Cancer cells frequently overexpress tissue factor (TF) and become procoagulant. This conversion may be driven by genetic transformation, including through the expression of the oncogenic epidermal growth factor receptor (EGFR) and its mutant, EGFRvIII, present in glioblastoma multiforme (GBM). Here we show that the EGFRvIII-dependent GBM cell transformation is associated with the onset of the simultaneous overexpression of TF, protease-activated receptors 1 and 2 (PAR1 and PAR2), and ectopic synthesis of factor VII (FVII). Efficient generation of factor Xa by these cells still requires exogenous FVIIa. However, as a result of EGFRvIII-dependent transformation, GBM cells become hypersensitive to TF/PAR-mediated signaling and produce ample angiogenic factors (vascular endothelial growth factor and interleukin-8) on exposure to FVIIa and PAR1- or PAR2-activating peptides. Thus, oncogenes may cause complex changes in the ability of GBM cancer cells to interact with the coagulation system, thereby exacerbating its influence on angiogenesis and disease progression.
Introduction
Tissue factor (TF) emerges as a common denominator of multiple processes associated with cancer progression and metastasis.1,–3 Interestingly, TF up-regulation on the surface of cancer cells and the release of microvesicular TF into the blood4,,–7 are both linked to oncogenic transformation.3,5 Notably, oncogenic epidermal growth factor receptor (EGFR) and the truncated, ligand-independent EGFRvIII mutant8 both up-regulate TF in various cancer settings, including glioblastoma multiforme (GBM),7 the most lethal type of primary brain tumor. GBM is associated with florid angiogenesis, thrombotic complications,9 and up-regulation of TF,10 to which both microenvironmental and genetic influences were found to contribute.7,11,12
TF is a 47-kDa cell-associated transmembrane protein that acts as the high-affinity receptor for factor VIIa (FVIIa)13 and a trigger of both coagulation14 and intracellular signaling.2,15 The latter aspect is mediated, at least in part, by the impact of the TF/VIIa complex on the G protein–coupled protease-activated receptors (PARs), either directly (PAR2) or via FXa (PAR1 and PAR2), or thrombin (PAR1).2
The significance of these events in cancer is underscored by the link between TF/PAR signaling and tumor angiogenesis,16,17 invasiveness,18 and progression,19 all of which are also a function of oncogenic transformation.3 It is therefore of interest to ask whether oncogenic and TF/PAR signaling pathways converge in some fashion in cancer cells and what the consequences are. Here we show that in GBM cells the EGFRvIII oncogene simultaneously up-regulates a cluster of functionally related elements of the TF/PAR pathway, including TF, PAR1, PAR2, and FVII, and renders cancer cells both procoagulant and hypersensitive to TF signaling.
Methods
Cells and treatments
Detailed description of cells, conditions, assays, and reagents is provided as supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Cells were maintained in Dulbecco modified Eagle medium with 10% serum.7 Treatments included 10nM of FVIIa (Enzyme Research Laboratories) and agonists of PAR1 (PAR1-AP: TFLLR-NH2) or PAR2 (PAR2-AP: SLIGKV-NH2), both from Bachem Biosciences.
Experimental procedures
TF procoagulant activity assay.
Cells were treated as indicated, followed by exposure to 5nM FVIIa, 150nM FX, and 5mM CaCl2 in Tris-buffered saline.20 The chromogenic reaction was triggered by addition of 2mM S-2765 (Chromogenix), stopped with 20 μL of 50% acetic acid, read at 405 nm, and the results expressed as arbitrary units normalized to the protein content.
Reverse-transcribed polymerase chain reaction analysis of gene expression.
Total RNA was extracted using TRIzol (Invitrogen), transcribed into cDNA, and amplified using a OneStep RT-PCR Kit (QIAGEN). Aliquots of 0.5 μg were used to carry out reverse transcription at 50°C for 30 minutes, followed by amplification.
Western blotting for protein detection.
Cell lysates were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis.5,7 On transfer, the polyvinylidene difluoride membranes were probed with primary antibodies, including: anti–human TF (1:1000; American Diagnostica 4503), β-actin (1:10 000; Invitrogen), PAR1 (1:500; R&D Systems), and PAR2 SAM11 (1:200; Santa Cruz Biotechnology). The signal was visualized using appropriate horseradish peroxidase-conjugated secondary antibodies and chemiluminescence (GE Healthcare).
Fluorescence-activated cell sorting for immunodetection of the TF antigen.
Single-cell suspensions were washed with phosphate-buffered saline containing 1% bovine serum albumin and 0.1% sodium azide before staining with mouse anti–human TF antibody (1:200; American Diagnostica 4509) and secondary goat anti–mouse antibody (1:200; Alexa Fluor 488 nm).
Enzyme-linked immunosorbent assay for detection of angiogenic factors.
Cell culture supernatants were processed and assayed as recommended by suppliers of the human vascular endothelial growth factor (VEGF) and interleukin-8 (IL-8) immunoassays (R&D Systems and BD-OptEIA).7 All experiments were independently reproduced at least 3 times. Data (mean ± SD) were calculated and Student t test used, as indicated (supplemental data).
Results and discussion
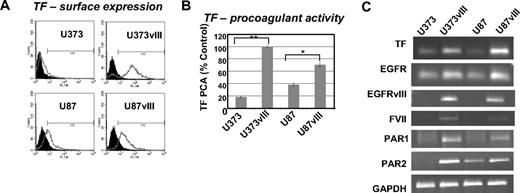
To extend our prior studies,7 we examined the expression and activity of TF in a panel of GBM cell lines expressing the EGFRvIII oncogene. Although the parental U373 and U87 cells express low to moderate amounts of TF antigen, mRNA, and procoagulant activity, these levels increase dramatically on the enforced expression of EGFRvIII (Figure 1A-C). This correlates with the onset of a highly tumorigenic and angiogenic phenotype, especially in the case of U373 cells, which in the absence of EGFRvIII are nontumorigenic in mice21 (and data not shown).
Up-regulation of TF, FVII, PAR1, and PAR2 in glioma cell lines expressing EGFRvIII oncogene. (A) TF antigen expression on the surface of 2 different GBM cell lines (U373 and U87) and their sublines transfected with EGFRvIII (fluorescence-activated cell sorting). (B) TF procoagulant activity assay (TF PCA) indicating the increased ability of EGFRvIII-transfected GBM cells to generate factor Xa (calibrated to the standard rabbit brain thromboplastin). Control (%) indicates the value obtained for U373vIII cells, which was the most procoagulant cell line in this panel (U373 and U373vIII, **P < .005; and U87 and U87vIII, *P < .05; N = 4). (C) The impact of EGFRvIII on the simultaneous expression of TF, PAR1, PAR2, and FVII mRNA in 2 different GBM cell lines U373 and U87 (reverse-transcribed polymerase chain reaction; “Experimental procedures” and supplemental data).
Up-regulation of TF, FVII, PAR1, and PAR2 in glioma cell lines expressing EGFRvIII oncogene. (A) TF antigen expression on the surface of 2 different GBM cell lines (U373 and U87) and their sublines transfected with EGFRvIII (fluorescence-activated cell sorting). (B) TF procoagulant activity assay (TF PCA) indicating the increased ability of EGFRvIII-transfected GBM cells to generate factor Xa (calibrated to the standard rabbit brain thromboplastin). Control (%) indicates the value obtained for U373vIII cells, which was the most procoagulant cell line in this panel (U373 and U373vIII, **P < .005; and U87 and U87vIII, *P < .05; N = 4). (C) The impact of EGFRvIII on the simultaneous expression of TF, PAR1, PAR2, and FVII mRNA in 2 different GBM cell lines U373 and U87 (reverse-transcribed polymerase chain reaction; “Experimental procedures” and supplemental data).
Interestingly, EGFRvIII-expressing GBM cells up-regulate not only TF, but also PAR1, PAR2, and (ectopically) FVII transcripts (Figure 1C). Thus, indolent U373 cells express TF and PAR1 at low levels and no detectable PAR2 transcript, whereas more aggressive U87 cells are positive for TF and PAR2. However, in both cell lines, the EGFRvIII-driven transformation causes a marked increase in the expression of TF, PAR1, PAR2, and FVII (Figure 1C).
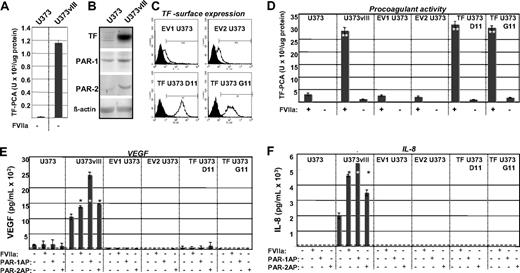
Cancer cells were previously found to ectopically produce FVII,22 and our study provides one possible (oncogenic) mechanism of such a conversion. The coexpression of TF and FVII in GBM cells also raises a possibility that such cells may be able to endogenously generate FXa (from FX), provided that FVII somehow becomes converted to FVIIa.22 We tested this by examining U373 (TF-low/FVII-negative) and U373vIII (TF-high/FVII-positive) cells in TF procoagulant activity (PCA) assays, in the absence of exogenous FVIIa. Although U373vIII cells generated higher TF PCA values than their U373 counterparts, the ability of both cell lines to generate FXa under these conditions was exceedingly low (Figure 2A vs D, where exogenous FVIIa was added). Hence, the efficient activation of coagulation by these cells depends on the availability of the exogenous FVIIa.
Functional impact of the EGFRvIII oncogene on the ability of the TF pathway to regulate procoagulant and angiogenic phenotype of glioblastoma cells. (A) Differential, but low-level FXa-generating activity exhibited by U373 and U373vIII cells in the absence of exogenous FVIIa (compare with panel D). In this setting, U373vIII cells exhibit greater ability to activate FX, possibly in relation to their production of endogenous FVII. (B) Simultaneous up-regulation of TF, PAR1, and PAR2 proteins, as a function of EGFRvIII-dependent cellular transformation of U373 glioblastoma cells (Western blot; β-actin is used as loading control). These changes correspond to those observed at the mRNA level, as shown in Figure 1C. (C) Fluorescence-activated cell sorter analysis documenting the results of the enforced expression of the exogenous human TF in parental U373 cells (human TF sequence was introduced using pcDNA3.1hygro vector). Viable TF U373D11 and TF U373G11 cells and cells transfected with empty vector (EV1 U373 and EV2 U373) were stained for surface TF antigen. Two of several similar clones in each category are shown. (D) Manifestation of a robust TF-dependent procoagulant activity (TF-PCA; generation of FXa) by EGFRvIII-transformed U373vIII cells expressing endogenous TF. Their EGFRvIII nonexpressing U373 counterparts transfected with TF (TF U373D11 and TF U373G11) exhibit comparable levels of TF PCA. In contrast, parental U373 cell line and several control transfectants (EV1 U373 and EV2 U373) display a negligible procoagulant activity. Unlike in the case of data presented in panel A, this assay was conducted in the presence of the exogenous recombinant FVIIa. **P < .005. N = 4. (E) VEGF release on addition of FVIIa or PAR agonistic peptides (PAR1-AP and PAR2-AP) to EGFRvIII, TF, and control U373-derived cells. VEGF in conditioned medium was detected by enzyme-linked immunosorbent assay. Dashed line represents the limit of the assay sensitivity, as defined by the supplier; dark bars, cells treated as indicated by “+”. Only in EGFRvIII-transformed U373vIII cells, but not in their parental (U373),TF transfected (TF U373D11 and TF U373G11), or mock-transfected (EV1 U373 and EV2 U373) counterparts, an appreciable increase in VEGF secretion was detected (24-hour stimulation with FVIIa 10nM or PAR1/2APs 100μM each). *P < .05. **P < .005. N = 2. (F) IL-8 up-regulation in glioma cell lines on activation of the TF/PAR pathway (test carried out using human IL-8 enzyme-linked immunosorbent assay; designations as in panel E). Appreciable increase in IL-8 production was observed on exposure to FVIIa, PAR1-AP, or PAR2-AP only in the case of EGFRvIII-transformed cells, but not in cells expressing TF in the absence of EGFRvIII (TF U373D11 and TF U373G11) or in cells with low levels of TF and PARs (U373, EV1 U373, and EV2 U373). All experimental conditions are as in panel E. Responses to treatments were compared with the corresponding untreated cells. *P < .05. **P < .005. N = 6 (“Experimental procedures” and supplemental data).
Functional impact of the EGFRvIII oncogene on the ability of the TF pathway to regulate procoagulant and angiogenic phenotype of glioblastoma cells. (A) Differential, but low-level FXa-generating activity exhibited by U373 and U373vIII cells in the absence of exogenous FVIIa (compare with panel D). In this setting, U373vIII cells exhibit greater ability to activate FX, possibly in relation to their production of endogenous FVII. (B) Simultaneous up-regulation of TF, PAR1, and PAR2 proteins, as a function of EGFRvIII-dependent cellular transformation of U373 glioblastoma cells (Western blot; β-actin is used as loading control). These changes correspond to those observed at the mRNA level, as shown in Figure 1C. (C) Fluorescence-activated cell sorter analysis documenting the results of the enforced expression of the exogenous human TF in parental U373 cells (human TF sequence was introduced using pcDNA3.1hygro vector). Viable TF U373D11 and TF U373G11 cells and cells transfected with empty vector (EV1 U373 and EV2 U373) were stained for surface TF antigen. Two of several similar clones in each category are shown. (D) Manifestation of a robust TF-dependent procoagulant activity (TF-PCA; generation of FXa) by EGFRvIII-transformed U373vIII cells expressing endogenous TF. Their EGFRvIII nonexpressing U373 counterparts transfected with TF (TF U373D11 and TF U373G11) exhibit comparable levels of TF PCA. In contrast, parental U373 cell line and several control transfectants (EV1 U373 and EV2 U373) display a negligible procoagulant activity. Unlike in the case of data presented in panel A, this assay was conducted in the presence of the exogenous recombinant FVIIa. **P < .005. N = 4. (E) VEGF release on addition of FVIIa or PAR agonistic peptides (PAR1-AP and PAR2-AP) to EGFRvIII, TF, and control U373-derived cells. VEGF in conditioned medium was detected by enzyme-linked immunosorbent assay. Dashed line represents the limit of the assay sensitivity, as defined by the supplier; dark bars, cells treated as indicated by “+”. Only in EGFRvIII-transformed U373vIII cells, but not in their parental (U373),TF transfected (TF U373D11 and TF U373G11), or mock-transfected (EV1 U373 and EV2 U373) counterparts, an appreciable increase in VEGF secretion was detected (24-hour stimulation with FVIIa 10nM or PAR1/2APs 100μM each). *P < .05. **P < .005. N = 2. (F) IL-8 up-regulation in glioma cell lines on activation of the TF/PAR pathway (test carried out using human IL-8 enzyme-linked immunosorbent assay; designations as in panel E). Appreciable increase in IL-8 production was observed on exposure to FVIIa, PAR1-AP, or PAR2-AP only in the case of EGFRvIII-transformed cells, but not in cells expressing TF in the absence of EGFRvIII (TF U373D11 and TF U373G11) or in cells with low levels of TF and PARs (U373, EV1 U373, and EV2 U373). All experimental conditions are as in panel E. Responses to treatments were compared with the corresponding untreated cells. *P < .05. **P < .005. N = 6 (“Experimental procedures” and supplemental data).
To further examine the consequences of EGFRvIII-dependent TF up-regulation, we verified it at the protein level (Figure 2B) and expressed TF exogenously in U373 cells (Figure 2C). Of several resulting clones, some (TF U373D11 and TF U373G11) produced TF at levels similar to those of U373vIII cells, from which they did not differ in terms of their procoagulant activity in the presence of recombinant FVIIa (Figure 2D).
Because EGFRvIII triggered not only the up-regulation of TF, but also of PAR1 and PAR2 (Figure 2B), we compared the signaling capacity of the TF/PAR pathway2 between parental, EGFRvIII-transformed, and TF-transfected, U373-derived cell lines. The cells were stimulated with FVIIa, PAR1, or PAR2 activating peptides (PAR1/2APs), and interrogated for production of angiogenic factors, such as IL-817 and VEGF.1,7 Indeed, very low/undetectable levels of IL-8 and VEGF were found in culture supernatants of U373 cells, and their control (EV1/2 U373), or TF-expressing transfectants (TF U373D11/G11), and this did not change markedly on stimulation. In contrast, U373vIII cells exhibited both constitutively high levels and a dramatic up-regulation of angiogenic factor production on stimulation with FVIIa, PAR1-AP, and PAR2-AP, although the responses differed between IL-8 and VEGF (Figure 2E-F).
In conclusion, our observations suggest that EGFRvIII and possibly other oncogenes may sensitize cancer cells to coagulation factor signaling, at least in part, because of simultaneous up-regulation of TF and PARs. This may precipitate proangiogenic and other biologic consequences related to the TF/PAR pathway. Targeting this pathway may therefore have therapeutic value in GBM, and the levels of TF, FVII, and PARs could be explored as biomarkers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Canadian Cancer Society (J.R.), Canadian Institutes of Health Research (J.R.), and infrastructure funds from Fonds de la recherche en santé du Quebec (J.R.). J.R. is the Jack Cole Chair in Pediatric Oncology.
Authorship
Contribution: N.M. and D.G. performed experiments; and N.M., D.G., and J.R. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Janusz Rak, Montreal Children's Hospital Research Institute, 4060 Ste Catherine West, Montreal, QC, H3Z 3Z2, Canada; e-mail: janusz.rak@mcgill.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal