Abstract
The immune response in heparin-induced thrombocytopenia is initiated by and directed to large multimolecular complexes of platelet factor 4 (PF4) and heparin (H). We have previously shown that PF4:H multimolecular complexes assemble through electrostatic interactions and, once formed, are highly immunogenic in vivo. Based on these observations, we hypothesized that other positively charged proteins would exhibit similar biologic interactions with H. To test this hypothesis, we selected 2 unrelated positively charged proteins, protamine (PRT) and lysozyme, and studied H-dependent interactions using in vitro and in vivo techniques. Our studies indicate that PRT/H and lysozyme/H, like PF4/H, show H-dependent binding over a range of H concentrations and that formation of complexes occurs at distinct stoichiometric ratios. We show that protein/H complexes are capable of eliciting high-titer antigen-specific antibodies in a murine immunization model and that PRT/H antibodies occur in patients undergoing cardiopulmonary bypass surgery. Finally, our studies indicate that protein/H complexes, but not uncomplexed protein, directly activate dendritic cells in vitro leading to interleukin-12 release. Taken together, these studies indicate that H significantly alters the biophysical and biologic properties of positively charged compounds through formation of multimolecular complexes that lead to dendritic cell activation and trigger immune responses in vivo.
Introduction
Heparin-induced thrombocytopenia (HIT) is an immune-mediated disorder caused by antibodies that recognize multimolecular complexes of platelet factor 4 (PF4), a positively charged platelet protein, and heparin (H), a negatively charged carbohydrate. We, and others, have shown that PF4 and H complexes assemble primarily through nonspecific electrostatic interactions governed by principles of colloidal chemistry.1-5 In colloidal systems, molecules of opposite charge “aggregate” or grow in size due to effects of charge neutralization. Particle interactions are frequently dependent on stoichiometric ratios of the 2 compounds, with the largest complexes occurring at molar ratios of the compounds leading to charge neutralization. When either compound is in molar excess, charge restabilization occurs and repulsive forces predominate, leading to reduced complex size and/or complex disassembly.
Studies to date indicate that PF4/H multimolecular complex formation is central to the pathogenesis of HIT. The characteristic bell-shaped curve seen with HIT antibody binding over a range of H concentrations coincides with H-dependent formation of multimolecular complexes.2,3 HIT antibody binding, as gauged by serologic assays or functional studies of platelet activation, is optimal when multimolecular complexes form at or near equimolar ratios of PF4:H. However, antibody binding is markedly reduced with increasing H concentrations, a phenomenon that can be directly attributed to loss of complex formation.2-4 Recent studies from our laboratory indicate that similar H-dependent changes affect the immunogenicity of PF4/H complexes in vivo.5,6 Our studies demonstrate that PF4/H complexes are immunogenic over a certain range of H concentrations associated with multimolecular complex formation and that the immune response is attenuated when PF4 or H is given alone or when H is in molar excess of PF4.5
H and H-like molecules bind several positively charged proteins in addition to PF4.7 These H-binding proteins (HBPs) are structurally and functionally diverse, and include, to name a few, nuclear proteins (protamine), enzymes (C1 esterase and lysozyme), adhesion molecules (fibronectin and vitronectin) growth factors (fibroblast growth factor), and lipid-binding proteins (apolipoprotein E and lipoprotein lipase). To date, it appears that a majority of HBP-H interactions are ionic in nature, with limited or no evidence for unique structural requirements, folding patterns or consensus H-binding regions in common.8-10
Early experimental studies of several H-binding proteins, including protamine (PRT) and lysozyme (Lys), indicate that H interacts stoichiometrically with these proteins to form complexes and/or aggregates.10-12 As noted with PF4/H complex formation,1 PRT and Lys interactions with H bear the hallmark of charge-dependent colloidal interactions, namely sensitivity to changes in pH and ionic strength of the buffer.10,12
The ubiquity of HBPs in organisms, the nonspecific nature of electrostatic interactions of HBPs with H and their similarity to PF4/H interactions, prompted us to investigate the biologic response to HBP/H complexes in vivo. These studies aim to characterize the multimolecular complexes formed between H and 2 structurally and functionally unrelated HBPs (PRT and Lys). Using in vitro and in vivo studies, we present data to show that H significantly enhances the immunogenicity of positively charged molecules through formation of protein-H multimolecular complexes that activate dendritic cells (DCs) and lead to an antigen-specific immune response in the host.
Methods
Biophysical studies of PRT/H and Lys/H complexes
Unless specified, reagents were purchased from Sigma-Aldrich. Solutions of PRT-sulfate (grade “X” amorphous powder from salmon sperm, molecular weight [Mw] 5.1 kDa) or Lys (chicken egg white, Mw 14.3 kDa) were mixed with varying concentrations of unfractionated heparin (UFH; 100 or 1000 U/mL; Heplock; Elkins-Sinn Inc) in Hanks balanced salt solution (Invitrogen) or H20. Murine PF4 (mPF4) was prepared from recombinant bacteria as previously described.5,6 For stoichiometric calculations involving UFH, we used previously published estimates of UFH specific activity at 140 U/mg3,5 and mean Mw of 15 kDa.3,5
Light absorbance of PRT/H and Lys/H solutions was measured in a Spectra Max Plus 384 Plate Reader (MDS Technologies) and analyzed using proprietary SoftMax Pro version 5.3. The electrophoretic mobility of HBP/H complexes was measured using a Zetasizer Nano ZS (Malvern) as previously described.5 Zeta potentials (ζ-potential), which are related to the surface charge of particles, were calculated using the Henry equation and analyzed using the accompanying Zetasizer software (Version 5.10; Malvern).
Murine immunization model
Immunizations were performed using modifications of a previously described immunization model.6 Protein or HBP/H concentrations for immunization were selected based on preliminary studies from biophysical data shown in Figure 1. Unless specified, mice were injected via retro-orbital plexus with PRT (125 μg/mL) + H (12.5 U/mL) or Lys (500 μg/mL) + H (12 U/mL) in a final volume of 100 μL daily for 5 days. Blood samples for enzyme-linked immunosorbent assay (ELISA) were collected in anesthetized mice from the retro-orbital blood plexus in acid-dextrose citrate (ACD) solution (ACD formula A; Baxter Healthcare Corporation) at baseline and at weekly intervals as specified after the start of immunizations. Animals injected with PRT/H or Lys/H were defined as seropositive if A450nm exceeds the mean A450nm + 3× the standard deviation (SD) of animals injected with the respective protein in the absence of H (PRT alone or Lys alone). All studies were performed with the approval of the Institutional Animal Care and Use Committee at Duke University.
H-dependent multimolecular complex formation with PRT and Lys. (A-B) Schematic representation of PF4 and H interactions as previously reported.5 PF4 in solution, in the absence of H, shows minimal light aborption (left side of curve in panel A) and displays positive charge (top part of curve in panel B). With increasing amounts of H (x-axis), charge neutralization occurs resulting in complexes of increasing size (peak in panel A) and neutral charge (inflection point in panel B). Addition of H beyond the concentration required for peak complexes results in complexes of reduced size (right side of curve in panel A) and increasingly negative charge (bottom of curve in panel B). (C-D) PRT (250-31 μg/mL) was incubated with increasing concentrations of H. Light absorption (panel C) and ζ-potential (panel D) were measured. E-F: Lys (1000-125 μg/mL) was incubated with increasing concentrations of H. Light absorption (panel E) and ζ-potential (panel F) were measured. Data are representative of 3 or more independent experiments.
H-dependent multimolecular complex formation with PRT and Lys. (A-B) Schematic representation of PF4 and H interactions as previously reported.5 PF4 in solution, in the absence of H, shows minimal light aborption (left side of curve in panel A) and displays positive charge (top part of curve in panel B). With increasing amounts of H (x-axis), charge neutralization occurs resulting in complexes of increasing size (peak in panel A) and neutral charge (inflection point in panel B). Addition of H beyond the concentration required for peak complexes results in complexes of reduced size (right side of curve in panel A) and increasingly negative charge (bottom of curve in panel B). (C-D) PRT (250-31 μg/mL) was incubated with increasing concentrations of H. Light absorption (panel C) and ζ-potential (panel D) were measured. E-F: Lys (1000-125 μg/mL) was incubated with increasing concentrations of H. Light absorption (panel E) and ζ-potential (panel F) were measured. Data are representative of 3 or more independent experiments.
PRT/H and Lys/H ELISAs
Murine antibodies to PRT/H and Lys/H complexes were measured by ELISA using a 96-well microtiter plate (Nunc Maxisorp; Nalge Nunc International) as previously described5,6 with the following exceptions. Antigen was coated overnight with 100 μL of antigen solution: PRT (31 μg/mL) + H (4 U/mL), Lys (125 μg/mL) + H (2.8 U/mL), mPF4 (10 μg/mL) + H (0.4 U/mL), or bovine serum albumin (BSA; 50 μg/mL) prepared in phosphate-buffered saline (PBS; Invitrogen). The protein:H ratios used for coating the plate were based on stoichiometric ratios obtained from biophysical data (Figure 1). Murine plasma was used at 1:100 and horseradish peroxidase–labeled goat anti-mouse IgG gamma was used at 1:500 for PRT/H antibodies and 1:2000 dilution for Lys/H antibodies.
Dendritic cell activation
DCs were isolated from nonimmunized C57BL/6 murine splenocytes using previously published protocols.13 Briefly, spleens were minced in Mg2+- and Ca2+-free Hanks balanced salt solution/5% fetal calf serum and digested with 1 mg/mL collagenase from Clostridium histolyticum and 0.2 mg/mL DNAse I for 30 minutes at 37°C. Cells were passed through 70-μm nylon mesh, washed with RPMI + 10% fetal calf serum/10mM EDTA (ethylenediaminetetraacetic acid)/20mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) and resuspended in 5 mL of the solution in a Petri dish, cells were harvested from plate and suspension-loaded over equal volume of 14.5% Nycodenz gradient. The interface was collected and further purified using CD11c MicroBeads (Miltenyi Biotec) following manufacturer's protocols. This procedure yielded DCs of > 94% purity as assessed by flow cytometry. Endotoxin-free buffers and/or reagents that tested low for endotoxin were used for cell-culture experiments.
After isolation, DCs were seeded into 96-well plates at 1 × 105 cells/well in RPMI media and incubated with media containing the following antigens: recombinant mPF4 (10 μg/mL) + H (0.4 U/mL) PRT (15.6 μg/mL) + H (2 U/mL) and Lys (125 μg/mL) + H (2.8 U/mL). Lipopolysaccharide (1 μg/mL) from Escherichia coli 055:B5 (Sigma-Aldrich) was added to the culture as positive control and cellular activation was measured 24 hours after addition of antigen using an interleukin-12/(IL-12)–optiELISA kit from BD Biosciences following manufacturer's directions.
Patient samples
Plasma from normal subjects (n = 45) was purchased from George King Biomedical Inc. Blood from patients undergoing cardiopulmonary bypass (CPB) surgery was collected at baseline, 5 days, and 30 days after the procedure using informed consent under an institutional review board–approved protocol. Human antibodies to PRT/H were measured using ELISA conditions described above for murine plasma with the exception of adding 0.05% Tween to buffers to reduce background binding. Antibodies to hPF4/H were detected using a commercial immunoassay (PF4 Enhanced; Genetics Technology Institute).
Statistical analysis
Antibody levels in murine and human ELISAs were compared using the Student t test for comparisons of 2 groups or by a 1-way ANOVA analysis for more than 2 groups. ELISA reactivity to various antigens was expressed as mean ± 1 SD and analyzed for significance using the Student t test. Statistical analyses were performed using GraphPad Prism (GraphPad Software Version 4.03). Differences were considered significant at P < .05.
Results
Biophysical characterization of PRT/H and Lys/H complexes
We have previously shown that PF4/H multimolecular complex formation occurs through colloidal interactions,5 which can be measured through changes in light scattering (absorbance) and surface charge or ζ-potential. In our previous studies, we showed that PF4 alone in solution displays minimal light absorption (Figure 1A right side of curve) on spectrophotometry and carries a positive charge as measured by ζ-potential (Figure 1B top of curve). With increasing concentrations of H, there is an accompanying increase in light transmission (peak curve Figure 1A) due to multimolecular assembly. In the ζ-potential, addition of H to a solution of PF4 lowers the surface charge of PF4, and leads to a negative or decreased slope (change from positive to negative, Figure 1B). The “peaks” in light transmission (Figure 1A) and the inflection points in the ζ-potential (Figure 1B) correlate closely with formation of large multimolecular complexes due to effects of charge neutralization.
To determine whether other positively charged proteins show similar colloidal interactions with H, we selected 2 HBPs, PRT sulfate and Lys, with known high affinity to H. To demonstrate multimolecular complex formation, we mixed various concentrations of PRT (31-250 μg/mL, Figure 1C-D) or Lys (125-1000 μg/mL, Figure 1E-F) with increasing amounts of H (0-100 U/mL) and measured light absorption (A280nm, Figure 1C and E) or ζ-potential (Figure 1D-F). As shown in Figure 1, increasing amounts of H altered the biophysical properties of PRT or Lys as indicated by changes in light transmission/absorption and ζ-potential. For a fixed protein concentration of PRT or Lys, we noted that addition of H results in complexes of increasing size, until a “peak” size is achieved; further increases in H result in decreased absorbance, which correlates with reduced size of complexes.5 As seen with PF4/H interactions, the H concentrations resulting in peak complex size for PRT or Lys, as measured by absorbance (A280nm, Figure 1C,E), were concordant with the H concentrations at which charge neutralization occurred (ζ-potential inflection points, Figure 1D-F) for each protein. The concentrations of protein and H resulting in the peak absorbance and/or neutral charge allowed us to approximate the stoichiometry of the 2 compounds necessary for multimolecular complex formation. For PRT, we approximated that peak complex size occurs at a molar ratio of approximately 3:1, whereas for Lys, peak complexes occur at molar ratios of approximately 5:1. The molar ratios for peak complex formation for these individual proteins remain constant for a given protein/H peak, irrespective of protein concentration (eg, for PRT, the peaks for 250 and 125 μg/mL curves occur at 32 and 16 U/mL H, respectively, both approximating a PHR molar ratio of 3:1).
Heparin modifies the immunogenicity of positively charged proteins in vivo
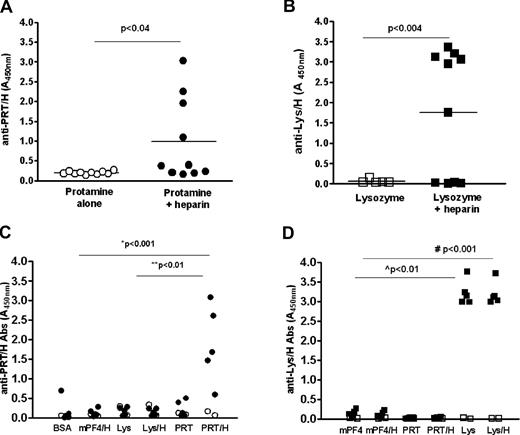
We have previously shown that mPF4/H complexes, but not mPF4 alone or H alone elicit robust immune responses in mice.5 To determine whether H modifies the immunogenicity of PRT and Lys similar to mPF4, we injected C57BL/6 mice with PRT + H or Lys + H as described in “Murine immunization model.” Similar to mPF4/H, mice injected with PRT/H or Lys/H, but not PRT alone or Lys alone, develop robust immune responses within 14 days of immunization (Figure 2A-B). When similar cohorts from replicate experiments were tested concurrently by ELISA (n = 30 for each antigen: PRT, PRT/H, Lys, or Lys/H), significant differences were seen in the mean absorbance (A450nm ± SD) of animals injected with PRT/H compared with PRT alone (PRT/H = 0.910 + 1.173 vs PRT = 0.1193 + 1.0193, P < .0001) and Lys/H compared with Lys alone (Lys/H = 1.537 + 1.5 vs Lys alone = 0.094 + 0.054; P < .0001; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In the combined cohort, seroconversions (as defined in “Murine immunization model”) occurred in 30% of animals injected with PRT/H (supplemental Figure 1A) and 53% of animals injected with Lys/H (supplemental Figure 1B). Antibody responses to PRT/H or Lys/H were highly specific for the immunizing antigen as antibodies formed to PRT/H (Figure 2C closed circles) or Lys/H (Figure 2D closed squares) did not crossreact with other antigens (mPF4 or BSA) or other HBP/H complexes (mPF4/H or PRT/H in mice expressing anti-Lys/H or vice versa; Figure 2C-D). Mice injected with PRT or Lys alone did not react to wells coated with PRT alone or Lys alone (supplemental Figure 2). Consistent with a polyclonal response, antibodies formed after PRT/H or Lys/H showed variable H-dependent reactivity by ELISA (Figure 2C-D and supplemental Figure 3). Whereas PRT/H antibodies showed binding to wells with increasing amounts of H (with minimal binding to PRT alone and increased binding to wells coated with PRT and variable amounts of H, supplemental Figure 3A), Lys/H antibodies showed no preferential H-dependent binding (supplemental Figure 3B).
Immune responses to PRT/H or Lys/H complexes. C57Bl/6 mice were immunized with PRT alone (125 μg/mL, n = 10) or PRT/H (125 μg/mL PRT and 12.5 U/mL H, n = 10) and Lys (500 μg/mL, n = 5) or Lys/H (500 μg/mL Lys and 12 U/mL, n = 10). (A-B) Peak antibody responses to PRT/H (panel A) and Lys/H (panel B) occurred within 14 days from start of immunization. (C-D) Specificity of representative seropositive PRT/H (panel C, •) and Lys/H (panel D, ■), and seronegative PRT/H (○) and Lys/H (□) are shown. P values were calculated for panels A and B using an unpaired t test with Welch correction and calculated for panels C and D using a 1-way ANOVA and Bonferroni posttest for calculating differences between conditions. *PRT/H vs BSA, mPF4/H, or Lys/H; **PRT/H vs PRT or Lys; #Lys/H vs BSA, mPF4/H, PRT, or PRT/H; L̂ys vs BSA, mPF4/H, PRT, or PRT/H. Data are representative of > 3 independent immunization experiments involving PRT/H or Lys/H.
Immune responses to PRT/H or Lys/H complexes. C57Bl/6 mice were immunized with PRT alone (125 μg/mL, n = 10) or PRT/H (125 μg/mL PRT and 12.5 U/mL H, n = 10) and Lys (500 μg/mL, n = 5) or Lys/H (500 μg/mL Lys and 12 U/mL, n = 10). (A-B) Peak antibody responses to PRT/H (panel A) and Lys/H (panel B) occurred within 14 days from start of immunization. (C-D) Specificity of representative seropositive PRT/H (panel C, •) and Lys/H (panel D, ■), and seronegative PRT/H (○) and Lys/H (□) are shown. P values were calculated for panels A and B using an unpaired t test with Welch correction and calculated for panels C and D using a 1-way ANOVA and Bonferroni posttest for calculating differences between conditions. *PRT/H vs BSA, mPF4/H, or Lys/H; **PRT/H vs PRT or Lys; #Lys/H vs BSA, mPF4/H, PRT, or PRT/H; L̂ys vs BSA, mPF4/H, PRT, or PRT/H. Data are representative of > 3 independent immunization experiments involving PRT/H or Lys/H.
Characterization of the in vivo immune response to PRT/H
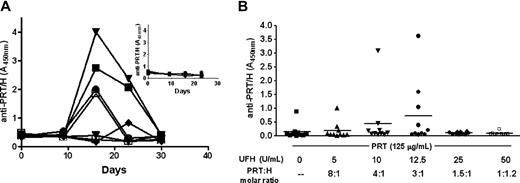
We undertook additional studies to determine whether the humoral response to PRT/H or Lys/H shares any important biologic features of mPF4/H immune response.6 As shown in Figure 3, the PRT/H immune response resembles mPF4/H seroconversions in terms of temporal course and stoichiometric requirements for multimolecular complex formation.5,6 Seroconversion is maximal by day 15 (D15) from start of immunization, with antibody levels declining to baseline levels by D30 (Figure 3A). Mice injected with PRT alone show no antibody responses to PRT/H over time (Figure 3A inset). A similar time course of seroconversions were seen with mice injected with Lys/H (supplemental Figure 4). As with PF4/H seroconversions,6 the immunogenicity of PRT/H complexes in vivo is linked to the stoichiometry of complex formation. Mice injected with PRT/H complexes at molar ratios of PRT:H of 3:1 showed a trend toward increased seroconversion, whereas mice injected with complexes at higher or lower molar ratios were less likely to seroconvert (Figure 3B, P = not significant [ns]). Other similarities with the PF4/H immune response were seen with respect to the doses of multimolecular complexes needed to elicit an immune response (supplemental Figure 5). These latter studies suggest that a critical mass amount of circulating multimolecular antigen is required, even if the complexes are formed at “optimal” stoichiometry.
Characterization of murine immune response to PRT/H. (A) Time course of murine immune response to PRT/H (n = 10). C57Bl/6 mice were immunized with PRT/H (PRT 125 μg/mL and H 12.5 U/mL) and antibody levels were followed over a time period of 30 days. Each line represents the seroconversion profile of an individual mouse injected with PRT/H. Figure insert shows the serologic response of individual mice injected with PRT alone over 30 days. (B) Stoichiometry of the immune response to PRT/H: Mice (n = 10/cohort) were immunized with a fixed amount of PRT (125 μg/mL) and varying H doses (0, 5, 10, 12.5, 25, and 50 U/mL) to yield varying PRT:H molar ratios as shown above (−, 8:1, 4:1, 3:1, 1.5:1, or 1:1.2). Results of antibody levels are shown at D15.
Characterization of murine immune response to PRT/H. (A) Time course of murine immune response to PRT/H (n = 10). C57Bl/6 mice were immunized with PRT/H (PRT 125 μg/mL and H 12.5 U/mL) and antibody levels were followed over a time period of 30 days. Each line represents the seroconversion profile of an individual mouse injected with PRT/H. Figure insert shows the serologic response of individual mice injected with PRT alone over 30 days. (B) Stoichiometry of the immune response to PRT/H: Mice (n = 10/cohort) were immunized with a fixed amount of PRT (125 μg/mL) and varying H doses (0, 5, 10, 12.5, 25, and 50 U/mL) to yield varying PRT:H molar ratios as shown above (−, 8:1, 4:1, 3:1, 1.5:1, or 1:1.2). Results of antibody levels are shown at D15.
PRT/H complexes and PF4/H complexes elicit DC activation
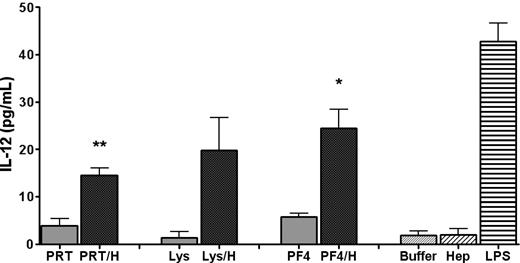
The similarity of the in vivo immune response to PRT/H and mPF4/H5,6 and the knowledge that both antigens formed multimolecular complexes with H, suggested the possibility that multimolecular complexes were more effective than uncomplexed protein at triggering cellular activation. To determine whether multimolecular complexes activated antigen presenting cells (APCs), DCs were harvested from nonimmunized C57Bl/6 mice and incubated with H or buffer, protein (mPF4, PRT, or Lys), or protein/H complexes. Cell supernatants were harvested and assayed for IL-12, a marker of DC activation. As shown in Figure 4, IL-12 was increased in wells containing HBP/H complexes compared with wells containing uncomplexed protein, buffer, or H. These studies show that antigen in the form of multimolecular complexes elicits potent cellular activation of DCs.
DC activation by protein/H complexes. Murine DCs were isolated from nonimmunized C57Bl/6 mice and incubated with PRT + H, Lys + H, mPF4 + H, buffer containing media (Buffer), H (0.4 U/mL) or lipopolysaccharide. IL-12 was measured from cell supernatants by ELISA. Shaded bars indicate protein/H complexes and light shade is uncomplexed protein. One-tailed paired t test was used for determining significance. *P < .01 for PF4 vs PF4/H; **P < .001 for PRT vs PRT/H; P = ns for Lys vs Lys/H.
DC activation by protein/H complexes. Murine DCs were isolated from nonimmunized C57Bl/6 mice and incubated with PRT + H, Lys + H, mPF4 + H, buffer containing media (Buffer), H (0.4 U/mL) or lipopolysaccharide. IL-12 was measured from cell supernatants by ELISA. Shaded bars indicate protein/H complexes and light shade is uncomplexed protein. One-tailed paired t test was used for determining significance. *P < .01 for PF4 vs PF4/H; **P < .001 for PRT vs PRT/H; P = ns for Lys vs Lys/H.
Antibodies to PRT/H can be demonstrated in human subjects undergoing CPB
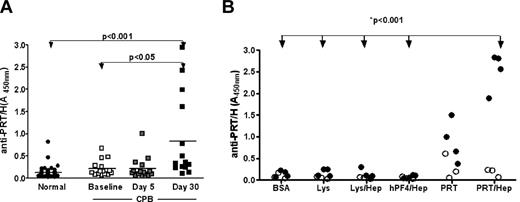
Because patients undergoing CPB are routinely exposed to high doses of PRT and H, and because our murine studies indicated that PRT/H complexes are immunogenic in vivo, we next asked whether patients undergoing CPB develop antibodies to PRT/H. To study the PRT/H immune response in humans, we analyzed samples from normal subjects (n = 45) and plasma from patients undergoing CPB (n = 15, for each patient, sample from baseline before CPB, 5 days and 30 days after CPB was evaluated). As shown in Figure 5A, plasma from normal subjects as well as plasma from CPB patients before surgery and 5 days after surgery showed minimal reactivity in the PRT/H ELISA. However, after 30 days, we observed that 4/15 patients (27%) developed significantly elevated levels of antibodies to PRT/H compared with normals, or their own plasma collected at baseline or 5D after surgery. Seropositive patients recognized PRT/H preferentially over PRT (P = ns) and did not crossreact with other antigens (Figure 5B, P < .001 for PRT/H vs BSA, Lys, Lys/H, or hPF4/H).
Antibodies to PRT/H in patients undergoing CPB. (A) Plasma from healthy subjects (“Normal,” n = 45) and patients undergoing CPB (n = 15) were screened for antibodies to PRT/H by ELISA at baseline, D5, and D30. (B) Specificity of antibodies to PRT/H. Plasma from 4 patients with antibodies to PRT/H (solid symbols) and 3 seronegative CPB patients (open symbols) were incubated with wells coated with BSA, hPF4/H, Lys, Lys/H, PRT, or PRT/H. P values were calculated for panels A and B using a 1-way ANOVA. For panel B, a Bonferroni posttest was applied for calculating differences between conditions. **P < .001 for PRT/H vs BSA, Lys, Lys/H, and hPF4/H.
Antibodies to PRT/H in patients undergoing CPB. (A) Plasma from healthy subjects (“Normal,” n = 45) and patients undergoing CPB (n = 15) were screened for antibodies to PRT/H by ELISA at baseline, D5, and D30. (B) Specificity of antibodies to PRT/H. Plasma from 4 patients with antibodies to PRT/H (solid symbols) and 3 seronegative CPB patients (open symbols) were incubated with wells coated with BSA, hPF4/H, Lys, Lys/H, PRT, or PRT/H. P values were calculated for panels A and B using a 1-way ANOVA. For panel B, a Bonferroni posttest was applied for calculating differences between conditions. **P < .001 for PRT/H vs BSA, Lys, Lys/H, and hPF4/H.
Discussion
In this study, we show that H forms multimolecular complexes with positively charged proteins other than PF4 and, in so doing, enhances the immunogenicity of these proteins in a murine model. We demonstrate that H-modified complexes are capable of activating DCs directly in vitro and that this process may lead to immune activation in vivo, as mice and humans undergoing CPB develop antigen-specific antibodies to PRT/H. These findings, and the similarity of the humoral response to varying H-modified complexes, suggest that antigen in the form of multimolecular complexes is potently immunizing in vivo.
The observation that H modifies the biophysical properties of proteins is not new. In 1935, Fischer showed that H-binding significantly alters the spectral properties of proteins.14 Subsequent studies have shown that H binds to a variety of proteins leading to alterations that affect not only protein function,9,15 but structural properties that enhance a proteins stability16,17 and/or its localization, as seen with PF4 or other cytokines.18 Crystallographic studies of several H-protein complexes do not reveal the presence of any consistent structural or sequential motifs in H-binding proteins, other than clusters of positively charged residues responsible for ionic interactions.8 Our earlier studies have shown that the biophysical alteration of PF4 by H significantly enhances the immunogenicity of PF4.5,6,19,20 To date, the immunologic consequences of H-binding to other positively charged proteins have not been examined.
For our studies, we selected 2 structurally and functionally dissimilar proteins, PRT and Lys, whose H-binding features have been partially characterized. PRT sulfate is a small (Mw 4100 Da), highly cationic DNA binding family of proteins found in sperm nuclei. PRTs replace sperm-specific histones during late spermatogenesis and compact DNA and inhibit its transcription and degradation.21 PRTs from varying species show conservation of arginine-rich domains (60%-80%), which account for the protein's high isoelectric point (pI) of 12,22 and conserved functional roles as a DNA-binding protein across species. The crystal structure of PRT or the PRT-H complex has not been solved; however, spectroscopy studies indicate that free PRT is unstructured in solution.21 Upon binding DNA, PRT binds to the phosphate groups in the major groove of the DNA helix allowing the DNA to coil tightly and condense.21
Lys, on the other hand, is a bactericidal enzyme (14 kDa) found in mucosal secretions (saliva and milk). The protein consists of 129 amino acids and its crystal structure reveals the presence of 5 helical regions consisting of 3 alpha helices and 5 regions of beta pleated sheets.23 Like PRT, Lys has a high pI (10.5) and a homogenous distribution of positive charges over the protein surface, to which H presumably binds.9 Bacteria use carbohydrate polyanions to bind Lys cationic sites and interfere with its catalytic activity. We considered the lack of any structural or functional similarities of these proteins to PF4 as proof-of-concept that multimolecular complex formation and immunogenicity of H-containing multimolecular complexes is a generalizable phenomenon that extends beyond PF4/H multimolecular complexes.
Using techniques to measure changes in absorbance and ζ-potential, we were able to confirm and extend previous observations on the biophysical interactions of both PRT and Lys. We demonstrated that both these proteins show H-dependent binding (Figure 1), with binding curves similar to that seen with PF4 and H.5 Specifically, these curves reveal that PRT/H and Lys/H interactions are charge dependent with PRT/H or Lys/H complexes increasing in size until a H concentration is reached at which charge neutralization occurs. The molar ratio leading to multimolecular complex assembly varies for each protein (mPF4:H approximately 2:1, PRT:H approximately 3-4:1, and Lys:H 5:1) and are comparable with findings reported in the literature using other techniques.9,10 These studies show that absorbance and ζ-potential are reliable, simple tools for assessing the stoichiometry of H-dependent complex formation with positively charged proteins.
We show that structural modification of a protein by H significantly alters its immunogenicity (Figure 2 and supplemental Figure 1). Mice injected with PRT or Lys alone do not make antibodies to these proteins (supplemental Figures 1-2), even though these proteins are foreign antigens in the murine system (salmon-derived PRT and chicken egg white–derived Lys). However, mice injected with H-modified PRT or Lys are more likely to develop high levels of antibodies to each H modified protein (Figure 2A-B and supplemental Figure 1). The similarity of the immune response with regards to the temporal kinetics (Figure 3 and supplemental Figure 4) and dependence on stoichiometric complex formation (Figure 3B), of disparate antigens as mPF4/H,6 PRT/H and Lys/H suggest that the immune response is likely triggered by formation of multimolecular complexes.
Despite use of syngeneic animals and fixed doses of antigen,5,6,19,20 we recognize that rates of seroconversion were not uniform in our experimental model. We noted an overall seroconversion rate of approximately 30%-50% of animals injected with PRT/H or Lys/H (supplemental data and supplemental Figure 1). Several variables likely account for nonuniform seroconversions in our murine model. First, we do not use adjuvant in our immunization strategy. It is known that most peptides and proteins, when injected alone, are poor immunogens.24 For this reason, adjuvant, such as alum or Freunds adjuvant, is typically used in animal models to enhance seroconversions. We purposefully avoided use of adjuvants in our model, because we were interested in demonstrating the “adjuvant” like properties of H. A second variable in this animal model is the use of UFH. UFH is a polydisperse compound containing molecules of variable chain length and charge.25 When UFH is mixed with PF4,5 PRT, or Lys, the polydispersity of UFH species is likely to result in complexes of variable size and/or charge density. Last, it is possible that complex size changes with time, as previously shown for complexes containing PF4 and H.5 While we strove to keep the injection conditions uniform, it is likely that size variation invariably occurred during the time required for sequential animal injections.
While PRT/H and Lys/H seroconversions share some essential features of mPF4/H seroconversions, with regards to timing (Figure 3A and supplemental Figure 4), stoichiometric requirements (Figure 3B) and dose dependency (supplemental Figure 5), it is important to recognize that the immune response to each antigen is highly specific. Antibodies to PRT/H do not crossreact with Lys/H or PF4/H and vice versa (Figure 2C-D). As well, there are some notable biologic differences with regards to the fine specificities of the polyclonal response to each immunogen. Whereas antibodies to PRT/H do not recognize PRT alone and show H-dependent binding by ELISA (Figure 2C and supplemental Figure 3A), antibodies formed after Lys/H exposure do not show H-dependent reactivity (Figure 2D and supplemental Figure 3B). We attribute these differences in serologic specificities to the polyclonal nature of the immune response to each antigen, possibly because of the types of neoepitopes that are displayed on the circulating antigen in vivo. Studies have shown that polyclonal antibodies from HIT patients can also show a range of binding specificities, with some antibodies binding to PF4 alone (26% in study by Pouplard et al26 ) while other antibodies show lack of H-dependent reactivity (4%-12% of antibodies in Warkentin et al27 and Whitlatch et al28,29 ). Taken together, differences in the H-dependent reactivity of Lys/H, PRT/H, and PF4/H antibodies in an ELISA, which is reflective of a polyclonal immune response, does not contradict our central observation that H modifies the immunogenicity of these individual proteins in vivo (Figure 2A-B and supplemental Figure 1).
The specificity of the immune response to each respective antigen implies an important role for adaptive immunity over innate immunity or T cell–independent immune responses.6,19,20 As adjuvant was not used in this or previous studies of our immunization model, it is presumed that multimolecular complexes exert an “adjuvant-like” effect on DCs.30 As shown in Figure 4, our studies confirm that protein/H complexes are capable of directly activating DCs. To what extent IL-12 production and/or DC activation contributes to the HIT immune response remains under investigation. Although our murine studies indicate a compelling role for T cells in PF4/H antibody production, the role of T cells in human HIT is less clear.31-34
The identification of a serologic response to PRT/H in humans undergoing CPB, in part, validates our in vitro and in vivo observations on the biologic consequences of circulating PRT/H multimolecular complexes. In the limited number of patients studied, we noted PRT/H antibodies in 4/15 patients undergoing CPB (Figure 5A). Patients undergoing CPB are routinely exposed to high doses of PRT (5-9 mg/kg, or an average dose of 350-630 mg in a 70-kg individual35 ) to reverse the anticoagulant effects of H (1.4-4.3 U/mL).36 While there are no studies of PRT levels in patients undergoing CPB, in healthy volunteers, a 250 mg dose (or 3.5 mg/kg dose in a 70-kg adult), is associated with a 10- to 50-μg/mL level of circulating PRT.37 The stoichiometric ratios seen in Figure 1A can likely be achieved in some CPB patients (Figure 5A) who may receive either higher doses of PRT or H and/or have the additional inflammatory stimulus of surgery.38
The clinical implications of a PRT/H immune response in humans are not clear. PRT allergy is well described in patients undergoing CPB,39 vasectomized men,40 and diabetic patients who receive insulin preparations containing PRT (neutral protamine Hagedorn [NPH] insulin).41,42 In most of these cases, the PRT allergy is associated with a type I hypersensitivity or anaphylactic reaction, associated with high-titer immunoglobulin (Ig)E and in some cases high-titer IgG.41-43 A more recent study documents the occurrence of anti-PRT antibodies in patients undergoing CPB, some of whom may be mistaken for HIT due to weak cross-reactivity of anti-PRT antibodies with PF4/H antibodies.44 Our studies, however, suggest that the PRT/H antibody response in humans is distinct from previously described PRT reactions (type I hypersensitivity) or delayed type hypersensitivity skin reactions (type IV) caused by subcutaneous H.45 The PRT/H seropositive patients in our cohort were asymptomatic and manifested a highly specific immune response to PRT/H (Figure 5B). Because samples from patients undergoing CPB were only available at D5 and D30, the exact timing of PRT/H seroconversions was not known. Because PRT is likely cleared from circulation by the time seroconversions occur, it is likely that any adverse outcomes associated with anti-PRT/H may be limited to patients who are in need of PRT re-exposure (ie, patients requiring another CPB or diabetic patients receiving NPH insulin).42,46 Additional studies in transgenic mice bearing platelet human FcγRIIR will be informative as to whether immune complexes containing anti-PRT/H and antigen are capable of eliciting platelet activation and thrombocytopenia.
In summary, our studies suggest that the immune response to H-modified complexes is a biologically conserved response. These findings have implications for understanding the pathogenesis of HIT and may provide insights into the existence of an immune response to other H-binding proteins capable of forming H-dependent multimolecular complexes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sharon Hall for assistance with obtaining patient samples.
This work was supported by the National Institutes of Health HL081395 (G.M.A.), UO1-HL072289 (T.L.O.), and U54-HL077878 (T.L.O.), U01-DD000014 (Centers for Disease Control and Prevention, T.L.O.) American Heart Association Grant-in-Aid (G.M.A.), US Environmental Protection Agency Science to Achieve Results award, and RD83241301 (M.R.W.), and the Howard Hughes Medical Institute grant 52005871 (S.L.C.).
National Institutes of Health
Howard Hughes Funding
Authorship
Contribution: S.L.C., B.E., M.R.W., and G.M.A. conceived and designed the study; I.J.W., B.E., M.R.W., and T.L.O. provided study materials or patients; S.L.C., B.E., F.H., M.J., R.Q., and G.A. collected and assembled data; S.L.C., B.E., F.H., R.Q., M.J., G.A., M.R.W., I.J.W., T.L.O., and G.M.A. analyzed and interpreted data; S.L.C., I.J.W., T.L.O., M.R.W., and G.M.A. wrote the manuscript; and S.L.C., B.E., F.H., R.Q., M.J., G.A., M.R.W., I.J.W., T.L.O., and G.M.A. approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gowthami M. Arepally, Duke University Health System, Box 3486, Rm 304 Sands Bldg, Durham, NC 27710; e-mail: arepa001@mc.duke.edu.