of genotype on sex-matched animals. For all other analyses, a Student t test was used to determine significance in cases where 2 groups are compared. For comparison of 3 or more groups, a 1-way analysis of variance (ANOVA) followed by the Bonferroni post test was used. Analysis of 2 or more groups over time was carried out using a 2-way ANOVA followed by the Bonferroni post test. Fibrinopeptide data were analyzed using a 2-way ANOVA with matching, followed by the Bonferroni post test. A nonlinear fit was applied to FpA curves, and best-fit values for k1 were compared by an F-test. An α value of 0.05 was set for all statistical tests. Specific P values are listed in all cases with the exception of post test comparisons after ANOVA analyses, where P is indicated as > or < .05, due to GraphPad output limitations. Data are presented as mean plus or minus SEM, unless otherwise indicated. All statistical analyses were performed in GraphPad Prism Statistical Software Version 5 (GraphPad Software).
Results
STAT5-deficiency increases susceptibility to thrombosis
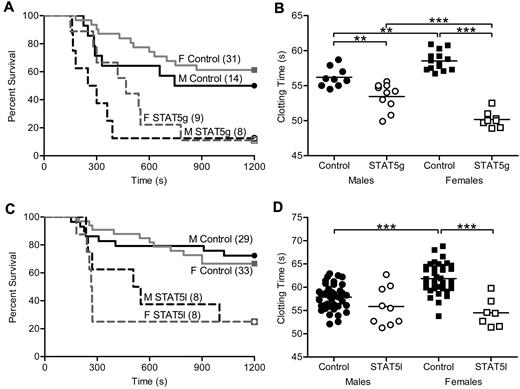
To determine the effect of GH signaling in thrombosis, we studied mice with an N-terminal disruption (deltaN STAT5) of Stat5a and Stat5b (referred to as STAT5g).5 Susceptibility to thrombosis in vivo was assessed with a well-characterized model of tissue factor (TF)–induced pulmonary embolism (PE).1,11,17,18 A weight-based dose of TF was injected into the inferior vena cava, and the end point measured was death. In this model, death has been demonstrated to be due to acute thrombosis and is dose-dependent.11 Sensitivity to TF differs between mouse strains (E.J.W., unpublished observations), and because STAT5g animals were maintained in a mixed (129Sv/J and C57Bl/6) background, we determined the median lethal dose (LD50) of TF in control male animals to allow for detection of both increased and decreased TF susceptibility in STAT5g mice. Male and female STAT5g mice were more susceptible to thrombosis in the PE model compared with sex-matched controls. Male STAT5g mice had 13% survival and a median survival time of 275 seconds compared with 50% and 972 seconds in control mice (n = 8, 14; P = .0273; Figure 1A). Female STAT5g mice had 11% survival and a median survival time of 540 seconds compared with 61% and 1200 seconds in control mice (n = 9, 31; P = .0011; Figure 1A).
In vivo thrombosis and in vitro coagulation in STAT5-deficient mice. (A) STAT5g male (- - -), STAT5g female (gray - - -), control male (—), and control female (gray —) mice were injected with 2.0 μL/g 1:40 TF dilution in a PE model of thrombosis. STAT5g males and females had reduced survival and shortened median survival times compared with sex-matched controls. No differences were found between genotype-matched males and females. (B) Whole blood was isolated from STAT5g male (○; n = 10), STAT5g female (□; n = 8), control male (●; n = 9), and control female (■; n = 14) mice. STAT5g mice had shortened TF-triggered clotting times compared with sex-matched controls. **P < .01, ***P < .001; 1-way ANOVA with Bonferroni post test. (C) STAT5l male (- - -), STAT5l female (gray - - -), control male (—), and control female (gray —) mice were injected with TF as in panel A. STAT5l males and females had reduced survival and shortened median survival times compared with sex-matched controls. No differences were found between genotype-matched males and females. (D) Whole blood was isolated from STAT5l male (○; n = 9), STAT5l female (□; n = 7), control male (●; n = 39), and control female (■; n = 38) mice. STAT5l mice had shortened TF-triggered clotting times compared with sex-matched controls. ***P < .001; 1-way ANOVA with Bonferroni post test.
In vivo thrombosis and in vitro coagulation in STAT5-deficient mice. (A) STAT5g male (- - -), STAT5g female (gray - - -), control male (—), and control female (gray —) mice were injected with 2.0 μL/g 1:40 TF dilution in a PE model of thrombosis. STAT5g males and females had reduced survival and shortened median survival times compared with sex-matched controls. No differences were found between genotype-matched males and females. (B) Whole blood was isolated from STAT5g male (○; n = 10), STAT5g female (□; n = 8), control male (●; n = 9), and control female (■; n = 14) mice. STAT5g mice had shortened TF-triggered clotting times compared with sex-matched controls. **P < .01, ***P < .001; 1-way ANOVA with Bonferroni post test. (C) STAT5l male (- - -), STAT5l female (gray - - -), control male (—), and control female (gray —) mice were injected with TF as in panel A. STAT5l males and females had reduced survival and shortened median survival times compared with sex-matched controls. No differences were found between genotype-matched males and females. (D) Whole blood was isolated from STAT5l male (○; n = 9), STAT5l female (□; n = 7), control male (●; n = 39), and control female (■; n = 38) mice. STAT5l mice had shortened TF-triggered clotting times compared with sex-matched controls. ***P < .001; 1-way ANOVA with Bonferroni post test.
In agreement with the in vivo findings, TF-triggered whole blood clotting times were significantly shorter in STAT5g versus control mice in vitro. Male STAT5g mice had a mean clotting time of 53.5 ± 0.6 seconds compared with 56.2 ± 0.5 seconds for controls (n = 10,9; P < .05; Figure 1B). Female STAT5g mice had a mean clotting time of 50.2 ± 0.4 seconds compared with 58.6 ± 0.4 seconds for controls (n = 8, 14; P < .05; Figure 1B). Similar results were found in platelet-poor plasma (supplemental Figure 1A), arguing against a significant effect of platelets or other circulating cells on the clotting phenotype of STAT5g mice.
Taken together, these results demonstrated that global STAT5 deficiency increased susceptibility to thrombosis in vivo and shortened clotting time in vitro.
Liver-specific deletion of STAT5 increases susceptibility to thrombosis
The liver is the principal site for biosynthesis and modification of most coagulation factors and related molecules; thus, to specifically determine the role of STAT5 in the liver in thrombosis, we analyzed animals with hepatocyte-specific deletion of the Stat5a-Stat5b locus (STAT5l).19 As with the observations in STAT5g mice, STAT5l animals were more susceptible to thrombosis in the PE model. Male STAT5l mice had 25% survival and a median survival time of 528 seconds compared with 72% and 1200 seconds for control (n = 8, 29; P = .0157; Figure 1C). Female STAT5l mice had 25% survival and a median survival time of 267 seconds compared with 67% and 1200 seconds for control mice (n = 8, 33; P = .0011; Figure 1C). In addition, TF-triggered whole blood clotting times were shortened in STAT5l mice compared with control. Male STAT5l mice had a mean clotting time of 55.9 ± 1.4 seconds versus 57.8 ± 0.4 seconds in control (n = 9, 39; P = not significant; Figure 1D), while female STAT5l mice had a mean clotting time of 54.5 ± 1.1 second compared with 61.9 ± 0.5 seconds in controls (n = 7, 38; P < .05; Figure 1D). Significant differences were found between control males and females, but not STAT5l males and females. Similar results were found in platelet-poor plasma (supplemental Figure 1B), again arguing against a significant effect of platelets or other circulating cells on the clotting phenotype of STAT5l mice.
These results demonstrated that hepatocyte-specific deletion of STAT5 shortened clotting times and increased susceptibility to thrombosis in a manner and magnitude that was nearly identical to the results observed in STAT5g mice. This is all the more remarkable because we observed nearly identical results in these 2 independent models of STAT5 deficiency created in 2 independent labs with 2 separate strategies (1 conditional and 1 global—1 a complete null and 1 a hypomorph). Because of the similar clotting phenotypes of STAT5g and STAT5l mice, STAT5l mice were used exclusively in subsequent in vitro experiments and are referred to as STAT5-deficient. In addition, male mice were used exclusively for subsequent in vitro experiments. However, animals were always compared with the appropriate littermate controls.
Elevated GH secretion does not cause altered thrombosis in STAT5-deficient mice
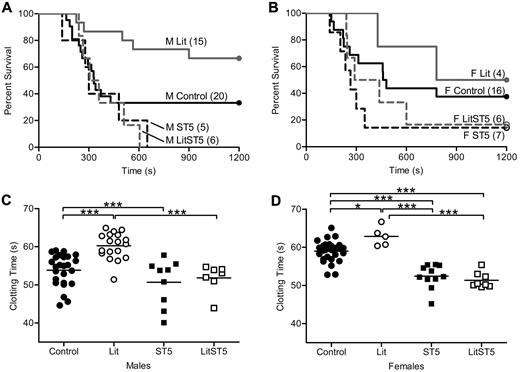
Previously published results from our laboratory showed that thrombosis susceptibility was reduced in GH-deficient little mice.1 Little mice are unable to respond to growth hormone releasing hormone (GHRH) because of a mutation in the GHRH receptor, and therefore do not secrete GH from the anterior pituitary gland. Animals homozygous for this mutation (litm/m) have no detectable serum GH, while hemizygous mice (litm/+) are indistinguishable from wild-type.20 In contrast to little mice, animals with either global or liver-specific disruption of STAT5 have increased GH secretion due to the loss of insulin-like growth factor 1–mediated feedback inhibition.4,19 Thus, the loss of STAT5 might impact clotting indirectly through secondary effects on GH secretion. To determine the influence of increased GH secretion on clotting in STAT5-deficient mice, we crossed STAT5g mice to little mice.20-22 Offspring from this cross were viable and born in expected ratios. Because all of our work with little and STAT5-deficient mice showed no differences between heterozygous-deficient and wild-type mice, we used them interchangeably and refer to them as control. The cross was designed to generate and compare animals of 4 genotypes including: Control/Control, litm/m/Control (Lit), Control/STAT5−/− (ST5), and litm/m/STAT5−/− (LitST5). We assessed thrombosis in vivo with the PE model and found that survival was nearly identical in male and female LitST5 and ST5 mice. Percent survival and median survival times in male mice were: Control: 30%, 330 seconds; Lit: 67%, 1200 seconds; ST5: 0%, 300 seconds; LitST5: 0%, 332 seconds (Figure 2A). Percent survival and median survival times in female mice were: Control: 38%, 470 seconds; Lit: 50%, 990 seconds; ST5: 14%, 265 seconds; LitST5: 17%, 364 seconds (Figure 2B). In addition, male LitST5 mice had TF-triggered whole blood clotting times that were significantly shorter than male Lit mice (n = 7, 18; P < .05) but comparable to ST5 mice (n = 7, 9; P > .05; Figure 2C). Likewise, female LitST5 mice had TF-triggered whole blood clotting times that were significantly shorter than both control and Lit female mice (n = 8, 28; P < .05) but comparable to ST5 mice (n = 8, 11; P > .05; Figure 2D). Mean clotting times and specific statistical comparisons are provided in supplemental Table 2. Taken together, these results indicated that the increased thrombosis observed in STAT5-deficient mice was not due to the secondary increase in GH secretion.
In vivo thrombosis and in vitro coagulation in STAT5-deficient and little intercrossed mice. (A) Control male (—; control/control), Lit male (gray —; litm/m/control), ST5 male (- - -; control/STAT5−/−), and LitST5 male (gray - - -; litm/m/STAT5−/−;) mice were injected with 3.0 μL/g 1:40 TF dilution in a PE model of thrombosis. Survival of male LitST5 was reduced compared with Lit and control male mice, but was similar to ST5. (B) Control female (—), Lit female (gray —), ST5 female (- - -), and LitST5 female (gray - - -) mice were injected with TF as in panel A. Survival of LitST5 female mice was reduced compared with Lit and control female mice, but was similar to ST5. (C) Whole blood was isolated from control male (●; control/control; n = 25), Lit male (○; n = 18), ST5 male (■; n = 9), and LitST5 male (□; n = 7) mice. TF-triggered whole blood clotting times were significantly shorter in LitST5 mice versus Lit, but were comparable to ST5. ***P < .001; 1-way ANOVA with Bonferroni post test. (D) Whole blood was isolated from control female (●; n = 28), Lit female (○; n = 5), ST5 female (■; n = 11), and LitST5 female (□; n = 8) mice. TF-triggered whole blood clotting times were significantly shorter in LitST5 mice versus Lit and control, but were comparable to ST5. *P < .05, ***P < .001; 1-way ANOVA with Bonferroni post test.
In vivo thrombosis and in vitro coagulation in STAT5-deficient and little intercrossed mice. (A) Control male (—; control/control), Lit male (gray —; litm/m/control), ST5 male (- - -; control/STAT5−/−), and LitST5 male (gray - - -; litm/m/STAT5−/−;) mice were injected with 3.0 μL/g 1:40 TF dilution in a PE model of thrombosis. Survival of male LitST5 was reduced compared with Lit and control male mice, but was similar to ST5. (B) Control female (—), Lit female (gray —), ST5 female (- - -), and LitST5 female (gray - - -) mice were injected with TF as in panel A. Survival of LitST5 female mice was reduced compared with Lit and control female mice, but was similar to ST5. (C) Whole blood was isolated from control male (●; control/control; n = 25), Lit male (○; n = 18), ST5 male (■; n = 9), and LitST5 male (□; n = 7) mice. TF-triggered whole blood clotting times were significantly shorter in LitST5 mice versus Lit, but were comparable to ST5. ***P < .001; 1-way ANOVA with Bonferroni post test. (D) Whole blood was isolated from control female (●; n = 28), Lit female (○; n = 5), ST5 female (■; n = 11), and LitST5 female (□; n = 8) mice. TF-triggered whole blood clotting times were significantly shorter in LitST5 mice versus Lit and control, but were comparable to ST5. *P < .05, ***P < .001; 1-way ANOVA with Bonferroni post test.
Thrombin generation is not altered in STAT5-deficient mice
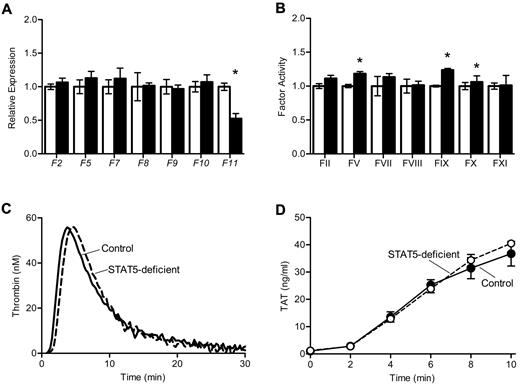
To determine the mechanism underlying increased thrombosis in STAT5-deficient mice, we measured the expression and activity of procoagulant factors II, V, VII, VIII, IX, X, and XI. Expression of the genes encoding these factors was comparable between genotypes, with the exception of reduced expression of F11 in STAT5-deficient mice (Figure 3A), which has been reported previously.7 Factor activities were quantified using modified 1-stage factor assays;1 there were modest increases in the activity of several factors in STAT5-deficient plasma versus control (Figure 3B). There was no significant difference in the activity of factor XIII between STAT5-deficient and control mice (1.57 ± 0.16 vs 1.34 ± 0.19; n = 3; P = .4028) as measured by the formation of fluorescently labeled cross-links.
Thrombin generation in STAT5-deficient plasma. (A) Expression of clotting factors: mRNA was isolated from control (open) and STAT5-deficient (filled) livers, and quantitative reverse transcription PCR was performed on cDNA. A raw GCN was calculated as described in the Methods, and each GCN was normalized to control mice. STAT5-deficient and control mice had comparable expression of coagulation factors, with the exception of F11 (n = 3, *P = .0069; Student t test). (B) Coagulation factor activity: plasma from control (open) and STAT5-deficient (filled) mice were mixed with the indicated human factor-deficient plasma; factor activities were measured as described in the Methods and normalized to control mice. STAT5-deficient mice had slightly increased FV, FIX, and FX activities, but were comparable to control for all other factor activities (n = 3; *P < .05; Student t test). (C) Thrombin generation: platelet-poor plasma was isolated from STAT5-deficient (- - -) and control mice (—). A fluorogenic thrombin substrate was added to plasma, and fluorescence was measured over time after the addition of TF. STAT5-deficient plasma had comparable endogenous thrombin potential to control mice.
Thrombin generation in STAT5-deficient plasma. (A) Expression of clotting factors: mRNA was isolated from control (open) and STAT5-deficient (filled) livers, and quantitative reverse transcription PCR was performed on cDNA. A raw GCN was calculated as described in the Methods, and each GCN was normalized to control mice. STAT5-deficient and control mice had comparable expression of coagulation factors, with the exception of F11 (n = 3, *P = .0069; Student t test). (B) Coagulation factor activity: plasma from control (open) and STAT5-deficient (filled) mice were mixed with the indicated human factor-deficient plasma; factor activities were measured as described in the Methods and normalized to control mice. STAT5-deficient mice had slightly increased FV, FIX, and FX activities, but were comparable to control for all other factor activities (n = 3; *P < .05; Student t test). (C) Thrombin generation: platelet-poor plasma was isolated from STAT5-deficient (- - -) and control mice (—). A fluorogenic thrombin substrate was added to plasma, and fluorescence was measured over time after the addition of TF. STAT5-deficient plasma had comparable endogenous thrombin potential to control mice.
We measured thrombin generation in mouse plasma using a fluorescent thrombin substrate. Thrombin generation curves were similar in STAT5-deficient and control plasma, with mean endogenous thrombin potentials of 431.4 ± 32.2 and 462.4 ± 15.8 (n = 4; P = .4208; Figure 3C). The formation of thrombin-antithrombin complexes was also measured at multiple time-points in plasma clotted in vitro; STAT5-deficient and control plasma had comparable levels of thrombin-antithrombin formation over time (n = 4; P = .5210; Figure 3D). Taken together, these findings indicated that changes in thrombin generation did not explain the significant increase in thrombosis susceptibility observed in STAT5-deficient animals.
Fibrin clot formation is altered in STAT5-deficient mice
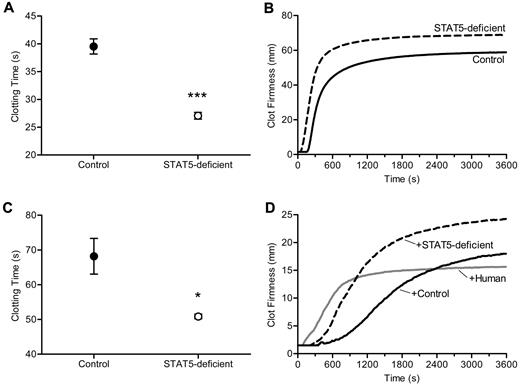
The shortened TF-triggered clotting times, the modest increase in the activities of multiple procoagulant factors, and the absence of an increase in thrombin generation led us to consider a change in fibrinogen and or fibrin assembly as the underlying cause of increased thrombosis in STAT5-deficient mice. To address this, we triggered clotting with the addition of thrombin and found mean clotting times of 27.1 ± 0.6 seconds vs 39.5 ± 1.4 seconds in STAT5-deficient and control plasma, respectively (n = 8, 6; P < .0001; Figure 4A). To further examine the dynamics of thrombin-triggered fibrin clot formation, we used thromboelastometry to quantify the development of clot strength over time. Here, we found shortened clotting times in STAT5-deficient samples compared with control (56.0 ± 3.2 seconds vs 177.0 ± 9.2 seconds; n = 3; P = .0002; Figure 4B) and greater maximum clot firmness (68.3 ± 1.2 mm vs 58.7 ± 2.7 mm; n = 3; P = .0316; Figure 4B). Next we measured liver expression of Fga by quantitative real-time PCR and found no difference between STAT5-deficient and control (78 303 ± 11 775 vs 64 278 ± 9 051 gene copy number [GCN]; n = 3; P = .3985). The concentration of fibrinogen protein was modestly, but not significantly increased in STAT5-deficient and control plasma (203.6 ± 37.7 μg/mL vs 151.6 ± 21.0 μg/mL; n = 4; P = .3282). The dramatically shortened thrombin-triggered clotting time together with comparable fibrinogen concentrations led us to speculate that the effect on clotting in STAT5-deficient mice might relate to an intrinsic modification of fibrinogen. Alternatively, the loss of STAT5 may change the concentration/activity of a circulating inhibitor affecting thrombin-catalyzed fibrinogen cleavage and/or fibrin polymerization.
Thrombin-triggered clotting. (A) Thrombin was added to platelet-poor plasma. Clotting times were 31.4% shorter in STAT5-deficient mice (○) compared with control (●; n = 8,6; *P < .0001; Student t test). (B) Thrombin-triggered fibrin clot formation and firmness were measured over time in whole blood isolated from STAT5-deficient (- - -) and control mice (—) using a thromboelastometer. This experiment was performed 3 times, and a representative curve is shown. The clotting times were shorter (P = .0002; n = 3; Student t test) and maximal clot firmness was greater (P = .0316; n = 3; Student t test) in STAT5-deficient plasma compared with control. (C) STAT5-deficient (○) and control (●) defibrinated plasma were supplemented with human fibrinogen to achieve a final concentration of 3.0 mg/mL. Thrombin-triggered clot times were 25.4% shorter in STAT5-deficient versus control samples (n = 3; *P = .0289; Student t test). (D) Thrombin-triggered fibrin clot formation and firmness were measured as in panel B using defibrinated pooled (n = 3) STAT5-deficient (- - -), defibrinated pooled control (n = 3; —), and defibrinated normal human plasma (gray —) mixed 1:1 with normal human plasma. This experiment was performed 2 times, and a representative curve is shown. The addition of STAT5-deficient plasma prolonged thrombin-triggered clot times by 185% compared with human, while control plasma prolonged clot times by 370% compared with human. Maximum clot firmness was increased by 20% with addition of control plasma and 60% with STAT5-deficient plasma.
Thrombin-triggered clotting. (A) Thrombin was added to platelet-poor plasma. Clotting times were 31.4% shorter in STAT5-deficient mice (○) compared with control (●; n = 8,6; *P < .0001; Student t test). (B) Thrombin-triggered fibrin clot formation and firmness were measured over time in whole blood isolated from STAT5-deficient (- - -) and control mice (—) using a thromboelastometer. This experiment was performed 3 times, and a representative curve is shown. The clotting times were shorter (P = .0002; n = 3; Student t test) and maximal clot firmness was greater (P = .0316; n = 3; Student t test) in STAT5-deficient plasma compared with control. (C) STAT5-deficient (○) and control (●) defibrinated plasma were supplemented with human fibrinogen to achieve a final concentration of 3.0 mg/mL. Thrombin-triggered clot times were 25.4% shorter in STAT5-deficient versus control samples (n = 3; *P = .0289; Student t test). (D) Thrombin-triggered fibrin clot formation and firmness were measured as in panel B using defibrinated pooled (n = 3) STAT5-deficient (- - -), defibrinated pooled control (n = 3; —), and defibrinated normal human plasma (gray —) mixed 1:1 with normal human plasma. This experiment was performed 2 times, and a representative curve is shown. The addition of STAT5-deficient plasma prolonged thrombin-triggered clot times by 185% compared with human, while control plasma prolonged clot times by 370% compared with human. Maximum clot firmness was increased by 20% with addition of control plasma and 60% with STAT5-deficient plasma.
Shortened clotting time in STAT5-deficient mice is due to changes in a plasma factor
To determine whether the observed differences in fibrin clot formation were due to changes intrinsic to fibrinogen and to control for modest differences in fibrinogen protein concentrations, we next defibrinated STAT5-deficient and control plasma and added back equal quantities of human fibrinogen. Defibrination reduced mouse fibrinogen levels by > 85%, and defibrinated plasma did not clot in the absence of fibrinogen supplementation. Importantly, the trace fibrinogen remaining in plasma after defibrination was equivalent between genotypes, as quantified by Western blot (supplemental Figure 2). Interestingly, when STAT5-deficient and control defibrinated plasma were supplemented with equal amounts of human fibrinogen, thrombin-triggered clot times remained significantly shorter in the STAT5-deficient samples; mean clot times were 50.9 ± 0.7 seconds and 68.2 ± 5.1 seconds in STAT5-deficient and control plasma, respectively, a shortening of 25% in STAT5-deficient samples (n = 3; P = .0289; Figure 4C). This strongly suggests that the shortened thrombin-triggered clotting in STAT5-deficient mice results from changes in the concentration or activity of a circulating plasma factor(s), rather than intrinsic changes in fibrinogen.
In addition, pooled defibrinated STAT5-deficient plasma, pooled defibrinated control plasma, and defibrinated human plasma were each mixed 1:1 with unaltered human reference plasma; thrombin-triggered clotting was studied by thromboelastometry. The addition of control plasma prolonged the clotting time by 370% compared with human plasma, while the addition of STAT5-deficient plasma only prolonged the clotting time by 185% compared with human plasma (Figure 4D). Maximum clot firmness was increased by 20% with addition of control plasma and 60% with STAT5-deficient plasma (Figure 4D). The changes in clot time with the addition of control and STAT5-deficient plasma suggested that control mouse plasma contains an inhibitor affecting thrombin-triggered fibrin polymerization and that this inhibitor is reduced in activity or concentration in STAT5-deficient animals, thus accounting for their increase in thrombosis relative to control mice.
The rate of fibrin polymerization is increased in plasma from STAT5-deficient mice
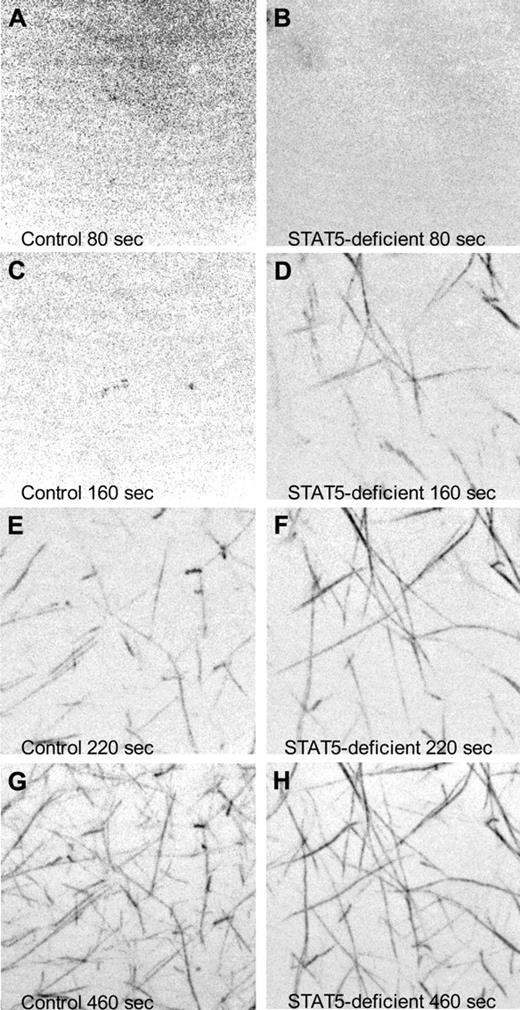
To observe the rate of fibrin polymerization in plasma from STAT5-deficient and control mice, we added a small amount of fluorescently labeled human fibrinogen to mouse plasma, triggered clotting with thrombin, and collected images over time via confocal microscopy. At 80 seconds after initiation, both samples showed diffuse fluorescence with no discernable fiber formation (Figure 5A-B and supplemental Videos 1-2). Fibers were first observed in STAT5-deficient plasma at 120 seconds, while fibers did not appear in control plasma until 180 seconds (Figure 5C-H and supplemental Videos 1-2). These experiments were repeated 3× and collectively showed that STAT5-deficient mice had significant differences in the rate of fibrin polymerization compared with control.
Confocal microscopy of fibrin polymerization. Platelet-poor plasma isolated from control (left panels) and STAT5-deficient (right panels) mice was supplemented with 80 μg/mL human fibrinogen labeled with AlexaFluor 488. Thrombin-triggered clotting was initiated in the center of a 35-mm glass-bottomed dish, and polymerization was recorded at 37°C using a Nikon Ti inverted microscope, 100×/1.49 Apo TIRF objective, Yokogowa CSU-X1 spinning disk confocal unit with 486 nm DPSS laser source, and a Photometrics Cascade II 512 camera. Images are maximum intensity projections of 5-μm stacks at the indicated time points. No discernable fibers were present at the beginning of capture (A-B). Fibrin fibrils first appeared at 120 seconds in STAT5-deficient plasma (C-D) and 180 seconds in control plasma (E-F). At 460 seconds, both control and STAT5-deficient plasma had established extensive fibrin networks (G-H).
Confocal microscopy of fibrin polymerization. Platelet-poor plasma isolated from control (left panels) and STAT5-deficient (right panels) mice was supplemented with 80 μg/mL human fibrinogen labeled with AlexaFluor 488. Thrombin-triggered clotting was initiated in the center of a 35-mm glass-bottomed dish, and polymerization was recorded at 37°C using a Nikon Ti inverted microscope, 100×/1.49 Apo TIRF objective, Yokogowa CSU-X1 spinning disk confocal unit with 486 nm DPSS laser source, and a Photometrics Cascade II 512 camera. Images are maximum intensity projections of 5-μm stacks at the indicated time points. No discernable fibers were present at the beginning of capture (A-B). Fibrin fibrils first appeared at 120 seconds in STAT5-deficient plasma (C-D) and 180 seconds in control plasma (E-F). At 460 seconds, both control and STAT5-deficient plasma had established extensive fibrin networks (G-H).
Thrombin-catalyzed release of fibrinopeptide B occurs more rapidly in plasma from STAT5-deficient mice
The results described in the previous section demonstrated an increase in the rate of thrombin-triggered fibrin polymerization in plasma from STAT5-deficient animals compared with control. This was independent of changes in thrombin generation or fibrinogen concentration. To explore the mechanism(s) underlying this clotting difference, we initiated clotting with the snake venom thrombin-like enzyme, reptilase. Unlike thrombin, which catalyzes the sequential release of FpA and FpB, reptilase exclusively catalyzes the release of FpA from fibrinogen.23 In stark contrast to the shortened thrombin-triggered clotting times observed in STAT5-deficient mice, reptilase-triggered clotting times were comparable between STAT5-deficient and control mice. STAT5-deficient mice had an average reptilase clotting time of 28.2 ± 0.5 seconds compared with 29.4 ± 0.6 seconds in control mice (n = 6; P = .1594).
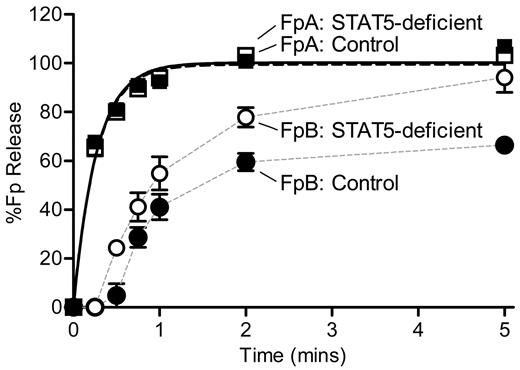
Because of the discrepant results of reptilase-triggered versus thrombin-triggered clotting times, we measured the rate of thrombin-catalyzed release of FpA and FpB by HPLC. STAT5-deficient and control plasma were defibrinated, supplemented with equal amounts of human fibrinogen, and mixed with thrombin. In preliminary experiments, we noted markedly reduced activity in mouse plasma samples compared with standard fibrinogen reactions performed in the absence of plasma. Specifically, release of FpA was markedly diminished, and no release of FpB was detected. However, using an increased concentration of thrombin (nearly 100-fold higher than standard fibrinogen reactions) we were able to detect release of both fibrinopeptides. It should be noted that there was no difference in the kinetics of FpA release between the STAT5-deficient and control samples at the lower thrombin concentration. Individual datasets describing the release of FpA over time were normalized to the calculated FpA maximum values. A direct comparison of FpA curves showed that STAT5-deficent and control mice had similar amounts of thrombin-catalyzed FpA release over time (n = 4; P = .6731; P > .05 at all time points; Figure 6). Normalized data were fit with a simple first-order reaction to determine the rate constant for release of FpA (k1), as described.16 STAT5-deficient and control mice had comparable FpA rate constants of 3.63 ± 0.24 minutes−1 and 3.87 ± 0.30 minutes−1, respectively (n = 4; P = .5705; Figure 6).
Kinetics of FpA and FpB release. Defibrinated control (●) and STAT5-deficient (○) plasma were supplemented with 1.0 mg/mL human fibrinogen. Reactions were initiated with thrombin and quenched at the indicated time points. FpA and FpB were quantified by HPLC analysis and normalized to the calculated FpA max values for each sample. FpA: STAT5-deficient (□) and control (■) mice had comparable release of FpA over time (n = 4; P = .6731; P > .05 at all time points; 2-way ANOVA with matching and Bonferroni post test). The rate constant, k1, was derived by fitting FpA curves to a first-order reaction (black lines); the rate of FpA release was similar between genotypes (n = 4; P = .5705; F test). FpB: In contrast, STAT5-deficient mice (○) demonstrated greater release of FpB over time compared with controls (●; n = 4; P = .0119; P < .05 at 0.5-, 2-, and 5-minute time points; 2-way ANOVA with matching and Bonferroni post test). Data points were plotted with a simple connecting line (gray lines).
Kinetics of FpA and FpB release. Defibrinated control (●) and STAT5-deficient (○) plasma were supplemented with 1.0 mg/mL human fibrinogen. Reactions were initiated with thrombin and quenched at the indicated time points. FpA and FpB were quantified by HPLC analysis and normalized to the calculated FpA max values for each sample. FpA: STAT5-deficient (□) and control (■) mice had comparable release of FpA over time (n = 4; P = .6731; P > .05 at all time points; 2-way ANOVA with matching and Bonferroni post test). The rate constant, k1, was derived by fitting FpA curves to a first-order reaction (black lines); the rate of FpA release was similar between genotypes (n = 4; P = .5705; F test). FpB: In contrast, STAT5-deficient mice (○) demonstrated greater release of FpB over time compared with controls (●; n = 4; P = .0119; P < .05 at 0.5-, 2-, and 5-minute time points; 2-way ANOVA with matching and Bonferroni post test). Data points were plotted with a simple connecting line (gray lines).
Individual datasets describing the release of FpB over time were normalized to their calculated FpA max values. A direct comparison of FpB curves showed that STAT5-deficent mice had a significant reduction of FpB release over time compared with control mice (n = 4; P = .0119; P < .05 at 0.5-, 2-, and 5-minute time points; Figure 6). Normalized FpB curves were fit to a first-order equation that assumes FpB release occurs only after FpA release, as is standard for fibrinogen reactions and has been described previously.16 However, we were unable to accurately fit the FpB release data to the conventional equation describing FpB release; thus, the FpB data in Figure 6 are not fit to any equation. It should be noted that the calculated rate constants (k2) from these curves showed that the rate of FpB release from STAT5-deficient mice (1.04 ± 0.08 minutes−1) was 2.3-fold faster than control mice (0.45 ± 0.05 minutes−1). Visual examination of the FpB release data suggests that the release of FpB from control animals was subject to inhibition; this is supported by the fact that maximum FpB release was 65% of the max FpA release in control samples compared with 94% in STAT5-deficient samples. Taken together, these data support the idea that there is an inhibitor of thrombin-catalyzed release of FpB in normal mouse plasma, and this inhibitor is reduced in STAT5-deficient plasma.
Discussion
Previously, we showed that thrombosis susceptibility was reduced in GH-deficient little mice.1 To explore the effects of GH signaling on thrombosis, we studied mice with global and hepatocyte-specific disruption of the Stat5a-Stat5b locus.5 These animals are largely GH-insensitive yet surprisingly, had increased thrombosis susceptibility. Given that STAT5-deficiency results in increased GH secretion, we crossed GH-deficient little mice to STAT5-deficient mice and determined that the thrombosis phenotype of STAT5-deficient mice was not due to the secondary increase in GH secretion. Importantly, there were no changes in thrombin generation, but there was a significant increase in the rate of fibrin polymerization in STAT5-deficient plasma compared with control. Fibrinogen depletion studies indicated that the effect on fibrin clot formation was not due to intrinsic changes in fibrinogen, but to changes in a circulating inhibitor. Mixing studies strongly suggested that this was an inhibitor and that it was reduced in STAT5-deficient compared with control plasma. While thrombin-triggered clot times were significantly shorter in STAT5-deficient plasma, reptilase-triggered clot times were unchanged. In accordance with these findings, there was an increase in thrombin-catalyzed release of FpB, but no difference in the rate of FpA release in STAT5-deficient plasma compared with control. Taken together, these studies demonstrated that the loss of STAT5 in the liver most likely led to a decrease in the concentration of a plasma inhibitor to thrombin-triggered cleavage of FpB. This resulted in an increase in the rate of fibrin polymerization and increased thrombosis susceptibility in STAT5-deficient animals.
Mice with hepatocyte-specific deletion of STAT5 have been reported to develop fatty liver beginning at 10 weeks of age, whereas mice studied at earlier time points had no evidence of lipid accumulation.24 GH resistance has also been associated with cirrhosis;25,26 however, a recent report showed no differences in fibrosis or other histological changes in the livers of 10-week-old mice with hepatocyte-specific deletion of STAT5.27 To minimize these potential confounders, the current experiments were conducted in mice between 6 and 8 weeks of age, past the age of sexual maturity but before the development of gross liver abnormalities. Furthermore, such pathologic changes in liver would be expected to decrease coagulation factor activities and prolong clotting times, neither of which was observed in our studies. Modest increases in fibrinogen protein have also been reported in STAT5-deficient mice.24,27 Our measurements did confirm a nonsignificant increase in Fga transcript and fibrinogen protein; however, clotting differences persisted in defibrinated STAT5-deficient and control plasma supplemented with equal amounts of human fibrinogen or mixed with human plasma. This strongly argues that the observed thrombotic phenotype was independent of the slight differences in fibrinogen concentration between STAT5-deficient and control mice.
Our model suggests that the loss of STAT5 leads to a reduction in the concentration of an inhibitor that affects the N terminus of the fibrinogen β-chain and normally impairs thrombin-catalyzed release of FpB. The human plasma mixing studies were particularly informative; mixing either control or STAT5-deficient plasma with normal human plasma resulted in a prolongation of the clotting times. There was a dramatic difference in the degree of prolongation between STAT5-deficient and control plasma. With the control plasma, the degree of prolongation was well beyond what we observed when we added defibrinated human plasma, while mixing STAT5-deficient plasma had a much smaller effect. This argues that mouse plasma normally contains an inhibitor that specifically delays the release of FpB and that the concentration or activity of this inhibitor is reduced in STAT5-deficient mice. The kinetics of fibrinopeptide release also strongly suggested the presence of an inhibitor in control plasma specifically affecting FpB release. As seen in Figure 6, there was a delay in FpB release, and the maximum FpB release was markedly reduced in the control versus STAT5-deficient samples. Importantly, this was not the case in the FpA release curves. This also likely explains why attempts to fit the control FpB release data to the conventional equation were not successful, as the equations used to model fibrinopeptide release do not account for the presence of an inhibitor.
These data are consistent with prior reports of patients with congenital dysfibrinogenemias affecting FpB cleavage and 2 reports describing patients with autoantibodies to fibrinogen specifically affecting FpB release. In all of these cases, the patients presented with prolonged thrombin times but normal reptilase times and had no clinical bleeding.28-34 In the 2 reports of patients with acquired inhibitors, FpB release was delayed, and the maximal FpB release was diminished by the addition of inhibitor.33,34 In our model, the loss of STAT5 resulted in a decrease in the concentration of inhibitor, shortened thrombin clotting times, normal reptilase times, an increase in the rate and magnitude of FpB release, and ultimately, increased susceptibility to thrombosis, relative to control.
The precise molecule affecting FpB release remains unknown. The fact that we observed a nearly identical phenotype in mice with global- and hepatocyte-specific deficiency of STAT5 leads us to presume that the inhibitor is normally synthesized or, at least, modified in the liver. Numerous proteins have been reported to interact with fibrin(ogen) to positively or negatively regulate clot formation. Recently described examples include the decorin and platelet factor-4.35,36 Furthermore, STAT5 is known to be involved in a number of signaling pathways including prolactin, interleukin-2 (IL-2), IL-3, IL-5, insulin, and epidermal growth factor signaling. Others have shown that there are dramatic changes in the expression of genes/proteins in the livers of STAT5-deficient mice.7,19 Therefore, the specific STAT5-dependent inhibitor that normally acts to inhibit fibrinogen cleavage could be the downstream target of numerous cytokines or hormones. Ongoing and future work in our laboratory is aimed at identifying this specific inhibitor and its primary regulatory pathway.
In addition to the clinical significance of altered FpB cleavage, STATs are increasingly important in many areas of biology and disease.37 Activation of the JAK/STAT pathway has been reported in many human cancers, and inhibitors of JAK/STAT signaling are now in various stages of clinical development.38 Our studies provide an important caution: if inhibition of STAT5 increases thrombosis susceptibility, targeting STAT5 in the treatment of cancer or inflammatory conditions may confer increased risk of thrombosis in this already at-risk population.
To our knowledge, this is the first study to identify a role for STAT5 in the regulation of fibrin clot formation and thrombosis. This is also the first report of a specific increase in the rate of FpB release without an effect on the release of FpA. Importantly, this is not just an isolated in vitro abnormality; mice deficient in STAT5 have a dramatic change in thrombosis susceptibility, in vivo. These findings mimic those observed in human patients with either genetic mutations in the FpB cleavage site or with autoantibodies directed against this site. The fact that patients with delayed FpB release are all characterized by a distinct absence of bleeding suggests that specifically targeting thrombin-catalyzed FpB release might provide a favorable balance of safety and efficacy in a potential antithrombotic therapy. Given the tremendous toll taken by thrombosis-related diseases, we hope that these and future studies will provide greater insight into the pathophysiology of thrombosis and may someday lead to novel strategies aimed at treating and preventing thrombosis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Oleg Gorkun for important discussions of the work and help analyzing data. We thank Lothar Hennighausen for providing conditional STAT5AB-deficient mice, Jim Ihle for providing global STAT5AB-deficient mice, Shaun Coughlin, Robin Shaw, and O. K. Gilman for important discussions of the work, and Tina Fong, Ting-Ting Hong, Katelyn Dow, and Maya Sukkari for outstanding technical assistance.
This work was supported in part by an American Society of Hematology Junior Faculty Scholar Award in Basic Science (E.J.W.) and National Institutes of Health grant R01 HL 31048 (S.T.L.). S.M.N. was supported by the National Heart, Lung, and Blood Institute (NHLBI) Training Grant in Pulmonary Diseases. J.D. was supported by a UCSF Office of Student Research Quarterly Research Fellowship
National Institutes of Health
Authorship
Contribution: S.M.N., S.T.L., and E.J.W. designed and performed experiments, interpreted and analyzed data, and wrote and edited the manuscript; B.A.H., B.C.S., R.E.L., and J.W.D. performed experiments and interpreted and analyzed data; and J.W.S. designed and performed experiments and interpreted and analyzed data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ethan J. Weiss, University of California San Francisco, Cardiovascular Research Institute, Rm 352Y, MC: 3120, 555 Mission Bay South Blvd, PO Box 589001, San Francisco, CA 94158-9001; e-mail: ethan.weiss@ucsf.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal