In this issue of Blood, Hartmann and colleagues report on the results of a high-resolution genomic profiling study in a large series of MCL. Copy number alteration and expression data are amalgamated with clinical outcome. Their data confirm the critical role of the cell-cycle and DNA damage-response pathways to MCL and also identify novel target genes within these. Specifically, Hippo signaling is recognized as a potentially novel pathogenetic pathway.1
Although the hallmark of mantle cell lymphoma (MCL) is overexpression of cyclin D1 related to the t(11;14) translocation, it is clear that there are a number of biologic factors synergizing in the disease process. Their identification has contributed to the provision of prognostic molecular markers, and such secondary genomic alterations are likely to account for the highly variable clinical behavior of MCL and may permit better risk stratification at diagnosis. Most of the additional abnormalities identified to date perturb the cell-cycle machinery and interfere with the cellular response to DNA damage.2 Our knowledge, however, of the biology of this disease is by no means complete. There have been a number of studies exploring copy number alteration.3,4 This research paper presents the largest dataset to date, using high-resolution techniques to power this study to combine genome-wide copy number alteration with gene expression and clinical data in a cohort of primary MCL cases. Importantly, this permitted identification of new survival-associated genetic alterations affecting prognosis and revealed potential new pathogenetic pathways.
This report confirms the complexity of the secondary genomic imbalances that occur in MCL. The few cases of cyclin D1–negative MCL included were found to carry the same types of abnormalities and complexity as cyclin D1–positive cases, confirming again that these represent the same pathogenic entity and raising the possibility that genetic profiling could be used as an aid to diagnosis when difficulties exist by providing a robust means of identifying this particular disease subtype.
By incorporating clinical survival with genomic data, this study nicely highlighted those genomic alterations that were most likely to influence the clinical course of the disease. Previous studies have identified key alterations relating to the genes that control cell cycle and response to DNA damage and these are confirmed here (summarized in Figure 1). Genomic amplification of cyclin-dependent kinase 4 (CDK4), deletion of cyclin-dependent kinase inhibitor 2A/p16 (CDKN2A), and overexpression of BIM1 (a transcriptional repressor of p16) all contribute to increasing the protein drive on cell cycling and proliferation.2 Loss of response to DNA damage through ATM and TP53 mutations further promotes cell cycling. Here Hartmann and colleagues provide good evidence that the regions of common loss are characterized by survival genes, associated with the proliferation signature5 that is critical to MCL pathogenesis. In this way, these data also suggest that subsets of genes encompassed by minor common regions of change impact on patients' outcome and have prognostic value. MCL is a “spectrum of diseases” with no clear best method for risk stratification besides continuous variables including MCL International Prognostic Index and Ki67. By identifying different molecular subsets this provides new clinically relevant prognostic stratification analogous to that seen with activated B cell–like and germinal center B cell–like gene array–identified subtypes of diffuse large B-cell lymphoma and may be an important basis for future clinical trial design.
Schematic diagram summarizing the cell-cycle and DNA damage response alterations in MCL, highlighting specific genomic copy number variations. The t(11;14)(q13;q32) translocation results in up-regulation of cyclin D1, an important regulator of the G1 phase of the cell cycle with its catalytic partner cyclin-dependent kinase 4 (CDK4). Overexpression of cyclin D1 maintains retinoblastoma (Rb) in a phosphorylated state leading to its inactivation and release of its suppression on the transcription factor E2F. E2F transactivates numerous S-phase gene promoters (cyclins D, E, A) and thus instigates DNA synthesis. Alterations described by Hartmann et al may act in the following ways. CUL4A binds to CDKN2A/p16 causing p16 activation. Loss of CUL4A prevents cell-cycle inhibition through p16. ING1 increases p21 expression by up-regulating the DNA damage-response gene p53. The loss of ING1 enhances cell-cycle progression through the G1/S checkpoint by removing the p21 brake. In MCL, MCPH1 is down-regulated resulting in increased cell cycling and a failure of apoptosis. Professional illustration by Debra T. Dartez.
Schematic diagram summarizing the cell-cycle and DNA damage response alterations in MCL, highlighting specific genomic copy number variations. The t(11;14)(q13;q32) translocation results in up-regulation of cyclin D1, an important regulator of the G1 phase of the cell cycle with its catalytic partner cyclin-dependent kinase 4 (CDK4). Overexpression of cyclin D1 maintains retinoblastoma (Rb) in a phosphorylated state leading to its inactivation and release of its suppression on the transcription factor E2F. E2F transactivates numerous S-phase gene promoters (cyclins D, E, A) and thus instigates DNA synthesis. Alterations described by Hartmann et al may act in the following ways. CUL4A binds to CDKN2A/p16 causing p16 activation. Loss of CUL4A prevents cell-cycle inhibition through p16. ING1 increases p21 expression by up-regulating the DNA damage-response gene p53. The loss of ING1 enhances cell-cycle progression through the G1/S checkpoint by removing the p21 brake. In MCL, MCPH1 is down-regulated resulting in increased cell cycling and a failure of apoptosis. Professional illustration by Debra T. Dartez.
Putative novel target genes were also identified on the basis of the frequency of their occurrence rather than effect on survival. In this way another layer of complexity has been added to the cell-cycle dysregulation inherent to MCL and further demonstrates how critical this is to this disease process (Figure 1). CUL4A, ING1, and MCPH1 have not previously been ascribed a role in lymphomagenesis, although they are known to have important functions in regulating the DNA damage-response pathways (see Figure 1 for a potential role in MCL cell-cycle dysregulation).
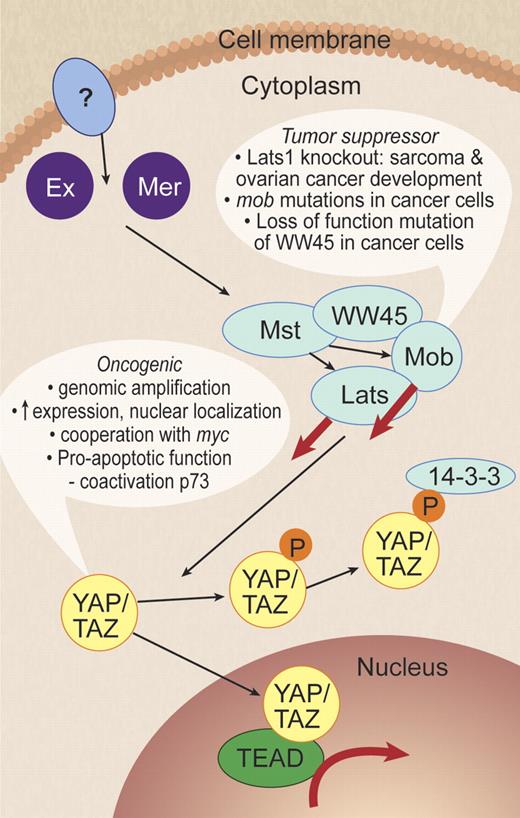
This report is of interest because it identifies Hippo pathway dysregulation for the first time in lymphoma. The Hippo pathway was originally discovered in Drosophila through its regulation of body and organ size by inhibiting cell proliferation and promoting apoptosis.6 Its role in cancer is increasingly being recognized with key components of the pathway acting as both oncogenes and tumor suppressors.7 Hartmann et al provide 2 lines of evidence to support a role for Hippo in MCL pathogenesis. This is an exciting development because it identifies for the first time the Hippo pathway as tumor suppressor genes contributing to lymphoma tumorigenesis. Decreased expression of Hippo members MOBKL2A, MOBKL2B, and LATS2 was associated with inferior survival. Second, loss of the genomic regions where these 3 genes are located was observed in almost 40% of MCL cases. MOBKL2A, MOBKL2B are homologues of the MOB1 gene that interacts with LATS in inhibition of YAP, a potent growth promoter. Evidence is growing to support the function of YAP as an oncogene as well as a tumor-suppressor function for its inhibitory upstream Hippo pathway components (see Figure 2 for summary of Hippo pathway). Thus, the findings of decreased expression of some of the Hippo members paves the way for further investigation of the Hippo pathway in lymphomagenesis. Is it perturbed in other B-cell lymphomas? This would be an interesting question to answer as well as raising the distinct possibility of a new therapeutic target, potentially with broad application.
The Hippo pathway. In mammalian cells, the Hippo pathway kinase cascade inhibits YAP by direct phosphorylation of YAP by Lats to generate a 14-3-3–binding site that induces YAP cytoplasmic translocation and inactivation. Evidence to support the role of YAP as an oncogene is listed. Ex indicates Expanded; Mer, Merlin, also called NF2; Mst, Mst1/2, also called STK4 and STK3, Hpo homolog; WW45, Sav homolog; Mob, Mps 1 binder kinase activator-like 1A/B, MOBKL1A/B, Mats homolog; Lats, Lats1/2, Wts homolog; YAP, Yes-associated protein, Yki homolog; TAZ, transcriptional coactivator with PDZ-binding motif, also called WWTR1, Yki homolog; and TEAD, TEA domain family member 1/2/3/4. Dashed arrows indicate unknown biochemical mechanism and question marks denote unknown components. Adapted from Zhao et al7 with permission. Professional illustration by Debra T. Dartez.
The Hippo pathway. In mammalian cells, the Hippo pathway kinase cascade inhibits YAP by direct phosphorylation of YAP by Lats to generate a 14-3-3–binding site that induces YAP cytoplasmic translocation and inactivation. Evidence to support the role of YAP as an oncogene is listed. Ex indicates Expanded; Mer, Merlin, also called NF2; Mst, Mst1/2, also called STK4 and STK3, Hpo homolog; WW45, Sav homolog; Mob, Mps 1 binder kinase activator-like 1A/B, MOBKL1A/B, Mats homolog; Lats, Lats1/2, Wts homolog; YAP, Yes-associated protein, Yki homolog; TAZ, transcriptional coactivator with PDZ-binding motif, also called WWTR1, Yki homolog; and TEAD, TEA domain family member 1/2/3/4. Dashed arrows indicate unknown biochemical mechanism and question marks denote unknown components. Adapted from Zhao et al7 with permission. Professional illustration by Debra T. Dartez.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal