Abstract
Hyperactivation of the transcription factor Stat5 leads to various leukemias. Stat5 activity is regulated by the protein phosphatase SHP-1 in a phospholipase C (PLC)–β3-dependent manner. Thus, PLC-β3–deficient mice develop myeloproliferative neoplasm, like Lyn (Src family kinase)– deficient mice. Here we show that Lyn/PLC-β3 doubly deficient lyn−/−;PLC-β3−/− mice develop a Stat5-dependent, fatal myelodysplastic/myeloproliferative neoplasm, similar to human chronic myelomonocytic leukemia (CMML). In hematopoietic stem cells of lyn−/−;PLC-β3−/− mice that cause the CMML-like disease, phosphorylation of SHP-1 at Tyr536 and Tyr564 is abrogated, resulting in reduced phosphatase activity and constitutive activation of Stat5. Furthermore, SHP-1 phosphorylation at Tyr564 by Lyn is indispensable for maximal phosphatase activity and for suppression of the CMML-like disease in these mice. On the other hand, Tyr536 in SHP-1 can be phosphorylated by Lyn and another kinase(s) and is necessary for efficient interaction with Stat5. Therefore, we identify a novel Lyn/PLC-β3–mediated regulatory mechanism of SHP-1 and Stat5 activities.
Introduction
Myelodysplastic/myeloproliferative neoplasms (MDS/MPNs) are de novo clonal myeloid neoplasms that exhibit dysplastic and proliferative features. Four subtypes of human MDS/MPNs have been identified, including chronic myelomonocytic leukemia (CMML) and juvenile myelomonocytic leukemia (JMML).1 CMML, a prototype of MDS/MPN, is characterized by persistent peripheral blood monocytosis, less than 20% blasts and dysplasia involving 1 or more myeloid lineages. While the cause of CMML is not fully understood yet, as many as 40% of CMML patients exhibit oncogenic mutations of KRAS or NRAS genes.2 JMML is clinically similar to CMML. Approximately 75% of JMML patients show mutations in KRAS, NRAS, NF1, or PTPN11. These data suggest that hyperactive RAS initiates CMML and JMML.3 This notion is supported by studies of Nf1,4 K-ras,5,6 N-ras,7 and Ptpn118 mutant mice, all of which develop myeloid disorders resembling JMML and CMML. Both CMML and JMML can progress to acute myeloid leukemia (AML) of the myelomonocytic and monocytic subtypes.
A small fraction (3.1% versus 19.6% with RAS mutations) of CMML patients harbors FLT3-ITD mutations. A knock-in mouse model harboring an internal tandem duplication (ITD) in the murine Flt3 locus developed MPN with monocytic features resembling CMML.9 Stat5 is activated by FLT3-ITD10 and is indispensable for the development of myeloproliferative disease induced by FLT3-ITD. STAT5 and other signaling pathways are activated by the TEL/PDGFβR fusion protein in CMML patients with the t(5;12) (q33;p13) chromosomal translocation, and STAT5 is critical for this MPN.11 This raises an immediate question about the role of Stat5 in the pathogenesis of CMML. A cellular characteristic of both JMML and CMML is the hyperesponsiveness to subsaturating concentrations of granulocyte macrophage colony-stimulating factor (GM-CSF),12 and activation of the JAK2-STAT5 pathway is an important signaling event after binding of GM-CSF to the GM-CSF receptor. Indeed, Kotecha et al observed a specific evoked STAT5 signaling signature in JMML, CMML, and M4/5 AML patients in response to low doses of GM-CSF, and levels of STAT5 Tyr694 phosphorylation were negatively correlated with clinical outcomes.13 In Nf1 mutant mice, the development of CMML-like disease requires GM-CSF14 and the common β chain of its receptor.15 In Ptpn11 E76K transduced bone marrow (BM) cells, phospho-Stat5 levels were dramatically increased.16 Furthermore, constitutively active Stat5 could induce Ba/F3 cells to differentiate into macrophages.17
Phospholipase C-β3 (PLC-β3) is a member of the PLC-β family enzymes that can produce diacylglycerol and inositol 1,4,5-trisphosphate (IP3) downstream of heterotrimeric G proteins. As diacylglycerol can activate several isoforms of protein kinase C, and IP3 can mobilize Ca2+, PLC-β is implicated in promoting cell proliferation.18 However, we recently reported that PLC-β3−/− mice develop a late-onset MPN.19 The mutant mice have increased numbers of hematopoietic stem cells (HSCs) with increased proliferative, survival, and myeloid-differentiative abilities. These properties are dependent on Stat5 and can be antagonized by the protein phosphatase SHP-1. PLC-β3 functions as a scaffold to interact with Stat5 and SHP-1 via its noncatalytic C-terminal domain to form the multimolecular SPS complex.19 PLC-β3 facilitates SHP-1–mediated dephosphorylation and inactivation of Stat5. Consistent with this, SHP-1 loss-of-function mutant (motheaten viable, mev) mice also develop MPN, phenocoping PLC-β3−/− mice.19 However, the MPN in mev/mev mice is more rapid and fatal than that in PLC-β3−/− mice. Moreover, mev/mev, but not PLC-β3−/−, mice develop anemia, a phenomenon often observed in MDS.20 These discrepancies have prompted us to study the regulation of SHP-1. Lyn, a Src family kinase, has been long thought to phosphorylate SHP-1.21 In this study, we investigate how Lyn interacts with PLC-β3 to regulate SHP-1 and Stat5 activities by analyzing a CMML-like disease in lyn−/−;PLC-β3−/− mice.
Methods
Mice, MPD, and MDS
PLC-β3−/− mice were described previously.22 Mev/mev and C57BL/6-Ly5.1 mice were purchased from The Jackson Laboratory. For the definition of MPD and MDS, we followed the criteria adopted by the Mouse Models of Human Cancers Consortium23 and by Passegue et al.24 The Animal Care and Use Committee of the La Jolla Institute for Allergy and Immunology approved all mouse experiments.
Transplantation of hematopoietic cells
BM cells (2 × 106 cells in 400 μL phosphate-buffered saline) were retro-orbitally injected into lethally (960 rad) irradiated recipient mice (C57BL/6-Ly5.1; 8 to 10 weeks old). For transplantation of HSCs and progenitors, fluorescence-activated cell-sorted CD34− KSL, CD34+ KSL, and myeloid progenitors (Lin−c-Kit+Sca-1−) were injected into lethally irradiated (960 rad) C57BL/6-Ly5.1 mice together with 1 × 105 C57BL/6-Ly5.1 helper BM cells. We also transplanted retrovirally transduced HSCs into lethally irradiated C57BL/6-Ly5.1 mice together with 2.5 × 105 Sca-1–depleted C57BL/6-Ly5.1 helper BM cells.
Analysis of phosphorylated signaling molecules
For analysis of phosphorylated signaling molecules, BM cells were left unstimulated or stimulated with interleukin-3 (IL-3; 100 ng/mL), then fixed with 1× Lyse/Fix buffer (BD Pharmingen) and permeabilizd with Perm buffer III (BD Pharmingen). Cells were stained with lineage markers revealed by PerCP-Cy5.5, anti–c-Kit (APC), anti–Sca-1 (PE), and the indicated phospho-specific antibodies (anti–phospho-Stat5A/B or anti–phospho-SHP-1) revealed by AlexaFluor 488-conjugated, goat anti–mouse or anti–rabbit secondary antibody. Samples were analyzed with FACSCalibur. Sca-1+c-Kit+ Lin− (KSL) cells were gated.
Cultures of BMMCs
BM cells from femurs were cultured in IL-3/stem cell factor (SCF)–containing medium for 4 weeks to obtain BM-derived mast cells (BMMCs). In some cases, CD34−KSL cells in BM were sorted and transduced with bicistronic retroviral vectors encoding SHP-1 or PLC-β3 CT, together with green fluorescent protein (GFP). Transduced (GFP+) cells were sorted and cultured in the presence of IL-3 and SCF for 4 weeks. The purity (> 95% c-Kit+FcϵRI+) of the resulting mast cells was confirmed by flow cytometry.
Coimmunoprecipitation and immunoblotting
For coimmunoprecipitation, transduced BMMCs deprived of IL-3 or splenocytes were stimulated with 100 ng/mL IL-3, and cell lysates were immunoprecipitated with anti-Lyn (44) or anti–PLC-β3 (C-20) antibodies (all from Santa Cruz Biotechnology) and analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by immunoblotting with anti-Lyn, anti–PLC-β3, or anti–SHP-1 (C-19) antibodies. For immunoblotting, cell lysates were analyzed by SDS-PAGE followed by immunoblotting with anti–p-SHP-1 (Tyr536; ECM Bioscience) or anti–p-SHP-1 (Tyr564; Cell Signaling Technologies) antibodies. Phosphorylated synthetic peptides corresponding to amino acids around Tyr536 and Tyr564 in human SHP1 were immunized in rabbits, and antibodies were purified using proprietary protocols.
Retroviral transduction
Sorted CD34− KSL cells (1000 cells/well) were incubated in α-minimum essential medium supplemented with 1% fetal bovine serum, SCF, IL-3, and IL-6 for 24 hours, and then transduced with a retrovirus vector (pMig-DN Stat5; pMIG-SHP-1, pMIG-PLC-β3 CT) in the presence of protamine sulfate (10 μg/mL) and recombinant fibronectin fragment (1 μg/mL) for 24 hours. Transduced cells were further subjected to liquid (in the presence of IL-3 and SCF) or semisolid cultures (MethoCult M3134 from StemCell Technologies) with the addition of IL-3.
SHP-1 phosphatase assay
SHP-1 phosphatase activity was measured using PTP assay kit (17-125; Millipore). Briefly, SHP-1 was immunoprecipitated from BMMCs and incubated with a synthetic phosphopeptide (R-R-L-I-E-D-A-E-pY-A-A-R-G). Then malachite green solution was added, and the absorbance was measured at 650 nm.
SHP-1 phosphorylation assay
Purified GST-SHP-1 was incubated at 37°C for 15 minutes with recombinant Lyn in the absence or presence of GST-PLC-β3 CT in kinase assay buffer (20mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.5, 10mM MnCl2, 10mM MgCl2, and 1mM ATP). Then the reaction was subjected to SDS-PAGE and immunoblotted with anti–phospho-SHP-1 antibodies.
Statistical analysis
Student t test was used for statistical analysis. P < .05 is considered significant.
Supplemental materials
Supplemental materials include supplemental Methods, 8 figures, and 3 tables (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Lyn−/−;PLC-β3−/− mice develop a severe MDS/MPN with monocytosis
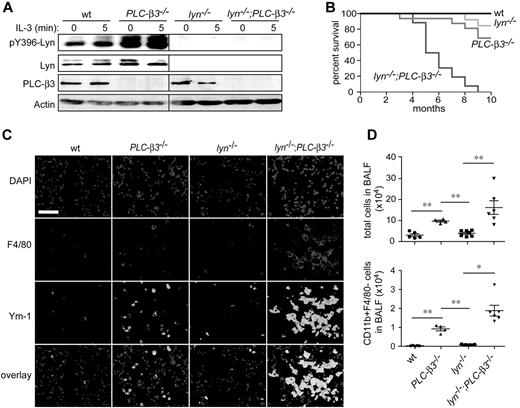
To investigate the molecular mechanism for increased Stat5 activity in PLC-β3−/− mice, we evaluated the function of Jak2 and Lyn, 2 kinases that can activate Stat5 and are predominantly expressed in BM cells among the members of Jak and Src family kinase, respectively. Although tyrosine phosphorylation of Jak2 was not significantly different between wild-type (wt) and PLC-β3−/− BM cells (data not shown), we observed dramatically increased phosphorylation of Lyn at Tyr396 in the activation loop in PLC-β3−/− BM cells (Figure 1A). To address if increased Lyn kinase activity is responsible for the higher level of Stat5 activity and contributes to the development of MPN in PLC-β3−/− mice, lyn−/−;PLC-β3−/− double knockout mice were generated. Surprisingly, lyn−/−;PLC-β3−/− mice all died within 10 months after birth, at which age approximately 70% of PLC-β3−/− and 90% of lyn−/− mice survived (Figure 1B). Histologically, severe inflammatory cell infiltration in the lungs was observed in lyn−/−;PLC-β3−/− mice, predominantly with F4/80+ macrophages and to a less extent with CD11b+F4/80− myeloid cells, similar to the phenotypes of lyn−/−;hck−/−,25 SHIP−/−,25-27 and mev/mev19 mice (Figure 1C-D). These macrophages expressed Ym-1, a surrogate marker for the M2-type macrophage. Myeloid cell infiltration was also seen in liver and kidney of lyn−/−;PLC-β3−/− mice (supplemental Figure 1).
Premature death and lung inflammation in lyn−/−;PLC-β3−/− mice. (A) Lyn phosphorylation at Tyr396 was measured in BM cells by immunoblotting. (B) Survival analysis of lyn−/−;PLC-β3−/− mice. The numbers of mice used: wt, 12; PLC-β3−/−, 36; lyn−/−, 16; lyn−/−;PLC-β3−/−, 32. (C) Immunofluorescent staining for F4/80 and Ym-1 in frozen sections of lung tissues. DAPI (4′,6-diamidino-2-phenylindole) is nuclear staining. Bar indicates 50 μm. (D) Cells in bronchoalveolar lavage fluid (BALF) were analyzed for CD11b and F4/80 expression by flow cytometry. The total cell and CD11b+F4/80− cell numbers were calculated (n = 4-7). Data are mean ± SD, *P < .05, **P < .01.
Premature death and lung inflammation in lyn−/−;PLC-β3−/− mice. (A) Lyn phosphorylation at Tyr396 was measured in BM cells by immunoblotting. (B) Survival analysis of lyn−/−;PLC-β3−/− mice. The numbers of mice used: wt, 12; PLC-β3−/−, 36; lyn−/−, 16; lyn−/−;PLC-β3−/−, 32. (C) Immunofluorescent staining for F4/80 and Ym-1 in frozen sections of lung tissues. DAPI (4′,6-diamidino-2-phenylindole) is nuclear staining. Bar indicates 50 μm. (D) Cells in bronchoalveolar lavage fluid (BALF) were analyzed for CD11b and F4/80 expression by flow cytometry. The total cell and CD11b+F4/80− cell numbers were calculated (n = 4-7). Data are mean ± SD, *P < .05, **P < .01.
These initial observations prompted us to further explore the hematologic phenotype of lyn−/−;PLC-β3−/− mice in comparison to wt, lyn−/−, and PLC-β3−/− mice. Flow cytometric analysis revealed a mild to moderate increase in the percentage of CD11b+Gr-1+ granulocytes in BM, spleen, and peripheral blood from PLC-β3−/− and lyn−/− mice at the age of 2-4 months, but this increase was more prominent in age-matched lyn−/−;PLC-β3−/− mice (Figure 2A-B). By this age, PLC-β3−/− mice did not develop obvious splenomegaly and lyn−/− mice showed a 2-fold increase in the size of spleen, but lyn−/−;PLC-β3−/− mice had a 5- to 10-fold larger spleen, whose structure was effaced with numerous myeloid cells and magekaryocytes, indicative of an extramedullary hematopoiesis (Figure 2C and supplemental Figure 1). Complete blood cell counts confirmed increased neutrophils in the peripheral blood from both PLC-β3−/− and lyn−/− mice. Lyn−/−;PLC-β3−/− mice, however, had even higher numbers of neutrophils (supplemental Tables 1-2). These neutrophils had multilobated nuclei, indicating mature cells (Figure 2D). Furthermore, lyn−/−;PLC-β3−/− mice had increased CD11b+Gr-1− monocytes in both percentage and absolute number in the peripheral blood (Figure 2A,D and supplemental Table 2). Intriguingly, all 6-month-old lyn−/−;PLC-β3−/− mice developed anemia and thrombocytopenia without evidence for increase of blasts (supplemental Table 2). The anemia and thrombocytopenia are suggestive of ineffective hematopoiesis, a hallmark for myelodysplasia. Consistent with this possibility, we observed increased megakaryocytes with monolobated and bilobated nuclei in the BM of lyn−/−;PLC-β3−/− mice, characteristics for dysmegakaryopoiesis (Figure 2E-F). Collectively, PLC-β3−/− and lyn−/− mice suffered from MPN, but lyn−/−;PLC-β3−/− mice had MDS/MPN with monocytosis, similar to human CMML.
MDS/MPN phenotype in lyn−/−;PLC-β3−/− mice. (A) Flow cytometric analysis of nucleated cells in BM, spleen (SPL), and peripheral blood leukocytes (PBL) at the age of 3 months. (B) Graphic summary of granulocytes and monocytes (CD11b+ cells) in these organs of 2- to 4-month-old lyn−/−;PLC-β3−/− mice (n = 12 per cohort). Data are mean ± SD, *P < .05. (C) Spleens at the age of 4 months. (D) Mature granulocytes and monocytes in blood smear at the age of 4 months. (E) Hematoxylin and eosin staining of monolobated megakaryocytes in the BM of lyn−/−;PLC-β3−/− mice, compared with multilobated ones in wt mice. (F) Graphic summary of megakaryocytes with the indicated numbers of nuclear lobes in BM at the age of 4-6 months (n = 3).
MDS/MPN phenotype in lyn−/−;PLC-β3−/− mice. (A) Flow cytometric analysis of nucleated cells in BM, spleen (SPL), and peripheral blood leukocytes (PBL) at the age of 3 months. (B) Graphic summary of granulocytes and monocytes (CD11b+ cells) in these organs of 2- to 4-month-old lyn−/−;PLC-β3−/− mice (n = 12 per cohort). Data are mean ± SD, *P < .05. (C) Spleens at the age of 4 months. (D) Mature granulocytes and monocytes in blood smear at the age of 4 months. (E) Hematoxylin and eosin staining of monolobated megakaryocytes in the BM of lyn−/−;PLC-β3−/− mice, compared with multilobated ones in wt mice. (F) Graphic summary of megakaryocytes with the indicated numbers of nuclear lobes in BM at the age of 4-6 months (n = 3).
The MDS/MPN in lyn−/−;PLC-β3−/− mice is BM cell-autonomous
To test if the MDS/MPN phenotype is transplantable, BM cells from the 4 genotypes (CD45.2+) at the age of 2 months were injected into lethally irradiated mice expressing the congenic maker CD45.1. Within 4 months after transplantation, recipients of PLC-β3−/− cells had only slightly increased neutrophils in BM, spleen, and peripheral blood (supplemental Figure 2A and data not shown), consistent with our previous report that only aged (8- to 10-month-old) PLC-β3−/− mice develop MPN.19 By contrast, MPN was observed in the recipients engrafted with lyn−/− cells. Importantly, lyn−/−;PLC-β3−/− recipient mice all developed severe MDS/MPN with increased monocytes, anemia, and thrombocytopenia, recapitulating the phenotype of lyn−/−;PLC-β3−/− mice (supplemental Figure 2A and supplemental Table 3). Similar to lyn−/−;PLC-β3−/− mice, these recipients died within 9 months after transplantation (supplemental Figure 2B). Collectively, these results demonstrate that the MDS/MPN in lyn−/−;PLC-β3−/− mice is largely BM cell-autonomous. However, platelet numbers dropped down at 6 months, but not 2 months, in lyn−/−;PLC-β3−/− mice and at 5 months after transplantation in lyn−/−;PLC-β3−/− recipient mice (supplemental Tables 1-3). There might be compensatory mechanisms going on in the stroma, although the reason why platelet counts dropped remains unknown.
HSCs and progenitors are increased in lyn−/−;PLC-β3−/− mice
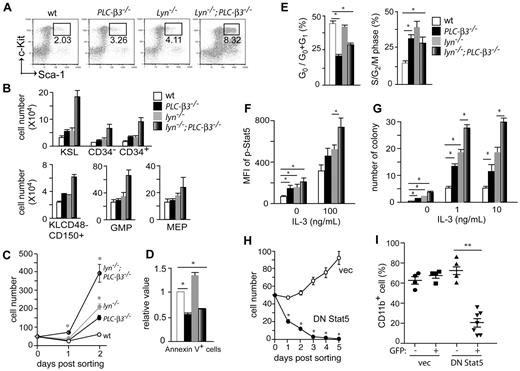
We next enumerated HSCs and progenitors by flow cytometric analysis. Both CD34− and CD34+ c-Kit+Sca-1+Lin− (KSL) cells, enriched for long-term HSCs (LT-HSCs) and short-term HSCs (ST-HSCs), respectively,28 were only slightly increased in PLC-β3−/− and lyn−/− mice at the age of 2 months (Figure 3A-B). However, these populations were increased approximately 4-fold in lyn−/−;PLC-β3−/− mice. Granulocyte-macrophage progenitors and megakaryocyte-erythroid progenitors, but not common myeloid progenitors or common lymphoid progenitors, were also increased in lyn−/−;PLC-β3−/− mice (Figure 3B and data not shown). CD150+CD48− cell population is enriched for HSCs.29 Thus, Lin−c-Kit+CD150+CD48− markers were also used to confirm the increase in HSCs in the BM of lyn−/−;PLC-β3−/− mice (Figure 3B).
Increased proliferation, decreased apoptosis and MPN-causing ability of lyn−/−;PLC-β3−/− HSCs are due to increased Stat5 activity. (A-B) BM cells were subjected to flow cytometric analysis of HSCs, myeloid, and erythroid progenitors. The absolute number was calculated based on the percentage of KSL cells and other progenitors in total BM cells. Results shown are representative of at least 3 measurements (2-4 months old, n = 12). (C) Sorted CD34−KSL cells (50 cells/well) were cultured in 96-well plates in the presence of IL-3 and SCF. (D) BM cells were stained for Lineage cocktail, c-Kit, Sca-1, and Annexin V, and subjected to flow cytometric analysis. Annexin V+ cells were counted in KSL populations (n = 4). (E) Sorted KSL cells were stained for either Pyronin-Y/Hoechst 33 342 (left) or propidium iodide (PI; right), and analyzed by flow cytometry. (F) BM cells were stimulated with IL-3, and phospho-Stat5 levels were analyzed by flow cytometry. Mean fluorescence intensity (MFI) of Stat5 phosphorylation in KSL cells is presented (n = 6). (G) Sorted CD34−KSL cells were cultured in methylcellulose in the presence or absence of IL-3. Ten days later, the number of colonies (including CFU-G, CFU-M, and CFU-GM) was counted. (H) Sorted CD34−KSL cells from BM of lyn−/−;PLC-β3−/− mice were transduced with a bicistronic retroviral vector encoding DN Stat5 or empty vector, together with GFP. Transduced (GFP+) cells were sorted into 96-well plates (50 cells/well) in the presence of IL-3 and SCF. (I) Sorted CD34−KSL cells from lyn−/−;PLC-β3−/− mice were transduced with DN Stat5 or empty vector and then adoptively transferred (without sorting) to lethally irradiated C57BL/6-Ly5.1 mice. Four months later, CD11b+ donor-derived (Ly5.2+) cells in peripheral blood were analyzed by flow cytometry. Data are mean ± SD, *P < .05, **P < .01.
Increased proliferation, decreased apoptosis and MPN-causing ability of lyn−/−;PLC-β3−/− HSCs are due to increased Stat5 activity. (A-B) BM cells were subjected to flow cytometric analysis of HSCs, myeloid, and erythroid progenitors. The absolute number was calculated based on the percentage of KSL cells and other progenitors in total BM cells. Results shown are representative of at least 3 measurements (2-4 months old, n = 12). (C) Sorted CD34−KSL cells (50 cells/well) were cultured in 96-well plates in the presence of IL-3 and SCF. (D) BM cells were stained for Lineage cocktail, c-Kit, Sca-1, and Annexin V, and subjected to flow cytometric analysis. Annexin V+ cells were counted in KSL populations (n = 4). (E) Sorted KSL cells were stained for either Pyronin-Y/Hoechst 33 342 (left) or propidium iodide (PI; right), and analyzed by flow cytometry. (F) BM cells were stimulated with IL-3, and phospho-Stat5 levels were analyzed by flow cytometry. Mean fluorescence intensity (MFI) of Stat5 phosphorylation in KSL cells is presented (n = 6). (G) Sorted CD34−KSL cells were cultured in methylcellulose in the presence or absence of IL-3. Ten days later, the number of colonies (including CFU-G, CFU-M, and CFU-GM) was counted. (H) Sorted CD34−KSL cells from BM of lyn−/−;PLC-β3−/− mice were transduced with a bicistronic retroviral vector encoding DN Stat5 or empty vector, together with GFP. Transduced (GFP+) cells were sorted into 96-well plates (50 cells/well) in the presence of IL-3 and SCF. (I) Sorted CD34−KSL cells from lyn−/−;PLC-β3−/− mice were transduced with DN Stat5 or empty vector and then adoptively transferred (without sorting) to lethally irradiated C57BL/6-Ly5.1 mice. Four months later, CD11b+ donor-derived (Ly5.2+) cells in peripheral blood were analyzed by flow cytometry. Data are mean ± SD, *P < .05, **P < .01.
MPN is caused by an LT-HSC–enriched population in lyn−/−;PLC-β3−/− mice
To determine the disease origin in lyn−/−;PLC-β3−/− mice, we sorted LT-HSCs, ST-HSCs and progenitors, then transplanted them into lethally irradiated CD45.1+ mice. Four months later, LT-HSCs from lyn−/−;PLC-β3−/− mice gave rise to MPN in recipients, as assessed by increased granulocytes in BM, spleen, and peripheral blood (supplemental Figure 3 and data not shown). By contrast, ST-HSCs or myeloid progenitors from lyn−/−;PLC-β3−/− mice failed to induce MPN after transplantation. These data collectively demonstrate that MPN is caused by an LT-HSC–enriched population in lyn−/−;PLC-β3−/− mice, similar to lyn−/− (supplemental Figure 3) and PLC-β3−/−19 mice.
Lyn−/−;PLC-β3−/− HSCs exhibit increased proliferation and reduced apoptosis
To evaluate the growth properties of HSCs, we cultured sorted CD34−KSL cells in the presence of IL-3 and SCF. PLC-β3−/− cells, similar to lyn−/− cells, grew more vigorously than wt cells. Lyn−/−;PLC-β3−/− cells grew 8-fold faster than wt cells, and 2- to 3-fold faster than PLC-β3−/− and lyn−/− cells, consistent with the in vivo phenotype (Figure 3C). The increased growth of lyn−/−;PLC-β3−/− cells could be due to increased cell cycling and/or reduced apoptosis. AnnexinV staining showed that lyn−/− KSL cells die by apoptosis more frequently than wt counterparts, suggesting that the increased apoptosis is overbalanced by increased proliferation in these cells. By contrast, PLC-β3−/− KSL cells were 40% less apoptotic, consistent with our published data.19 Lyn−/−;PLC-β3−/− KSL cells had an intermediate degree of apoptosis, but the percentage of apoptotic cells was still lower than wt cells (Figure 3D). Cell cycle analysis revealed more lyn−/− KSL cells in the S/G2/M phases but comparable numbers in the G0 phase compared with wt cells, indicating that Lyn is a negative regulator for the G1-S transition, but has no effects on the G0-G1 checkpoint (Figure 3E). On the other hand, compared with wt cells, both PLC-β3−/− and lyn−/−;PLC-β3−/− KSL cells cycled more frequently into G1 and S/G2/M phases and stayed less frequently in the G0 phase. These results suggest that PLC-β3 is essential for regulation of the G0-G1 transition. Interestingly, slightly but significantly more lyn−/−;PLC-β3−/− KSL cells were found in the G0 phase than PLC-β3−/− cells, suggesting that lyn−/−;PLC-β3−/− HSCs might exhaust more slowly than PLC-β3−/− HSCs. This interpretation could explain the higher number of HSCs in lyn−/−;PLC-β3−/− mice compared with PLC-β3−/− mice (Figure 3B).
Increased Stat5 activity in lyn−/−;PLC-β3−/− HSCs is required for increased in vitro growth and MPN development
To dissect the molecular mechanisms of MDS/MPN development in lyn−/−;PLC-β3−/− mice, we performed flow cytometric analysis to quantify the activities of several signaling pathways, including Jak-Stat, PI-3 kinase/Akt, MAPK, and NF-κB. First, we confirmed that Stat5 phosphorylation at Tyr694 was increased in PLC-β3−/− KSL cells as reported previously.19 It was also increased in lyn−/− KSL cells, and reached to the highest level in lyn−/−;PLC-β3−/− KSL cells before and after cytokine stimulation (Figure 3F and supplemental Figure 4), suggesting that both PLC-β3 and Lyn inhibit Stat5 phosphorylation. By contrast, no consistent differences were found with respect to the other tested signaling molecules, including phospho-Jak2 and phospho-Stat3 levels somewhat to our surprise (data not shown). Increased sensitivity to growth factors is a hallmark of MPNs.12 To address this possibility, colony formation assays were performed. Lyn−/−, PLC-β3−/−, and lyn−/−;PLC-β3−/− KSL cells generated a few colonies in a cytokine-independent manner, indicating that these populations contain transformed cells (Figure 3G). Under low concentrations of IL-3 or GM-CSF, wt KSL cells gave rise to small numbers of colonies, and colony-forming efficiencies were significantly higher in lyn−/− and PLC-β3−/− KSL cells. As expected, lyn−/−;PLC-β3−/− KSL cells generated the highest number of colonies, in line with their highest Stat5 activity among the 4 genotypes (Figure 3G and supplemental Figure 5).
To test if Stat5 is required for the increased in vitro cell growth and MDS/MPN development, sorted CD34−KSL cells from lyn−/−;PLC-β3−/− mice were transduced with dominant-negative Stat5 (DN Stat5). As shown in Figure 3H, DN Stat5-transduced lyn−/−;PLC-β3−/− CD34−KSL cells died out after 5 days even in the medium containing IL-3 and SCF, suggesting that Stat5 is essential for the survival of these cells. We also transplanted infected CD34−KSL cells into lethally irradiated CD45.1+ mice. Only empty vector-, but not DN Stat5-harboring CD34−KSL cells caused MPN in recipients (Figure 3I), suggesting that Stat5 is required for MPN development in lyn−/−;PLC-β3−/− mice.
The MDS/MPN phenotype of mev/mev mice is more similar to that of lyn−/−;PLC-β3−/− mice than that of PLC-β3−/− mice
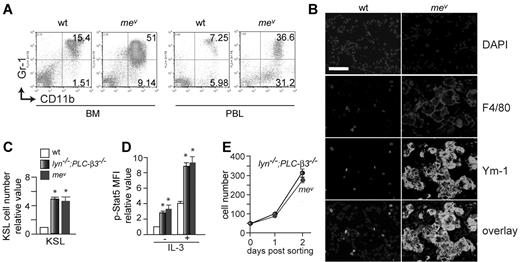
PLC-β3 functions as an adaptor to recruit SHP-1 and Stat5 to form the SPS complex, and facilitates SHP-1 to dephosphorylate Stat5 at Tyr694.19 In line with this, mev/mev mice develop MPN as well.19 However, in comparison to PLC-β3−/− mice, mev/mev mice developed a more severe MPN (Figure 4A) with an earlier onset, accompanied by severe inflammatory cell infiltration in lung, liver, and kidney (Figure 4B and supplemental Figure 1). Furthermore, mev/mev mice had similar numbers of KSL cells with comparable levels of Stat5 phosphorylation as lyn−/−;PLC-β3−/− mice (Figure 4C-D). CD34−KSL cells from mev/mev and lyn−/−;PLC-β3−/− mice grew at a similar rate in vitro (Figure 4E), and these cells gave rise to MPN upon transplantation into lethally irradiated mice (supplemental Figure 3). Therefore, together with the reported anemia in mev/mev mice,20 the MDS/MPN phenotype of mev/mev mice is closer to that of lyn−/−;PLC-β3−/− mice than that of PLC-β3−/− mice, implying a similar level of defects in the same signaling pathways in mev/mev and lyn−/−;PLC-β3−/− mice.
Phenotypic similarities between lyn−/−;PLC-β3−/− and mev/mev mice. (A) Flow cytometric analysis of granulocytes and monocytes in BM and peripheral blood from 6-week-old mev/mev mice. (B) Immunofluorescent staining for F4/80 and Ym-1 in lung tissues. Bar indicates 50 μm. (C) BM cells were subjected to flow cytometric analysis for HSCs/progenitors. The absolute number was calculated based on the percentage of KSL cells in BM. (D) BM cells were stimulated with IL-3 and then phospho-Stat5 levels in KSL cells were analyzed by flow cytometry (n = 4). (E) Sorted CD34−KSL cells (50 cells/well) were cultured in 96-well plates in the presence of IL-3 and SCF. Data are mean ± SD, *P < .05.
Phenotypic similarities between lyn−/−;PLC-β3−/− and mev/mev mice. (A) Flow cytometric analysis of granulocytes and monocytes in BM and peripheral blood from 6-week-old mev/mev mice. (B) Immunofluorescent staining for F4/80 and Ym-1 in lung tissues. Bar indicates 50 μm. (C) BM cells were subjected to flow cytometric analysis for HSCs/progenitors. The absolute number was calculated based on the percentage of KSL cells in BM. (D) BM cells were stimulated with IL-3 and then phospho-Stat5 levels in KSL cells were analyzed by flow cytometry (n = 4). (E) Sorted CD34−KSL cells (50 cells/well) were cultured in 96-well plates in the presence of IL-3 and SCF. Data are mean ± SD, *P < .05.
Both PLC-β3 and Lyn physically interact with SHP-1 and are involved in the regulation of SHP-1 phosphorylation at Tyr536 and Tyr564
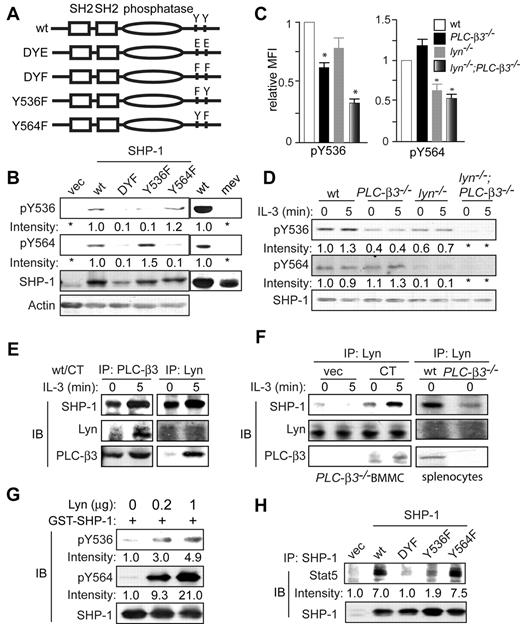
The molecular basis for the regulation of SHP-1 activity is incompletely understood.30,31 The C-terminal phosphorylation sites have been mapped to Tyr536 and Tyr564 residues32 and putative tyrosine kinases that catalyze these phosphorylations include Lyn,33,34 Lck,35 Src,36 and insulin receptor.32 To study Tyr536 and Tyr564 phosphorylation, we first tested the quality of newly developed phospho-specific antibodies against Tyr536 and Tyr564. To this end, wt and phosphorylation mutant (Y536F, Y564F, and double-mutant DYF) SHP-1 vectors (Figure 5A) were introduced into mev/mev BM cells. We chose mev/mev cells, because SHP-1 phosphorylation at Tyr536 and Tyr564 was lost in these cells and was recovered by wt SHP-1 transduction, as detected by immunoblotting with the new antibodies (Figure 5B). As expected, the level of phospho-Tyr536 was drastically reduced in SHP-1 Y536F- and DYF-, but not in Y564F-expressing cells; the level of phospho-Tyr564 was drastically reduced in SHP-1 Y564F- and DYF-, but not in Y536F-expressing cells (Figure 5B), confirming that these 2 antibodies are indeed specific for phospho-Tyr536 and phospho-Tyr564 residues, respectively.
Lyn and PLC-β3 regulate SHP-1 phosphorylation at Tyr536 and Tyr564. (A) Scheme of a panel of SHP-1 retroviral constructs. (B) CD34−KSL cells from mev/mev mice were retrovirally transduced with empty vector or SHP-1 mutants. GFP+ cells were sorted and cultured in the presence of IL-3 and SCF for 4 weeks and were analyzed by immunoblotting. Right 2 lanes indicate results with nontransduced cells. (C) BM cells were analyzed by flow cytometry for phospho-Tyr536 and phospho-Tyr564. MFI of SHP-1 phosphorylation in KSL cells was shown (n = 6). Data are mean ± SD, *P < .05 versus wt. (D) BMMCs were analyzed by immunoblotting. (E) wt CD34−KSL cells were retrovirally transduced with PLC-β3 CT. GFP+ cells were sorted and cultured in the presence of IL-3 and SCF for 4 weeks. Interactions among PLC-β3, Lyn, and SHP-1 were examined by coimmunoprecipitation followed by immunoblotting. (F) Left, PLC-β3−/− CD34−KSL cells were retrovirally transduced with PLC-β3 CT. GFP+ cells were sorted and cultured in the presence of IL-3 and SCF for 4 weeks. The interaction among PLC-β3, Lyn, and SHP-1 was examined by coimmunoprecipitation followed by immunoblotting. Right, splenocytes were used for coimmunoprecipitation and immunoblotting. (G) GST-SHP-1 (0.5 μg) was incubated with various amounts of recombinant Lyn protein and analyzed by immunoblotting. (H) mev/mev BMMCs expressing the indicated SHP-1 proteins were immunoprecipitated with anti–SHP-1 and followed by immunoblotting.
Lyn and PLC-β3 regulate SHP-1 phosphorylation at Tyr536 and Tyr564. (A) Scheme of a panel of SHP-1 retroviral constructs. (B) CD34−KSL cells from mev/mev mice were retrovirally transduced with empty vector or SHP-1 mutants. GFP+ cells were sorted and cultured in the presence of IL-3 and SCF for 4 weeks and were analyzed by immunoblotting. Right 2 lanes indicate results with nontransduced cells. (C) BM cells were analyzed by flow cytometry for phospho-Tyr536 and phospho-Tyr564. MFI of SHP-1 phosphorylation in KSL cells was shown (n = 6). Data are mean ± SD, *P < .05 versus wt. (D) BMMCs were analyzed by immunoblotting. (E) wt CD34−KSL cells were retrovirally transduced with PLC-β3 CT. GFP+ cells were sorted and cultured in the presence of IL-3 and SCF for 4 weeks. Interactions among PLC-β3, Lyn, and SHP-1 were examined by coimmunoprecipitation followed by immunoblotting. (F) Left, PLC-β3−/− CD34−KSL cells were retrovirally transduced with PLC-β3 CT. GFP+ cells were sorted and cultured in the presence of IL-3 and SCF for 4 weeks. The interaction among PLC-β3, Lyn, and SHP-1 was examined by coimmunoprecipitation followed by immunoblotting. Right, splenocytes were used for coimmunoprecipitation and immunoblotting. (G) GST-SHP-1 (0.5 μg) was incubated with various amounts of recombinant Lyn protein and analyzed by immunoblotting. (H) mev/mev BMMCs expressing the indicated SHP-1 proteins were immunoprecipitated with anti–SHP-1 and followed by immunoblotting.
Next, these antibodies were used to measure the phosphorylation levels at Tyr536 and Tyr564 in KSL cells by flow cytometry and in BMMCs and splenocytes by immunoblotting. As shown in Figure 5C-D, Tyr536 phosphorylation was reduced in both lyn−/− and PLC-β3−/− cells, and was abolished in lyn−/−;PLC-β3−/− cells. On the other hand, Tyr564 phosphorylation was not affected in PLC-β3−/− cells, but reduced or almost abrogated in lyn−/− and lyn−/−;PLC-β3−/− cells. These data suggest that Lyn is the predominant kinase that phosphorylates Tyr564, and PLC-β3 may help tyrosine kinases including Lyn to phosphorylate Tyr536.
We previously showed that the C-terminal domain (CT) of PLC-β3 interacts with SHP-1 in Ba/F3 and splenic cells.19 PLC-β3 coimmunoprecipitation with SHP-1 was not only confirmed in BMMCs overexpressing PLC-β3 CT, but also PLC-β3 coimmunoprecipitation with Lyn was newly found in these cells (Figure 5E). Similar to PLC-β3/SHP-1 interactions, PLC-β3/Lyn interactions were enhanced by IL-3 stimulation. Furthermore, coimmunoprecipitation of Lyn with SHP-1 was also revealed in wt splenocytes and PLC-β3 CT-overexpressing PLC-β3−/− BMMCs. Lyn/SHP-1 interactions were also enhanced by IL-3 stimulation. Interestingly, Lyn/SHP-1 interactions were only weakly detected in PLC-β3−/− splenocytes and BMMCs (Figure 5F), suggesting that PLC-β3 CT facilitates the interaction between Lyn and SHP-1.
Reduced SHP-1 phosphorylation at Tyr536 and Tyr564 in lyn−/− cells (Figure 5C-D) and physical interaction between Lyn and SHP-1 (Figure 5E-F) raised the possibility that Lyn phosphorylates these SHP-1 residues. We tested this possibility by in vitro kinase assays. As shown in Figure 5G, SHP-1 phosphorylation at Tyr564 and Tyr536 was increased in a Lyn concentration-dependent manner, indicating that Lyn can directly phosphorylate both residues.
Phosphorylation of Tyr564, but not Tyr536, is critical for SHP-1 phosphatase activity
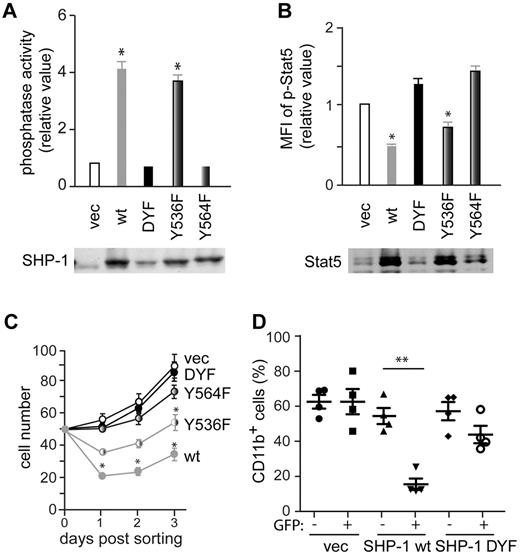
To investigate the effect of Tyr536 and Tyr564 phosphorylation on SHP-1 phosphatase activity, wt, Y536F, Y564F or doubly YF mutant (DYF) SHP-1 vectors were introduced into mev/mev CD34−KSL cells. As expected, wt SHP-1 increased a phosphatase activity by 4-fold over empty control vector (note that aberrant mev/mev SHP-1 proteins exhibit 10%-20% activity of wt enzyme). Y536F mutant exhibited a phosphatase activity similar to wt enzyme. By contrast, Y564F and DYF mutants had activities similar to vector control (Figure 6A). These results strongly suggest that Tyr564 phosphorylation is critical for SHP-1 phosphatase activity. Moreover, increased SHP-1 phosphatase activity in wt- and Y536F-expressing cells correlated well with reduced Stat5 phosphorylation, while low phosphatase activities in vector-, Y564F- and DYF-expressing cells with higher Stat5 phosphorylation (Figure 6B). These relationships were further extended to lower-to-higher in vitro growth rates in the order of wt-, Y536F-, Y564F-, DYF-, and vector-expressing cells (Figure 6C). Furthermore, wt SHP-1-expressing mev/mev CD34−KSL cells lost their MPN-causing ability upon transplantation, whereas DYF SHP-1-expressing mev/mev cells retained it (Figure 6D).
Tyr564 phosphorylation is critical for catalytic activity and biologic functions of SHP-1. (A) CD34−KSL cells from mev/mev mice were retrovirally transduced with empty vector or various SHP-1 mutants. Sorted GFP+ cells were cultured in the presence of IL-3 and SCF for 4 weeks and then measured for SHP-1 phosphatase activity. Data are mean ± SD, *P < .05 versus vector control. SHP-1 levels shown are taken from Figure 5B. (B) Stat5 phosphorylation in the cells from panel A was analyzed by flow cytometry. Stat5 levels in the cell panel were assessed by immunoblotting. (C) CD34−KSL cells from mev/mev mice were transduced with a bicistronic retroviral vector encoding wt SHP-1, DYF SHP-1, Y536F SHP-1, Y564F SHP-1, or empty vector, together with GFP. Transduced cells were sorted and cultured in the presence of IL-3 and SCF. (D) Transduced mev/mev CD34−KSL cells were adoptively transferred (without sorting) to lethally irradiated C57BL/6-Ly5.1 mice. Donor-derived (Ly5.2+) CD11b+ cells in peripheral blood were analyzed by flow cytometry.
Tyr564 phosphorylation is critical for catalytic activity and biologic functions of SHP-1. (A) CD34−KSL cells from mev/mev mice were retrovirally transduced with empty vector or various SHP-1 mutants. Sorted GFP+ cells were cultured in the presence of IL-3 and SCF for 4 weeks and then measured for SHP-1 phosphatase activity. Data are mean ± SD, *P < .05 versus vector control. SHP-1 levels shown are taken from Figure 5B. (B) Stat5 phosphorylation in the cells from panel A was analyzed by flow cytometry. Stat5 levels in the cell panel were assessed by immunoblotting. (C) CD34−KSL cells from mev/mev mice were transduced with a bicistronic retroviral vector encoding wt SHP-1, DYF SHP-1, Y536F SHP-1, Y564F SHP-1, or empty vector, together with GFP. Transduced cells were sorted and cultured in the presence of IL-3 and SCF. (D) Transduced mev/mev CD34−KSL cells were adoptively transferred (without sorting) to lethally irradiated C57BL/6-Ly5.1 mice. Donor-derived (Ly5.2+) CD11b+ cells in peripheral blood were analyzed by flow cytometry.
The Tyr536 residue of SHP-1 is critical for interaction with Stat5
Despite the normal catalytic activity, Y536F SHP-1 exhibited lower suppression of Stat5 phosphorylation and in vitro cell growth in mev/mev BMMCs and HSCs, compared with wt SHP-1 (Figure 6). This could be due to weaker interactions between Y536F SHP-1 and Stat5. Indeed, coimmunoprecipitation of SHP-1 with Stat5 was drastically reduced in mev/mev BMMCs expressing Y536F or DYF SHP-1 (Figure 5H). By contrast, wt and Y564F SHP-1 were well coimmunoprecipitated with Stat5. These results suggest that SHP-1 phosphorylation of Tyr536 is critical for interaction with Stat5.
SHP-1 phosphorylation at Tyr564 is critical for suppression of Stat5 phosphorylation, in vitro cell growth, and MDS/MPN development
The above results suggest that SHP-1 phosphatase activity is inversely correlated with Stat5 activity, in vitro cell growth, and MPN-causing abilities of HSCs. To test whether these observations can be generalized, we measured the enzymatic activity of SHP-1 in BMMCs derived from wt, lyn−/−, PLC-β3−/−, lyn−/−;PLC-β3−/−, and mev/mev mice. Consistent with our observation that Lyn phosphorylates SHP-1 at the critical residue Tyr564, SHP-1 phosphatase activity was reduced by 40% in lyn−/− cells (Figure 7A). Importantly, lyn−/−;PLC-β3−/− cells had a very low level of SHP-1 phosphatase activity, similar to mev/mev cells (Figure 7A), showing that the reduced SHP-1 phosphatase activity is a common signaling defect shared by mev/mev and lyn−/−;PLC-β3−/− mice. To test the possibility that reduced SHP-1 phosphatase activity might be responsible for the increased in vitro growth of HSCs and MPN development in lyn−/−;PLC-β3−/− mice, we retrovirally transduced CD34−KSL cells with SHP-1. Although overexpression of wt SHP-1 suppressed the in vitro growth of mev/mev CD34−KSL cells (Figure 6C) as well as wt, lyn−/−, and PLC-β3−/− cells (supplemental Figure 6), it had little effect on lyn−/−;PLC-β3−/− cells (supplemental Figure 6 and Figure 7D). Wild-type SHP-1–overexpressing CD34−KSL cells from lyn−/−;PLC-β3−/− mice still caused MPN upon transplantation (Figure 7E). Consistent with these results, the transduced wt SHP-1 was not phosphorylated at Tyr536 or Tyr564 in lyn−/−;PLC-β3−/− cells (supplemental Figure 7). Therefore, we introduced Y536E/Y564E (DYE) mutations into SHP-1 to mimic phosphorylation. Indeed, transduction of lyn−/−;PLC-β3−/− cells with DYE SHP-1 increased SHP-1 phosphatase activity by 10-fold in lyn−/−;PLC-β3−/− cells, in contrast to a mere 2-fold increase by wt SHP-1 expression (Figure 7B). Importantly, DYE-transduced cells exhibited substantially lower levels of Stat5 phosphorylation and in vitro growth rate (Figure 7C-D). Furthermore, MPN-causing properties of the DYE-transduced cells were lost upon transplantation (Figure 7E). These results suggest that severely reduced SHP-1 phosphatase activity is responsible for the MDS/MPN-associated phenotypes observed in lyn−/−;PLC-β3−/− mice.
Reduced SHP-1 phosphatase activity is responsible for MDS/MPN development in lyn−/−;PLC-β3−/− mice. (A) CD34−KSL cells were cultured in the presence of IL-3 and SCF for 4 weeks and were analyzed for SHP-1 phosphatase activity. Data are mean ± SD, *P < .05 versus vector control. Similar expression levels of SHP-1 among the cells can be seen in Figure 5D. (B) CD34−KSL cells from lyn−/−;PLC-β3−/− mice were retrovirally transduced with empty vector or various SHP-1 mutants. Sorted GFP+ cells were cultured in the presence of IL-3 and SCF for 4 weeks and then measured for SHP-1 phosphatase activity. (C) Stat5 phosphorylation in the cells from panel B was measured by flow cytometry. Expression of SHP-1 (C) and Stat5 (D) was assessed by immunoblotting. (D) CD34−KSL cells from lyn−/−;PLC-β3−/− mice were transduced with a bicistronic retroviral vector encoding wt SHP-1, DYE SHP-1, or empty vector, together with GFP. Transduced GFP+ cells were sorted and cultured in the presence of IL-3 and SCF. (E) Transduced lyn−/−;PLC-β3−/−CD34−KSL cells were adoptively transferred (without sorting) to lethally irradiated C57BL/6-Ly5.1 mice. Donor-derived (Ly5.2+) CD11b+ cells in peripheral blood were analyzed by flow cytometry. **P < .01.
Reduced SHP-1 phosphatase activity is responsible for MDS/MPN development in lyn−/−;PLC-β3−/− mice. (A) CD34−KSL cells were cultured in the presence of IL-3 and SCF for 4 weeks and were analyzed for SHP-1 phosphatase activity. Data are mean ± SD, *P < .05 versus vector control. Similar expression levels of SHP-1 among the cells can be seen in Figure 5D. (B) CD34−KSL cells from lyn−/−;PLC-β3−/− mice were retrovirally transduced with empty vector or various SHP-1 mutants. Sorted GFP+ cells were cultured in the presence of IL-3 and SCF for 4 weeks and then measured for SHP-1 phosphatase activity. (C) Stat5 phosphorylation in the cells from panel B was measured by flow cytometry. Expression of SHP-1 (C) and Stat5 (D) was assessed by immunoblotting. (D) CD34−KSL cells from lyn−/−;PLC-β3−/− mice were transduced with a bicistronic retroviral vector encoding wt SHP-1, DYE SHP-1, or empty vector, together with GFP. Transduced GFP+ cells were sorted and cultured in the presence of IL-3 and SCF. (E) Transduced lyn−/−;PLC-β3−/−CD34−KSL cells were adoptively transferred (without sorting) to lethally irradiated C57BL/6-Ly5.1 mice. Donor-derived (Ly5.2+) CD11b+ cells in peripheral blood were analyzed by flow cytometry. **P < .01.
Discussion
We show that lyn−/−;PLC-β3−/− mice develop a Stat5-dependent, CMML-like disease that originates from an LT-HSC–enriched population. Mechanistically, in the absence of Lyn and PLC-β3, reduced SHP-1 phosphorylation at Tyr536 and Tyr564 causes drastically reduced SHP-1 phosphatase activity, resulting in constitutive activation of Stat5. This is the first study suggesting PLC-β3 involvement in MDS/MPN, while recent studies, which are not replicated in another study, suggest that mono-allelic deletion of the PLC-β1 gene encoding another member of the PLC-β family, is associated with a worse clinical course in MDS patients, including higher risk of AML evolution.37,38 It will be interesting to compare how PLC-β1 and PLC-β3, both expressed in hematopoietic and nonhematopoietic cells, are involved in leukemogenesis.
Constitutive activation of Stat5 leads to hematologic malignancies.39-41 Stat5 is activated by several oncoproteins, such as BCR-ABL,42-45 FLT3-ITD,46 and JAK2 V617F47 and is likely required for the pathogenesis of leukemia/MPN caused by these oncoproteins. The present study suggests that reduced expression of Lyn, PLC-β3, or SHP-1 might contribute to the development of JMML, CMML, or M4/5 AML. Thus, polymorphisms, such as single-nucleotide polymorphisms or deletions/additions, which affect expression or structures of these genes could potentially link to increased risk of the disease development. Hyperphosphorylation of STAT5 in response to subsaturating concentrations of GM-CSF has been reported in leukemias, including JMML, CMML, and M4/5 AML that have hyperactivation of the Ras/ERK signaling pathway,13 raising the intriguing possibility that Ras activation is upstream of Stat5 activation and supporting an important role of Stat5 in the development of these myelomonocytic leukemias. In line with this, lyn−/−;PLC-β3−/−, mev/mev, lyn−/−;hck−/−, and SHIP−/− mice25 develop a fatal MPN with monocytic features accompanied by increased Stat5 activity. Interestingly, macrophages in these mice exhibit an alternatively activated or M2 phenotype, suggesting that hyperactivated Stat5 might be involved in the M2 programming. The M2 phenotype can be induced by cytokines produced by T helper type 2 cells,48 and Stat5 is required for differentiation of T helper type 2 cells49 and M2 macrophages.50 Thus, it will be interesting to investigate if the macrophages (or monocytes) in myelomonocytic leukemic patients are of the M2 phenotype.
Stimulation with various cytokines and growth factors induces activation of Jak2, which in turn activates Stat5 to promote cell proliferation and survival.51 Consistent with this, we observed completely abrogated Stat5 phosphorylation in Jak2−/− fetal live cells, as shown previously.52,53 However, we report here that Lyn in HSCs and myeloid cells inhibits Stat5 activation in vivo by phosphorylating and activating SHP-1, a critical negative regulator of Stat5. SHP-1 was shown to inhibit Stat5 activation indirectly by dephosphorylating Jak2 via the interaction with Grb254 and to directly dephosphorylate and inhibit Stat5 in a PLC-β3-dependent manner.19
Here we demonstrate that PLC-β3 also facilitates Lyn-mediated phosphorylation and activation of SHP-1. We found the differential regulation of SHP-1 phosphorylation at Tyr536 and Tyr564 by PLC-β3 and Lyn: Lyn directly phosphorylates Tyr564 and activates the SHP-1 catalytic activity (supplemental Figure 8). However, PLC-β3 is only required for the phosphorylation at Tyr536, but not Tyr564. In this regard, Lyn and another kinase(s) phosphorylate Tyr536 in a PLC-β3-dependent manner. Using semisynthetic SHP-1 proteins containing different phosphonate analogues at Tyr536 and Tyr564, Zhang et al. showed that phosphotyrosine mimetics at Tyr536 lead to approximately 4- to 8-fold stimulation of SHP-1 phosphatase activity over the nonphosphorylated enzyme, whereas phosphotyrosine mimetics at Tyr564 lead to 1.6-fold stimulation.55 However, our mutagenesis studies clearly showed that (1) phosphorylation at Tyr564, but not Tyr536, is critical for SHP-1 phosphatase activity and that (2) Stat5 activity and in vitro growth of mev/mev cells were inhibited by Y536F mutant, but not Y564F. The method used by Zhang et al. was different from our mutagenesis approach and not extensively verified in other phosphorylation studies. Importantly, our results demonstrate a strong inverse correlation between SHP-1 phosphatase activity and Stat5 activity, in vitro cell growth and MPD development in lyn−/−;PLC-β3−/− cells expressing wt or phosphomimetic DYE mutant SHP-1. Thus, a potential model to explain the role of Tyr564 in determining SHP-1 catalytic activity is that the phosphorylated Tyr564 residue has the potential to engage the N-SH2 domain intramolecularly and expose the catalytic domain (supplemental Figure 8). What is the role of Tyr536 phosphorylation? Phosphorylated Tyr536 residue may recruit Grb2.30 Another intriguing possibility is that phosphorylation of Tyr536 can strengthen the interaction between SHP-1 and its substrates. Indeed, Tyr536 was critical for interaction with Stat5 (supplemental Figure 8). Consistent with this, the inhibitory effect of Y536F on Stat5 phosphorylation and cell growth was inferior to wt SHP-1, although Y536F SHP-1 had a similar level of phosphatase activity to wt SHP-1.
An interesting finding in this study is the loss of SHP-1 phosphorylation at both Tyr536 and Tyr564 in mev/mev cells. A T-to-A transversion point mutation at a splice donor site of SHP-1 allele in these mice results in the use of 2 cryptic splice donor sites56 : a splicing event involving the cryptic site 15 bp upstream from the normal site results in a transcript encoding a protein lacking 5 amino acids; the other results in another transcript containing a 69-bp intronic sequence encoding 23 amino acids. Both splicing events affect the structure of the phosphatase core domain. Therefore, the amino acid deletion or addition might directly reduce SHP-1 phosphatase activity. Alternatively, it might cause conformational changes that prevent phosphorylation at Tyr536 and Tyr564. The similarities in severely reduced SHP-1 phosphatase activity and Tyr536 and Tyr564 phosphorylation between lyn−/−;PLC-β3−/− cells and mev/mev cells are consistent with the latter possibility.
When wt SHP-1 was transduced into lyn−/−;PLC-β3−/− cells, SHP-1 phosphatase activity was increased by 2-fold, but with little effect on Stat5 activity, in vitro cell growth, and MPD-inducing capabilities. This suggests that the adaptor function of PLC-β3 is necessary for SHP-1 to function properly, but this dependency can be overridden by very high levels of SHP-1 phosphatase activity achieved by DYE SHP-1 transduction in lyn−/−;PLC-β3−/− cells. Our previous study demonstrated the presence of the SPS complex.19 This study now adds Lyn as a putative new member of this complex; Lyn regulates phosphatase activity of SHP-1 by directly phosphorylating Tyr564 and, together with PLC-β3, by directly and indirectly increasing Tyr536 phosphorylation. SHP-1 catalytic activity is inversely correlated with Stat5 phosphorylaiton and activity. Therefore, the SPS complex is the platform on which SHP-1 and Stat5 activities are finely regulated. Our study thus suggests that enhancing SHP-1 phosphatase activity may be a potential strategy to inhibit Stat5 activity and to treat MPN, MPN/MDS, and myeloid leukemia.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Xiaowu Zhang (Cell Signaling Technology) for kindly providing reagents.
This study was supported in part by grants from the National Institutes of Health to T.K. W.X. was supported in part by funds from the Diabetes and Immune Disease National Research Institute. This is Publication 1277 from the La Jolla Institute for Allergy and Immunology.
National Institutes of Health
Authorship
Contribution: W.X., T.A., and H.-Y.W. performed experiments; W.X., Y.K., and T.K. designed experiments and analyzed results; and W.X. and T.K. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Toshiaki Kawakami, Division of Cell Biology, La Jolla Institute for Allergy and Immunology, 9420 Athena Cir, La Jolla, CA 92037; e-mail: toshi@liai.org.