Tissue factor (TF) is the primary activator of the coagulation cascade. During endotoxemia, TF expression leads to disseminated intravascular coagulation. However, the relative contribution of TF expression by different cell types to the activation of coagulation has not been defined. In this study, we investigated the effect of either a selective inhibition of TF expression or cell type-specific deletion of the TF gene (F3) on activation of coagulation in a mouse model of endotoxemia. We found that inhibition of TF on either hematopoietic or nonhematopoietic cells reduced plasma thrombin-antithrombin (TAT) levels 8 hours after administration of bacterial lipopolysaccharide (LPS). In addition, plasma TAT levels were significantly reduced in endotoxemic mice lacking the TF gene in either myeloid cells (TFflox/flox,LysMCre mice) or in both endothelial cells (ECs) and hematopoietic cells (TFflox/flox,Tie-2Cre mice). However, deletion of the TF gene in ECs alone had no effect on LPS-induced plasma TAT levels. Similar results were observed in mice lacking TF in vascular smooth muscle cells. Finally, we found that mouse platelets do not express TF pre-mRNA or mRNA. Our data demonstrate that in a mouse model of endotoxemia activation of the coagulation cascade is initiated by TF expressed by myeloid cells and an unidentified nonhematopoietic cell type(s).
Introduction
During endotoxemia and sepsis, activation of the coagulation cascade leads to disseminated intravascular coagulation.1 Pharmacologic inhibition of tissue factor (TF) or a genetic reduction of TF expression has been shown to reduce coagulation and mortality in animal models of endotoxemia and sepsis.2,–4 These results indicate that TF is the primary activator of coagulation in these models. However, the relative contribution of TF expression by different cell types to the activation of coagulation cascade is unknown.
TF is constitutively expressed by cells within and surrounding the blood vessel wall, such pericytes and adventitial fibroblasts.5,6 It has been proposed that TF expressed by these cell types forms a hemostatic envelope that limits bleeding after vessel injury.5 Low levels of TF have also been observed in vascular smooth muscle cells (VSMCs).7 In addition, in vitro studies demonstrated that lipopolysaccharide (LPS) stimulation of VSMCs increases TF expression.8
In vitro studies demonstrated that activated endothelial cells (ECs) express TF.9,10 However, only a limited number of studies were able to demonstrate TF expression by ECs in vivo. One study found colocalization of TF and the EC marker CD31 in the kidney of endotoxemic mice.11 Another study demonstrated the presence of TF protein only on ECs within the splenic microvasculature of septic baboons.12 More recently, TF protein was observed on ECs at aortic branch points of septic baboons.13 However, the TF associated with ECs was restricted to granular structures some of which were also positive for the leukocyte marker P-selectin glycoprotein ligand-1 (PSGL-1), suggesting that leukocyte-derived microparticles (MPs) may deliver TF to activated ECs in vivo.13
Early studies indicated that circulating blood cells do not normally express TF in healthy humans.5 However, a recent study found very low levels of TF antigen in a small subset of CD14-positive monocytes.14 LPS stimulation of monocytes and monocytic cells induces TF expression in vitro and in vivo.5,14,,–17 Furthermore, we and others have shown that TF expression by hematopoietic cells contributes to activation of coagulation in endotoxemic mice.4,18 Other studies have reported TF expression by neutrophils and eosinophils.19,20 However, more recent studies found that neither neutrophils nor eosinophils express TF but can acquire TF by binding monocyte-derived MPs.21,22
TF expression by platelets is controversial. In 2001, Engelman and colleagues reported that activation of platelets results in translocation of TF from α-granules to the cell surface.23 Other studies found that quiescent and stimulated platelets express variable levels of TF mRNA and protein.24,25 Recently, we demonstrated that human platelets contained TF pre-mRNA that is spliced into TF mRNA and translated into protein.26 In contrast, other groups have failed to detect any TF protein or activity on resting platelets or calcium ionophore-stimulated platelets.27,–29 Nevertheless, platelets may acquire TF by binding monocyte-derived MPs. For instance, it has been shown that monocyte-derived MPs can bind and fuse with activated platelets via an interaction between CD15 or PSGL-1 on MPs with P-selectin on platelets.29,30
In this study, we investigated the effect of either selective inhibition of TF or cell type-specific deletion of the TF gene on the activation of coagulation in a mouse model of endotoxemia. We demonstrated that both hematopoietic and nonhematopoietic cell TF contributes to activation of coagulation in this model. Moreover, deletion of the TF gene in myeloid but not ECs is associated with a significant reduction in levels of plasma thrombin-antithrombin (TAT). Finally, deletion of the TF gene in either megakaryocytes/platelets or VSMCs did not reduce coagulation in endotoxemic mice. These results indicate that TF expression by myeloid cells and an unidentified nonhematopoietic cell type(s) leads to activation of coagulation during endotoxemia.
Methods
Mice
Generation of human TF (HTF) mice and TFflox/flox mice has been described.31 Mice expressing Cre recombinase under control of lysozyme M (LysM), tyrosine kinase with immunoglobulin-like and EGF-like domains 2 (Tie-2), platelet factor 4 (PF4) and smooth muscle 22α (SM22α) promoters have been described.32,,–35 All mice used for the study were backcrossed to the C57BL/6 genetic background for 6 generations. Littermate controls were used for all mouse studies. All studies were approved by the Animal Care and Use Committee of the different institutions and comply with National Institutes of Health guidelines.
Mouse model of endotoxemia
The mouse model of endotoxemia used in these studies consisted of a single intraperitoneal injection of 5 mg/kg Escherichia coli LPS, serotype 0111:B4 (Sigma-Aldrich). For analysis of plasma TAT levels, blood was collected from the inferior vena cava into sodium citrate (final concentration, 0.38%) 8 hours after LPS injection (R.P. and N.M., unpublished data, 2009, showed that plasma TAT levels peak at this time point). Blood was centrifuged (4000g for 10 minutes at 4°C) to obtain plasma, which was stored at −80°C until analysis. For experiments using cH36 antibody,36 mice were injected intraperitoneally with cH36 (5 mg/kg) or control human IgG (5 mg/kg) 30 minutes before LPS injection. We determined that this dose of cH36 did not cause bleeding in endotoxemic HTF mice.
Bone marrow transplantation
Recipient mice (8 weeks old) were irradiated with 13 Gy (2 doses of 650 rad 4 hours apart) using a cesium 137 irradiator (Gammacell 40; Atomic Energy of Canada) to ablate endogenous bone marrow–derived cells. Irradiated mice were injected via the retro-orbital sinus with 2 × 106 bone marrow cells isolated from donor animals as described.4 Mice were allowed to recover for 6 weeks. Blood cells were used for genotyping to demonstrate reconstitution with the donor bone marrow. Polymerase chain reaction (PCR) analysis of genomic DNA was performed using the following primers: human TF forward primer: 5′-ATACATTCGAGTGCTCTGAAGTGCAT-3′ and reverse primer: 5′-CATGGAGTCCAAAGATGAATGAATC-3′ (663 base pairs); mouse TF forward primer: 5′-ATGAGGAGCTGTGTTAAAGGGTCGCAGAA-3′ and reverse primer: 5′-TGCAGTAAATGCACGTGTCTGCCAT-3′ (559 base pairs); Cre recombinase forward primer: 5′-GACGGAAATCCATCGCTCGACCAG-3′ and reverse primer: 5′-GACATGTTCAGGGATCGCCAGGCG-3′ (570 base pairs).
Isolation and culture of mouse peritoneal macrophages
Mouse peritoneal macrophages (PMs) were isolated by peritoneal lavage 3 days after intraperitoneal injection with 2 mL of a 3% thioglycollate solution. Cells were cultured in RPMI 1640 supplemented with 2mM l-glutamine, 10mM HEPES, 50 U/mL penicillin, 50 μg/mL streptomycin, and 10% heat-inactivated fetal calf serum at 37°C in 5% CO2. After 2 hours of incubation in 6 well plates, nonadherent cells were removed by washing, and the adherent PMs were allowed to recover overnight in fresh medium. To induce TF expression PMs (5 × 106 cells/ well) were stimulated with 1 μg/mL LPS. Cells were collected 5 hours after LPS treatment to analyze TF activity.
Isolation of mouse platelets and leukocytes
Blood from C57BL/6 mice was drawn into acid-citrate-dextrose via cannulation of the carotid artery. Samples were diluted with warm Pipes Saline Glucose (PSG) and centrifuged at 115g for 10 minutes to separate cells and plasma. Platelet-rich plasma was recentrifuged at 500g for an additional 10 minutes in the presence of 300μM prostaglandin E1 (PGE1; Cayman Chemicals). The supernatant was discarded and the platelet-rich pellet was resuspended in warm PSG supplemented with PGE1. Platelets were then incubated for 20 minutes with CD45 and Ter-119 microbeads (Miltenyi Biotec) to remove leukocytes and red blood cells, respectively, by positive selection. The negative fraction, which contained purified platelets (ie, < 1 leukocyte per 10 000 platelets), was centrifuged and the cells were resuspended in M199 culture media.
To obtain peripheral blood leukocytes, the red blood cell/leukocyte rich fraction was exposed to hypotonic lysis buffer to disrupt the red blood cells, centrifuged, and the remaining pellet, which contained leukocytes, was resuspended in M199.
For RNA isolation, platelets, leukocytes and RAW 264.7 mouse macrophages (ATCC) were incubated with or without 100 ng/mL LPS for 1 hour and then lysed with TRIzol (Invitrogen).
PCR analysis of TF mRNA isolated from platelets and leukocytes
RNA was isolated following a standard TRIzol extraction protocol. One μg of RNA was used in each reverse transcription reaction to generate cDNA using oligo dT primers. PCR was performed as follows: 94°C 5 minutes, a 3-step cycle of 94°C 30 seconds, 61°C 30 seconds, 72°C 1 minute (repeated 40 times), and an extra extension time of 72°C 10 minutes. Primer sets for mouse TF were targeted to exon 4 and 5 to detect both unspliced and spliced RNA.26 The mouse TF primer sets were as follows: primer set A, forward 5′-TGGACAGCCAGTAATTCAGCAGT-3′ and reverse 5′-TCCTTCTTCCACATCAATCGAGA-3′ (unspliced 901 base pairs, spliced 223 base pairs); primer set B, forward 5′-ACTTATCGGAAAGGCTCAAGCAC-3′ and reverse 5′-TCCTTCTTCCACATCATCGAGA-3′ (unspliced 759 base pairs, spliced 81 base pairs). The mouse integrin αIIb primers were complimentary to sequences in exon 27 (5′-TGGGGCTGTCCAGCCTCCG-3′) and exon 29 (5′-GCCAAAACTAGCAGGGTCAGC-3′; 232 base pairs).
Real-time PCR analysis of TF mRNA from blood cells
Total RNA was isolated from whole blood using a Mouse RiboPure-Blood RNA Isolation Kit (Applied Biosystems/Ambion). cDNA was synthesized from 1 μg of RNA using a RETROscript Kit (Applied Biosystems/Ambion) on a MyCycler themal cycler (Bio-Rad). Real-time PCR was then performed using SYBR Green RealMasterMix (Eppendorf AG) on a Mastercycler ep realplex machine (Eppendorf AG). Mouse TF mRNA was detected using forward primer spanning exon 4 and 5 (5′-TCAAGCACGGGAAAGAAAAC-3′) and reverse primer located within exon 5 (5′-CTGCTTCCTGGGCTATTTTG-3′), which generated a 137 base pairs product.37 TF expression was normalized to the expression of hypoxanthine-guanine phosphoribosyltransferase (forward, 5′-GTGGTGAAAAGGACCTCTCG-3′ and reverse 5′-TGAAGTACTCATTATAGTCAAGGGGA-3′), and relative expression levels were calculated using the comparative Ct method.
PCR analysis of DNA from blood cells
Genomic DNA from blood cells was purified using a Flexigene DNA Kit (QIAGEN). PCR amplification was performed using a MyCycler themal cycler (Bio-Rad) with primers spanning the mouse TF gene promoter from nucleotide −1097 to −539 which contains the 5′ loxP site.31 These primers generate a fragment of 559 base pairs for wild type (WT) allele and 611 base pairs for the floxed allele: forward, 5′-ATGAGGAGCTGTGTTAAAGGGTCGCAGAA-3′ and reverse, 5′-TGCAGTAAATGCACGTGTCTGCCAT-3′. The mouse interleukin-2 (IL-2) gene was used as a control: forward primer, 5′-CTAGGCCACAGAATTGAAAGATCT-3′ and reverse primer: 5′-GTAGGTGGAAATTCTAGCATCATCC-3′ (324 base pairs).
Detection of TF gene deletion rate in endothelial cells isolated from the lungs of TFflox/flox Tie-2Cre mice
Lungs were aseptically removed from 7-day-old, anesthetized WT C57BL/6, TFflox/flox,or TFflox/flox,Tie-2Cre pups, washed once with phosphate-buffered saline (PBS), and then finely minced using a razor blade. Lung tissue was incubated in 1 mL of prewarmed collagenase solution, containing 0.25 mg of soybean trypsin inhibitor (Calbiochem), 2 mg of bovine serum albumin, 2 mg of collagenase Type I (Worthington Biochemical), and 20mM HEPES in Hanks buffered salt solution. After 1-hour incubation at 37°C, the digestion mixture was passed through a 70-μm cell strainer and washed with PBS. Total cells were then FcBlocked (BD Pharmingen) and stained with PE-labeled rat anti–mouse CD31 (BD Pharmingen) and Alexa 488-labeled rat-anti–mouse CD102 (eBioscience) antibodies. Double-positive (CD31+,CD102+) ECs (∼ 110 000 per animal) were sorted using a MoFlo XDP (Beckman Coulter) cell sorting machine. Cells were then lysed in 30 μL of lysis buffer (10mM Tris-HCl, 50mM KCl, 2.5 mM MgCl2, 0.45% nonident NP-40, 0.45% Tween-20 and 0.2 mg/mL proteinase K, pH 8.3) for 30 minutes at 55°C, followed by 10 minutes incubation at 95°C to deactivate proteinase K activity. Two μL of cell lysate was used amplify the mouse TF gene, mouse IL-2 gene and the cre recombinase.
One-stage clotting assay
Cell pellets (5 × 106 cells) were solubilized at 37°C for 15 minutes using 15mM n-Octyl-β-D-glucopyranoside and incubated with cH36 (50 μg/mL) or control IgG (50 μg/mL) for 10 minutes. The procoagulant activity of cell lysates was measured using a 1-stage clotting assay as described previously38 with a Start4 clotting machine (Diagnostica Stago). Clotting times were converted to procoagulant activity by comparison with a standard curve generated with mouse brain extract. The procoagulant activity of each sample was normalized to total protein concentration determined using a Bio-Rad DC protein assay (Bio-Rad).
Immunohistochemistry
Paraffin sections (4-μm thick) were obtained from the lung of control and LPS-treated (8 hours) mice. Before staining, sections were subjected to antigen retrieval solution (10mM citrate buffer, pH = 6.0) for 25 minutes at 95°C. TF staining was performed using a rat anti–mouse TF monoclonal antibody (28μg/mL; 1H1).7 Sections were subsequently incubated with a biotinylated anti–rat secondary antibody (Vector Laboratories), followed by LSAB2 Strepavidin-HRP (Dako). Slides were developed using Liquid DAB (3,3′-diaminobenzidine) and substrate chromagen system (Dako). Images were captured with the Olympus DP70 digital camera (Olympus America) attached to the Olympus DX51WI microscope using the 40× Olympus UPlanFln aperture that resulted in a final magnification of 400. Images were acquired using DP controller software (Olympus).
Analysis of plasma TAT levels
Plasma TAT levels were determined using an Enzygnost TAT micro enzyme-linked immunosorbent assay (Siemens Healthcare Diagnostics).
Statistical analysis
All statistical analyses were performed using SigmaStat 4 (SPSS). All measurements are represented as means plus or minus SEM. For 2-group comparison of parametric data, a Student t test was performed. Kruskal-Wallis One Way ANOVA on Ranks, with a Dunn post hoc analysis, was performed on comparisons of more than 2 groups. The criterion for significance for all experiments was P less than .05.
Results
Selective inhibition of human TF using an anti–human TF monoclonal antibody
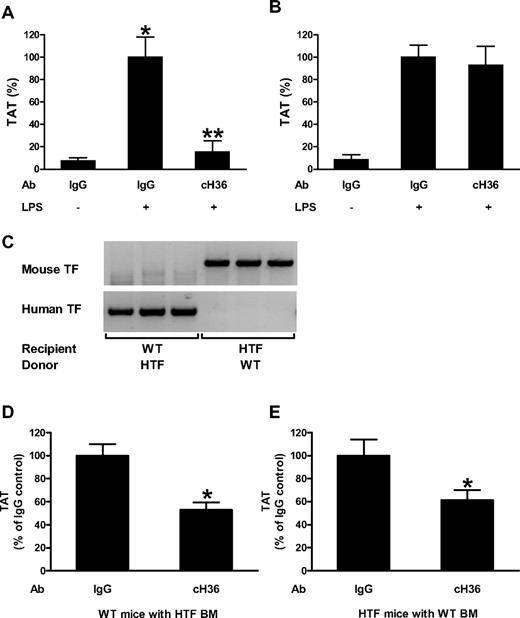
To determine the specificity of an anti–human TF monoclonal antibody (cH36), we analyzed its ability to either inhibit human or mouse TF. cH36 completely inhibited LPS-induced human TF activity of PMs isolated from mice expressing only human TF (HTF mice; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) but did not inhibit mouse TF activity of PMs isolated from WT C57BL/6 mice (supplemental Figure 1B). We also analyzed the ability of cH36 to inhibit the activation of coagulation in endotoxemic HTF and WT mice. Animals were injected intraperitoneally with cH36 or control human IgG 30 minutes before LPS injection. cH36 significantly reduced (86%) plasma TAT levels in endotoxemic HTF compared with mice receiving the IgG controls (Figure 1A). In contrast, cH36 did not reduce TAT levels in endotoxemic WT mice (Figure 1B). These data indicate that cH36 selectively inhibits human TF activity without affecting mouse TF activity.
Contribution of TF expressed by hematopoietic and nonhematopoietic cells to the activation of coagulation. (A) Effect of cH36 (50 μg/mL) or IgG (50 μg/mL) control antibody on LPS-induced plasma TAT levels in HTF mice (mean ± SEM for IgG, IgG/LPS and cH36/LPS groups were 2.6 ± 0.9, 34.2 ± 6.2 and 5.3 ± 3.4 ng/mL, respectively) 8 hours after LPS injection. (B) Effect of cH36 (50 μg/mL) or IgG (50 μg/mL) control antibody on LPS-induced plasma TAT levels in WT mice (mean ± SEM for IgG, IgG/LPS and cH36/LPS groups were 3.3 ± 1.7, 38.9 ± 4.2 and 36.1 ± 6.6 ng/mL, respectively) 8 hours after LPS injection (n = 3 for all treatment conditions). *Statistically significant difference (P < .05) between IgG- and IgG/LPS-treated group. **Statistically significant difference (P < .05) between IgG/LPS- and cH36/LPS-treated group. (C) PCR analysis of DNA isolated from blood cells from mice that underwent bone marrow transplantation to demonstrate reconstitution of the donor mice with recipient bone marrow 6 weeks after the transplantation. (D) Plasma TAT levels were analyzed in WT mice transplanted with bone marrow from HTF mice after treatment with IgG (n = 13; mean ± SEM 55.9 ± 5.7 ng/mL) or cH36 (n = 13; mean ± SEM 29.6 ± 3.6 ng/mL) 8 hours after LPS injection. *P < .05. (E) Plasma TAT levels were analyzed 8 hours after LPS injection in HTF mice transplanted with WT bone marrow after treatment with IgG (n = 12; mean ± SEM 46.6 ± 6.5 ng/mL) or cH36 (n = 13; mean ± SEM 28.5 ± 4.1 ng/mL;). *P < .001.
Contribution of TF expressed by hematopoietic and nonhematopoietic cells to the activation of coagulation. (A) Effect of cH36 (50 μg/mL) or IgG (50 μg/mL) control antibody on LPS-induced plasma TAT levels in HTF mice (mean ± SEM for IgG, IgG/LPS and cH36/LPS groups were 2.6 ± 0.9, 34.2 ± 6.2 and 5.3 ± 3.4 ng/mL, respectively) 8 hours after LPS injection. (B) Effect of cH36 (50 μg/mL) or IgG (50 μg/mL) control antibody on LPS-induced plasma TAT levels in WT mice (mean ± SEM for IgG, IgG/LPS and cH36/LPS groups were 3.3 ± 1.7, 38.9 ± 4.2 and 36.1 ± 6.6 ng/mL, respectively) 8 hours after LPS injection (n = 3 for all treatment conditions). *Statistically significant difference (P < .05) between IgG- and IgG/LPS-treated group. **Statistically significant difference (P < .05) between IgG/LPS- and cH36/LPS-treated group. (C) PCR analysis of DNA isolated from blood cells from mice that underwent bone marrow transplantation to demonstrate reconstitution of the donor mice with recipient bone marrow 6 weeks after the transplantation. (D) Plasma TAT levels were analyzed in WT mice transplanted with bone marrow from HTF mice after treatment with IgG (n = 13; mean ± SEM 55.9 ± 5.7 ng/mL) or cH36 (n = 13; mean ± SEM 29.6 ± 3.6 ng/mL) 8 hours after LPS injection. *P < .05. (E) Plasma TAT levels were analyzed 8 hours after LPS injection in HTF mice transplanted with WT bone marrow after treatment with IgG (n = 12; mean ± SEM 46.6 ± 6.5 ng/mL) or cH36 (n = 13; mean ± SEM 28.5 ± 4.1 ng/mL;). *P < .001.
TF expression by both hematopoietic and nonhematopoietic cells contributes to activation of the coagulation cascade during endotoxemia
We determined the effect of selectively inhibiting either hematopoietic or nonhematopoietic cell TF on the activation of coagulation during endotoxemia. We generated 2 groups of mice: group 1 expressed human TF from hematopoietic cells (WT recipient mice with bone marrow from HTF mice) and group 2 expressed human TF from nonhematopoietic cells (HTF recipient mice with bone marrow from WT mice). PCR analysis of blood cell DNA demonstrated successful reconstitution with donor bone marrows (Figure 1C). Both groups of mice were treated with either cH36 or IgG control antibody via a single intraperitoneal injection 30 minutes before LPS injection. cH36 reduced LPS-induced plasma TAT levels in mice expressing human TF in hematopoietic or nonhematopoietic cells by 47% and 39%, respectively (Figure 1D-E). These data indicate that TF expression by both hematopoietic and nonhematopoietic cells contributes to activation of coagulation in mice 8 hours after LPS administration.
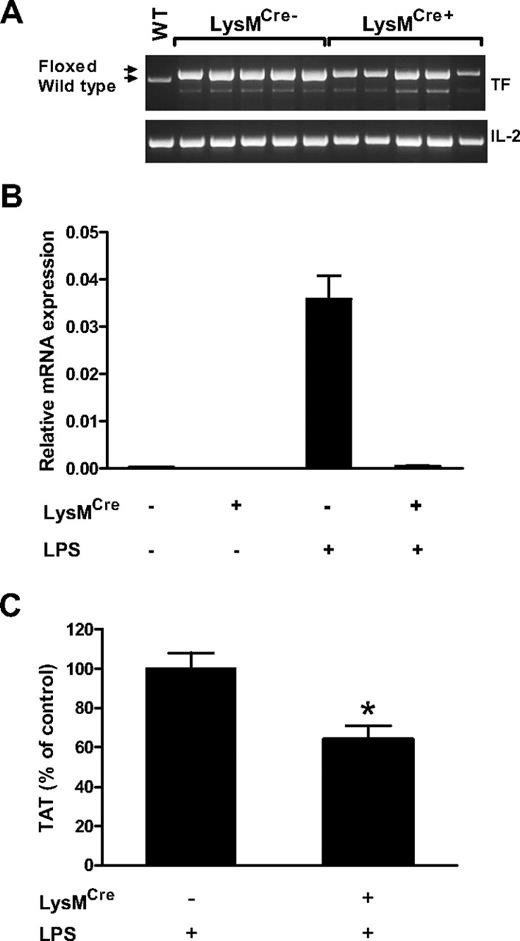
Effect of deletion of the TF gene in myeloid cells on activation of coagulation during endotoxemia
To analyze the TF-positive cell type within the hematopoietic cell population that activated coagulation, we generated mice with the TF gene deleted in myeloid cells (TFflox/flox,LysMCre mice) using mice expressing the Cre recombinase under the control of the myeloid-specific LysM promoter. LPS-induced procoagulant activity of PMs isolated from TFflox/flox,LysMCre mice was reduced by 93% compared with the procoagulant activity of LPS-treated PMs isolated from TFflox/flox mice (7.2 ± 3.4% vs 100 ± 11.4%, respectively; n = 3 for both groups, P < .01). Next, we analyzed the deletion rate of the TF gene in blood cells collected from LPS-treated TFflox/flox,LysMCre and TFflox/flox mice at both the DNA and mRNA levels. PCR analysis of DNA from blood cells demonstrated only a partial deletion (∼ 50%) of the TF gene in blood cells collected from TFflox/flox,LysMCre mice (Figure 2A). This result was expected because we have found that myeloid cells represent approximately 30% to 40% of all white blood cells in mice (data not shown). Importantly, TF mRNA expression was almost completely abolished in blood cells collected from endotoxemic TFflox/flox,LysMCre mice (Figure 2B). These data indicate that levels of TF expression in myeloid cells are dramatically reduced in TFflox/flox,LysMCre mice and that this cell population is the major source of cellular TF in the blood.
Analysis of TFflox/flox, LysMCre mice. (A) PCR analysis of mouse TF DNA isolate from blood cells demonstrates partial deletion of TF gene. Arrows indicate PCR products for wild type and floxed alleles. Note a nonspecific band below the TF band. The mouse interleukin-2 (IL-2) gene was used as a loading control. WT indicates DNA from a WT mouse. (B) Real-time PCR analysis of TF mRNA in blood cells isolated from TFflox/flox and TFflox/flox,LysMCre mice before (n = 2 per group) and after (n = 4 per group) LPS injection (2 hours). (C) Plasma TAT levels were analyzed in TFflox/flox (n = 35; mean ± SEM 19.4 ± 1.6 ng/mL) and TFflox/flox,LysMCre (n = 36; mean ± SEM 12.4 ± 1.4 ng/mL) mice 8 hours after LPS injection. *P < .01.
Analysis of TFflox/flox, LysMCre mice. (A) PCR analysis of mouse TF DNA isolate from blood cells demonstrates partial deletion of TF gene. Arrows indicate PCR products for wild type and floxed alleles. Note a nonspecific band below the TF band. The mouse interleukin-2 (IL-2) gene was used as a loading control. WT indicates DNA from a WT mouse. (B) Real-time PCR analysis of TF mRNA in blood cells isolated from TFflox/flox and TFflox/flox,LysMCre mice before (n = 2 per group) and after (n = 4 per group) LPS injection (2 hours). (C) Plasma TAT levels were analyzed in TFflox/flox (n = 35; mean ± SEM 19.4 ± 1.6 ng/mL) and TFflox/flox,LysMCre (n = 36; mean ± SEM 12.4 ± 1.4 ng/mL) mice 8 hours after LPS injection. *P < .01.
To determine the effect of deletion of the TF gene in myeloid cells on the activation of coagulation cascade in endotoxemic mice, we analyzed levels of plasma TAT in TFflox/flox,LysMCre mice and TFflox/flox control mice 8 hours after LPS injection. We found that plasma TAT levels in TFflox/flox,LysMCre mice were significantly reduced (36%) compared with plasma TAT levels in TFflox/flox littermate controls (Figure 2C). These results indicate that TF expression by myeloid cells contributes to the activation of coagulation during endotoxemia.
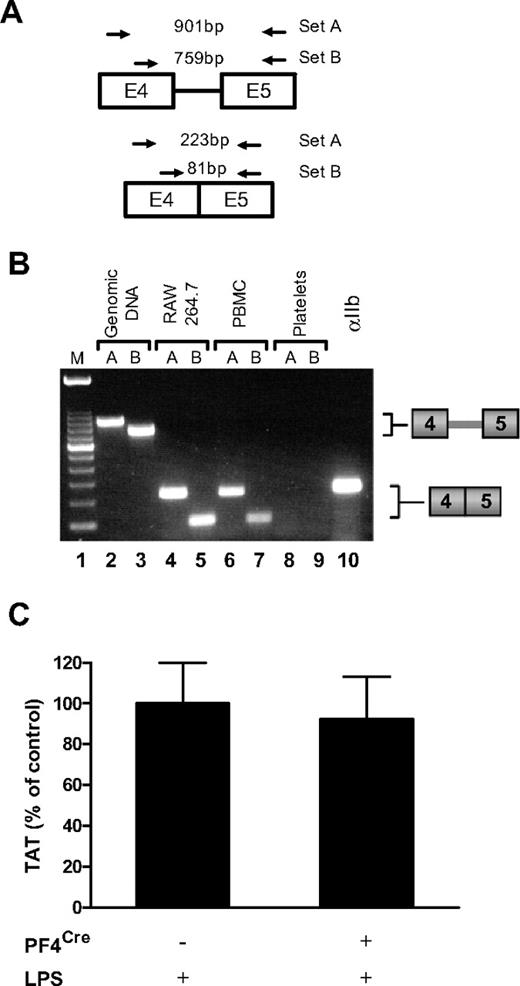
Does platelet TF contribute to activation of coagulation in endotoxemic mice?
Quiescent human platelets express TF precursor-mRNA (pre-mRNA) and, in response to different agonists, splice the pre-mRNA into a mature, translatable message.26 We used a similar strategy to attempt to detect TF pre-mRNA and mRNA in platelets isolated from WT mice (Figure 3A). Figure 3B shows that LPS-stimulated mouse platelets, which possess mRNA for integrin αIIb (lane 10), do not express TF pre-mRNA or spliced mRNA (lanes 8–9). Similarly, TF pre-mRNA was not present in unstimulated mouse platelets (data not shown). In contrast, LPS stimulated mouse RAW 264.7 macrophages (lanes 4–5) and mouse peripheral blood leukocytes (lanes 6–7) expressed spliced TF mRNA (Figure 3B). Mouse genomic DNA was used as a positive control for detection of the intronic message (Figure 3B lanes 2–3). Together these data indicate that isolated mouse platelets do not express TF pre-mRNA or mRNA. This result does not exclude the possibility that megakaryocytes transfer low levels of TF protein to platelets that contributes to activation of coagulation in vivo. Therefore, we crossed TFflox/flox mice with mice expressing Cre recombinase from the megakaryocyte/platelet specific promoter PF4 to generate TFflox/flox,PF4Cre mice. However, we did not observed any differences in plasma TAT levels between LPS-treated TFflox/flox,PF4Cre and TFflox/flox mice (Figure 3C).
Mouse platelets do not express TF. (A) Diagram showing the position of 2 sets of primers used to detect TF pre-mRNA and mRNA. The expected size of PCR products for primer sets A and B are shown. (B) PCR analysis of TF was performed on either mouse genomic DNA or cDNA from LPS stimulated RAW 264.7 macrophages, LPS stimulated mouse peripheral blood leukocytes, or LPS stimulated mouse platelets. Lane 10 contains platelet cDNA amplified with primers for integrin αIIb. This gel is representative of 11 independent experiments. (C) Plasma TAT levels were analyzed in TFflox/flox (n = 13; mean ± SEM 18.9 ± 2.1 ng/mL) and TFflox/flox,PF4Cre (n = 13; mean ± SEM 17.5 ± 2.2 ng/mL) mice 8 hours after LPS injection.
Mouse platelets do not express TF. (A) Diagram showing the position of 2 sets of primers used to detect TF pre-mRNA and mRNA. The expected size of PCR products for primer sets A and B are shown. (B) PCR analysis of TF was performed on either mouse genomic DNA or cDNA from LPS stimulated RAW 264.7 macrophages, LPS stimulated mouse peripheral blood leukocytes, or LPS stimulated mouse platelets. Lane 10 contains platelet cDNA amplified with primers for integrin αIIb. This gel is representative of 11 independent experiments. (C) Plasma TAT levels were analyzed in TFflox/flox (n = 13; mean ± SEM 18.9 ± 2.1 ng/mL) and TFflox/flox,PF4Cre (n = 13; mean ± SEM 17.5 ± 2.2 ng/mL) mice 8 hours after LPS injection.
Effect of deletion of the TF gene in ECs and hematopoietic cells on activation of coagulation during endotoxemia
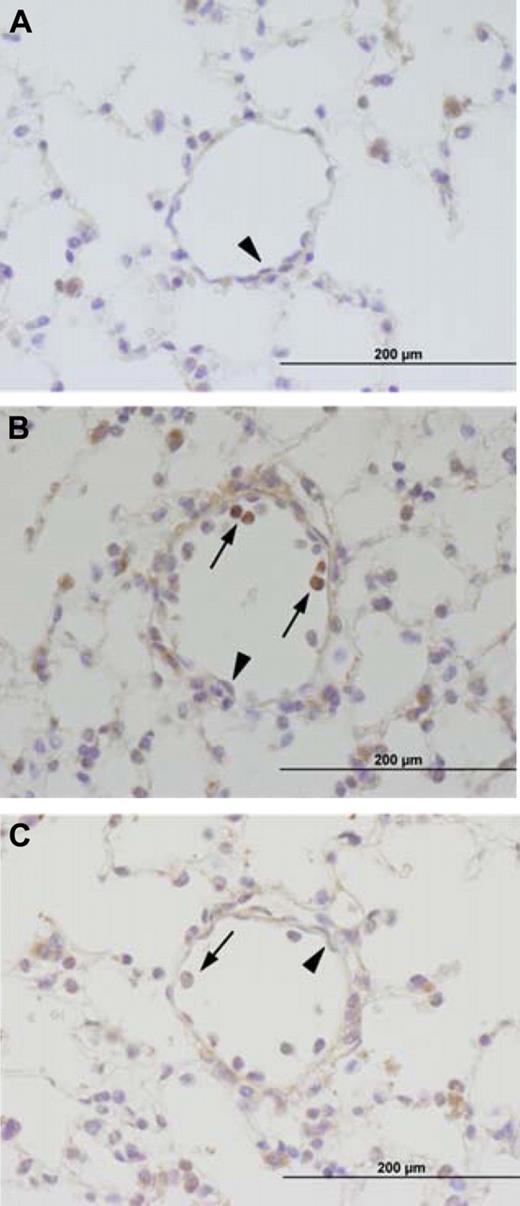
Tie-2-mediated expression of the Cre recombinase leads to deletion of floxed genes in both ECs and hematopoietic cells.33,39,40 To investigate the contribution of TF expression by these cells to the activation of coagulation during endotoxemia, we generated TFflox/flox,Tie-2Cre mice. We found that LPS induced TF expression in a subset of leukocytes but not ECs in the lung (Figure 4). Importantly, TF-positive leukocytes were not observed in endotoxemic TFflox/flox,Tie-2Cre mice (Figure 4). Next, we analyzed the deletion rate of the TF gene in hematopoietic cells. In 1-stage clotting assay, LPS-induced procoagulant activity of PMs isolated from TFflox/flox,Tie-2Cre mice was reduced by 96% compared with TFflox/flox controls (4 ± 2.7% vs 100 ± 27%, respectively; n = 3 for both groups, P < .05). PCR analysis of DNA isolated from the blood cells collected from LPS-treated TFflox/flox,Tie-2Cre mice demonstrated almost complete deletion (∼ 95%) of the TF gene (Figure 5A). In addition, TF mRNA expression was dramatically reduced in blood cells collected from endotoxemic TFflox/flox,Tie-2Cre mice compared with TFflox/flox mice (Figure 5B). These data indicate that LPS-induced TF expression in hematopoietic cells is dramatically reduced in TFflox/flox,Tie-2Cre mice. Finally, we determined the deletion rate of the TF gene in lung ECs. We used flow cytometry to isolate a population of ECs positive for 2 EC markers (CD31 and CD102; supplemental Figure 2). This cells population represents 6% of the total number of cells obtained after digestion of lung tissue (supplemental Figure 2). PCR analysis of the DNA isolated from the double-positive cells demonstrated almost complete deletion of the floxed fragment of TF gene in 3 of the 4 independent cell preparations from TFflox/flox,Tie-2Cre mice (Figure 5C). These data indicate that the TF gene is efficiently deleted in in TFflox/flox,Tie-2Cre mice.
Immunohistochemical analysis of TF expression. TF expression was analyzed in lungs of untreated TFflox/flox mice (A) or TFflox/flox (B) and TFflox/flox,Tie-2Cre (C) mice 8 hours after LPS injection. TF-positive cells are brown. ↑ indicates leukocytes and  indicates ECs. Incubation of serial sections with control rat IgG demonstrated no staining (data not shown).
indicates ECs. Incubation of serial sections with control rat IgG demonstrated no staining (data not shown).
Immunohistochemical analysis of TF expression. TF expression was analyzed in lungs of untreated TFflox/flox mice (A) or TFflox/flox (B) and TFflox/flox,Tie-2Cre (C) mice 8 hours after LPS injection. TF-positive cells are brown. ↑ indicates leukocytes and  indicates ECs. Incubation of serial sections with control rat IgG demonstrated no staining (data not shown).
indicates ECs. Incubation of serial sections with control rat IgG demonstrated no staining (data not shown).
Analysis of TFflox/flox, Tie-2Cre mice. (A) PCR analysis of mouse TF DNA isolated from blood cells demonstrates deletion of the TF gene. Arrows indicate PCR products for wild type and floxed alleles. A nonspecific band is present below the TF band. The mouse IL-2 gene was used as a loading control. WT indicates DNA from a WT mouse. (B) Real-time PCR analysis of TF mRNA in blood cells isolated from TFflox/flox and TFflox/flox,Tie-2Cre mice before (n = 2 per group) and after (n = 4 per group) LPS injection (2 hours). (C) PCR analysis of mouse TF DNA isolated from ECs demonstrates deletion of the TF gene. Arrows indicate PCR products for wild type and floxed alleles. The mouse IL-2 gene was used as a loading control. Presence or absence of Cre recombinase (Cre) in different mouse groups is shown. Low levels of fibroblast contamination likely explain the residual TF-positive band in 1 of the 4 cell preparations from TFflox/flox,Tie-2Cre mice[b]. (D) Plasma TAT levels were analyzed in TFflox/flox (n = 19; mean ± SEM 15.2 ± 1.7 ng/mL) and TFflox/flox,Tie-2Cre (n = 21; mean ± SEM 7.7 ± 0.9 ng/mL) mice 8 hours after LPS injection. *P < .001.
Analysis of TFflox/flox, Tie-2Cre mice. (A) PCR analysis of mouse TF DNA isolated from blood cells demonstrates deletion of the TF gene. Arrows indicate PCR products for wild type and floxed alleles. A nonspecific band is present below the TF band. The mouse IL-2 gene was used as a loading control. WT indicates DNA from a WT mouse. (B) Real-time PCR analysis of TF mRNA in blood cells isolated from TFflox/flox and TFflox/flox,Tie-2Cre mice before (n = 2 per group) and after (n = 4 per group) LPS injection (2 hours). (C) PCR analysis of mouse TF DNA isolated from ECs demonstrates deletion of the TF gene. Arrows indicate PCR products for wild type and floxed alleles. The mouse IL-2 gene was used as a loading control. Presence or absence of Cre recombinase (Cre) in different mouse groups is shown. Low levels of fibroblast contamination likely explain the residual TF-positive band in 1 of the 4 cell preparations from TFflox/flox,Tie-2Cre mice[b]. (D) Plasma TAT levels were analyzed in TFflox/flox (n = 19; mean ± SEM 15.2 ± 1.7 ng/mL) and TFflox/flox,Tie-2Cre (n = 21; mean ± SEM 7.7 ± 0.9 ng/mL) mice 8 hours after LPS injection. *P < .001.
To determine the effect of a combined deficiency of TF in ECs and hematopoietic cells on activation of coagulation, we analyzed plasma TAT levels in TFflox/flox,Tie-2Cre mice and TFflox/flox littermate controls. We found that plasma TAT levels in TFflox/flox,Tie-2Cre mice were significantly reduced by 50% compared with plasma TAT levels in TFflox/flox mice (Figure 5D). These results indicate that deletion of the TF gene in TFflox/flox,Tie-2Cre mice reduces the activation of coagulation in endotoxemic mice.
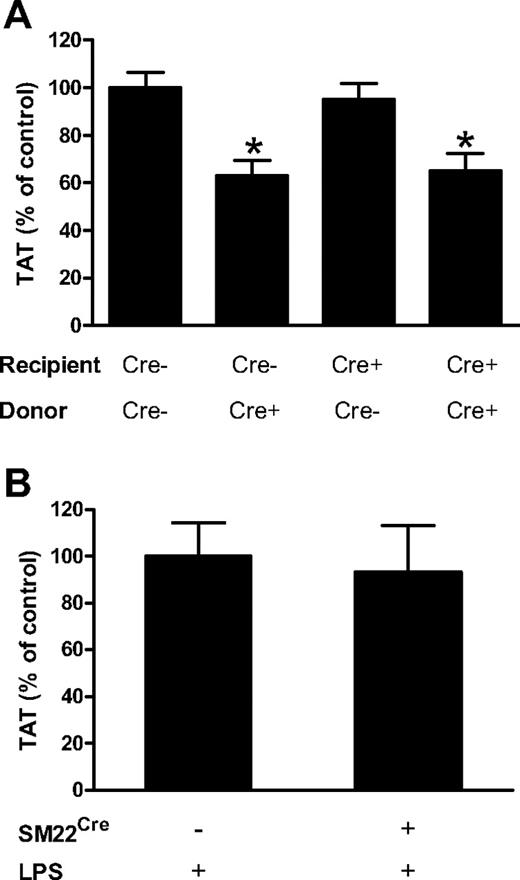
Selective deletion of TF gene in either ECs or VSMCs does not reduce activation of coagulation in endotoxemic mice
To determine whether TF expressed by EC contributes to the activation of coagulation during endotoxemia, we transplanted bone marrow from TFflox/flox mice into TFflox/flox,Tie-2Cre mice to selectively delete the TF gene in ECs. We also generated 3 groups of mice as controls: (1) TFflox/flox mice with bone marrow from TFflox/flox,Tie-2Cre mice (TF deleted in hematopoietic cells), (2) TFflox/flox,Tie-2Cre mice with bone marrow from TFflox/flox,Tie-2Cre mice (TF deleted in EC and hematopoietic cells), and (3) TFflox/flox mice with bone marrow from TFflox/flox mice (normal levels of TF). PCR analysis of blood cell DNA for levels of the Cre recombinase gene demonstrated successful reconstitution with the different donor bone marrows (data not shown). All 4 groups of mice were challenged with LPS and plasma TAT levels were analyzed 8 hours later. Importantly, we did not observe a significant reduction in plasma TAT levels in mice with the TF gene selectively deleted in ECs (Figure 6A). Consistent with our earlier experiment (Figure 1D), a selective reduction of TF expression in hematopoietic cells was associated with a significant reduction of TAT levels by 37% (Figure 6A). Furthermore, a combined deletion of the TF gene in both ECs and hematopoietic cells showed a similar reduction of plasma TAT levels (35%). These data suggest that deletion of TF gene in ECs has no significant effect on activation of coagulation 8 hours after treatment of mice with LPS.
Deletion of the TF gene in ECs or VSMCs has no effect on activation of coagulation. (A) Plasma TAT levels were analyzed 8 hours after LPS injection in TFflox/flox transplanted with TFflox/flox bone marrow (n = 18, mean ± SEM 35.9 ± 2.3 ng/mL), TFflox/flox transplanted with TFflox/flox,Tie-2Cre bone marrow (n = 12, mean ± SEM 22.7 ± 2.3 ng/mL), TFflox/flox,Tie-2Cre transplanted with TFflox/flox bone marrow (n = 13, mean ± SEM 34.1 ± 2.5 ng/mL) and TFflox/flox,Tie-2Cre transplanted with TFflox/flox,Tie-2Cre bone marrow (n = 13, mean ± SEM 23.4 ± 2.6 ng/mL). (B) Plasma TAT levels were analyzed 8 hours after LPS injection in TFflox/flox (n = 17; mean ± SEM 24.5 ± 3.5 ng/mL) and TFflox/flox,SM22Cre (n = 19; mean ± SEM 22.9 ± 3.4 ng/mL). In panel A the presence or absence of Cre recombinase in recipient and donor mice is indicated by Cre+ and Cre−. *P < .05 compared with control group expressing normal levels of TF (first bar in panel A).
Deletion of the TF gene in ECs or VSMCs has no effect on activation of coagulation. (A) Plasma TAT levels were analyzed 8 hours after LPS injection in TFflox/flox transplanted with TFflox/flox bone marrow (n = 18, mean ± SEM 35.9 ± 2.3 ng/mL), TFflox/flox transplanted with TFflox/flox,Tie-2Cre bone marrow (n = 12, mean ± SEM 22.7 ± 2.3 ng/mL), TFflox/flox,Tie-2Cre transplanted with TFflox/flox bone marrow (n = 13, mean ± SEM 34.1 ± 2.5 ng/mL) and TFflox/flox,Tie-2Cre transplanted with TFflox/flox,Tie-2Cre bone marrow (n = 13, mean ± SEM 23.4 ± 2.6 ng/mL). (B) Plasma TAT levels were analyzed 8 hours after LPS injection in TFflox/flox (n = 17; mean ± SEM 24.5 ± 3.5 ng/mL) and TFflox/flox,SM22Cre (n = 19; mean ± SEM 22.9 ± 3.4 ng/mL). In panel A the presence or absence of Cre recombinase in recipient and donor mice is indicated by Cre+ and Cre−. *P < .05 compared with control group expressing normal levels of TF (first bar in panel A).
Endotoxemia is associated with an increase in vascular permeability which would expose TF-positive cells within the vessel wall to blood.41 To analyze the contribution of VSMCs TF to the activation of coagulation, TFflox/flox,SM22αCre mice were generated using mice expressing Cre recombinase under control of the VSMC-specific promoter SM22α. The SM22α promoter is expressed in VSMCs in major arteries.42 We previously showed that levels of TF mRNA and activity are reduced by 96% and 95%, respectively, in TFflox/flox,SM22αCre mice.43 We did not observe any differences in plasma TAT levels between LPS-treated TFflox/flox,SM22αCre and TFflox/flox mice (Figure 6B). These data indicate that TF expression by VSMCs does not contribute to LPS-induced activation of coagulation at 8 hours.
Discussion
Despite the well-documented role of TF in the pathophysiology of endotoxemia and sepsis, the cellular sources of TF that contribute to activation of coagulation during endotoxemia and sepsis have not been investigated. We and others have previously demonstrated that a genetic reduction of TF expression in hematopoietic cells significantly reduced the activation of coagulation in a mouse model of endotoxemia.4,18 In this study, we extended this observation by showing that inhibition of TF expressed by hematopoietic cells reduced coagulation by 47%. In addition, we found that endotoxemic TFflox/flox mice with bone marrow from TFflox/flox,Tie-2Cre mice had a significant reduction in plasma TAT levels compared with controls. Finally, we demonstrated that the reduction in plasma TAT levels in mice lacking TF expression in myeloid cells is similar to the changes observed in mice lacking TF in hematopoietic cells (supplemental Figure 3). This indicates that myeloid cells are the major source of TF within the hematopoietic cell population. LysM-dependent expression of Cre recombinase leads to deletion of floxed genes in monocytes/macrophages and neutrophils.32 Therefore, we cannot determine which cell type within the myeloid cell population is the source of TF during endotoxemia. However, based on previous studies,14,,–17 we propose that monocytes are likely to be the primary source of TF within the myeloid cell population that activates coagulation in endotoxemic mice.
We and others have previously demonstrated that human platelets express functional TF.24,–26 Moreover, we found that freshly isolated platelets from septic patients more frequently express mature TF mRNA compared with healthy controls, indicating splicing of TF pre-mRNA in vivo (Matthew Rondina, Hansjörg Schwertz, A.S.W., unpublished data). Surprisingly, we failed to detect any TF pre-mRNA or mRNA in unstimulated or activated mouse platelets. Moreover, plasma TAT levels in TFflox/flox,PF4Cre mice were not different from those observed in TFflox/flox littermate controls. These results indicate that there are species-specific differences in platelet TF expression.
We found that TF expressed by nonhematopoietic cells contributes to 39% of the TF-dependent activation of coagulation cascade during endotoxemia in HTF mice. These data are consistent with our previous study that showed a greater reduction in TAT levels in endotoxemic low TF mice compared with endotoxemic WT mice containing low TF bone marrow.4 Based on in vitro studies, ECs were the most likely TF-positive candidate cell type within this cell population. However, we found that deletion of TF gene in ECs did not reduce activation of coagulation during endotoxemia at 8 hours. There are several possible explanations for this result. For instance, TF may only be expressed by a small subset of ECs or at very low levels. This may explain why we and others did not detect TF expression on ECs in LPS treated mice, rats, and rabbits44,,–47 (Figure 4). Alternatively, EC TF may contribute to activation of coagulation at a different time.
Interestingly, a recent study examined TAT levels in endotoxemic mice that overexpressed a dominant negative IκBα in an EC-specific manner.11,48 These mice exhibited reduced TF expression in ECs within the kidney vasculature, as well as reduce plasma TAT and inflammation.11,48 However, the reduced TAT levels could be due to either reduced TF expression in ECs or reduced inflammation with a secondary reduction in coagulation. It has been previously demonstrated that reducing inflammation attenuates activation of coagulation in animal models of sepsis.49,50 Our results suggest that the reduction in plasma TAT levels observed by Song and colleagues11 was not a direct result of reduced TF expression by ECs but rather was due to a general reduction in the inflammatory response.
In endotoxemic animals, TF expression is increased in many organs, such as the lung and kidney.11,45 EC-specific inhibition of the NF-κB pathway failed to reduce LPS-induced TF expression in the lung and kidneys, indicating that during endotoxemia ECs are a minor source of TF in these organs.11 The absence of a role for TF expression by ECs in the activation of coagulation in our model indicates that other nonhematopoietic cell types must express TF in endotoxemic mice. It is known that vascular permeability is increased during endotoxemia.41 We speculated that exposure of TF in the vessel wall to blood may lead to activation of coagulation. However, we found that deletion of TF in VSMCs did not reduce activation of coagulation. Therefore, other nonhematopoietic cell types have to be considered as a source of TF that contribute to increased TAT levels. Parenchymal cells, such as perivascular fibroblast, astrocytes in the brain, cardiomyocytes in the heart and epithelial cells in the lung and kidney, all express TF and are in direct contact with the endothelium.6,31 It is likely that during endotoxemia and sepsis, a loss of ECs barrier function will expose many of these TF-positive cells to blood. Therefore, we speculate that parenchymal cells in many different organs may be the source of the nonhematopoietic TF that activates coagulation in endotoxemic mice. Deleting the TF gene in one of these cell types may not be sufficient to significantly reduce coagulation.
Taken together, our results demonstrate that TF expressed by both hematopoietic and nonhematopoietic cells contributes to activation of the coagulation cascade in a mouse model of endotoxemia. Within the hematopoietic cell population, monocytes appear to be the predominant cell type expressing TF with no contribution from megakaryocytes or platelets in this mouse model. Finally, deletion of the TF gene in either ECs or VSMCs did not reduce TAT levels at 8 hours after LPS, indicating that TF expressed by other nonhematopoietic cells contributes to activation of coagulation in endotoxemic mice. A limitation of the study is that we analyzed a single time point (8 hours) after LPS injection and it is possible that the relative contribution of TF expressed by different cell types may change over time. For example, it is known that during sepsis the number of monocytes in the circulation is reduced over time. Therefore, it would be expected that TF expressed by this cell type will have a larger contribution to the activation of coagulation at earlier time points. Conversely, the contribution of TF expressed by nonhematopoietic cells may play a major role in the activation of coagulation at later time points, due to progressive damage of the endothelium and increased permeability. Our studies indicate that selective inhibition of monocyte TF may be a novel way to reduce disseminated intravascular coagulation in endotoxemia and sepsis.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rachel E. Tilley, Todd Holscher, and David Manly for excellent technical support, Hing Wong for the generous gift of cH36, and Daniel Kirchhofer for generous gift of 1H1.
This work was funded by the National Institutes of Health (A.S.W. and N.M.).
National Institutes of Health
Authorship
Contribution: R.P. and N.M. designed experiments and wrote the manuscript; R.P., J.-G.W., A.P.O., J.W., S.A., and M.T. performed experiments; J.W.R. and E.N.L. performed in vitro platelets experiments; A.S.W. supervised in vitro platelets experiments; T.L. provided input for experimental design; and all authors gave final approval of the manuscript for submission.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nigel Mackman, 98 Manning Dr, CB7035, Chapel Hill, NC 27599; e-mail: nmackman@med.unc.edu.

![Figure 5. Analysis of TFflox/flox, Tie-2Cre mice. (A) PCR analysis of mouse TF DNA isolated from blood cells demonstrates deletion of the TF gene. Arrows indicate PCR products for wild type and floxed alleles. A nonspecific band is present below the TF band. The mouse IL-2 gene was used as a loading control. WT indicates DNA from a WT mouse. (B) Real-time PCR analysis of TF mRNA in blood cells isolated from TFflox/flox and TFflox/flox,Tie-2Cre mice before (n = 2 per group) and after (n = 4 per group) LPS injection (2 hours). (C) PCR analysis of mouse TF DNA isolated from ECs demonstrates deletion of the TF gene. Arrows indicate PCR products for wild type and floxed alleles. The mouse IL-2 gene was used as a loading control. Presence or absence of Cre recombinase (Cre) in different mouse groups is shown. Low levels of fibroblast contamination likely explain the residual TF-positive band in 1 of the 4 cell preparations from TFflox/flox,Tie-2Cre mice[b]. (D) Plasma TAT levels were analyzed in TFflox/flox (n = 19; mean ± SEM 15.2 ± 1.7 ng/mL) and TFflox/flox,Tie-2Cre (n = 21; mean ± SEM 7.7 ± 0.9 ng/mL) mice 8 hours after LPS injection. *P < .001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/5/10.1182_blood-2009-12-259267/5/m_zh89991054770005.jpeg?Expires=1767900696&Signature=2LIJ3EFKFerxuvyoVgcuLmzfrB71dL3DCXEamc3-JusmQ1I3D0Q19s9Qr2B71ksTh0rFA6XDTJCpmIBtM2zx7~gHuYisYxl9SKuBiI6du682o8SZrbHZky~4PSRFVz9bBl6knouHKoZSgCf~RZExb1vNpO4otcxlKTSfvb3tjy3~NDLDhnDz3c4FI3Ay85PAV7kIDxRjiYrZD-7F5Ct3fbg~rppbWxco6shqpgj-iMf---La-yTkSpDZRqymDcKPwOKif8I8cVELZQ~icWKJhlxeQJcbEu-uXKgOIVHIrg8duIOM3k~MUmHm4eqC3NvIQuAfw60yLF1z-iJhiQoUGA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)