Abstract
Mouse bone marrow erythropoiesis is homeostatic, whereas after acute anemia, bone morphogenetic protein 4 (BMP4)–dependent stress erythropoiesis develops in the spleen. The aim of this work was to compare spleen stress erythropoiesis and bone marrow erythropoiesis in a mouse model of zymosan-induced generalized inflammation, which induces long-lasting anemia and to evaluate the ability of erythropoietin (Epo) injections to correct anemia in this setting. The effects of zymosan and/or Epo injections on erythroid precursor maturation and apoptosis, serum interferon-γ levels, hematologic parameters, and spleen BMP4 expression were analyzed, as well as the effect of zymosan on red blood cell half-life. We found that bone marrow erythropoiesis is suppressed by inflammation and does not respond to Epo administration, despite repression of erythroblast apoptosis. On the contrary, a robust erythropoietic response takes place in the spleen after Epo injections in both control and zymosan-induced generalized inflammation mice. This specific response implies Epo-mediated induction of BMP4 expression by F4/80+ spleen macrophages, proliferation of stress burst-forming units-erythroid, and increased number of spleen erythroblasts. It allows only partial recovery of anemia, probably because of peripheral destruction of mature red cells. It is not clear whether similar BMP4-dependent stress erythropoiesis can occur in human bone marrow after Epo injections.
Introduction
In mice, bone marrow erythropoiesis is primarily homeostatic, whereas “stress erythropoiesis” develops rapidly in the spleen after acute anemia.1 This spleen erythropoiesis requires bone morphogenetic protein 4 (BMP4) expression to initiate expansion of a specialized population of resident immature erythroid progenitors and their differentiation into erythropoietin (Epo)-responsive stress burst-forming units-erythroid (BFU-E). These stress BFU-E respond to high levels of Epo2-4 and differentiate rapidly into erythroblasts. However, very little is known on the response of this extramedullary erythropoiesis during anemia of inflammation, also called anemia of chronic diseases (ACD). ACD develops during chronic inflammatory syndromes, such as chronic infections, neoplastic disorders, or autoimmune diseases, and is the most frequent type of anemia in hospitalized patients. Several factors contribute to the development of ACD, such as diminished proliferation and differentiation of erythroid progenitor cells, reduced red blood cell (RBC) half-life, reduced formation, and biologic activity of endogenous Epo and deregulation of iron homeostasis.5 In chronic inflammatory states, T cells and monocytes induce immune effector mechanisms, thereby producing proinflammatory cytokines, which play a major role in the onset of anemia. Among these, interleukin-6 (IL-6) alone can rapidly induce hepcidin synthesis, resulting in inhibition of iron efflux from macrophages and consequently to hypoferremia.6 This leads to impaired iron availability for erythroid progenitor cells and iron-restricted erythropoiesis.7 Both interferon-gamma (IFN-γ) and tumor necrosis factor-α directly inhibit erythroid progenitor colony formation and appear to have a prominent role in causing the anemia associated with chronic diseases.8-10 Epo is the crucial regulator of RBC production and delivers growth, differentiation, and survival signals from the CFU-E to the proerythroblast stage. Recombinant human Epo has been indicated for more than 20 years for correction of anemia in chronic kidney disease,11 and its therapeutic indications have subsequently been extended to ACD. The administration of Epo has been shown to reduce the need for transfusions in different clinical settings. However, in intensive care patients, randomized control trials showed little to no benefit of Epo injections in the early course of intensive care hospitalization with regards to blood transfusion requirements.12 In these patients, anemia is very frequent and may persist for as long as 6 months after discharge, with persistent decrease in Epo concentrations.13
To get a better insight into the link between prolonged inflammation and anemia, we used the zymosan-induced generalized inflammation (ZIGI) model as a model of critical illness. Peritoneal injection of zymosan activates macrophages and induces the production of a wide range of proinflammatory mediators. After a first phase of severe illness, the animals appear to return to normal before developing multiple organ failure in a third phase. We have previously shown that this phase of generalized inflammation induces a persistent, long-lasting anemia.14 We also showed that stimulation of erythropoiesis by Epo injections favors iron mobilization from tissue iron stores, although we did not evaluate the erythropoietic response in this context. The aim of this work was to study the relationship between inflammation, anemia, and erythropoiesis in this model of generalized inflammation and to evaluate the respective response to Epo of spleen and bone marrow erythropoiesis. Our findings highlight major differences between bone marrow and spleen erythropoiesis in terms of sensitivity to proinflammatory cytokines and response to Epo injections. We also demonstrate that spleen macrophages synthesize BMP4 in response to Epo, thereby inducing proliferation of stress BFU-E.
Methods
Mice
For all experiments, 8-week-old C57BL/6 male mice were used (Center d'élevage Janvier, Le Genest St Isle, France). Before use, animals were allowed to acclimatize for 5 days. Animals were cared for in accordance with criteria outlined in the European Convention for the Protection of Laboratory Animals. All experiments were performed on groups of 4 to 8 mice, unless stated otherwise. Animal studies received approval from the review board of Hôpital Bichat.
ZIGI
The ZIGI model has been widely used to study systemic inflammation in relation to organ failure.15 Inflammation is first induced by a intraperitoneal injection of 40 μg of lipolysaccharides (from Escherichia coli 0128-B12) diluted in 200 μL of saline solution, followed 6 days later by intraperitoneal injection of zymosan A (Sigma-Aldrich; 16 mg diluted in 500 μL of saline). The day of zymosan injection is referred to as Z1. ZIGI mice were killed at various time points: Z5, Z9, Z12, and Z17 (Figure 1).
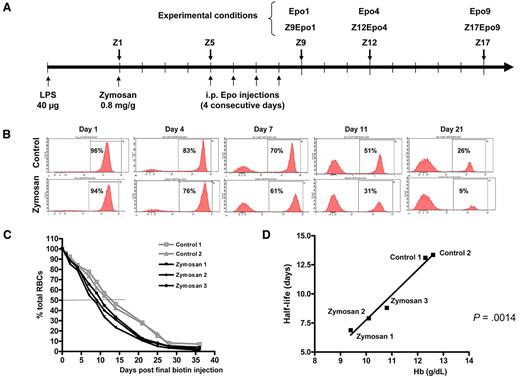
ZIGI mouse model: experimental scheme and measure of RBC half-life. (A) Experimental scheme. Inflammation was obtained by a first intraperitoneal injection of 40 μg of LPS followed 6 days later by a single intraperitoneal injection of 0.8 mg/g zymosan. The day of zymosan injection is considered as day 1 and referred to as Z1. ZIGI mice were killed at various time points: Z5, Z9, Z12, and Z17. Intraperitoneal injections of 200 IU of Epo were performed on 4 consecutive days starting at Z5. Mice were analyzed one day (Z9Epo1), 4 days (Z12Epo4), or 9 days (Z17Epo9) after the final injection. Control naive mice received the Epo injections on 4 consecutive days (days 1-4) and were analyzed 1 day (Epo1) or 4 days (Epo4) after the final injection. (B-D) RBC turnover after zymosan injection. Biotin was injected on 3 consecutive days, and zymosan was injected 1 day after (day 1). At the indicated times, a small aliquot of blood was analyzed by streptavidin labeling to detect the decay of biotinylated erythrocytes over time; n = 2 for control mice and n = 3 for ZIGI mice. (B) Representative histograms of data from one mouse in each group are shown for day 1 to day 21. (C) Remaining biotinylated RBCs at different time points after zymosan injection, expressed as percentage of the total circulating RBCs. The decay curves are shown for 2 control mice and 3 ZIGI mice. (D) Correlation between Hb level of the 2 control and 3 ZIGI mice (at Z5) and the measured RBC half-life. Statistical significance was analyzed by linear regression.
ZIGI mouse model: experimental scheme and measure of RBC half-life. (A) Experimental scheme. Inflammation was obtained by a first intraperitoneal injection of 40 μg of LPS followed 6 days later by a single intraperitoneal injection of 0.8 mg/g zymosan. The day of zymosan injection is considered as day 1 and referred to as Z1. ZIGI mice were killed at various time points: Z5, Z9, Z12, and Z17. Intraperitoneal injections of 200 IU of Epo were performed on 4 consecutive days starting at Z5. Mice were analyzed one day (Z9Epo1), 4 days (Z12Epo4), or 9 days (Z17Epo9) after the final injection. Control naive mice received the Epo injections on 4 consecutive days (days 1-4) and were analyzed 1 day (Epo1) or 4 days (Epo4) after the final injection. (B-D) RBC turnover after zymosan injection. Biotin was injected on 3 consecutive days, and zymosan was injected 1 day after (day 1). At the indicated times, a small aliquot of blood was analyzed by streptavidin labeling to detect the decay of biotinylated erythrocytes over time; n = 2 for control mice and n = 3 for ZIGI mice. (B) Representative histograms of data from one mouse in each group are shown for day 1 to day 21. (C) Remaining biotinylated RBCs at different time points after zymosan injection, expressed as percentage of the total circulating RBCs. The decay curves are shown for 2 control mice and 3 ZIGI mice. (D) Correlation between Hb level of the 2 control and 3 ZIGI mice (at Z5) and the measured RBC half-life. Statistical significance was analyzed by linear regression.
RBC turnover
RBC life span in control and in ZIGI mice was assayed by in vivo biotinylation followed by fluorescence-activated cell sorter (FACS) analysis. Mice received retro-orbital intravenous injection of N-hydroxysuccinimide-biotin (40 μg/g body weight; Pierce Biotechnology) on 3 consecutivedays, followed by zymosan injection on the fourth day; lipopolysaccharides (LPSs) were administrated 6 days before zymosan injection. Aliquots of blood drawn in phosphate-buffered saline supplemented with 0.1% glucose (PBS-G) were then collected on the day of zymosan injection (day 1) and then every 2 or 3 days. Washed erythrocytes (adjusted to 3 million cells) were incubated with antistreptavidin fluorescein isothiocyanate antibody (BD Biosciences) at 25°C for 1 hour. Cells were washed twice in PBS-G, and the percentage of labeled RBCs was determined on FACSCanto (BD Biosciences). The percentage of biotinylated cells was calculated as a ratio of positive cells to all RBCs.
Hematologic parameters
Mice were anesthetized by isoflurane inhalation. Blood was collected on ethylenediaminetetraacetic acid by puncture of the orbital sinus. Hemoglobin (Hb) level, reticulocytes, and RBCs were measured on the XE alpha 2100 analyzer (Sysmex; Roche Diagnostics). In some cases, blood was collected on dry tube and centrifuged, and serum was frozen at −20°C until assayed for cytokine levels.
In vivo Epo treatment
Intraperitoneal Epo injections were performed with 200 IU of human biosynthetic Epoα (epoetin alfa, Eprex; Janssen-Cilag) diluted in 100 μL of saline solution. Epo was injected in control mice on 4 consecutive days, and mice were analyzed 1 day (Epo1) or 4 days (Epo4) after the final injection.
Epo injections in ZIGI mice were performed on 4 consecutive days, starting at Z5. Mice were analyzed one day (Z9Epo1), 4 days (Z12Epo4), or 9 days (Z17Epo9) after the final injection.
Flow cytometry
Splenocytes were isolated by mechanical dissociation of the spleen, bone marrow cells were flushed out of femurs and tibias, and cells were resuspended in PBS/2mM ethylenediaminetetraacetic acid/0.5% bovine serum albumin.
After preincubation with 1 μg/mL mouse IgG (Negative/Isotype control; AbD Serotec), cells were immunostained for 20 minutes at 4°C with fluorescein isothiocyanate–conjugated anti-Ter119 (BD Biosciences) and phycoerythrin-conjugated anti-CD71 (AbD Serotec) antibodies. Propidium iodide was used to exclude dead cells, and apoptosis was assessed using the Annexin V FITC Apoptosis Detection kit (BD Biosciences). Flow cytometric analysis was carried out on a BD FACSCalibur.
mRNA quantification
Total RNA was extracted from spleen using the RNAplus extraction Kit (Quantum Biotechnologies) according to the manufacturer's instructions. Total RNA was reverse transcribed into cDNA using SuperScript Reverse transcriptase (Invitrogen). Real-time polymerase chain reaction quantification of transcripts was performed on Lightcycler 480 instrument (Roche Diagnostics). Primer sequences starting from the 5′ end were as follows: BMP4 forward, TGCTGGCGAGCCCGCTTCTG; BMP4 reverse, TGCGTCGCTCCGAATGGCACTAC; S14 forward, CAGGACCAAGACCCCTGGA; S14 reverse, ATCTTCATCCCAGAGCGAGC.
BMP4 mRNA levels were normalized using the expression of the mouse ribosomal S14 mRNA as a housekeeping gene.
Cytokine quantification
Determination of serum concentrations of Epo and IFN-γ were carried out using commercially available enzyme-linked immunosorbent assay kits obtained from R&D Systems or eBioscience, respectively, according to the manufacturer's instructions
Colony assays for BFU-E
Splenocytes and bone marrow cells were isolated from control, Z, Epo, and Z + Epo mice. Then, 1 × 105/mL nucleated bone marrow cells and 2 × 106/mL nucleated splenocytes were plated in methylcellulose media (StemCell Technologies) containing 3 U/mL Epoα (epoetin alfa, Eprex; Janssen-Cilag) with (bone marrow cells) or without (splenocytes) 10 ng/mL IL-3 (Sigma-Aldrich). For assays examining the effect of IFN-γ on BFU-E proliferation, cells were plated in methylcellulose in the conditions described above, supplemented with 2000 U/mL IFN-γ (BD Biosciences). Photographs of BFU-E colonies were done with the camera Control Pro (Nikon).
Immunostaining
Spleens were harvested at different time points after treatment and fixed in 10% paraformaldehyde, and serial sections were cut from the paraffin-embedded tissue.
The expression of BMP4 was analyzed with anti-BMP4 antibody (AbCys SA) at 1:100 dilution, and expression of F4/80 was analyzed with anti-F4/80 antibody (Serotec) at 1:500 dilution using the Envision+ Kit (Dako North America). Pictures were obtained using a Leica DM2500 microscope (Leica Systems), using either a 10×/0.25SL or a 20×/0.40SL objective. Images were acquired with a Leica DFC300FX camera and processed with TRIBVN ICS software (TRIBVN France).
Immunofluorescence analysis of tissues was done on paraffin sections. Sections were permeabilized with 0.1% Triton X-100; they were then washed with PBS and blocked with PBS-bovine serum albumin 3%. Sections were stained with anti-F4/80 antibody at 1:50 dilution (Abcam) and anti-BMP4 at 1:10 dilution (Vector Laboratories). Secondary antibodies were Alexa 488–conjugated goat antimouse and Alexa 555-conjugated goat antirat antibodies (Invitrogen). Images were acquired using a Zeiss confocal microscope LSM510 MET (Carl Zeiss) with a 63×/1.4 NA oil immersion objective and processed with Zeiss LSM Image Browser software.
Statistical analysis
All statistics were calculated using the Statgraphics plus, Version 6.0 software. Data are reported as mean ± SEM. Statistical analyses were performed using Mann-Whitney or Student t test as appropriate.
Results
Zymosan shortens RBC survival and induces anemia
In the ZIGI mouse model,14 inflammation was obtained by an initial intraperitoneal LPS injection thought to trigger the immune system and to prevent an excessive response to zymosan, followed by a single intraperitoneal zymosan injection 6 days later, considered as day 1 (Z1, Figure 1A). This ZIGI model has previously been shown to induce a sustained inflammatory response with prolonged cytokine production.15 These mice displayed marked anemia as early as Z5 with reduced number of RBCs and lower Hb levels (Table 1). Anemia was still present at day 17 (Z17).
Hematologic parameters, spleen weight, and serum Epo levels at different time points for each experimental condition
| Condition . | RBCs, ×106/μL . | Reticulocytes, ×103/μL . | Hb, g/dL . | MCV, fL . | Spleen weight, mg . | Serum Epo, pg/mL . |
|---|---|---|---|---|---|---|
| Controls | 9.45 ± 0.67 | 28 ± 4.7 | 14.1 ± 1.07 | 48.51 ± 3.05 | 81 ± 9 | 94 |
| Zymosan | ||||||
| Z5 | 7.30 ± 0.8* | 11 ± 1.52* | 10.8 ± 0.86* | 44.47 ± 0.57 | 189 ± 37* | 290 |
| Z9 | 6.76 ± 0.7* | 20 ± 5.46 | 9.8 ± 1.01* | 47.22 ± 3.11 | 322 ± 18.9* | |
| Z12 | 7.29 ± 0.76* | 32 ± 8.7 | 10.1 ± 0.97* | 48.28 ± 0.31 | 419 ± 150* | 499 |
| Z17 | 6.23 ± 0.2* | 47 ± 0.5* | 7.8 ± 0.22* | 45.03 ± 0.79 | 481 ± 112* | |
| Epo | ||||||
| Epo1 | 10.71 ± 0.61* | 83 ± 12.79* | 16.5 ± 1.16* | 49.34 ± 1.46 | 266 ± 42.2* | 1060 |
| Epo4 | 11.24 ± 0.5* | 29 ± 10.38* | 17.4 ± 0.91* | 50.80 ± 0.97 | 80 ± 15.15 | 100 |
| Zymosan + Epo | ||||||
| Z9Epo1 | 7.43 ± 0.29* | 40 ± 7.37*† | 10.8 ± 0.45* | 46.63 ± 0.80 | 355 ± 74* | 284 |
| Z12Epo4 | 8.20 ± 0.36* | 33 ± 6.34 | 11.9 ± 0.85* | 49.05 ± 2.08 | 445 ± 130* | 192 |
| Z17Epo9 | 7.68 ± 0.8*‡ | 51 ± 12.78* | 11.2 ± 1.18*‡ | 44.55 ± 1.4 | 269 ± 39.1*‡ | 111 |
| Condition . | RBCs, ×106/μL . | Reticulocytes, ×103/μL . | Hb, g/dL . | MCV, fL . | Spleen weight, mg . | Serum Epo, pg/mL . |
|---|---|---|---|---|---|---|
| Controls | 9.45 ± 0.67 | 28 ± 4.7 | 14.1 ± 1.07 | 48.51 ± 3.05 | 81 ± 9 | 94 |
| Zymosan | ||||||
| Z5 | 7.30 ± 0.8* | 11 ± 1.52* | 10.8 ± 0.86* | 44.47 ± 0.57 | 189 ± 37* | 290 |
| Z9 | 6.76 ± 0.7* | 20 ± 5.46 | 9.8 ± 1.01* | 47.22 ± 3.11 | 322 ± 18.9* | |
| Z12 | 7.29 ± 0.76* | 32 ± 8.7 | 10.1 ± 0.97* | 48.28 ± 0.31 | 419 ± 150* | 499 |
| Z17 | 6.23 ± 0.2* | 47 ± 0.5* | 7.8 ± 0.22* | 45.03 ± 0.79 | 481 ± 112* | |
| Epo | ||||||
| Epo1 | 10.71 ± 0.61* | 83 ± 12.79* | 16.5 ± 1.16* | 49.34 ± 1.46 | 266 ± 42.2* | 1060 |
| Epo4 | 11.24 ± 0.5* | 29 ± 10.38* | 17.4 ± 0.91* | 50.80 ± 0.97 | 80 ± 15.15 | 100 |
| Zymosan + Epo | ||||||
| Z9Epo1 | 7.43 ± 0.29* | 40 ± 7.37*† | 10.8 ± 0.45* | 46.63 ± 0.80 | 355 ± 74* | 284 |
| Z12Epo4 | 8.20 ± 0.36* | 33 ± 6.34 | 11.9 ± 0.85* | 49.05 ± 2.08 | 445 ± 130* | 192 |
| Z17Epo9 | 7.68 ± 0.8*‡ | 51 ± 12.78* | 11.2 ± 1.18*‡ | 44.55 ± 1.4 | 269 ± 39.1*‡ | 111 |
Data are mean ± SEM. Data correspond to the results obtained from a pool of 4 sera from 4 different mice.
MCV indicates mean corpuscular volume.
P < .02 compared with controls.
P < .03 compared with Z9.
P < .007 compared with Z17.
Because impaired erythrocyte life cycle is one of the factors contributing to ACD, we tested the possibility that RBC turnover could be affected in our model. We measured the erythrocyte life span in vivo in control and in ZIGI mice. Zymosan injection induced a rapid decline in biotinylated RBCs compared with control mice (Figure 1B). RBC half-life was reduced from 13.2 days in control mice to less than 8 days in ZIGI mice (Figure 1C). In both control and ZIGI mice, there was a significant correlation between the Hb level at Z5 and RBC half-life (Figure 1D), suggesting that the rapid drop in RBC number (Table 1) after zymosan injection probably results from intravesicular or extravascular hemolysis. Activated macrophages probably exhibit increased phagocytic activity of RBCs, but we failed to demonstrate the contribution of this mechanism to zymosan-induced reduction in RBC half-life. However, intravascular hemolysis is also a contributing factor because we found a strong increase in bilirubin level in ZIGI mice (2.3μM at Z2 compared with 0.2μM in control mice).
Bone marrow erythropoiesis is repressed by inflammation and does not respond to Epo injections
To investigate the effect of inflammation on erythropoiesis, we compared the percentage of Ter119-positive (Ter119+) cells and the number of erythroblasts at different maturation stages in ZIGI and control mice, in the bone marrow and the spleen. We used a flow cytometry assay based on cell surface expression of Ter119 and CD71 together with the forward scatter parameter.16 Cell viability was determined by propidium iodide exclusion and was always greater than 90%. Ter119 has been identified as an erythroid lineage-specific marker expressed from the pro-erythroblast to the mature RBC stage, whereas CD71 (transferrin receptor) expression changes with the degree of maturation. This method allows the identification of 3 well-resolved erythroblast subpopulations corresponding to 3 progressive stages of maturation. The larger Ter119+CD71+ cells are basophilic immature erythroblasts (Ery A), the smaller Ter119+CD71+ cells polychromatic intermediate erythroblasts (Ery B) and the Ter119+CD71− cells acidophilic late erythroblasts (Ery C; supplemental data, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
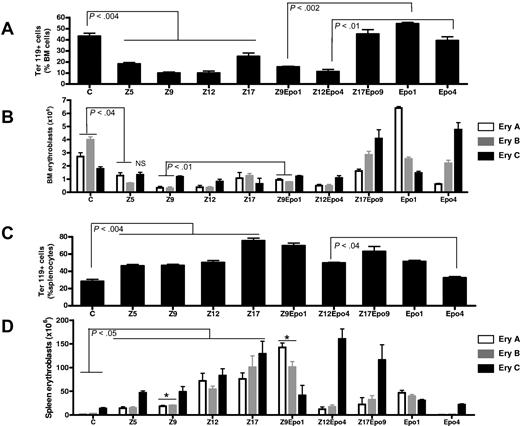
In the bone marrow, erythroid cells represented almost 45% of the total cell population at baseline. There was a significant decrease in this proportion in the inflammatory state, between days 5 and 12 (Figure 2A). This was accompanied by a concomitant increase in the proportion of CD45+ cells, consisting mostly of cells from the macrophage-granulocyte lineages (CD11b+ cells; supplemental data). At Z17, the proportion of Ter119+ cells was still significantly lower than in control mice but was starting to rise again. The total number of erythroblasts in one femur was reduced from (8.6 ± 1.8) × 106 to (3.4 ± 0.6) × 106 at Z5. This resulted mostly from a reduction in the number of immature (Ery A and Ery B) erythroblasts, with little change in the number of mature erythroblasts (Figure 2B).
Differential effect of inflammation and Epo injections on spleen and bone marrow erythroblast maturation. The proportion of Ter119+ cells (A,C) and the number of erythroblasts at different stages of maturation (B,D) were analyzed in bone marrow (A-B) and spleen (C-D) cells. The proportion of erythroblasts at each stage of maturation was determined by FACS (supplemental data), and the corresponding number of erythroblasts was calculated based on the observation that a femur contains 20 × 106 cells and a spleen contains 106 cells/mg wet weight. The 3 stages cor-respond to early (Ery A), intermediate (Ery B), and late erythroblasts (Ery C). Results are shown for control mice (C), ZIGI mice killed 5 days (Z5), 9 days (Z9), 12 days (Z12), and 17 days (Z17) after zymosan injection, Epo-treated control mice (Epo1 and Epo4), and ZIGI mice treated with Epo (Z9Epo1; Z12Epo4; Z17Epo9). Data are mean ± SEM for 4 to 8 mice per group per time point. *P < .005 between Z9 and Z9Epo1 for Ery A and Ery B.
Differential effect of inflammation and Epo injections on spleen and bone marrow erythroblast maturation. The proportion of Ter119+ cells (A,C) and the number of erythroblasts at different stages of maturation (B,D) were analyzed in bone marrow (A-B) and spleen (C-D) cells. The proportion of erythroblasts at each stage of maturation was determined by FACS (supplemental data), and the corresponding number of erythroblasts was calculated based on the observation that a femur contains 20 × 106 cells and a spleen contains 106 cells/mg wet weight. The 3 stages cor-respond to early (Ery A), intermediate (Ery B), and late erythroblasts (Ery C). Results are shown for control mice (C), ZIGI mice killed 5 days (Z5), 9 days (Z9), 12 days (Z12), and 17 days (Z17) after zymosan injection, Epo-treated control mice (Epo1 and Epo4), and ZIGI mice treated with Epo (Z9Epo1; Z12Epo4; Z17Epo9). Data are mean ± SEM for 4 to 8 mice per group per time point. *P < .005 between Z9 and Z9Epo1 for Ery A and Ery B.
These data show that inflammation represses bone marrow erythropoiesis by significantly decreasing the number of Ter119+ cells and by altering the balance between the different erythroblast subpopulations.
Because Epo is one of the treatments of anemia and is also known to suppress proinflammatory cytokine expression, we wanted to test the effect of Epo injections on bone marrow erythropoiesis in zymosan-treated mice.
We performed 4 consecutive daily injections of Epo and killed the mice the day after the last injection. In control mice, this treatment triggered a moderate increase in proportion of Ter119+ cells in the bone marrow. However, we observed that the number of early erythroblasts (Ery A) had increased from (2.71 ± 0.76) × 106 in controls to (6.41 ± 0.27) × 106 at Epo1 (Figure 2B). This was followed 3 days later (Epo 4) by a corresponding increase in mature erythroblasts from (1.78 ± 0.4) × 106 in controls to (4.76 ± 1.2) × 106 in Epo-treated mice (Figure 2B). By contrast, bone marrow erythropoiesis did not respond when inflammation and Epo injections were combined, Ter119+ cells representing only 15% of live cells in the bone marrow at Z9Epo1 (Figure 2A). The increase in number of early erythroblasts observed in control mice at Epo1 or in late erythroblasts at Epo4 was fully blunted in Z9Epo1 or Z12Epo4 conditions, respectively (Figure 2B). However, a highly significant increase in the number of late erythroblasts was observed by Z17Epo, compared with either controls or Z17 mice.
Spleen erythropoiesis is moderately stimulated by ACD and strongly enhanced by Epo injections in the presence of inflammation
In the spleen of control mice, Ter119+ cells represented 25% of splenocytes (Figure 2C), the major part of these Ter119+ cells consisting of mature cells (Ery C). However, unlike the bone marrow, in the spleen, inflammation led to a significant 50% increase in the erythroid population at Z5, with a 30% reduction in CD45+ cells (Supplemental data). This increase persisted at Z9 and Z12 and peaked at Z17. Combined with a progressive increase in spleen size (Table 1), this led to a progressive increase in the number of erythroblasts at all stages of maturation, from (1.1 ± 0.8) × 106 in controls to (76 ± 26)× 106 at Z17 for Ery A, from (3.0 ± 1.0) × 106 to (100 ± 48) × 106 for Ery B and from (13.9 ± 3.8) × 106 to (129 ± 52) × 106 for Ery C (Figure 2D).
In ZIGI mice, Epo injections induced a sustained erythropoietic response in the spleen, even higher than in control mice (Figure 2B), Ter119+ cells representing more than 70% of splenocytes at Z9Epo1. Regarding the different populations of erythroblasts, Epo injections in ZIGI mice allowed a significant and marked increase in immature Ery A and Ery B at Z9Epo1, followed 3 days later (Z12Epo4) by a similar increase in the number of mature erythroblasts (Figure 2D), a situation reminiscent of what was observed after Epo injections in the bone marrow of control mice. At that stage, immature erythroblasts were significantly lower than in Z12 mice, probably because the anemia had been partially corrected (Hb = 11.9 ± 0.85 g/dL in Z12Epo4 vs 10.1 ± 0.97 g/dL in Z12).
Together, these data show that, in inflammatory conditions, the spleen and the bone marrow react in a very different way. Indeed, bone marrow erythropoiesis is suppressed, whereas in the spleen ACD moderately stimulates erythropoiesis.
The erythroid response to Epo injections is blunted in the bone marrow in the presence of inflammation. On the contrary, spleen erythropoiesis responds to Epo injections despite the presence of inflammation. This appears as another notable difference between the 2 sites of erythropoiesis.
Apoptosis distribution in bone marrow and spleen during inflammation and Epo injections
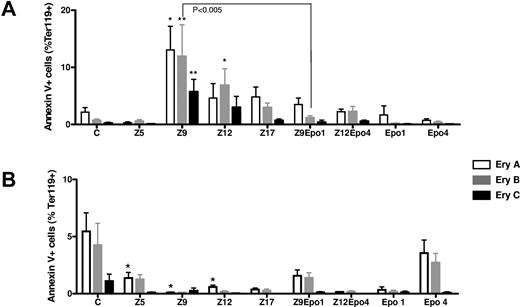
To determine whether the decreased production of bone marrow early-stage erythroblasts (Ery A and Ery B) observed during inflammation was a result of apoptosis, we examined annexin V-positive cells among the different subpopulations of erythroblasts (Figure 3). Although in bone marrow the percentage of apoptotic cells was approximately 2% at basal state, this proportion was significantly increased at Z9 for all 3 stages of maturation (Figure 3A). This increase in proportion of apoptotic erythroblasts could participate in repressing bone marrow erythropoiesis. On the contrary, in the spleen, during inflammation, the percentage of apoptotic erythroblasts was lower in zymosan-treated mice than in control mice, at all stages of inflammation (Figure 3B).
Effects of inflammation and Epo injections on erythroblast apoptosis. The proportion of annexin V+ cells was measured in each subset of erythroblast in bone marrow (A) and spleen (B). Results are shown for control mice (C), ZIGI mice sacrificed 5 days (Z5), 9 days (Z9), 12 days (Z12), and 17 days (Z17) after zymosan injection, Epo-treated control mice (Epo1 and Epo4), and ZIGI mice treated with Epo (Z9Epo1; Z12Epo4). The 3 stages correspond to early (Ery A), intermediate (Ery B), and late erythroblasts (Ery C). Bars represent mean ± SEM. *P < .04, **P < .005 when each value was compared to control mice (C).
Effects of inflammation and Epo injections on erythroblast apoptosis. The proportion of annexin V+ cells was measured in each subset of erythroblast in bone marrow (A) and spleen (B). Results are shown for control mice (C), ZIGI mice sacrificed 5 days (Z5), 9 days (Z9), 12 days (Z12), and 17 days (Z17) after zymosan injection, Epo-treated control mice (Epo1 and Epo4), and ZIGI mice treated with Epo (Z9Epo1; Z12Epo4). The 3 stages correspond to early (Ery A), intermediate (Ery B), and late erythroblasts (Ery C). Bars represent mean ± SEM. *P < .04, **P < .005 when each value was compared to control mice (C).
Epo is known to exert antiapoptotic effects on erythroid precursors. Indeed, there was a notable reduction in percentage of annexin V+ in bone marrow cells after Epo injections in inflammatory conditions (Figure 3A), especially in the most immature erythroblasts one day after the final Epo injection (Z9Epo1). However, this antiapoptotic effect of Epo was not sufficient to restore bone marrow erythropoiesis.
In the spleen, apoptosis was repressed whatever the situation (Z, Epo, or Z + Epo) compared with control mice (Figure 3B).
Late increase in IFN-γ in the ZIGI mice and partial repression by Epo injections
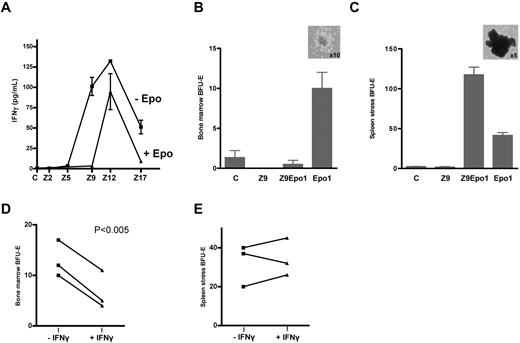
To determine the role of IFN-γ in the inhibition of growth and differentiation of erythroid precursors, we measured the serum level of this proinflammatory cytokine. There was a sharp rise in IFN-γ concentrations between Z5 and Z12, with a maximum 130-fold increase at Z12 compared with control mice (Figure 4A). This late increase in IFN-γ levels suggests that this cytokine induced bone marrow apoptosis, which was maximal at Z9. However, bone marrow erythropoiesis was already fully suppressed at Z5 when IFN-γ was only moderately increased (3.8 pg/mL vs 0.7 pg/mL), suggesting that other proinflammatory cytokines might also contribute to the repression of erythropoiesis. These results suggest that, despite a dramatic increase in IFN-γ serum levels in inflammatory conditions, this cytokine, which is known to be the most potent inhibitor of erythropoiesis, is not able to suppress spleen erythropoiesis during inflammation.
Effect of inflammation and Epo injections on serum IFN-γ levels and BFU-E numbers in bone marrow and spleen. (A) Serum concentrations of IFN-γ in ZIGI mice, either nontreated (−Epo) or after Epo injections (+Epo). In the latter case, serum IFN-γ determinations were performed at the usual time points (Z9Epo1, Z12Epo4, and Z17Epo9). Data are the mean from 2 to 6 pools of 2 sera each. Errors bars represent SEM. (B-E) Analysis of BFU-E expansion from bone marrow (B-D) and spleen (C-E) in different groups of mice. (B-C) A total of 1.5 × 104 bone marrow (B) and 3 × 105 splenocytes (C) cells were plated in methylcellulose media containing 3 U/mL Epo (spleen) or 3 U/mL Epo plus 10 ng/mL IL-3 (bone marrow) and BFU-E were scored after 5 days (spleen) or 8 days (bone marrow). The pictures (inset) show typical BFU-E colony from an Epo1 bone marrow (B) or from a Z9Epo1 spleen (C). Photographs of BFU-E colonies were taken with a camera Control Pro (Nikon). (D-E) To test the IFN-γ effect on BFU-E proliferation, a total of 1.5 × 104 bone marrow cells (D) and 3 × 105 splenocytes (E) cells from 3 different Epo1 mice were plated in methylcellulose media containing 3 U/mL Epo (spleen) or 3 U/mL Epo plus 10 ng/mL IL-3 (bone marrow), in the presence (+IFN-γ) or absence (−IFN-γ) of 2000 U/mL IFN-γ in the media. Three independent mice were analyzed for each condition. Errors bars represent SEM. Significant differences were found only in the bone marrow when analyzed by Student paired t test.
Effect of inflammation and Epo injections on serum IFN-γ levels and BFU-E numbers in bone marrow and spleen. (A) Serum concentrations of IFN-γ in ZIGI mice, either nontreated (−Epo) or after Epo injections (+Epo). In the latter case, serum IFN-γ determinations were performed at the usual time points (Z9Epo1, Z12Epo4, and Z17Epo9). Data are the mean from 2 to 6 pools of 2 sera each. Errors bars represent SEM. (B-E) Analysis of BFU-E expansion from bone marrow (B-D) and spleen (C-E) in different groups of mice. (B-C) A total of 1.5 × 104 bone marrow (B) and 3 × 105 splenocytes (C) cells were plated in methylcellulose media containing 3 U/mL Epo (spleen) or 3 U/mL Epo plus 10 ng/mL IL-3 (bone marrow) and BFU-E were scored after 5 days (spleen) or 8 days (bone marrow). The pictures (inset) show typical BFU-E colony from an Epo1 bone marrow (B) or from a Z9Epo1 spleen (C). Photographs of BFU-E colonies were taken with a camera Control Pro (Nikon). (D-E) To test the IFN-γ effect on BFU-E proliferation, a total of 1.5 × 104 bone marrow cells (D) and 3 × 105 splenocytes (E) cells from 3 different Epo1 mice were plated in methylcellulose media containing 3 U/mL Epo (spleen) or 3 U/mL Epo plus 10 ng/mL IL-3 (bone marrow), in the presence (+IFN-γ) or absence (−IFN-γ) of 2000 U/mL IFN-γ in the media. Three independent mice were analyzed for each condition. Errors bars represent SEM. Significant differences were found only in the bone marrow when analyzed by Student paired t test.
We next determined the effect of Epo injections on IFN-γ production. As expected, Epo injections in control mice did not induce synthesis of this cytokine (data not shown). Epo injections in ZIGI mice exerted an anti-inflammatory effect, thereby dramatically reducing IFN-γ concentration one day after the final injection (Z9Epo1), but this repression was not sufficient to restore bone marrow erythropoiesis. Surprisingly, 4 days after Epo injections (Z12Epo4), serum IFN-γ levels started to rise again, although to a lesser extent than in the absence of Epo. This delayed increase in IFN-γ did not induce a burst in apoptotic erythroblasts in the bone marrow (Figure 3A).
Epo injections enhances spleen stress erythropoiesis
Because spleen erythropoiesis responded differently to inflammation from bone marrow erythropoiesis, we wondered whether spleen erythropoiesis presented the hallmarks of stress erythropoiesis. Therefore, we first measured the number of stress BFU-E by plating splenocytes in methylcellulose in the presence of Epo alone; bone marrow cells were grown in the presence of both Epo and IL-3. The bone marrow and the spleen contained very few BFU-E in basal conditions. In ZIGI mice at Z9, no colonies were detected in the bone marrow. In the spleen, the number of BFU-E was not significantly different from controls. This suggests that the proliferation of spleen erythroblasts in inflammatory conditions does not result from increased BFU-E formation. By contrast, Epo injections induced a moderate increase in the number of BFU-E in the bone marrow (from 2 or 3 to 10; Figure 4B) and a marked increase in the spleen (from 2 or 3 to 40; Figure 4C). The effect of Epo injections was fully suppressed in the bone marrow when inflammation was installed, whereas Epo injections in ZIGI mice induced a 3-fold increase in spleen stress BFU-E compared with Epo injections in controls. The colonies derived from stress BFU-E also exhibited a different morphology from bone marrow colonies: they were larger and redder and contained only one cluster of clumped cells with no satellite colonies.
Because IFN-γ is known to inhibit the growth of erythroid precursors, and considering that the Epo-mediated increase in BFU-E is blunted in the presence of inflammation, in the bone marrow but not in the spleen, we decided to plate bone marrow and spleen cells in methylcellulose media supplemented or not with IFN-γ. To achieve a sufficient number of colonies, we performed this assay on bone marrow and spleen cells from Epo1 mice. Our results show that in vitro IFN-γ significantly suppressed bone marrow BFU-E proliferation (Figure 4D), whereas it did not affect spleen stress BFU-E proliferation (Figure 4E).
These data demonstrate that, in an inflammatory situation, the spleen develops a stress erythropoiesis response after Epo injections, whereas the response is fully blunted in the bone marrow.
Epo induces BMP4 expression by spleen macrophages
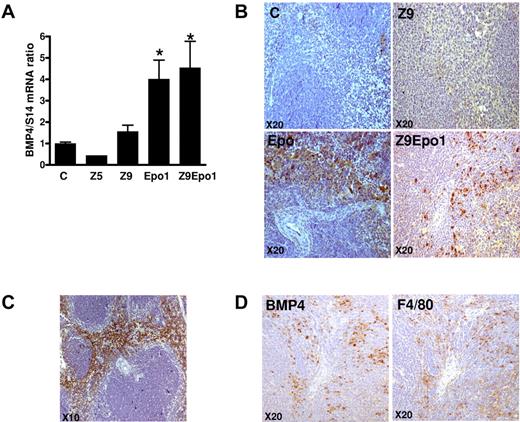
Given that we witnessed an erythropoietic response (increased number of BFU-E growing in methylcellulose in 3 days in the presence of Epo alone) in the spleen, after Epo injections in mice with chronic inflammation, we evaluated whether these stress BFU-E resulted from an increased expression of BMP4, as has been described in acute anemia or hypoxia. Therefore, we determined BMP4 expression at the mRNA and protein level in our model of chronic inflammation with or without Epo injections.
In the spleen, BMP4 mRNA was not increased by inflammation alone (Figure 5A), which is compatible with the low number of spleen BFU-E in this condition (Figure 4C). However, BMP4 mRNA was significantly increased by Epo injections whether or not inflammation was present (Figure 5A). Similar results were obtained at the protein level when BMP4 expression was investigated by immunohistochemistry (Figure 5B). In untreated mice and in mice with zymosan-induced inflammation, BMP4 was not detected. On the contrary, in Epo-injected mice, with or without inflammation, BMP4 was highly expressed (Figure 5B), the staining being localized in the red pulp of the perifollicular zone (Figure 5C).
Analysis of spleen BMP4 expression at the mRNA and protein levels. (A) Quantification of spleen BMP4 mRNA by quantitative reverse-transcribed polymerase chain reaction, normalized to S14 mRNA. *P < .001 compared with control mice. Errors bars represent SEM. (B-D) Immunochemistry. (B-C) Spleens were isolated, fixed in 10% paraformaldehyde, paraffin-embedded, and cut, and sections were stained with anti-BMP4 antibody (original magnification ×20). (C) Lower magnification of an Epo1 mouse is shown (original magnification ×10). (D) Serial spleen sections from one Z9Epo1 mouse were stained with either anti-BMP4 or anti-F4/80. Pictures are representative of at least 3 independent spleens. BMP4 and F4/80 staining was visualized using an Olympus microscope (original magnification ×20).
Analysis of spleen BMP4 expression at the mRNA and protein levels. (A) Quantification of spleen BMP4 mRNA by quantitative reverse-transcribed polymerase chain reaction, normalized to S14 mRNA. *P < .001 compared with control mice. Errors bars represent SEM. (B-D) Immunochemistry. (B-C) Spleens were isolated, fixed in 10% paraformaldehyde, paraffin-embedded, and cut, and sections were stained with anti-BMP4 antibody (original magnification ×20). (C) Lower magnification of an Epo1 mouse is shown (original magnification ×10). (D) Serial spleen sections from one Z9Epo1 mouse were stained with either anti-BMP4 or anti-F4/80. Pictures are representative of at least 3 independent spleens. BMP4 and F4/80 staining was visualized using an Olympus microscope (original magnification ×20).
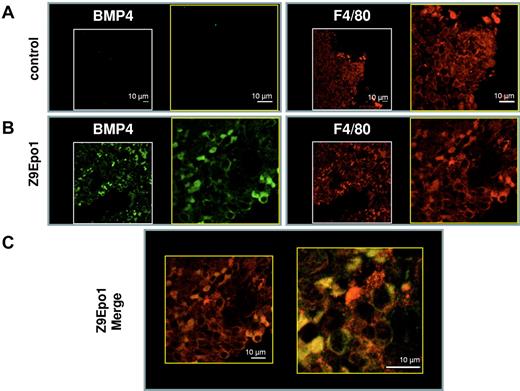
BMP4 is known to be synthesized both by the stroma and by stress erythroid precursors in an autocrine fashion. To identify more precisely the BMP4-producing cells, we performed double immunolabeling experiments on serial spleen sections, using F4/80 as a marker of the macrophage lineage. These experiments showed that, in the Z + Epo condition, BMP4 seemed to be synthesized by macrophages (Figure 5D). To confirm these results, we tested for colocalization of BMP4 and F4/80 by confocal immunofluorescence (Figure 6). In a control spleen, only F4/80 labeling was observed (Figure 6A). In the spleen from a Z9Epo1 mouse, the results clearly showed both BMP4 and F4/80 labeling (Figure 6B). The majority of spleen macrophages expressing F4/80 also expressed BMP4, and the merging of both fluorescent images demonstrated colocalization of BMP4 with F4/80. Our data demonstrate, for the first time, that macrophages from red pulp of the spleen can synthesize BMP4 in response to Epo injections. This pathway contributes to the stress erythropoietic response by stimulating the proliferation of BMP4-responsive progenitors that subsequently differentiate into stress BFU-E sensitive to high levels of Epo.
Confocal analysis of BMP4 and F4/80 colocalization in spleen macrophages. For immunofluorescence procedure, spleen sections of control mice (A) and Z9Epo1 (B-C) mice were stained with both anti-BMP4 and anti-F4/80 antibodies followed by an Alexa 488–conjugated goat anti–rabbit (BMP4; green) and an Alexa 555–conjugated goat anti–rat (F4/80; red). (C) The merge between the green and the red fluorescence at 2 different magnifications reveals yellow color corresponding to colocalization of F4/80 with BMP4 protein in a Z9Epo1 mouse. Most BMP4+ cells appear to be F4/80-expressing cells. At least 3 independent spleens were analyzed in each condition. Scale bar represents 10 μm.
Confocal analysis of BMP4 and F4/80 colocalization in spleen macrophages. For immunofluorescence procedure, spleen sections of control mice (A) and Z9Epo1 (B-C) mice were stained with both anti-BMP4 and anti-F4/80 antibodies followed by an Alexa 488–conjugated goat anti–rabbit (BMP4; green) and an Alexa 555–conjugated goat anti–rat (F4/80; red). (C) The merge between the green and the red fluorescence at 2 different magnifications reveals yellow color corresponding to colocalization of F4/80 with BMP4 protein in a Z9Epo1 mouse. Most BMP4+ cells appear to be F4/80-expressing cells. At least 3 independent spleens were analyzed in each condition. Scale bar represents 10 μm.
Epo injections in ZIGI mice allow partial recovery from severe anemia
We have seen that the rapid destruction of RBCs after zymosan injection induced a rapid drop in Hb levels. However, stimulation of proliferation and differentiation of erythroblasts triggered by anemia in the spleen of ZIGI mice was not sufficient to prevent the development of a long-lasting anemia that could be considered as severe up to Z17 (Hb 7.8 g/dL vs 14.1 g/dL in controls). This anemia is aregenerative until Z12, with a low reticulocyte count despite a 4-fold increase in serum Epo concentrations (Table 1) and despite the important increase in number of spleen erythroblasts. This response seemed to be insufficient to correct anemia because the number of circulating mature erythrocytes remained low: (7.29 ± 0.76) × 106/μL at Z12 versus (9.45 ± 0.67) × 106/μL in controls, suggesting persisting peripheral destruction of RBCs. Epo injections in ZIGI mice allowed a partial recovery from the inflammation-induced anemia. Indeed, 1, 4, and 9 days after the final Epo injection, reticulocytes and Hb concentrations were higher in Z + Epo mice compared with Z mice alone, although they remained below controls levels (Table 1). Therefore, Epo injections in ZIGI mice were able to partially correct anemia.
Discussion
Inflammation is known to be responsible for resistance to Epo therapy in several clinical conditions. Here, using the zymosan-induced septic shock-like syndrome mouse model,14 we show that in mice, similar to what is observed in humans, bone marrow erythropoiesis is repressed by inflammation and does not respond to Epo injections, contrary to spleen erythropoiesis. These results highlight the differences that exist between steady-state bone marrow erythropoiesis and spleen stress erythropoiesis, both in terms of sensitivity to the inhibitory actions of pro-inflammatory cytokines and of differential response after Epo injections in the presence of inflammation.
Mice have hematologic and inflammatory systems similar to humans,17,18 although they exhibit specific attributes, including the fact that they are capable of extensive extramedullar erythropoiesis. Our data support an important role for extramedullar erythropoiesis in response to Epo injections during inflammation, with the following sequence of events: (1) production of BMP4 by macrophages, (2) massive proliferation of spleen stress BFU-E, (3) increase in spleen erythroblast, and (4) production of an efficient reticulocytosis associated with partial correction of anemia. Such a specific response by the spleen is probably the result of 2 factors: a microenvironment unique to the spleen on the one hand and a different sensitivity of the resident spleen erythroid precursors to various cytokines on the other hand.
Macrophages are essential actors in the stromal microenvironment playing a dual role in the control of erythropoiesis, by having a supportive role in erythroblast development but also by producing proinflammatory cytokines that can suppress erythropoiesis. Macrophages interact directly with developing erythroblasts in the erythroblastic islands of the bone marrow where they provide cell-cell interactions important for control of apoptosis, proliferation, differentiation, and enucleation. Interestingly, we observed that in the spleen, especially in inflammatory condition, macrophages that synthesize BMP4 in response to Epo injections are F4/80+. This murine antigen allows the differentiation of macrophages that are central to erythroblastic islands in the bone marrow from other subsets of resident macrophages.19 It is intriguing that it is the same subset of F4/80-positive macrophages that produce BMP4, a factor required for the proliferation of stress erythroid progenitors, suggesting that they are part of spleen erythroblastic islands. Our results support a central role of macrophages in erythroid development, through the expression of BMP4. These data are in accordance with previous work from Sadahira et al showing that depletion of red pulp stromal F4/80+ macrophages leads to suppression of erythropoietic activity.20
The spleen harbors a specialized population of BMP4-responsive progenitors deriving from bone marrow cells. These progenitors are able to differentiate into stress BFU-E in the spleen microenvironment in the presence of BMP4, stem cell factor and hypoxia.21 Our in vitro experiments point to an absence of sensitivity of these spleen BFU-E to the inhibitory action of pro-inflammatory cytokines such as IFN-γ.
Our results also highlight the importance of Epo in the control of BMP4 expression. So far, hypoxia was considered as the major regulator of BMP4 expression, and HIF-2α binding sites were recently identified in the upstream regulatory region of the BMP4 gene.22 Clearly, hypoxia associated with ACD is not sufficient to trigger a BMP4 response; accordingly, there was no increase in spleen BFU-E. However, the activation of BMP4 expression observed both at the mRNA and protein levels after Epo injections, in control and in ZIGI mice, together with the concomitant increase in the number of Epo-responsive spleen stress BFU-E demonstrate a direct role of Epo in stimulating BMP4 expression by macrophages. The mechanism underlying this Epo-mediated increase in BMP4 gene expression remains to be determined.
Bone marrow and spleen erythroblasts also exhibited a different behavior after zymosan-induced inflammation. In the inflammatory condition alone, despite the lack of stress BFU-E in the spleen, there was a moderate increase in the number of erythroblasts, probably resulting from the reduced apoptosis of early and intermediate stages. Conversely, inflammation induced apoptosis of immature erythroblasts in the bone marrow, probably mediated by the strong increase in IFN-γ. The delayed and reduced serum IFN-γ levels induced by Epo injections might also account for the reduction in apoptosis of bone marrow erythroblasts. Indeed, it has been shown that Epo offers protection against systemic inflammation23 by suppressing the production of proinflammatory cytokines.24
Our data suggest that Epo injections in the context of ACD allow an increase in Hb levels and prevent the emergence of severe anemia over time (Table 1). However, this increase is delayed and incomplete, although it reaches the target Hb level normally expected for erythropoiesis-stimulating agents.25,26
Several factors could contribute to this partial response. We have shown that proinflammatory stimulus reduced RBC survival; and although we were unable to evaluate the possible beneficial effect of Epo on peripheral destruction of RBCs, it is tempting to speculate that Epo protected mature RBCs from prolonged hemolysis. Another possibility is that the erythropoietic response in ZIGI mice is iron restricted because of a hepcidin-driven iron sequestration. It is now well demonstrated that inflammation triggers an important IL-6-mediated increase in hepcidin synthesis6 and elevated serum hepcidin levels in turn induce iron retention within macrophages and iron-restricted erythropoiesis. Recently, monoclonal antihepcidin antibodies given in combination with erythropoiesis-stimulating agents were shown to correct anemia induced by injection of heat-killed Brucella abortis.27 In ZIGI mice, we previously showed that liver IL-6 and hepcidin mRNAs were increased at the early stages of the inflammatory response, but that stimulation of erythropoiesis by Epo injections suppressed hepcidin expression and favored mobilization of iron stores from the spleen.14 Furthermore, the anemia of ZIGI mice is moderately microcytic (Table 1) and the red cell volume returns to normal after Epo injections, suggesting that restricted iron delivery to the developing erythroblasts is not a contributing factor to the partial Epo efficiency. However, in some human ACD situations, such as anemia related to cancer, intravenous iron was able to increase the Epo response,28 suggesting that low iron stores can also contribute to Epo resistance.
Our study is the first to evaluate the effect of inflammation on murine stress erythropoiesis. All studies performed so far have demonstrated that spleen stress erythropoiesis develops in response to acute anemia and hypoxia, but our results point to a direct role of Epo in the initiation of this response.
It is unclear how our data relate to the human situation where, at the adult stage, erythropoiesis takes place almost exclusively in the bone marrow. The absence of a splenic compensation mechanism in humans may be a factor in the anemia of chronic inflammation. However, it is possible to speculate that the human bone marrow is able to mount a BMP4-dependent stress erythropoietic response after Epo injections or in acute stress conditions and that this response has remained undetected up until now. Recently, BMP4 was shown to be required for maintenance of hematopoietic stem cell number and function in mouse adult bone marrow,29 but these data have not been confirmed in humans yet. In addition, some specific pathologic conditions in humans lead to extramedullary erythropoiesis in particular thalassemia intermedia or myelofibrosis. It is unclear whether, in these particular situations, erythropoiesis shares specific features with mouse spleen stress erythropoiesis. For instance, in sickle cells disease, an accelerated erythroid differentiation pathway has been described.30
It would be interesting to determine whether bone marrow macrophages in humans can synthesize BMP4 in response to high doses of Epo and induce a medullar stress erythropoiesis, thereby representing a potential therapeutic target for ACD.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Marie Kurtz and Frederic Madec for their technical assistance, Françoise Cluzeau for her help with the confocal experiments, and Ivan Moura and Nicholas Heming for their comments on the paper.
S.M. was supported by the Association pour la Recherche sur le Cancer (PhD fellowship).
Authorship
Contribution: S.M. performed most of the experiments and analyzed data; S.M. and C.B. conceived the project and wrote the manuscript; V.A. analyzed the hematologic data and helped with FACS experiments; P.L. provided assistance with the mice experiments; S. Lyoumi helped setting the RBC survival experiments; M.H.-N. helped with the FACS experiments; O.T. performed the histologic experiments; S.B. and Z.K. helped with the confocal microscopy analysis; J.-L.C. analyzed and discussed the data; S. Lasocki initiated the project and discussed the data; and C.B. supervised the study and corrected the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carole Beaumont, Inserm U773, Faculté Xavier Bichat, 16 rue Henri Huchard, 75018 Paris, France; e-mail: carole.beaumont@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal