Proteasome and HDAC inhibitors interact strongly to promote cell death in multiple myeloma and other human cancer cells. This study challenges current assumptions about the mechanisms underlying these interactions.
Proteasome inhibitors (PIs) are the most active therapies for multiple myeloma (MM), but they do not produce cures, and there is currently an aggressive effort to identify PI-based combinations that produce greater clinical activity.1 Among the candidates identified in preclinical studies, combinations of PIs and histone deacetylase inhibitors (HDACis) appear to be among the most potent, producing synergistic cytotoxicity in preclinical MM models2,3 and in a variety of other human solid and hematologic cancer cell lines and xenografts.4 These studies prompted the initiation of 2 phase 1 clinical trials to evaluate the effects of combination therapy with bortezomib plus vorinostat (also known as SAHA, a pan HDACi) in refractory MM. Although the results should be treated as preliminary until phase 2 data are available, overall response rates in both trials were about 50%,1 suggesting that there will be benefit from combining PIs and HDACis in patients. Bortezomib appears to have greater single-agent activity than HDACis,1 supporting the notion that HDACis work by enhancing bortezomib's cytotoxic activity, and not vice versa.
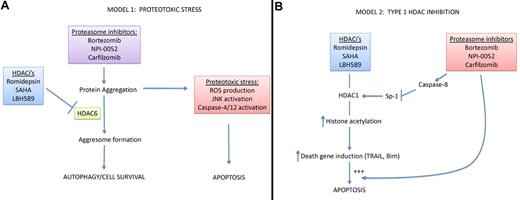
Two mechanistic explanations for PI-HDACi synergy. (A) HDAC inhibitors promote PI-induced proteotoxic stress. By blocking the proteasome, PIs promote the accumulation of damaged and misfolded proteins that are prone to aggregation, and it is this protein aggregation that serves as the primary cytotoxic stress, causing downstream reactive oxygen species (ROS) accumulation, JNK activation, and ER caspase (4 and 12) activation. HDACis promote this proteotoxic stress by blocking HDAC6, which is required for “aggresome” formation and the transfer of protein aggregates to lysosomes via autophagy. (B) Proteasome inhibitors promote type I HDAC inhibition. In this model, inhibition of type I HDACs serves as the primary cytotoxic stimulus, perhaps by promoting expression of “death genes” such as TNF-related apoptosis-inducing ligand (TRAIL) and Bim, a BH3-only member of the BCL-2 family. PIs synergize with HDAC inhibitors by promoting caspase-8 activation, cleavage and inactivation of Sp-1, and subsequent down-regulation of type I HDAC expression. Importantly, other studies have demonstrated that PIs promote TRAIL- and Bim-dependent apoptosis, so they may also interact with the HDACi pathway downstream of their effects on Sp-1.
Two mechanistic explanations for PI-HDACi synergy. (A) HDAC inhibitors promote PI-induced proteotoxic stress. By blocking the proteasome, PIs promote the accumulation of damaged and misfolded proteins that are prone to aggregation, and it is this protein aggregation that serves as the primary cytotoxic stress, causing downstream reactive oxygen species (ROS) accumulation, JNK activation, and ER caspase (4 and 12) activation. HDACis promote this proteotoxic stress by blocking HDAC6, which is required for “aggresome” formation and the transfer of protein aggregates to lysosomes via autophagy. (B) Proteasome inhibitors promote type I HDAC inhibition. In this model, inhibition of type I HDACs serves as the primary cytotoxic stimulus, perhaps by promoting expression of “death genes” such as TNF-related apoptosis-inducing ligand (TRAIL) and Bim, a BH3-only member of the BCL-2 family. PIs synergize with HDAC inhibitors by promoting caspase-8 activation, cleavage and inactivation of Sp-1, and subsequent down-regulation of type I HDAC expression. Importantly, other studies have demonstrated that PIs promote TRAIL- and Bim-dependent apoptosis, so they may also interact with the HDACi pathway downstream of their effects on Sp-1.
As “targeted” agents, PIs and HDACis are “dirty” drugs that no doubt work by many different mechanisms.4 Early studies suggested that PIs might kill MM and other cancer cells by blocking the inflammation- and cell survival–associated transcription factor, NFκB,4 but more recent data have challenged this notion.5 Rather, there is greater consensus for the idea that PIs induce MM cell death by promoting proteotoxic protein build-up and aggregation, mimicking certain neurodegenerative diseases.4 MM cells display a uniquely high protein synthetic load and possess very well-developed endoplasmic reticular-Golgi networks, which may explain why PIs display such uniquely high antitumor activity in the disease.
Histone deacetylases can be grouped into 3 major subfamilies (type I, type II, and sirtuins) based on structural and functional homologies.6 The most familiar are the type I HDACs (HDACs 1-3), which regulate chromatin structure by promoting histone deacetylation and chromatin compaction, although type I HDACs can also alter the acetylation of nonhistone proteins. Less is known about the functions of most of the type II HDACs. The exception is HDAC6, which is localized primarily in the cytosol and plays important roles in microtubule-mediated trafficking via its ability to promote tubulin deacetylation.4 Among the sirtuins, most attention has been paid to SIRT1, which is known for its role in promoting longevity, regulating metabolism and autophagy, and modulating the acetylation of several well-known transcription factors (p53, FOXO3A, NFκB-p65, and HSF1).7 The popular natural product resveratrol inhibits SIRT1, but the HDACis that are currently being evaluated clinically do not. Importantly, the effects of HDAC6 on tubulin acetylation mediate the coordination of protein turnover via the proteasome and autophagy, presumably by facilitating the trafficking of protein aggregates to prelysosomal structures known as “aggresomes.”4 Thus, HDACis that target HDAC6 or HDAC6 siRNAs disrupt aggresome formation and promote death in MM cells8 and other cancer cells exposed to PIs (see figure panel A),4 providing an attractive molecular explanation for the cytotoxic effects of PI-HDACi combinations.
The results of the study by Kikuchi et al in this issue turn this model on its head.9 The authors showed that bortezomib increased histone acetylation by decreasing expression of HDACs 1-3 (but not HDAC6 or SIRT1) in MM cell lines and primary tumor isolates, and they linked these effects to the synergy observed in cells exposed to bortezomib plus the potent pan-HDAC inhibitor, romidepsin (see figure panel B). Bortezomib's effects were mediated via caspase-8–dependent cleavage and inactivation of the transcription factor Sp-1, which functions as a potent inducer of type I HDAC expression. Importantly, overexpression of HDAC1 inhibited bortezomib-induced cell death, whereas HDAC1 knockdown promoted cytotoxicity, indicating that HDAC1 inhibition was sufficient to explain the cytotoxic effects observed. The data are reminiscent of earlier findings by Miller et al, who showed that another clinically relevant PI (NPI-0052) increased histone acetylation via a caspase-8–dependent mechanism.10 A central role for type I HDACs is consistent with data obtained with the type I–selective drug, MS-275 (now SNDX-275), which also synergizes with PIs in hematopoietic tumor cells11 even though it does not inhibit HDAC6.
In the end, given that both classes of drug are “dirty,” the molecular mechanisms involved in their cytotoxic effects are likely to be very complicated and context-dependent. There is still good reason to be attracted to the “proteotoxicity” model (see figure panel A) given the work emerging from the neurodegenerative disease literature and the fact that the immediate target of proteasome inhibitors is protein degradation. Within this context, PI-induced, caspase-8–dependent HDAC inhibition could reinforce type I HDAC–dependent processes that contribute to cell killing. Furthermore, given the broad role Sp-1 and its relatives play in driving gene expression, the effects of bortezomib should extend well beyond the type I HDACs to include a variety of other survival-associated transcriptional targets. It could be argued that the true test of both models will be whether they can help identify the subsets of patients who would benefit most from combination therapy. For example, the proteotoxicity model predicts that sensitivity might be linked to baseline ER stress, rates of protein synthesis (translation), and/or the efficiency of nonproteosomal degradative pathways (autophagy), whereas in the HDAC inhibition model sensitivity might be linked to type I HDAC (and particularly HDAC1) levels. Fortunately, both models can now be tested. In the end, as is most often the case in biology, the answer will probably involve a combination of the 2 models plus mechanisms we have not yet identified.
Conflict-of-interest disclosure: D.M. has received research support from Astra-Zeneca, Nereus Pharmaceuticals, Syndax Pharmaceuticals. Scientific Advisory Board: Apocell Inc. Equity (stock): Apocell Inc. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal