Abstract
Although platelets appear by embryonic day 10.5 in the developing mouse, an embryonic role for these cells has not been identified. The SYK–SLP-76 signaling pathway is required in blood cells to regulate embryonic blood-lymphatic vascular separation, but the cell type and molecular mechanism underlying this regulatory pathway are not known. In the present study we demonstrate that platelets regulate lymphatic vascular development by directly interacting with lymphatic endothelial cells through C-type lectin-like receptor 2 (CLEC-2) receptors. PODOPLANIN (PDPN), a transmembrane protein expressed on the surface of lymphatic endothelial cells, is required in nonhematopoietic cells for blood-lymphatic separation. Genetic loss of the PDPN receptor CLEC-2 ablates PDPN binding by platelets and confers embryonic lymphatic vascular defects like those seen in animals lacking PDPN or SLP-76. Platelet factor 4-Cre–mediated deletion of Slp-76 is sufficient to confer lymphatic vascular defects, identifying platelets as the cell type in which SLP-76 signaling is required to regulate lymphatic vascular development. Consistent with these genetic findings, we observe SLP-76–dependent platelet aggregate formation on the surface of lymphatic endothelial cells in vivo and ex vivo. These studies identify a nonhemostatic pathway in which platelet CLEC-2 receptors bind lymphatic endothelial PDPN and activate SLP-76 signaling to regulate embryonic vascular development.
Introduction
Blood and vessels arise simultaneously and function together to nourish the developing embryo and mature animal.1,2 Later in development, the close relationship of blood and vessels is reflected by the emergence of the first definitive hematopoietic cells from endothelial cells in the aorta.3 Studies in adult animals have further suggested that blood cells may give rise directly to endothelial cells through circulating endothelial precursors during active angiogenesis,4 but the role of such cells remains controversial,5 and the cell types and molecular mechanisms by which blood cells regulate endothelial and vessel growth remain poorly defined.
The finding that mice lacking the hematopoietic proteins SYK, SLP-76, or PLCg2 develop lethal lymphatic vascular defects during embryonic development provided new insight into how blood cells may regulate vessel growth and development.6,7 SYK, SLP-76 and PLCg2 function in a linear pathway downstream of immune-type receptors in blood cells.8 Previous studies have shown that this signaling pathway is required in blood and not in endothelial cells to prevent newly forming lymphatic vessels from connecting to preexisting blood vessels.9 Deciphering how this blood cell signaling pathway controls embryonic vascular development has promised to define a novel mechanism by which blood cells control endothelial and vascular growth.
Initial studies to identify the blood cell type in which SYK and SLP-76 signaling are required for vascular development suggested that this pathway might be used by blood cells that contribute directly to lymphatic endothelium in the developing embryo.9 However, subsequent studies of lymphatic development did not show evidence of blood cell–derived endothelial cells in early lymphatic vessels,10 leaving the basis for lymphatic vascular regulation by this pathway uncertain. More recently, vascular phenotypes in mice lacking PODOPLANIN (PDPN), a lymphatic endothelial surface protein known to bind C-type lectin-like receptor 2 (CLEC-2) receptors on platelets, have led to the hypothesis that platelets might use this pathway to control vascular development.11-13 However, genetic studies suggesting that platelets are not required for vascular development have argued against such a mechanism,14 and whether platelets use CLEC-2 receptors for such a nonhemostatic function is not known.
In the present study we demonstrate that CLEC-2 receptors and platelet SLP-76 are required for regulation of normal lymphatic vascular development, and we use genetic lineage tracing to definitively exclude a role for circulating endothelial precursors in this process. These genetic studies define a molecular pathway by which platelets recognize lymphatic endothelial cells and a novel, nonhemostatic role for platelets in regulating embryonic vascular development.
Methods
Mice
Vav-Cre, PF4-Cre, Slp-76−/−, and Slp-76fl/fl animals have been previously described.15-18 Pdpn+/− and Clec-2+/− mice were generated with the use of gene targeting of RF8 SV/129 embryonic stem cells (a kind gift of Dr Robert Farese, University of California, San Francisco) as shown in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and Figure 2. F1 generation Pdpn+/− and Clec-2+/− animals (50% 129;50% C57Bl/6) were intercrossed, and phenotypic analysis was conducted on Pdpn−/− and Clec-2−/− animals and wild-type littermates. Tie2-Cre transgenic animals and R26RYFP mice were obtained from The Jackson Laboratory. All mice were maintained on a mixed genetic background. Animal protocols were approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Histologic analyses
Paraffin-embedded tissue sections were stained with anti–lymphatic vessel endothelial hyaluronan receptor 1 (LYVE-1) polyclonal9 plus hematoxylin and developed using diaminobenzidine peroxidase substrate (Vector Laboratories). Immunofluorescence was performed with CD41 and CD62P monoclonal antibodies (mAbs; BD PharMingen), a polyclonal for green fluorescent protein/yellow fluorescent protein (GFP/YFP; Abcam), 4′-6′-diamidino-2-phenylindole (DAPI; Invitrogen), an anti–mouse thrombocyte polyclonal (Inter-Cell Technologies Inc) and Alexa Fluor 488 and 647 Monoclonal Antibody Labeling Kits (Invitrogen). Detailed protocols for immunofluorescence are available online at http://www.med.upenn.edu/mcrc/histology_core/. Histologic images were acquired with a Nikon Eclipse 80i microscope using a 40×/0.45 numeric aperture (NA) dry objective. Images in Figure 5A and B (20×/0.45 NA dry objective) and Figure 6C (10×/0.30 NA dry objective) were acquired using a Nikon Eclipse TE-2000-U microscope. Images in Figure 7 were acquired with a Leica DM 6000B confocal microscope with a 10×/0.40 NA dry objective using a 2× zoom. All images were edited using Photoshop verison 9.0 (Adobe Systems).
Bone marrow reconstitution studies
Flow cytometry
Whole blood cells were harvested from mice as previously described.20 Immunostaining was performed with antibodies to CD41 and CD45 (clone 30-F11), both from BD PharMingen. Recombinant Human Podoplanin/Fc Chimera protein (R&D Systems) was incubated with whole blood cells at room temperature for 1 hour. Cells were fixed for 5 minutes in 2% paraformaldehyde and then stained as usual. AffinPure Rabbit anti–human immunoglobulin G (Fcγ) was used to aggregate PDPN/Fc (Jackson ImmunoResearch Laboratories) and fluorescein isothiocyanate polyclonal anti–rabbit immunoglobulin (PharMingen) was used for detection. Binding of Alexa Fluor 647–conjugated human fibrinogen (Invitrogen) was used to measure platelet activation.
Statistics
P values were calculated with an unpaired 2-tailed Student t test or χ2 analysis as indicated.
Blood and lymphatic endothelial cell culture
Blood endothelial cells (BECs; Lonza) harvested from human umbilical veins or adult human dermal lymphatic microvascular endothelial cells (LECs; Lonza) were maintained with the use of standard cell culture techniques in EGM-2 MV media (Lonza). At confluency, cells were detached with the use of 0.05% Trypsin-EDTA (ethylenediaminetetraacetic acid; Gibco).
Static platelet adhesion and activation
Lab-Tek II 8-chamber slides (Thermo Fisher Scientific) were coated with 0.3 mg/mL fibronectin (BD PharMingen) in phosphate-buffered saline (PBS; 0.008M Na2HPO4, 0.002M KH2PO4, 0.14M NaCl, and 0.01M KCl) for 2 hours at room temperature and rinsed with PBS. BECs or LECs at 0.8 × 105 cells/well were plated in fibronectin-coated 8-chamber slides and cultured overnight in EGM-2 MV media (Lonza). Washed platelets from wild-type or Slp-76−/− mice were diluted to 1.5 to 3 × 108 cells/mL in EGM-2 MV media, and 0.15 mL of platelet suspension was added to the endothelial cell culture and incubated at 37°C under 5% CO2 for 1 hour. Cells were rinsed 3 times with PBS to remove nonadherent platelets and fixed with 2% paraformaldehyde for 10 minutes. Platelet adhesion and activation were detected by biotin-conjugated anti–mouse CD41 mAb followed by Texas Red–conjugated streptavidin and fluorescein isothiocyanate–conjugated anti–mouse CD62P, respectively. Endothelial cells were visualized by DAPI staining.
Whole blood flow over endothelial cell monolayer
Glass microscope slides (25 × 75 × 1 mm; Fisher Scientific) were coated with 0.3 mg/mL fibronectin (BD PharMingen) in PBS (0.008M Na2HPO4, 0.002M KH2PO4, 0.14M NaCl, and 0.01M KCl) for 2 hours at room temperature and rinsed with PBS. BECs or LECs (7 × 105) were plated on the top of fibronectin-coated slides in 1 mL of media and allowed to adhere for 2 hours. An additional 10 mL of media was added, and cells were cultured overnight. A tapered-wall parallel plate flow chamber was assembled as previously described21 and placed on the stage of an inverted microscope (Nikon Eclipse TE2000-U). Heparinized whole blood was collected from mice as previously described21 and labeled with Alexa Fluor 488–conjugated CD41 mAb. Blood was perfused over the BEC- or LEC-coated glass slide at a controlled flow rate of 0.214 mL/min with the use of a syringe pump (Model 11′ Plus; Harvard Apparatus) at room temperature for 5 minutes. Shear rates were calculated as previously described and ranged from approximately 200 to 650 s−1, which are equivalent to shear stresses ranging between 8 and 26 dynes/cm2.21 The adhesion of fluorescently labeled platelets was monitored continuously by epifluorescence microscopy. Images were captured with a CCD camera (C9300-201; Hamamatsu) with a 300-W Xenon lamp (PerkinElmer Optoelectronics) through a Lambda DG-4 high-speed filter changer (Sutter Instruments) used at 470 nm Ex/525 nm Em.
Cell viability assay
LECs were plated in a 12-well plate at 0.18 × 106 cells/well and cultured overnight. Washed platelets from wild-type mice were diluted to 1.5 to 3 × 108 cells/mL in EGM-2 MV media, and 0.5 mL of platelet suspension was added to the endothelial cell culture and incubated at 37°C under 5% CO2 for 2 hours. The supernatant was removed, and cells were rinsed 3 times with PBS and detached from the plate with 0.05% trypsin-EDTA. Cells were stained with propidium iodide to assay cell viability and analyzed by flow cytometry.
Bromodeoxyuridine incorporation assay
LECs were plated in a 6-well plate at 0.18 × 106 cells/well and cultured overnight. Washed platelets from wild-type mice were diluted to 1.5 to 3 × 108 cells/mL in EGM-2 MV media, and 0.5 mL of platelet suspension was added to the endothelial cell culture and incubated at 37°C under 5% CO2 for 2 hours. The supernatant was removed, and cells were rinsed 3 times with PBS and then incubated in EGM-2 MV media containing 10μM bromodeoxyuridine (BrdU) for 2 hours. Media without BrdU served as negative control. After incubation, the cells were detached from the plate with 0.05% trypsin-EDTA, and the BrdU incorporation was detected with the BD PharMingen BrdU Flow Kit according to the manufacturer's protocol.
Cell migration assay
LECs were plated in a 6-well plate at 0.18 × 106 cells/well and cultured overnight. A scratch was made across the endothelial cell confluent monolayer with the use of a p200 pipet tip, and the width of the scratch was recorded at time 0. Washed platelets from wild-type mice were diluted to 1.5 to 3 × 108 cells/mL in EGM-2 MV media, and 0.5 mL of platelet suspension was added to the endothelial cell culture and incubated at 37°C under 5% CO2 for 20 hours. Platelet-free media served as a negative control. The widths of the scratches were measured again at 20 hours to estimate the effect of the platelets on endothelial cell migration.
Results
The lymphatic endothelial surface protein PDPN functions in a nonhematopoietic cell type to regulate lymphatic vascular development
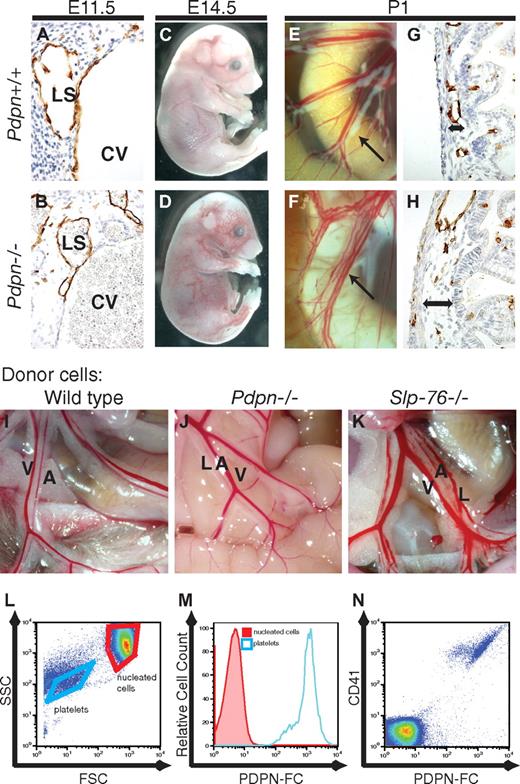
Two recent studies have reported a vascular mixing phenotype similar to that of SLP-76–deficient mice in animals lacking the lymphatic endothelial surface protein PDPN.11,12 To compare this phenotype to that of mice lacking SLP-76 we generated mice that carry a null Pdpn allele due to insertion of a Cre cDNA into exon 1 (supplemental Figure 1). SLP-76–deficient embryos develop blood-filled lymph sacs at embryonic day 11.5 (E11.5) and blood-filled cutaneous vessels and edema at E14 to E16.6 Neonatal mice lacking SLP-76 exhibit a severe vascular phenotype in the small intestine characterized by the presence of chylous ascites, mixing of chyle and blood in mesenteric and intestinal vessels, and edema of the intestinal wall.6,19 Similar phenotypes were observed in all Pdpn−/− embryos and neonates (Figure 1A-H), suggesting that PDPN and SLP-76 might operate in a common pathway required for blood-lymphatic vascular separation. PDPN and SLP-76 could function together in a single hematopoietic cell type, or PDPN could be required in LECs, and SLP-76 could be required in hematopoietic cells. To determine whether PDPN is required in hematopoietic cells we reconstituted lethally irradiated wild-type mice with PDPN-deficient fetal liver cells, a method that has previously been shown to confer the intestinal vascular phenotype to wild-type animals when SYK or SLP-76–deficient donor cells were used.6,19,22 Animals reconstituted with wild-type (N = 5) or PDPN-deficient (N = 8) hematopoietic cells did not develop intestinal phenotypes (Figure 1I-J). In contrast, most animals reconstituted with SLP-76–deficient cells exhibited blood-filled mesenteric and intestinal lymphatics characteristic of vascular mixing (16 of 21; P < .001 by χ2 analysis; Figure 1K). Thus, PDPN is required in nonhematopoietic cells to regulate blood-lymphatic vascular separation and probably functions in LECs.
Podoplanin is required in nonhematopoietic cells for blood-lymphatic separation. (A-H) Pdpn−/− animals exhibit vascular mixing phenotypes identical to those of Slp-76−/− animals. (A-B) Development of blood-filled lymph sacs in E11.5 Pdpn−/− embryos. (C-D) Blood-filled cutaneous lymphatics are visible in E14.5 Pdpn−/− embryos. (E-F) Blood-filled mesenteric lymphatics in Pdpn−/− neonatal animals (arrows) are shown. (G-H) Histologic evidence of intestinal edema in neonatal animals (double arrows) is shown. Immunostaining for LYVE-1+ lymphatic endothelium is shown (A-B,G-H). CV indicates cardinal vein; and LS, lymph sac. (I-K) Blood-filled mesenteric lymphatic vessels arise in lethally irradiated wild-type mice after reconstitution with Slp-76−/− (K) but not wild-type (I) or Pdpn−/− (J) donor hematopoietic cells. A indicates artery; V, vein; and L, lymphatic. (L-N) PDPN-Fc fusion protein selectively binds platelets in whole blood. Binding of PDPN-Fc to anuclear platelets vs nucleated blood cells is shown with the use of forward and side scatter (FSC and SSC) on a log scale to discriminate the 2 cell populations (L; gated cell populations are indicated by purple boxes). All PDPN-bound cells express the platelet-specific integrin CD41 (M-N).
Podoplanin is required in nonhematopoietic cells for blood-lymphatic separation. (A-H) Pdpn−/− animals exhibit vascular mixing phenotypes identical to those of Slp-76−/− animals. (A-B) Development of blood-filled lymph sacs in E11.5 Pdpn−/− embryos. (C-D) Blood-filled cutaneous lymphatics are visible in E14.5 Pdpn−/− embryos. (E-F) Blood-filled mesenteric lymphatics in Pdpn−/− neonatal animals (arrows) are shown. (G-H) Histologic evidence of intestinal edema in neonatal animals (double arrows) is shown. Immunostaining for LYVE-1+ lymphatic endothelium is shown (A-B,G-H). CV indicates cardinal vein; and LS, lymph sac. (I-K) Blood-filled mesenteric lymphatic vessels arise in lethally irradiated wild-type mice after reconstitution with Slp-76−/− (K) but not wild-type (I) or Pdpn−/− (J) donor hematopoietic cells. A indicates artery; V, vein; and L, lymphatic. (L-N) PDPN-Fc fusion protein selectively binds platelets in whole blood. Binding of PDPN-Fc to anuclear platelets vs nucleated blood cells is shown with the use of forward and side scatter (FSC and SSC) on a log scale to discriminate the 2 cell populations (L; gated cell populations are indicated by purple boxes). All PDPN-bound cells express the platelet-specific integrin CD41 (M-N).
PDPN-Fc proteins selectively bind platelets
The only known receptor for PDPN is CLEC-2, a C-type lectin-like receptor of unknown biologic function that is expressed selectively by platelets and known to couple to SYK and SLP-76 signaling through a single intracellular YxxL motif.23-25 To determine whether any hematopoietic cell type other than platelets interacts with PDPN, and therefore might be the cell type in which SLP-76 signaling is required during lymphatic vascular development, we examined binding to a PDPN-Fc fusion protein. PDPN-Fc strongly bound CD41+ platelets but no other cell types in the circulating blood (Figure 1L-N).
CLEC-2 receptor is required for PDPN to bind and activate platelets
The studies suggested that PDPN on the surface of LECs might activate SYK and SLP-76 signaling through the platelet CLEC-2 receptor. To directly test the role of CLEC-2 in PDPN-mediated platelet responses and vascular development we generated Clec2−/− mice with the use of gene targeting to delete the first exon and promoter of Clec-2 (Figure 2A). Clec-2+/− megakaryocytes and platelets expressed reduced levels of CLEC-2 protein and Clec-2 mRNA, whereas Clec-2−/− megakaryocytes and platelets lacked both (Figure 2B-C). To determine the role of CLEC-2 in mediating PDPN responses we tested PDPN-Fc fusion protein interaction with wild-type and CLEC-2–deficient platelets. PDPN-Fc fusion proteins were unable to bind Clec-2−/− platelets (Figure 2D). Cross-linking of PDPN-Fc proteins potently activated wild-type platelets but not Clec-2−/− platelets, although Clec-2−/− platelets exhibited normal activation responses to convulxin, a potent agonist for the glycoprotein VI receptor that also signals through SYK and SLP-7626 (Figure 2E). These studies identify that CLEC-2 is the receptor by which PDPN binds and activates platelets.
CLEC-2–deficient platelets do not interact with PDPN. (A) Gene targeting strategy for Clec-2. The deleted sequence in exon 1 encodes the entire intracellular domain of the CLEC-2 receptor, including the YxxL motif required to activate SYK, as well as the transcriptional and translational start sites. (B) Clec-2−/− platelets lack CLEC-2 protein. Immunoblotting was used to measure CLEC-2 and actin proteins in cell lysate derived from brain (left lane) and platelet-rich plasma (right lanes). CLEC-2 levels in Clec-2+/− platelets were reduced by approximately 50% compared with those in Clec-2+/+ platelets (second and third lanes from left, lysate from 0.75 mL of blood loaded). BecauseClec-2−/− mice die in the perinatal period, CLEC-2 levels were measured in platelets derived from lethally irradiated wild-type mice that had been reconstituted with Clec-2+/+ or Clec-2−/− fetal liver cells (right 4 lanes, lysate from 0.1 mL of blood loaded in each lane). (C) In situ hybridization of E14.5 mouse embryos for Clec-2. Clec-2 expression is indicated in red; DAPI nuclear staining is shown in blue. (D) PDPN-Fc proteins bind Clec-2+/+ but not Clec-2−/− platelets. (E) PDPN-Fc proteins activate Clec-2+/+ but not Clec-2−/− platelets. Platelet activation was measured by the binding of allophycocyanin-conjugated fibrinogen. Platelet activation by the glycoprotein VI receptor agonist convulxin is shown as a positive control.
CLEC-2–deficient platelets do not interact with PDPN. (A) Gene targeting strategy for Clec-2. The deleted sequence in exon 1 encodes the entire intracellular domain of the CLEC-2 receptor, including the YxxL motif required to activate SYK, as well as the transcriptional and translational start sites. (B) Clec-2−/− platelets lack CLEC-2 protein. Immunoblotting was used to measure CLEC-2 and actin proteins in cell lysate derived from brain (left lane) and platelet-rich plasma (right lanes). CLEC-2 levels in Clec-2+/− platelets were reduced by approximately 50% compared with those in Clec-2+/+ platelets (second and third lanes from left, lysate from 0.75 mL of blood loaded). BecauseClec-2−/− mice die in the perinatal period, CLEC-2 levels were measured in platelets derived from lethally irradiated wild-type mice that had been reconstituted with Clec-2+/+ or Clec-2−/− fetal liver cells (right 4 lanes, lysate from 0.1 mL of blood loaded in each lane). (C) In situ hybridization of E14.5 mouse embryos for Clec-2. Clec-2 expression is indicated in red; DAPI nuclear staining is shown in blue. (D) PDPN-Fc proteins bind Clec-2+/+ but not Clec-2−/− platelets. (E) PDPN-Fc proteins activate Clec-2+/+ but not Clec-2−/− platelets. Platelet activation was measured by the binding of allophycocyanin-conjugated fibrinogen. Platelet activation by the glycoprotein VI receptor agonist convulxin is shown as a positive control.
CLEC-2 is required for normal lymphatic vascular development
Mice heterozygous for the null Clec-2 allele were healthy and fertile, but all Clec-2−/− animals developed mid-gestation and neonatal lymphatic vascular phenotypes similar to those of mice lacking PDPN, SYK, or SLP-76 (Figure 3A-L). Like animals lacking PDPN or SYK, all CLEC-2–deficient mice died within the first several weeks after birth (N = 30). Analysis of Clec-2 expression in the E14.5 mouse embryo with the use of in situ hybridization showed strong expression in multinucleated fetal liver megakaryocytes with no expression in the developing vasculature or other organs (Figure 2C; data not shown). To determine whether CLEC-2 is required in blood cells to regulate lymphatic vessel growth, lethally irradiated wild-type mice were reconstituted with wild-type or CLEC-2–deficient fetal liver cells. In contrast to animals reconstituted with PDPN-deficient fetal liver cells, but similar to animals reconstituted with SYK-deficient or SLP-76-deficient hematopoietic cells, animals reconstituted with CLEC-2–deficient fetal liver cells developed vascular mixing phenotypes characterized by blood-filled intestinal and mesenteric lymphatics (N = 12; Figure 3M-O). These findings show that the PDPN receptor CLEC-2 is required in blood cells for normal lymphatic vascular development.
CLEC-2 is required in blood cells for normal lymphatic vascular development. (A-L) Clec-2−/− animals exhibit blood-lymphatic vascular mixing phenotypes. (A-F) Clec-2−/− embryos exhibit blood-filled lymphatics like those of Slp-76−/− animals during mid-gestation. Whole embryo images show blood-filled cutaneous lymphatics and edema (above). Transverse sections show anti-LYVE-1 immunostaining (brown) of lymph sacs adjacent to the cardinal vein (below). (G-L) Clec-2−/− neonates exhibited blood-filled mesenteric vessels (G,I,K) and edema of the intestine wall (H,J,L) like those of Slp-76−/− animals. (M-O) Blood-filled mesenteric lymphatic vessels arise in lethally irradiated wild-type mice after reconstitution with Clec-2−/− (N-O) but not wild-type (M) donor hematopoietic cells. A indicates artery; V, vein; and L, lymphatic.
CLEC-2 is required in blood cells for normal lymphatic vascular development. (A-L) Clec-2−/− animals exhibit blood-lymphatic vascular mixing phenotypes. (A-F) Clec-2−/− embryos exhibit blood-filled lymphatics like those of Slp-76−/− animals during mid-gestation. Whole embryo images show blood-filled cutaneous lymphatics and edema (above). Transverse sections show anti-LYVE-1 immunostaining (brown) of lymph sacs adjacent to the cardinal vein (below). (G-L) Clec-2−/− neonates exhibited blood-filled mesenteric vessels (G,I,K) and edema of the intestine wall (H,J,L) like those of Slp-76−/− animals. (M-O) Blood-filled mesenteric lymphatic vessels arise in lethally irradiated wild-type mice after reconstitution with Clec-2−/− (N-O) but not wild-type (M) donor hematopoietic cells. A indicates artery; V, vein; and L, lymphatic.
SLP-76 signaling is required in platelets for normal lymphatic vascular development
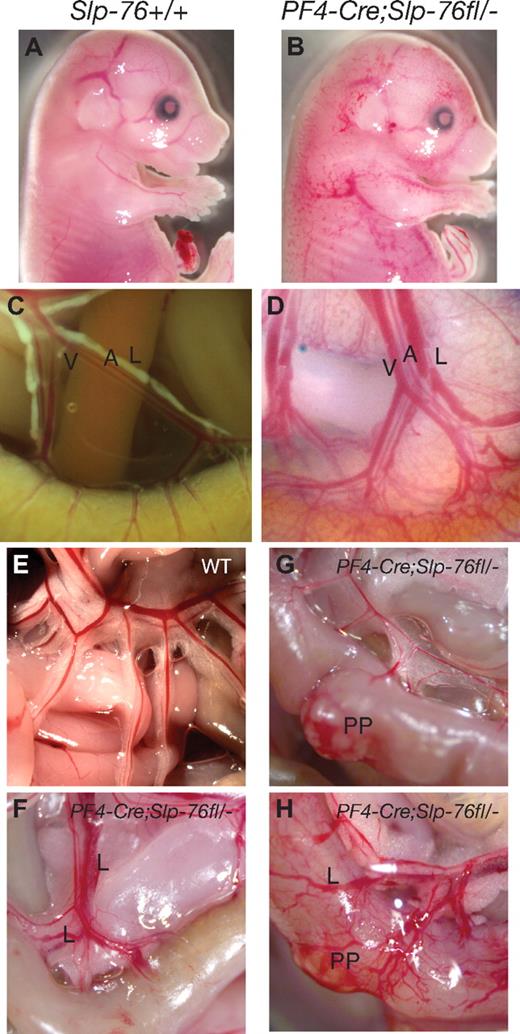
Our results support a mechanism in which PDPN on LECs binds CLEC-2 receptors on platelets and activates SYK and SLP-76 signaling to regulate blood-lymphatic vascular separation. However, mice deficient in the nuclear factor–erythroid-derived 2 (NF-E2) transcription factor lacking almost all platelets fail to develop a similar phenotype,14 and CLEC-2 expression has also been reported in neutrophils.27 To functionally test the role of platelets in blood-lymphatic vascular separation we deleted Slp-76 in platelets and megakaryocytes with the use of a platelet factor 4 (PF4) Cre transgene.16 PF4-Cre;Slp-76fl/−;R26RYFP embryos and neonates developed the visual and histologic signs of blood-lymphatic mixing characteristic of mice lacking SYK, SLP-76, or PDPN (Figure 4A-D; data not shown). Vascular phenotypes were not detected in all PF4-Cre;Slp-76fl/−;R26RYFP neonates (14 of 20), but by age 12 weeks all PF4-Cre;Slp-76fl/−;R26RYFP animals exhibited blood-filled lymphatic vessels in the intestine and mesentery (Figure 4E-H).
SLP-76 is required in platelets for normal lymphatic vascular development. (A-B) PF4-Cre;Slp-76fl/− embryos exhibit blood-filled cutaneous lymphatics. (C-D) PF4-Cre;Slp-76fl/− neonates exhibit blood-filled mesenteric and intestinal lymphatic vessels. A indicates artery; V, vein; and L, lymphatic. (E-H) Twelve-week-old PF4-Cre;Slp-76fl/− animals exhibit vascular mixing phenotypes that range in severity from blood-filled Peyer patches (PP, left) to disruption of the normal vascular pattern of the intestine wall and blood-filled mesenteric lymphatic vessels (L, right).
SLP-76 is required in platelets for normal lymphatic vascular development. (A-B) PF4-Cre;Slp-76fl/− embryos exhibit blood-filled cutaneous lymphatics. (C-D) PF4-Cre;Slp-76fl/− neonates exhibit blood-filled mesenteric and intestinal lymphatic vessels. A indicates artery; V, vein; and L, lymphatic. (E-H) Twelve-week-old PF4-Cre;Slp-76fl/− animals exhibit vascular mixing phenotypes that range in severity from blood-filled Peyer patches (PP, left) to disruption of the normal vascular pattern of the intestine wall and blood-filled mesenteric lymphatic vessels (L, right).
To determine the level of SLP-76 function required in platelets to regulate lymphatic vascular development we next measured SLP-76 function in the platelets of PF4-Cre;Slp-76fl/− animals with the use of single cell activation responses to convulxin.28 The platelets of all (9 of 9) 12-week-old PF4-Cre;Slp-76fl/− animals with lymphatic vascular defects exhibited a complete loss of SLP-76 function (supplemental Figure 2A-B). In contrast, the platelets of neonatal PF4-Cre;Slp-76fl/−;R26RYFP animals without vascular phenotypes exhibited incomplete loss of SLP-76 function (supplemental Figure 2C). These studies suggest that lymphatic vascular defects arise only when the loss of platelet SLP-76 signaling is complete. To determine the cellular specificity of PF4-Cre–mediated recombination during embryonic development we performed immunostaining for YFP in E14.5 PF4-Cre;Slp-76fl/−;R26RYFP embryos. PF4-Cre–activated YFP expression was detected only in the fetal liver where it was restricted to mature, multinucleated megakaryocytes (supplemental Figure 3A). Analysis of YFP expression in circulating blood cells showed that PF4-Cre–mediated recombination was restricted to CD41+ platelets and did not take place in GR1+ neutrophils, the only other cell type reported to express CLEC-227 (supplemental Figure 3B). These studies functionally define the platelet as the cell type in which SLP-76 signaling is required for normal lymphatic vascular development.
Platelet aggregates form on the surface of LECs in a SLP-76–dependent manner in vitro and in vivo
Our genetic studies predict an interaction between platelets and LECs during embryonic vascular development. To further characterize platelet interaction with LECs, BECs harvested from human umbilical veins and LECs harvested from human dermis were cultured in the presence of platelets. Platelets adhered strongly to LECs but not to BECs, and LEC-adherent platelets were activated, indicated by the expression of P-selectin on the platelet surface and by the formation of platelet aggregates (Figure 5A). In contrast, SLP-76–deficient platelets adhered to LECs under static conditions but were not activated and did not form aggregates on the LEC surface (Figure 5A). When heparinized whole blood was flowed over confluent endothelial cell monolayers under venous shear forces, wild-type platelets did not adhere to BECs but rapidly formed aggregates on the LEC surface (Figure 5B). PDPN-deficient platelets formed aggregates on LECs under flow, but SLP-76–deficient platelets did not (Figure 5B). These studies show that platelets are activated by and form aggregates on the surface of LECs through a SLP-76–dependent pathway.
Platelets are activated by and form aggregates on LECs in a SLP-76–dependent manner in vitro and in vivo. (A) Wild-type platelets bind and are activated on the surface of LECs but not BECs. Slp-76−/− platelets adhere to LECs but do not form aggregates or express P-selectin. CD41 staining identifies platelets, and P-selectin expression identifies activated platelets. (B) Flow of heparinized wild-type blood at a shear level of 8 dynes/cm2 results in platelet aggregate formation on LECs but not on BECs (left 2 panels). PDPN-deficient platelets adhere and form aggregates on LECs, but SLP-76–deficient platelets fail to form aggregates (right 2 panels). Platelets are visualized with Alexa Fluor 488–conjugated anti-CD41 antibody after 5 minutes of flow. (C) Identification of platelet aggregates on LYVE-1+ LECs in Slp-76+/+ but not Slp-76−/− developing embryos. Immunostaining of E11.5 transverse embryo sections with antibodies against platelets (red) and the LEC LYVE-1 protein (green) are shown. Arrows indicate platelet aggregates on the endothelial cell surface. CV indicates cardinal vein; and LS, lymph sac. (D-E) Injection of wild-type platelets into Slp-76−/− animals results in the formation of platelet aggregates on the surface of LECs. Wild-type platelets were injected into the vitelline vein of E18 Slp-76−/− embryos (D) or into the retroorbital sinus of P21 Slp-76−/− animals (E). Platelet aggregates (red) and lymphatic endothelium (anti–LYVE-1, green) were detected by immunostaining of tissues harvested 12 hours after injection.
Platelets are activated by and form aggregates on LECs in a SLP-76–dependent manner in vitro and in vivo. (A) Wild-type platelets bind and are activated on the surface of LECs but not BECs. Slp-76−/− platelets adhere to LECs but do not form aggregates or express P-selectin. CD41 staining identifies platelets, and P-selectin expression identifies activated platelets. (B) Flow of heparinized wild-type blood at a shear level of 8 dynes/cm2 results in platelet aggregate formation on LECs but not on BECs (left 2 panels). PDPN-deficient platelets adhere and form aggregates on LECs, but SLP-76–deficient platelets fail to form aggregates (right 2 panels). Platelets are visualized with Alexa Fluor 488–conjugated anti-CD41 antibody after 5 minutes of flow. (C) Identification of platelet aggregates on LYVE-1+ LECs in Slp-76+/+ but not Slp-76−/− developing embryos. Immunostaining of E11.5 transverse embryo sections with antibodies against platelets (red) and the LEC LYVE-1 protein (green) are shown. Arrows indicate platelet aggregates on the endothelial cell surface. CV indicates cardinal vein; and LS, lymph sac. (D-E) Injection of wild-type platelets into Slp-76−/− animals results in the formation of platelet aggregates on the surface of LECs. Wild-type platelets were injected into the vitelline vein of E18 Slp-76−/− embryos (D) or into the retroorbital sinus of P21 Slp-76−/− animals (E). Platelet aggregates (red) and lymphatic endothelium (anti–LYVE-1, green) were detected by immunostaining of tissues harvested 12 hours after injection.
Lymphatic vascular development begins when endothelial cells lining the cardinal vein acquire lymphatic identity and sprout to form the primary lymph sacs.10,29 To determine whether platelets interact with LECs during this process we performed immunostaining of transverse sections of E11.5 mouse embryos to detect platelets and LYVE-1+ LECs. Platelet aggregates were detected on the surface of LECs in the cardinal vein in 17 of 80 sections from wild-type embryos, but in only 1 of 240 sections from Slp-76−/− embryos (Figure 5C). Most of these aggregates were composed of 1 or 2 layers of platelets on the surface of individual LECs (Figure 5C arrows). Similar platelet-LEC interactions could not be detected in the intestine or elsewhere at later time points in embryos or neonates (data not shown). However, infusion of wild-type platelets into SLP-76–deficient embryos (Figure 5D) or neonates (Figure 5E), in which blood circulates in contact with lymphatic endothelium, resulted in the rapid formation of platelet aggregates on the surface of LECs. These studies show that platelets contact LECs during their specification in the cardinal vein and form aggregates on the LEC surface in a SLP-76–dependent manner in vivo.
Platelets do not affect endothelial viability, proliferation, or migration in vitro
To determine how platelets might regulate lymphatic vascular development we cocultured primary LECs with human platelets and measured endothelial viability, proliferation, and migration. Platelet coculture did not cause LEC death or slow the proliferation of LECs in vitro (Figure 6A-B). Platelets also did not alter the rate of LEC migration in an in vitro scratch assay (Figure 6C). These studies suggest that platelet activation on the surface of LECs does not affect lymphatic vascular development through major changes in cell viability, proliferation, or migration.
Platelets do not affect LEC viability, proliferation, or migration in vitro.(A) Overnight culture of platelets with LECs does not affect endothelial cell viability measured by nuclear uptake of propidium iodide. Control indicates cells not treated with propidium iodide. (B) Overnight culture of platelets with LECs does not affect endothelial cell proliferation measured by uptake of BrdU after a 2-hour pulse. Control indicates cells not treated with BrdU. (C) Culture of platelets with LECs does not alter migration in a scratch assay. N = 3 for each group. Error bars indicate SD.
Platelets do not affect LEC viability, proliferation, or migration in vitro.(A) Overnight culture of platelets with LECs does not affect endothelial cell viability measured by nuclear uptake of propidium iodide. Control indicates cells not treated with propidium iodide. (B) Overnight culture of platelets with LECs does not affect endothelial cell proliferation measured by uptake of BrdU after a 2-hour pulse. Control indicates cells not treated with BrdU. (C) Culture of platelets with LECs does not alter migration in a scratch assay. N = 3 for each group. Error bars indicate SD.
Genetic lineage tracing shows that blood cells do not contribute to lymphatic endothelium at sites of vascular mixing
These studies collectively support a mechanism in which platelets regulate lymphatic endothelial and vessel growth through a PDPN–CLEC-2 interaction. In contrast, previous studies have suggested that SLP-76 might be required in circulating endothelial precursors that directly contribute to vascular endothelium to prevent blood-lymphatic vascular mixing.9 To definitively test this mechanism we used a Vav-Cre transgene that drives highly specific and efficient recombination in definitive hematopoietic cells15 to simultaneously delete Slp-76 and permanently activate YFP expression in the blood cells of Vav-Cre;Slp-76fl/−;R26RYFP mice. Vav-Cre;Slp-76fl/−;R26RYFP embryos and neonates exhibited defects in blood-lymphatic vascular separation identical to those of SLP-76–deficient embryos, indicating Cre activity in the causal cell type (supplemental Figure 4). To determine whether circulating hematopoietic cells contribute to lymphatic vascular endothelium, we examined expression of YFP in Vav-Cre;R26RYFP animals in the intestinal submucosa, which is the site of greatest vascular disruption in animals lacking PDPN, CLEC-2, SYK, or SLP-76.6 Confocal analysis failed to show any YFP+LYVE-1+ LECs in Vav-Cre;R26RYFP animals (0 of 596 endothelial cells in 221 vessels; Figure 7A-D). By contrast, YFP+LYVE-1+ endothelial cells were readily detected in the intestine of Tie2-Cre;R26RYFP neonates in which Cre is expressed in all endothelial cells (Figure 7E-F). These studies definitively exclude a role for circulating endothelial precursors in this regulatory pathway and are consistent with a mechanism in which LEC PDPN activates platelet CLEC-2 and SLP-76 signaling.
Blood cells do not contribute to lymphatic endothelium at the site of vascular mixing in mice lacking PDPN, CLEC-2, or SLP-76. Vav-Cre–activated YFP expression was used to detect hematopoietic contribution to lymphatic endothelium in the intestine, the site of greatest vascular mixing in mice lacking PDPN, CLEC-2, or SLP-76. (A-D) Confocal microscopy of sections of Vav-Cre;R26RYFP neonates immunostained for the lymphatic endothelial surface protein LYVE-1 (red) and YFP (green) fails to show YFP+LYVE-1+ endothelial cells. Arrows indicate LYVE-1+ endothelial cells. Arrowheads indicate YFP+ hematopoietic cells that are frequently adjacent to but not incorporated within LYVE-1+ endothelium. (E-F) Analysis of Tie2-Cre;R26RYFP neonates shows numerous true LYVE-1+YFP+ endothelial cells (arrows).
Blood cells do not contribute to lymphatic endothelium at the site of vascular mixing in mice lacking PDPN, CLEC-2, or SLP-76. Vav-Cre–activated YFP expression was used to detect hematopoietic contribution to lymphatic endothelium in the intestine, the site of greatest vascular mixing in mice lacking PDPN, CLEC-2, or SLP-76. (A-D) Confocal microscopy of sections of Vav-Cre;R26RYFP neonates immunostained for the lymphatic endothelial surface protein LYVE-1 (red) and YFP (green) fails to show YFP+LYVE-1+ endothelial cells. Arrows indicate LYVE-1+ endothelial cells. Arrowheads indicate YFP+ hematopoietic cells that are frequently adjacent to but not incorporated within LYVE-1+ endothelium. (E-F) Analysis of Tie2-Cre;R26RYFP neonates shows numerous true LYVE-1+YFP+ endothelial cells (arrows).
Discussion
In the present study we have used rigorous genetic approaches to define a role for CLEC-2 on platelets in regulating lymphatic vascular development. These studies identify the cellular and molecular pathways underlying the lymphatic vascular defects in mice lacking SYK, SLP-76, and PDPN, and they establish CLEC-2 as a novel platelet receptor that functions during vascular development. The demonstration of a role for platelets in regulating vascular development provides an explanation for why these cells appear as early as E10.5 in mouse embryonic development30 even though prior studies have not identified a necessary role for platelet-mediated hemostasis in utero.31
Mammalian platelets are highly specialized, anuclear cells required for hemostasis after traumatic vessel injury. Although platelets participate in inflammatory responses that affect endothelial and vascular function32 and numerous studies have proposed a role for platelets in regulating angiogenesis,33 a platelet-specific molecular mechanism for directing endothelial and vessel growth has not been identified. Our studies identify a molecular pathway by which platelets directly regulate lymphatic vascular development. The CLEC-2 receptor is one of a family of receptors with single YxxL motifs that are known to couple to SYK and SLP-76.23,25 The discovery of platelet CLEC-2 as a PDPN receptor raised the possibility that platelets might interact with PDPN-expressing cell types such as LECs and therefore might be the cell type in which SYK and SLP-76 signaling is required for normal lymphatic vascular development.11-13 However, functional genetic studies argued strongly against such a mechanism. NF-E2–deficient mice lacking almost all platelets do not exhibit the lymphatic vascular defects observed in mice lacking SYK or SLP-76,14 and vascular development is normal in the large number of genetically modified mice that lack receptors such as the aIIbb3 integrin that are required for hemostatic platelet responses.34 The findings that loss of the platelet-specific CLEC-2 receptor and platelet-specific deletion of Slp-76 confer lymphatic vascular defects provide unambiguous proof of this novel and unexpected role for platelets in vascular development and identify a platelet receptor with a developmental role.
If platelets are required to regulate lymphatic vascular development, why do NF-E2–deficient animals lacking platelets develop normally? The resolution of these discrepant genetic observations most probably lies in the observations that (1) NF-E2–deficient mice do generate low numbers of abnormal platelets with functional activation responses,35 and (2) that animals in which Slp-76 has been conditionally deleted do not develop vascular phenotypes until virtually all SLP-76 function is lost (supplemental Figure 2). These findings suggest that a very low threshold of platelet function is sufficient for this vascular function.
A key question raised by our studies is how platelet CLEC-2 receptors regulate lymphatic vascular development and maintain separation of the blood and lymphatic vascular networks. Although the answer to this question is not yet clear, several important points are evident from our genetic studies and known platelet biology. First, the requirements for SYK and SLP-76 indicate that platelets must be activated by PDPN binding to CLEC-2 receptors to perform this vascular regulatory function. Second, platelets cannot exit the blood vascular space without encountering strong activating stimuli such as collagen or thrombin generated by coagulation proteases. Thus, for naive platelet CLEC-2 receptors to be activated by LEC-bound PDPN proteins they must encounter LECs in an intact blood vessel. The detection of platelet aggregates on the surface of LECs in the E11.5 cardinal vein is consistent with such a mechanism and suggests that platelets might control blood-lymphatic connections simply by “clotting off” budding lymphatics. However, this mechanism does not explain the development of a more extensive vascular phenotype in the intestine of older deficient embryos, a site where LECs arise by sprouting from preexisting lymphatics that are not in contact with blood.10 This model also fails to explain how platelets lacking essential aggregation machinery such as the aIIbb3 integrin could successfully perform this function. An alternative mechanism is one in which migrating LECs encounter and penetrate blood vessels where they become decorated by activated platelets that inhibit further growth, perhaps because of the effect of locally released platelet granule contents such as thrombospondin and PF4 that are antiangiogenic.36,37 Because our in vitro studies do not show a dramatic effect of platelet activation on LEC function and we have not been able to identify platelet-covered LECs in the vessels of the neonatal intestine, such a mechanism is entirely speculative at this time. Future studies addressing the role of distinct platelet activation end points such as degranulation will be required to understand how this pathway instructs lymphatic development.
A final question is whether platelet CLEC-2 receptors have evolved exclusively to regulate lymphatic vascular development and growth. A recent study of mice in which CLEC-2 receptors were pharmacologically depleted by anti–CLEC-2 antibodies has suggested a role for CLEC-2 in thrombosis that appears independent of any endothelial interaction.38 Thus, CLEC-2 receptors may recognize ligand(s) other than PDPN that is exposed during platelet responses to known hemostatic stimuli such as collagen. In addition, PDPN is expressed on numerous cell types other than LECs to which bloodborne platelets would not normally be exposed, including pulmonary epithelial cells,39 mesothelial cells,40 glomerular podocytes,39,41 and numerous types of tumor cells.42 It is therefore possible that PDPN–CLEC-2 interaction may mediate a novel mechanism for extravascular platelet activation that functions during hemostasis after trauma. The ability of PDPN to activate platelets through CLEC-2 receptors has also been proposed as a mechanism by which tumor cells may activate platelets to regulate tumor growth and metastasis in vivo.13,43,44 Future studies addressing these nondevelopmental roles for CLEC-2 and PDPN are likely to yield new insights into platelet biology.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Nancy Speck, Celeste Simon, Steve DiNardo, Mitch Weiss, Doug Epstein, Benjamin Kleaveland, and other members of the Kahn laboratory for valuable insights.
This work was supported by National Institutes of Health grant HL072798 (M.L.K.), by American Heart Association grant 0715222U (C.C.B.), and by the Department of Medicine at the University of Pennsylvania.
National Institutes of Health
Authorship
Contribution: C.C.B., A.A.S., P.R.H., A.W.F., J.S.M., G.A.K., and M.L.K. designed and performed the experiments shown and wrote the paper; P.M., Z.Z., M.C., C.-Y.C., B.X., M.-M.L., D.Z., E.S., M.T.S., and D.J.M. helped perform the experiments; and M.S., R.S., J.S.M., and T.G. contributed critical reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mark L. Kahn, Department of Medicine and Cardiovascular Institute, University of Pennsylvania, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: markkahn@mail.med.upenn.edu.