Abstract
Definitive risk factors for the development of adult T-cell leukemia (ATL) among asymptomatic human T-cell leukemia virus type I (HTLV-1) carriers remain unclear. Recently, HTLV-1 proviral loads have been evaluated as important predictors of ATL, but a few small prospective studies have been conducted. We prospectively evaluated 1218 asymptomatic HTLV-1 carriers (426 males and 792 females) who were enrolled during 2002 to 2008. The proviral load at enrollment was significantly higher in males than females (median, 2.10 vs 1.39 copies/100 peripheral blood mononuclear cells [PBMCs]; P < .001), in those 40 to 49 and 50 to 59 years of age than that of those 40 years of age and younger (P = .02 and .007, respectively), and in those with a family history of ATL than those without the history (median, 2.32 vs 1.33 copies/100 PBMCs; P = .005). During follow-up, 14 participants progressed to overt ATL. Their baseline proviral load was high (range, 4.17-28.58 copies/100 PBMCs). None developed ATL among those with a baseline proviral load lower than approximately 4 copies. Multivariate Cox analyses indicated that not only a higher proviral load, advanced age, family history of ATL, and first opportunity for HTLV-1 testing during treatment for other diseases were independent risk factors for progression of ATL.
Introduction
Human T-cell leukemia virus type I (HTLV-1), the first human retrovirus to be identified, is etiologically associated with adult T-cell leukemia (ATL), HTLV-1–associated myelopathy/tropical spastic paraparesis (HAM/TSP), and HTLV-1 uveitis/HTLV-1–associated uveitis (HU/HAU).1-3 Worldwide, endemic areas for the virus are unevenly distributed, which include southwest Japan, the Caribbean islands, South America, and a part of Central Africa.4 In Japan, the number of HTLV-1 carriers was estimated to be approximately 1.2 million people during the late 1980s.5 The majority of HTLV-1 carriers remain asymptomatic throughout their lives. The lifetime risks of developing ATL and HAM/TSP are estimated to be approximately 2.5% to 5%6,7 and 0.3% to 2%,8,9 respectively.
Several molecular biologic studies have reported that various cellular dysfunctions induced by viral genes (eg, tax and HBZ), genetic and epigenetic alterations, and the host immune system may be involved in the leukemogenesis of ATL.10-12 Clinical and epidemiologic studies have also reported a variety of possible risk factors for ATL, including vertical transmission of HTLV-1 infection, male gender, a long latent period, increased leukocyte counts or abnormal lymphocyte counts, and higher levels of anti–HTLV-1 antibody titers and soluble interleukin-2 receptor-α.13-19 However, there are no clear determinants that separate those who develop ATL from those who remain healthy carriers.
Recently, HTLV-1 proviral load levels have been evaluated as important predictors of development of ATL and HAM/TSP. Some cross-sectional studies showed that HTLV-1 proviral load levels were higher in ATL and HAM/TSP compared with asymptomatic HTLV-1 carriers.20,21 However, the proviral load levels of asymptomatic HTLV-1 carriers exhibited a very wide range,20,22,23 and these levels may vary by sex, race, habitats, and comorbidities.24 The proviral load levels of asymptomatic HTLV-1 carriers were also examined serially in some prospective studies; however, the number of reported cases was very small.25-28 Although these previous studies suggest a possible important role for HTLV-1 proviral load in the development of ATL and HAM/TSP, the association between HTLV-1 proviral load and diseases development remains unclear.
The identification of risk factors for developing ATL among virus carriers is necessary to prevent these diseases in HTLV-1 endemic areas. To investigate detailed viral- and host-specific determinants of disease development, larger and longer prospective studies are warranted. In 2002, we established a nationwide cohort study for asymptomatic HTLV-1 carriers in Japan named the Joint Study on Predisposing Factors of ATL Development (JSPFAD).29 The main objective of this project is to establish reliable predisposing factors for developing ATL by prospectively following a large number of asymptomatic HTLV-1 carriers. Here, for the first-time, we report the study method, baseline demographic characteristics, and distribution characteristics of baseline HTLV-1 proviral load of asymptomatic HTLV-1 carriers. We have also evaluated progression to ATL and its risk predictors.
Methods
Participants and study design
The JSPFAD is a nationwide prospective study of HTLV-1 carriers, which was approved by the Ministry of Education, Culture, Sports, Science and Technology of Japan. The project was established in August 2002 by Japanese clinicians and basic researchers of 41 institutions composed of 14 university hospitals and 27 educational hospitals located in various areas of Japan (supplemental Appendix, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Objectives of the project are to establish reliable predisposing factors for development of ATL by prospectively following a large number of asymptomatic HTLV-1 carriers. This includes performing clinical examinations and biomarker assays, as well as establishing a biomaterial resource bank of plasma, viable peripheral blood mononuclear cells (PBMCs), frozen PBMCs pellets, and genomic DNA from PBMCs of HTLV-1–infected persons for the future evaluations with new molecular biology techniques.
Hematologists at the collaborating institutions were responsible for enrolling participants after receiving approval from their Institutional Review Boards. The study protocol was approved by the Ministry of Education, Culture, Sports, Science and Technology of Japan. Eligible participants were those who had known of their HTLV-1 infection and had confirmed the HTLV-1–positive serology at any of the medical institutions. Potential participants visited any of the collaborating institutions directly or via the website of the JSPFAD (www.htlv1.org/). They received adequate explanations for the enrollment procedure from the hematologists at the collaborating institutions. Enrollment was conditional on participants giving written informed consent in accordance with the Declaration of Helsinki. The primary participants were asymptomatic HTLV-1 carriers. A small number of patients with definite ATL, HAM/TSP, and HU/HAU were also enrolled as controls.
Data collection and sample storage
After providing written informed consent, participants were expected to fill out a questionnaire regarding demographic information, to provide peripheral blood samples, and to periodically visit the institution for follow-up. After reconfirming the asymptomatic HTLV-1 carrier status of the participants, hematologists at the collaborating institutions assigned a unique identification number to each participant and subsequently sent all materials (individual questionnaire sheets, clinical data, and blood samples drawn into ethylenediaminetetraacetic acid and heparin tubes) to the JSPFAD office (Department of Medical Genome Sciences, Laboratory of Tumor Cell Biology, Graduate School of Frontier Sciences, University of Tokyo, Japan).
The self-administered questionnaire included items on demographic characteristics, birthplaces of the participants and their mothers, family history regarding HTLV-1 status and HTLV-1–associated diseases, length of marriage, partner's HTLV-1 status, first opportunity for HTLV-1 testing, and histories of disease manifestations other than HTLV-1–associated diseases. Additional questionnaire items, information on prior blood transfusion, and smoking habits (present, past, or nonsmoking) were also included after April 2008.
Clinical data included information on the date of visit, complete blood cell count, differential cell counts (including abnormal lymphocytes per 100 leukocytes), lactate dehydrogenase, HTLV-1 serologic test, comorbidities other than HTLV-1–associated diseases, and the development of any HTLV-1–associated diseases during follow-up. Blood samples were collected at enrollment, annually thereafter (in principal), and as needed. Blood samples sent to the study office at the University of Tokyo were separated into plasma, PBMCs, and genomic DNA and then used for viral marker assays at the University of Tokyo or stored for the biomaterial bank at the Japanese Red Cross Fukuoka Blood Center.
Viral marker assays
HTLV-1 proviral load of PBMC samples was measured by real-time polymerase chain reaction (PCR) using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems Japan), as previously described with minor modifications.30,31 Genomic DNA from PBMCs was isolated using a QIAGEN Blood Kit (QIAGEN). Quantitative real-time PCR was performed using multiplex PCR with 2 sets of primers specific for the HTLV-1 provirus and the human gene encoding the RNase P enzyme. The primers and the probe for the gene encoding RNase P were purchased from Applied Biosystems; those for the pX region of the HTLV-1 provirus were described previously.30,31 Genomic DNA of normal control PBMCs mixed with a plasmid DNA, which contained almost the whole genome of the HTLV-1 provirus (SacI site of 5′-LTR to SacI site of 3′-LTR), was used as control template. The copy number of the plasmid DNA was calculated based on the size and weight of the plasmid DNA, as measured by spectrophotometry. The proviral loads were expressed as copy numbers per 100 PBMCs, based on the assumption that infected cells harbored 1 copy of the integrated HTLV-1 provirus per cell. Samples with a higher proviral load (> 20 copies/100 PBMCs) were subjected to Southern blot analysis to examine the clonality of the infected cells. Assays to detect the integrated band of HTLV-1 provirus genome were described previously.32 Genomic DNA samples (10 mg) were digested with PstI or EcoRI restriction enzymes and were size-fractionated on 0.7% agarose gels. They were then transferred onto a nylon membrane by the Southern blot technique. Hybridization to randomly primed 32P-labeled DNA probes for the whole proviral genome (SacI to SacI fragment of the HTLV-1 proviral genome) was performed, followed by appropriate stringency washing steps and autoradiography. Soluble interleukin-2 receptor was measured by a commercial laboratory (SRL Inc) using an enzyme-linked immunosorbent assay (Endogen) and reported as units per milliliter.
Statistical analysis
Analyses were performed for participants who enrolled as of December 2008. Age at enrollment was categorized into 5 groups: younger than 40, 40 to 49, 50 to 59, 60 to 69, and 70 years or older. Geographic location was divided into 4 areas: northern (Hokkaido and Tohoku), metropolitan (Tokyo, Osaka, and Nagoya), southern (Kyushu and Okinawa), and others (supplemental Figure 1). First opportunity for HTLV-1 testing was divided into 3 categories: by screening for HTLV-1 (regional-mass, multiphasic, blood donor, and maternal screenings), by the presence of HTLV-1–infected family members (including spouse), and by the patient status under treatment for diseases unrelated to HTLV-1. A positive family history was considered to be present when participants had information on first-degree relatives (parents, siblings, or offspring) who were HTLV-1 carriers or had HTLV-1–associated diseases (ie, ATL, HAM/TSP, and HU/HAU). Any leukemia and/or lymphoma other than ATL were also taken into consideration. A positive comorbidity at enrollment was considered to be present when any information on diseases other than HTLV-1–associated diseases was available at enrollment. HTLV-1 proviral loads (copy numbers/100 PBMCs) were used as a continuous variable (raw and the power-transformed data) or by categorizing them into quartiles. We applied a square-root transformation to the raw data of proviral loads to reduce the skewness. Continuous data were presented as median (range) values and compared using a Mann-Whitney test. Categorical data were compared using a χ2 test or Fisher exact test. We calculated person-years of follow-up for each participant from the date of enrollment to the date of ATL diagnosis, the date of last follow-up, or September 30, 2009, whichever came first. Cumulative progression to ATL was estimated using Kaplan-Meier curves. To estimate the effect of baseline HTLV-1 proviral load and selected demographic factors on ATL development, we performed Cox proportional hazards analyses, and expressed as hazard ratios (HR) and 95% confidence intervals (CI), which were calculated by robust sandwich variance estimates. To check for possible incompleteness in the multivariate model, we also performed analyses using sub-datasets. All statistical analyses were performed using SAS Version 9.1 (SAS Institute Japan) with a 2-tailed significance level of .05.
Results
Baseline demographic characteristics
From August 2002 to December 2008, 1259 participants of asymptomatic HTLV-1 carriers were enrolled in this study. However, HTLV-1 proviral load was not measured for 41 participants. Thus, a total of 1218 participants (426 males and 792 females) were included in this analysis. Demographic characteristics of the participants at enrollment are shown in Table 1. The median ages at enrollment in the cohort were 59.6 years (range, 6.9-92.8 years) for males or 58.3 years (range, 17.8-90.3 years) for females. The largest percentage of study participants was from the southern area, which is a well-known HTLV-1 endemic area in Japan, followed by the metropolitan area. The southern area also had the largest percentage for birthplaces for most participants and their mothers.
Baseline demographic characteristics of asymptomatic HTLV-1 carriers
| Variable . | Male, no. (%) . | Female, no. (%) . |
|---|---|---|
| Total | 426 | 792 |
| Age, y | ||
| Younger than 40 | 48 (11.3) | 119 (15.0) |
| 40-49 | 70 (16.4) | 130 (16.4) |
| 50-59 | 99 (23.2) | 174 (22.0) |
| 60-69 | 88 (20.7) | 172 (21.7) |
| 70 or older | 121 (28.4) | 197 (24.9) |
| Place of enrollment | ||
| Northern area | 10 (2.3) | 32 (4.0) |
| Metropolitan area | 75 (17.6) | 144 (18.1) |
| Southern area | 333 (78.2) | 597 (75.4) |
| Other areas | 8 (1.9) | 19 (2.4) |
| Birthplace of participants | ||
| Northern area | 18 (4.2) | 33 (4.2) |
| Metropolitan area | 30 (7.0) | 80 (10.1) |
| Southern area | 240 (56.3) | 400 (50.5) |
| Other areas | 20 (4.7) | 54 (6.8) |
| Unknown | 118 (27.7) | 225 (28.4) |
| Birthplace of participants' mothers | ||
| Northern area | 16 (3.8) | 32 (4.0) |
| Metropolitan area | 13 (3.1) | 39 (4.9) |
| Southern area | 247 (58.0) | 426 (53.8) |
| Other areas | 28 (6.6) | 64 (8.1) |
| Unknown | 122 (28.6) | 231 (29.2) |
| First opportunity for HTLV-1 testing | ||
| Screening for HTLV-1 | 209 (49.1) | 452 (57.1) |
| Regional mass screening | 77 | 164 |
| Multiphasic screening | 24 | 44 |
| Blood donor screening | 108 | 128 |
| Maternal screening | 0 | 116 |
| Revelation of HTLV-1–positive family | 33 (7.7) | 101 (12.7) |
| During treatment of other diseases | 117 (27.5) | 148 (18.7) |
| Unknown | 67 (15.7) | 91 (11.5) |
| Family history of HTLV-1–associated diseases* | ||
| Absent | 98 (23.0) | 154 (19.5) |
| Absent for a first-degree relative but having an infected spouse | 6 (1.4) | 23 (2.9) |
| Carrier only | 27 (6.3) | 74 (9.3) |
| HU/HAU only | 2 (0.5) | 1 (0.1) |
| HAM | 2 (0.5) | 7 (0.9) |
| ATL | 34 (8.0) | 74 (9.3) |
| Leukemia or lymphoma | 9 (2.1) | 26 (3.3) |
| Unknown family history | 248 (58.2) | 433 (54.7) |
| Comorbidity† | ||
| Absent | 331 (77.7) | 630 (79.5) |
| Present | 95 (22.3) | 162 (20.5) |
| Infectious diseases | 20 | 25 |
| Autoimmune diseases | 3 | 26 |
| Malignant diseases | 36 | 44 |
| Skin diseases | 8 | 8 |
| Other disease | 28 | 59 |
| Variable . | Male, no. (%) . | Female, no. (%) . |
|---|---|---|
| Total | 426 | 792 |
| Age, y | ||
| Younger than 40 | 48 (11.3) | 119 (15.0) |
| 40-49 | 70 (16.4) | 130 (16.4) |
| 50-59 | 99 (23.2) | 174 (22.0) |
| 60-69 | 88 (20.7) | 172 (21.7) |
| 70 or older | 121 (28.4) | 197 (24.9) |
| Place of enrollment | ||
| Northern area | 10 (2.3) | 32 (4.0) |
| Metropolitan area | 75 (17.6) | 144 (18.1) |
| Southern area | 333 (78.2) | 597 (75.4) |
| Other areas | 8 (1.9) | 19 (2.4) |
| Birthplace of participants | ||
| Northern area | 18 (4.2) | 33 (4.2) |
| Metropolitan area | 30 (7.0) | 80 (10.1) |
| Southern area | 240 (56.3) | 400 (50.5) |
| Other areas | 20 (4.7) | 54 (6.8) |
| Unknown | 118 (27.7) | 225 (28.4) |
| Birthplace of participants' mothers | ||
| Northern area | 16 (3.8) | 32 (4.0) |
| Metropolitan area | 13 (3.1) | 39 (4.9) |
| Southern area | 247 (58.0) | 426 (53.8) |
| Other areas | 28 (6.6) | 64 (8.1) |
| Unknown | 122 (28.6) | 231 (29.2) |
| First opportunity for HTLV-1 testing | ||
| Screening for HTLV-1 | 209 (49.1) | 452 (57.1) |
| Regional mass screening | 77 | 164 |
| Multiphasic screening | 24 | 44 |
| Blood donor screening | 108 | 128 |
| Maternal screening | 0 | 116 |
| Revelation of HTLV-1–positive family | 33 (7.7) | 101 (12.7) |
| During treatment of other diseases | 117 (27.5) | 148 (18.7) |
| Unknown | 67 (15.7) | 91 (11.5) |
| Family history of HTLV-1–associated diseases* | ||
| Absent | 98 (23.0) | 154 (19.5) |
| Absent for a first-degree relative but having an infected spouse | 6 (1.4) | 23 (2.9) |
| Carrier only | 27 (6.3) | 74 (9.3) |
| HU/HAU only | 2 (0.5) | 1 (0.1) |
| HAM | 2 (0.5) | 7 (0.9) |
| ATL | 34 (8.0) | 74 (9.3) |
| Leukemia or lymphoma | 9 (2.1) | 26 (3.3) |
| Unknown family history | 248 (58.2) | 433 (54.7) |
| Comorbidity† | ||
| Absent | 331 (77.7) | 630 (79.5) |
| Present | 95 (22.3) | 162 (20.5) |
| Infectious diseases | 20 | 25 |
| Autoimmune diseases | 3 | 26 |
| Malignant diseases | 36 | 44 |
| Skin diseases | 8 | 8 |
| Other disease | 28 | 59 |
HTLV-1 indicates human T-cell leukemia virus type 1; HU, HTLV-1 uveitis; HAU, HTLV-1–associated uveitis; HAM, HTLV-1 myelopathy; and ATL, adult T-cell leukemia.
Family history was restricted to a first-degree relative. “Present” indicates that participants have a parent, sibling, or offspring diagnosed with HTLV-1-associated diseases. Family members with HAM and HU/HAU were included into the category of “HAM.” Family members with ATL and HAM and/or HU/HAU were included into the category of “ATL.”
Comorbidity indicates that participants have any diseases other than HTLV-1–associated diseases at enrollment.
One-half of the participants came to know of their HTLV-1 infections through screening for HTLV-1, and one-fourth was informed of their infections while receiving treatments for diseases other than HTLV-1–associated diseases. More than half of the participants did not know their family status of HTLV-1 infection. Only 119 female participants knew about the HTLV-1 infection status of their husbands, of whom 53 (45%) of the husbands were positive for HTLV-1 (data not shown). However, we were not able to obtain reliable information on male-to-female transmission for the female participants. We obtained information on comorbidities at enrollment from 257 participants, of which 45 had comorbid infectious diseases (eg, strongyloidiasis, chronic bronchitis, hepatitis C virus infection, lymphadenitis), 29 had autoimmune diseases (rheumatoid arthritis, chronic thyroiditis, Sjögren syndrome, and other autoimmune or chronic inflammatory diseases), 80 had a variety of definite malignant diseases other than ATL (non-Hodgkin lymphoma, acute myeloid leukemia, gastric cancer, lung cancer, or other malignancies), 16 had skin diseases, and 87 had other common diseases (eg, hypertension, diabetes).
Distributions of baseline HTLV-1 proviral load
Figure 1 shows distribution of baseline HTLV-1 proviral load in 1218 participants. There was a wide range of skewness in the raw data, with a median of 1.60 copies/100 PBMCs (range, 0-55.8 copies/100 PBMCs; 25th-75th percentile, 0.29-4.54 copies/100 PBMCs; Figure 1A). The square-root transformation reduced the skew in the raw data, with a median of 1.26 copies/100 PBMCs (range, 0-7.47 copies/100 PBMCs; 25th-75th percentile, 0.54-2.13 copies/100 PBMCs; Figure 1B). Figure 1C shows the frequency of participants in each quartile of proviral load.
Distribution of baseline HTLV-1 proviral load levels among 1218 asymptomatic HTLV-1 carriers. (A) Scatter plot of raw data of proviral load (left) and the vertical box and whiskers plot (right): the box delineates 25th percentile (0.29 copies/100 periopheral blood mononuclear cells [PBMCs]), median (1.60 copies/100 PBMCs), and 75th percentiles (4.54 copies/100 PBMCs), and the whiskers delineate the minimum (0 copies/100 PBMCs) and maximum (55.8 copies/100 PBMCs). (B) Scatter plot of square-root transformed values of the raw proviral load (left) and the vertical box and whiskers plot (right): the box delineates 25th percentile (0.54 copies/100 PBMCs), median (1.26 copies/100 PBMCs), and 75th percentiles (2.13 copies/100 PBMCs), and the whiskers delineate the minimum (0 copies/100 PBMCs) and maximum (7.47 copies/100 PBMCs). (C) The frequency of participants in the quartile distributions of proviral load. Q1 indicates quartile 1 (< 25th percentile); Q2, quartile 2 (25th percentile to median); Q3, quartile 3 (median to 75th percentile); Q4: quartile 4 (> 75th percentile); Sqrt, square-root transformation; and N, number of participants.
Distribution of baseline HTLV-1 proviral load levels among 1218 asymptomatic HTLV-1 carriers. (A) Scatter plot of raw data of proviral load (left) and the vertical box and whiskers plot (right): the box delineates 25th percentile (0.29 copies/100 periopheral blood mononuclear cells [PBMCs]), median (1.60 copies/100 PBMCs), and 75th percentiles (4.54 copies/100 PBMCs), and the whiskers delineate the minimum (0 copies/100 PBMCs) and maximum (55.8 copies/100 PBMCs). (B) Scatter plot of square-root transformed values of the raw proviral load (left) and the vertical box and whiskers plot (right): the box delineates 25th percentile (0.54 copies/100 PBMCs), median (1.26 copies/100 PBMCs), and 75th percentiles (2.13 copies/100 PBMCs), and the whiskers delineate the minimum (0 copies/100 PBMCs) and maximum (7.47 copies/100 PBMCs). (C) The frequency of participants in the quartile distributions of proviral load. Q1 indicates quartile 1 (< 25th percentile); Q2, quartile 2 (25th percentile to median); Q3, quartile 3 (median to 75th percentile); Q4: quartile 4 (> 75th percentile); Sqrt, square-root transformation; and N, number of participants.
The median proviral load and a frequency of subjects in each quartile of proviral load by demographic characteristics are shown in Table 2. Males and females were significantly different in proviral load levels, with a median value of 2.10 copies/100 PBMCs (range, 0-46.6 copies/100 PBMCs) for males and that of 1.39 copies/100 PBMCs (range, 0-55.8 copies/100 PBMCs) for females (P < .001). Males were probably distributed in the highest quartile of proviral load level than females.
HTLV-1 VL levels by demographic characteristics
| Demographic characteristics . | No. . | Median VL (range) (copies/100 PBMCs) . | Frequency of subjects by VL level, n (% of row) . | |||
|---|---|---|---|---|---|---|
| Quartile 1 (VL: < 0.29)‡ . | Quartile 2 (VL: 0.29-1.60) . | Quartile 3 (VL: 1.60-4.54) . | Quartile 4 (VL: ≥ 4.54) . | |||
| Total | 1.60 (0-55.8) | 303 | 306 | 304 | 305 | |
| Sex | ||||||
| Male | 426 | 2.10 (0-46.6)* | 84 (19.7) | 100 (23.5) | 93 (21.8) | 149 (35.0) |
| Female | 792 | 1.39 (0-55.8)† | 219 (27.7) | 206 (26.0) | 211 (26.6) | 156 (19.7) |
| Age, y | ||||||
| Younger than 40 | 167 | 1.37 (0-16.4)† | 49 (29.3) | 43 (25.8) | 50 (29.9) | 25 (15.0) |
| 40-49 | 200 | 1.77 (0-41.7)* | 43 (21.5) | 52 (26.0) | 51 (25.5) | 54 (27.0) |
| 50-59 | 273 | 1.84 (0-36.1)* | 64 (23.4) | 64 (23.4) | 63 (23.1) | 82 (30.4) |
| 60-69 | 260 | 1.56 (0-46.6) | 66 (25.4) | 66 (25.4) | 61 (23.5) | 67 (25.8) |
| 70 or older | 318 | 1.52 (0-55.8) | 81 (25.5) | 81 (25.5) | 79 (24.8) | 77 (24.2) |
| First opportunity for HTLV-1 testing | ||||||
| Screening | 661 | 1.46 (0-55.8)† | 182 (27.5) | 160 (24.2) | 175 (26.5) | 144 (21.8) |
| Revelation of HTLV-1–positive family | 134 | 1.45 (0-46.6) | 31 (23.1) | 40 (29.9) | 39 (29.1) | 24 (17.9) |
| During treatment for other diseases | 265 | 1.93 (0-41.7)* | 56 (21.1) | 66 (24.9) | 57 (21.5) | 86 (32.5) |
| Unknown | 158 | 2.08 (0-30.3)* | 34 (21.5) | 40 (25.3) | 33 (20.9) | 51 (32.3) |
| Family history of HTLV-1–related diseases | ||||||
| Absence or carrier/HU/HAU only | 385 | 1.33 (0-32.4)† | 100 (26.0) | 105 (27.2) | 100 (26.0) | 80 (20.8) |
| HAM/TSP | 9 | 3.85 (1.2-9.4)* | 0 | 1 (11.1) | 5 (55.6) | 3 (33.3) |
| ATL | 108 | 2.32 (0-46.6)* | 18 (16.7) | 26 (24.1) | 33 (30.6) | 31 (28.7) |
| Leukemia or lymphoma | 35 | 2.47 (0-12.8)* | 3 (8.6) | 9 (25.7) | 11 (31.4) | 12 (34.3) |
| Unknown family history | 681 | 1.55 (0-55.8) | 182 (26.7) | 165 (24.2) | 155 (22.8) | 179 (26.3) |
| Comorbidity | ||||||
| Absence | 961 | 1.65 (0-55.8)† | 241 (25.1) | 234 (24.4) | 244 (25.4) | 242 (25.2) |
| Infectious diseases | 45 | 2.75 (0-28.6)* | 7 (15.6) | 8 (17.8) | 13 (28.9) | 17 (37.8) |
| Autoimmune diseases | 29 | 1.33 (0-41.7) | 10 (34.5) | 7 (24.1) | 4 (13.8) | 8 (27.6) |
| Malignant diseases | 80 | 1.57 (0-19.4) | 19 (23.8) | 21 (26.3) | 23 (28.8) | 17 (21.3) |
| Skin diseases | 16 | 0.60 (0.07-14.6) | 6 (37.5) | 5 (31.3) | 3 (18.8) | 2 (12.5) |
| Other disease | 87 | 1.17 (0-22.0) | 20 (23.0) | 31 (35.6) | 17 (19.5) | 19 (21.8) |
| Demographic characteristics . | No. . | Median VL (range) (copies/100 PBMCs) . | Frequency of subjects by VL level, n (% of row) . | |||
|---|---|---|---|---|---|---|
| Quartile 1 (VL: < 0.29)‡ . | Quartile 2 (VL: 0.29-1.60) . | Quartile 3 (VL: 1.60-4.54) . | Quartile 4 (VL: ≥ 4.54) . | |||
| Total | 1.60 (0-55.8) | 303 | 306 | 304 | 305 | |
| Sex | ||||||
| Male | 426 | 2.10 (0-46.6)* | 84 (19.7) | 100 (23.5) | 93 (21.8) | 149 (35.0) |
| Female | 792 | 1.39 (0-55.8)† | 219 (27.7) | 206 (26.0) | 211 (26.6) | 156 (19.7) |
| Age, y | ||||||
| Younger than 40 | 167 | 1.37 (0-16.4)† | 49 (29.3) | 43 (25.8) | 50 (29.9) | 25 (15.0) |
| 40-49 | 200 | 1.77 (0-41.7)* | 43 (21.5) | 52 (26.0) | 51 (25.5) | 54 (27.0) |
| 50-59 | 273 | 1.84 (0-36.1)* | 64 (23.4) | 64 (23.4) | 63 (23.1) | 82 (30.4) |
| 60-69 | 260 | 1.56 (0-46.6) | 66 (25.4) | 66 (25.4) | 61 (23.5) | 67 (25.8) |
| 70 or older | 318 | 1.52 (0-55.8) | 81 (25.5) | 81 (25.5) | 79 (24.8) | 77 (24.2) |
| First opportunity for HTLV-1 testing | ||||||
| Screening | 661 | 1.46 (0-55.8)† | 182 (27.5) | 160 (24.2) | 175 (26.5) | 144 (21.8) |
| Revelation of HTLV-1–positive family | 134 | 1.45 (0-46.6) | 31 (23.1) | 40 (29.9) | 39 (29.1) | 24 (17.9) |
| During treatment for other diseases | 265 | 1.93 (0-41.7)* | 56 (21.1) | 66 (24.9) | 57 (21.5) | 86 (32.5) |
| Unknown | 158 | 2.08 (0-30.3)* | 34 (21.5) | 40 (25.3) | 33 (20.9) | 51 (32.3) |
| Family history of HTLV-1–related diseases | ||||||
| Absence or carrier/HU/HAU only | 385 | 1.33 (0-32.4)† | 100 (26.0) | 105 (27.2) | 100 (26.0) | 80 (20.8) |
| HAM/TSP | 9 | 3.85 (1.2-9.4)* | 0 | 1 (11.1) | 5 (55.6) | 3 (33.3) |
| ATL | 108 | 2.32 (0-46.6)* | 18 (16.7) | 26 (24.1) | 33 (30.6) | 31 (28.7) |
| Leukemia or lymphoma | 35 | 2.47 (0-12.8)* | 3 (8.6) | 9 (25.7) | 11 (31.4) | 12 (34.3) |
| Unknown family history | 681 | 1.55 (0-55.8) | 182 (26.7) | 165 (24.2) | 155 (22.8) | 179 (26.3) |
| Comorbidity | ||||||
| Absence | 961 | 1.65 (0-55.8)† | 241 (25.1) | 234 (24.4) | 244 (25.4) | 242 (25.2) |
| Infectious diseases | 45 | 2.75 (0-28.6)* | 7 (15.6) | 8 (17.8) | 13 (28.9) | 17 (37.8) |
| Autoimmune diseases | 29 | 1.33 (0-41.7) | 10 (34.5) | 7 (24.1) | 4 (13.8) | 8 (27.6) |
| Malignant diseases | 80 | 1.57 (0-19.4) | 19 (23.8) | 21 (26.3) | 23 (28.8) | 17 (21.3) |
| Skin diseases | 16 | 0.60 (0.07-14.6) | 6 (37.5) | 5 (31.3) | 3 (18.8) | 2 (12.5) |
| Other disease | 87 | 1.17 (0-22.0) | 20 (23.0) | 31 (35.6) | 17 (19.5) | 19 (21.8) |
HTLV-1 indicates human T-cell leukemia virus type 1; VL, HTLV-1 proviral load; PBMCs, peripheral blood mononuclear cells; HU, HTLV-1 uveitis; HAU, HTLV-1–associated uveitis; HAM, HTLV-1 myelopathy; TSP, tropical spastic paraparesis; and ATL, adult T-cell leukemia.
Mann-Whitney test revealed a statistically significant difference in the VL level compared with the reference group.
Reference group.
The VL was categorized based on quartile cutoff points (the 25th, 50th, and 75th percentiles of the VL distribution) in 1218 HTLV-1 carriers. The unit of VL was copies/100 PBMCs.
Among age groups, the median proviral load of those 40 to 49 and 50 to 59 years of age was significantly higher than that of those less than or equal to 40 years (P = .02 and P = .007, respectively). Both age groups were probably distributed in the highest quartile of proviral load levels. Because we found a significantly different median proviral load by sex, we additionally evaluated the proviral load level by age group in each sex. The highest median value was found in those 50 to 59 years of age (2.89 copies/100 PBMCs) in males, but in 40 to 49 years of age (1.49 copies/100 PBMCs) in females, although there were no statistical differences by age group for both sexes (data not shown).
Among the categories for the first opportunity for HTLV-1 testing, the proviral load level was significantly higher (P = .002) in participants informed of their infection during treatment for diseases unrelated to HTLV-1 compared with those who came to know of their infection by screenings (Table 2). Participants informed of their infection during treatment for diseases unrelated to HTLV-1 were probably distributed in the highest quartile of proviral load levels. There was no difference in the proviral load level between those who came to know of their infection by the presence of HTLV-1–positive family members and those who came to know of their infection by screenings.
When we evaluated the proviral load level by family history status, participants who had no family history of HTLV-1 infection, who had only HTLV-1 carriers in the family, who had only an HTLV-1 carrier husband, and who had only HU/HAU in the family were grouped together as a reference category. The proviral load levels of those with a family history of HAM/TSP (median 3.85 copies/100 PBMCs) and ATL (median 2.32 copies/100 PBMCs) were significantly higher (P = .01 and P = .005, respectively) compared with those of the reference group (Table 2). Indeed, those with a family history of HAM/TSP and ATL were probably distributed in the third and fourth quartiles of proviral load levels. Of interest, the median proviral load level of those with a family history of leukemia or lymphoma was also significantly higher (P = .009) compared with those of the reference group.
Among the categories for comorbidity, there was no statistical difference in the proviral load levels when we simply compared between those with and without comorbidity at enrollment (data not shown). However, when we compared those without comorbidity and those with infectious diseases at enrollment, the median proviral load of the latter was significantly higher than that of the former (P = .05; Table 2).
Prognosis
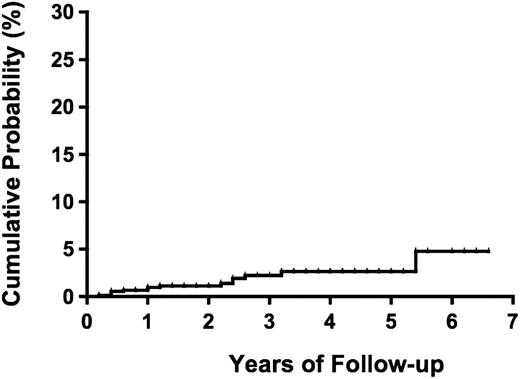
During a median follow-up period of 1.0 year (range, 0-6.6 years) and a total of 1981.2 person-years, 14 (1.1%) participants (4 males and 10 females) progressed to overt ATL (2 acute, 2 lymphoma, and 10 smoldering types; Table 3). The incidence rate of ATL was 7.1 per 1000 person-years for all types of ATL and 2.0 per 1000 person-years for the aggressive types (acute and lymphoma) of ATL. The median duration from date of enrollment to date of diagnosis of ATL was 13.8 months (range, 2.8-64.4 months). The cumulative probability of progression to ATL was reached 4.8% (95% CI, 1.9%-11.8%) at 5.4 years (Figure 2).
Cases who developed ATL from HTLV-I carrier status
| Case no. . | Demographic characteristics . | Baseline clinical and biologic values . | ATL development . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex . | Age, y . | Place of birth . | First opportunity for HTLV-1 testing . | Family history of HTLV-1–related disease . | Comorbidity at enrollment . | HTLV-1 VL, copies/100 PBMCs . | sIL-2R, U/mL . | Abnormal lymphocytes, percentage . | LDH, IU/L . | WBC, ×103/mm3 . | Clinical type . | Duration from enrollment, mo . | |
| Progression to aggressive type of ATL | |||||||||||||
| 1 | M | 79.9 | Southern | ATL family | ATL | None | 5.47 | 479 | 2 | 157 | 4200 | Acute | 7.4 |
| 2 | F | 70.3 | Southern | ATL family | ATL | None | 4.73 | 904 | 0 | 365 | 9130 | Acute | 38.6 |
| 3 | M | 71.9 | Southern | Other disease | None | Skin disease | 4.17 | 1450 | 0 | 351 | 5140 | Lymph | 4.6 |
| 4 | F | 75.8 | Southern | Unknown | Unknown | None | 10.52 | 2080 | 3 | 308 | 3600 | Lymph | 30.6 |
| Progression to indolent type of ATL | |||||||||||||
| 5 | F | 60.0 | Southern | Other disease | Unknown | None | 9.12 | 340 | 14 | 192 | 5100 | Sm | 6.0 |
| 6 | F | 71.9 | Southern | Multiphasic screening | None | None | 10.60 | 1320 | 2 | 199 | 4000 | Sm | 29.8 |
| 7 | F | 59.5 | Southern | Multiphasic screening | None | None | 21.90 | 635 | 4.5 | 188 | 4100 | Sm | 12.0 |
| 8 | F | 74.0 | Southern | Other disease | Unknown | Gallbladder cancer | 10.11 | 1110 | 2 | 240 | 2700 | Sm | 26.8 |
| 9 | F | 54.1 | Southern | Other disease | Unknown | None | 18.85 | 971 | 2 | 198 | 5660 | Sm | 29.0 |
| 10 | F | 43.3 | Southern | Pregnancy | ATL | None | 13.90 | 372 | 1 | ND | 5400 | Sm | 64.4 |
| 11 | F | 62.2 | Southern | Other disease | Unknown | Eye disease | 6.86 | 1560 | ND | 508 | 12100 | Sm | 6.0 |
| 12 | M | 57.6 | Southern | Other disease | Unknown | None | 7.67 | ND | 2 | 234 | 5500 | Sm | 15.4 |
| 13 | F | 41.0 | Metropolitan | Pregnancy | None | None | 12.14 | 349 | 2.5 | 189 | 7690 | Sm | 12.2 |
| 14 | M | 66.1 | Southern | Other disease | None | Prostatitis | 28.58 | 2660 | 0 | 158 | 8500 | Sm | 2.8 |
| Case no. . | Demographic characteristics . | Baseline clinical and biologic values . | ATL development . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex . | Age, y . | Place of birth . | First opportunity for HTLV-1 testing . | Family history of HTLV-1–related disease . | Comorbidity at enrollment . | HTLV-1 VL, copies/100 PBMCs . | sIL-2R, U/mL . | Abnormal lymphocytes, percentage . | LDH, IU/L . | WBC, ×103/mm3 . | Clinical type . | Duration from enrollment, mo . | |
| Progression to aggressive type of ATL | |||||||||||||
| 1 | M | 79.9 | Southern | ATL family | ATL | None | 5.47 | 479 | 2 | 157 | 4200 | Acute | 7.4 |
| 2 | F | 70.3 | Southern | ATL family | ATL | None | 4.73 | 904 | 0 | 365 | 9130 | Acute | 38.6 |
| 3 | M | 71.9 | Southern | Other disease | None | Skin disease | 4.17 | 1450 | 0 | 351 | 5140 | Lymph | 4.6 |
| 4 | F | 75.8 | Southern | Unknown | Unknown | None | 10.52 | 2080 | 3 | 308 | 3600 | Lymph | 30.6 |
| Progression to indolent type of ATL | |||||||||||||
| 5 | F | 60.0 | Southern | Other disease | Unknown | None | 9.12 | 340 | 14 | 192 | 5100 | Sm | 6.0 |
| 6 | F | 71.9 | Southern | Multiphasic screening | None | None | 10.60 | 1320 | 2 | 199 | 4000 | Sm | 29.8 |
| 7 | F | 59.5 | Southern | Multiphasic screening | None | None | 21.90 | 635 | 4.5 | 188 | 4100 | Sm | 12.0 |
| 8 | F | 74.0 | Southern | Other disease | Unknown | Gallbladder cancer | 10.11 | 1110 | 2 | 240 | 2700 | Sm | 26.8 |
| 9 | F | 54.1 | Southern | Other disease | Unknown | None | 18.85 | 971 | 2 | 198 | 5660 | Sm | 29.0 |
| 10 | F | 43.3 | Southern | Pregnancy | ATL | None | 13.90 | 372 | 1 | ND | 5400 | Sm | 64.4 |
| 11 | F | 62.2 | Southern | Other disease | Unknown | Eye disease | 6.86 | 1560 | ND | 508 | 12100 | Sm | 6.0 |
| 12 | M | 57.6 | Southern | Other disease | Unknown | None | 7.67 | ND | 2 | 234 | 5500 | Sm | 15.4 |
| 13 | F | 41.0 | Metropolitan | Pregnancy | None | None | 12.14 | 349 | 2.5 | 189 | 7690 | Sm | 12.2 |
| 14 | M | 66.1 | Southern | Other disease | None | Prostatitis | 28.58 | 2660 | 0 | 158 | 8500 | Sm | 2.8 |
ATL indicates adult T-cell leukemia; HTLV-1, human T-cell leukemia virus type 1;VL, HTLV-1 proviral load; PBMCs, peripheral blood mononuclear cells; sIL-2R, soluble interleukin-2 receptor; LDH, lactate dehydrogenase; WBC, white blood cell count; Sm, smoldering type; and ND, not done.
Probability of progression to ATL among 1218 asymptomatic HTLV-1 carriers.
The median proviral load at enrollment for these 14 participants was 10.3 copies/100 PBMCs (range, 4.17-28.58 copies/100 PBMCs), which was significantly higher than those who did not develop ATL (1.56 copies/100 PBMCs; range, 0-55.8 copies/100 PBMCs; P < .001). Of interest, the median proviral load level at enrollment was significantly higher for those who developed smoldering types of ATL than for those who developed aggressive types of ATL (11.4 and 5.1 copies/100 PBMCs, respectively, P = .02), whereas the median entry age was significantly younger for the former than for the latter (59.8 and 73.9 years, respectively, P = .02). Distribution of the 14 participants who developed ATL by demographic characteristics and by quartile of proviral load levels is shown in Table 4. Among 14 ATLs, 13 occurred in the highest quartile of baseline proviral load (> 4.54 copies/100 PBMCs) and 1 occurred in the third quartile (1.60-4.54 copies/100 PBMCs), whereas no ATL developed in quartiles 1 and 2 (< 1.60 copies/100 PBMCs). A high frequency of ATL was also seen in older age group, those with first opportunity for HTLV-1 testing during treatment of other diseases and those with a family history of ATL. Therefore, we decided to include the baseline HTLV-1 proviral load (the square-root transformed continuous value), age, first opportunity for HTLV-1 testing, and family history into Cox hazard analyses as covariates to test the effects on the development of ATL.
Frequency of subjects who developed ATL by demographic characteristics and by VL level
| Demographic characteristics . | No. of subjects . | No. of ATLs (% of subjects) . | Frequency of ATL by VL level, n (% of subjects in each quartile in Table 2) . | ||
|---|---|---|---|---|---|
| Quartiles 1 and 2 (VL: < 1.60)* . | Quartile 3 (VL: 1.60-4.54) . | Quartile 4 (VL: ≥ 4.54) . | |||
| Total | 1218 | 14 (1.1) | 0 | 1 (0.3) | 13 (4.3) |
| Sex | |||||
| Male | 426 | 4 (0.9) | 0 | 1 (1.1) | 3 (2.0) |
| Female | 792 | 10 (1.3) | 0 | 0 | 10 (6.4) |
| Age, y | |||||
| Younger than 40 | 167 | 0 | — | — | — |
| 40-49 | 200 | 2 (1.0) | 0 | 0 | 2 (3.7) |
| 50-59 | 273 | 3 (1.1) | 0 | 0 | 3 (3.7) |
| 60-69 | 260 | 3 (1.2) | 0 | 0 | 3 (4.5) |
| 70 or older | 318 | 6 (1.9) | 0 | 1 (1.3) | 5 (6.5) |
| First opportunity for HTLV-1 testing | |||||
| Screening | 661 | 4 (0.6) | 0 | 0 | 4 (2.8) |
| Revelation of HTLV-1-positive family | 134 | 2 (1.5) | 0 | 0 | 2 (8.3) |
| During treatment for other diseases | 265 | 7 (2.6) | 0 | 1 (1.8) | 6 (7.0) |
| Unknown | 158 | 1 (0.6) | 0 | 0 | 1 (2.0) |
| Family history of HTLV-1–related diseases | |||||
| Absence or carrier/HU/HAU only | 385 | 5 (1.3) | 0 | 1 (1.0) | 4 (5.0) |
| HAM/TSP | 9 | 0 | — | — | — |
| ATL | 107 | 3 (2.8) | 0 | 0 | 3 (9.7) |
| Leukemia or lymphoma | 36 | 0 | 0 | 0 | 0 |
| Unknown family history | 681 | 6 (0.9) | 0 | 0 | 6 (3.4) |
| Comorbidity | |||||
| Absence | 961 | 10 (1.0) | 0 | 0 | 10 (4.1) |
| Infectious diseases | 45 | 1 (2.2) | 0 | 0 | 1 (5.9) |
| Autoimmune diseases | 29 | 0 | — | — | — |
| Malignant diseases | 80 | 1 (1.3) | 0 | 0 | 1 (5.9) |
| Skin diseases | 16 | 1 (6.3) | 0 | 1 (33.3) | 0 |
| Other disease | 87 | 1 (1.1) | 0 | 0 | 1 (5.3) |
| Demographic characteristics . | No. of subjects . | No. of ATLs (% of subjects) . | Frequency of ATL by VL level, n (% of subjects in each quartile in Table 2) . | ||
|---|---|---|---|---|---|
| Quartiles 1 and 2 (VL: < 1.60)* . | Quartile 3 (VL: 1.60-4.54) . | Quartile 4 (VL: ≥ 4.54) . | |||
| Total | 1218 | 14 (1.1) | 0 | 1 (0.3) | 13 (4.3) |
| Sex | |||||
| Male | 426 | 4 (0.9) | 0 | 1 (1.1) | 3 (2.0) |
| Female | 792 | 10 (1.3) | 0 | 0 | 10 (6.4) |
| Age, y | |||||
| Younger than 40 | 167 | 0 | — | — | — |
| 40-49 | 200 | 2 (1.0) | 0 | 0 | 2 (3.7) |
| 50-59 | 273 | 3 (1.1) | 0 | 0 | 3 (3.7) |
| 60-69 | 260 | 3 (1.2) | 0 | 0 | 3 (4.5) |
| 70 or older | 318 | 6 (1.9) | 0 | 1 (1.3) | 5 (6.5) |
| First opportunity for HTLV-1 testing | |||||
| Screening | 661 | 4 (0.6) | 0 | 0 | 4 (2.8) |
| Revelation of HTLV-1-positive family | 134 | 2 (1.5) | 0 | 0 | 2 (8.3) |
| During treatment for other diseases | 265 | 7 (2.6) | 0 | 1 (1.8) | 6 (7.0) |
| Unknown | 158 | 1 (0.6) | 0 | 0 | 1 (2.0) |
| Family history of HTLV-1–related diseases | |||||
| Absence or carrier/HU/HAU only | 385 | 5 (1.3) | 0 | 1 (1.0) | 4 (5.0) |
| HAM/TSP | 9 | 0 | — | — | — |
| ATL | 107 | 3 (2.8) | 0 | 0 | 3 (9.7) |
| Leukemia or lymphoma | 36 | 0 | 0 | 0 | 0 |
| Unknown family history | 681 | 6 (0.9) | 0 | 0 | 6 (3.4) |
| Comorbidity | |||||
| Absence | 961 | 10 (1.0) | 0 | 0 | 10 (4.1) |
| Infectious diseases | 45 | 1 (2.2) | 0 | 0 | 1 (5.9) |
| Autoimmune diseases | 29 | 0 | — | — | — |
| Malignant diseases | 80 | 1 (1.3) | 0 | 0 | 1 (5.9) |
| Skin diseases | 16 | 1 (6.3) | 0 | 1 (33.3) | 0 |
| Other disease | 87 | 1 (1.1) | 0 | 0 | 1 (5.3) |
ATL indicates adult T-cell leukemia; HTLV-1, human T-cell leukemia virus type 1; VL, HTLV-1 proviral load; HU, HTLV-1 uveitis; HAU, HTLV-1–associated uveitis; HAM, HTLV-1 myelopathy; TSP, tropical spastic paraparesis; and —, not applicable.
The VL was categorized based on quartile cutoff points (the 25th, 50th, and 75th percentiles of the VL distribution) in 1218 HTLV-1 carriers. The unit of VL was copies/100 PBMCs.
We identified that baseline proviral load was strongly associated with the risk of progression to ATL on both univariate and multivariate Cox analyses. In the multivariate analysis, the adjusted HR for the square-root transformed proviral load per unit increase was 3.57 (95% CI, 2.25-5.68; Table 5). We also found that advanced age, family history of ATL, and first opportunity to learn of HTLV-1 infection during treatment of other diseases were independently associated with the development of ATL, after adjusting the effect of proviral load. The adjusted HR for developing ATL per 5-year increase of age from 40 years was 1.67 (95% CI, 1.12-2.50). HTLV-1 carriers having a family history of ATL had 12 times higher risk of developing ATL compared with those not having the history (adjusted HR = 12.1; 95% CI, 2.26-64.7), and those who came to know their HTLV-1 infection during treatment for other diseases had 4 times higher risk of developing ATL compared with references (adjusted HR = 4.16; 95% CI, 1.37-12.6), although the CIs were wide because of the smaller group sizes (Table 5). Of interest, male gender was not a significant risk factor for developing ATL, even though the median proviral load was significantly higher in males than in females (Table 2).
Cox proportional hazards modeling of risk factors for ATL development
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Male sex (vs female) | 0.74 (0.23-2.37) | .61 | 0.38 (0.12-1.18) | .09 |
| Square-root transformed VL per unit increase | 2.55 (1.91-3.41) | < .001 | 3.57 (2.25-5.68) | < .001 |
| Age per 5-year increase from 40 y | 1.20 (0.94-1.53) | .15 | 1.67 (1.12-2.50) | .012 |
| Family history of ATL (vs others) | 2.68 (0.80-8.98) | .11 | 12.1 (2.26-64.7) | .004 |
| First opportunity for HTLV-1 testing during treatment of other diseases (vs others) | 3.40 (1.12-10.28) | .03 | 4.16 (1.37-12.6) | .012 |
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| Male sex (vs female) | 0.74 (0.23-2.37) | .61 | 0.38 (0.12-1.18) | .09 |
| Square-root transformed VL per unit increase | 2.55 (1.91-3.41) | < .001 | 3.57 (2.25-5.68) | < .001 |
| Age per 5-year increase from 40 y | 1.20 (0.94-1.53) | .15 | 1.67 (1.12-2.50) | .012 |
| Family history of ATL (vs others) | 2.68 (0.80-8.98) | .11 | 12.1 (2.26-64.7) | .004 |
| First opportunity for HTLV-1 testing during treatment of other diseases (vs others) | 3.40 (1.12-10.28) | .03 | 4.16 (1.37-12.6) | .012 |
Analyses were performed using robust sandwich variance estimates.
ATL indicates adult T-cell leukemia; HTLV-1, human T-cell leukemia virus type 1; VL, HTLV-1 proviral load; HR, hazard ratio; and CI, confidence interval.
Because the distribution of proviral load was skewed even after the value was square-root transformed, it was possible that ATL events in subjects with skewed high proviral loads contributed to results. To check the possibility, we performed a multivariate analysis using a sub-dataset that excluded subjects with skewed proviral load (> 16 copies in Figure 1C; n = 39, including 3 who developed ATL). Nevertheless, we observed similar results as the original dataset, although age factor was no longer statistically significant (P = .07; supplemental Table 1). It is also possible that effects of some of the risk factors are weighted because of only 1 patient with an event because only 14 were analyzed as events in the multivariate analyses. To check the possibility, we performed 14 leave-one-out analyses, omitting 1 of 14 cases at a time from the original dataset. The Jackknifed coefficient of each parameter revealed the stability, which indicated that none of 14 cases affected the original model (data not shown).
Discussion
Previous studies reported no significant differences in the HTLV-1 proviral load by sex and age in asymptomatic HTLV-1 carriers.21,22,24,33 In the present study, however, we found that there were significant differences in the proviral load by sex and age (Table 2). The median HTLV-1 proviral load was significantly higher in males than females. The median HTLV-1 proviral load for those 40 to 49 and 50 to 59 years of age was significantly higher than for those less than or equal to 40 years. The discrepancy between results of previous studies and those of the present study may be primarily explained by the differences in study population characteristics. We also found sex differences in age distributions of HTLV-1 proviral load; in male subjects, the median proviral load level was the highest at 50 to 59 years of age, whereas in female subjects it was highest at 40 to 49 years of age, although there were no statistical differences. These distribution characteristics of HTLV-1 proviral load are of interest when we consider the differences in sex and age at onset between ATL and HAM/TSP. ATL occurs predominantly in older males (∼ 60 years), whereas HAM/TSP occurs predominantly in middle-aged females (∼ 45-55 years). Thus, the proviral load levels of asymptomatic HTLV-1 carriers might be the highest in the age groups approximately 5 to 10 years before the average age at onset of ATL and HAM/TSP. These distribution characteristics may be related to differences in host immune responses to HTLV-1 and other unknown host factors.34
The present study revealed that the median proviral load level of those with a family history of ATL or HAM/TSP was significantly higher than for those with no family history (Table 2). These results support previous studies indicating that HTLV-1–infected blood donors and asymptomatic carriers with familial HAM/TSP or ATL tend to have a higher HTLV-1 proviral load than those without family history.21,33 In the present study, the proviral loads were also higher in those with a family history of leukemia or lymphoma than those without such history. We assume that the family history of leukemia or lymphoma may have included some ATL cases because some participants provided a diagnosis as just unknown leukemia or lymphoma. Although the present study was a large cohort, data collection regarding family history of HTLV-1–associated diseases was insufficient because one-half of the participants did not know their family HTLV-1 status. Further detailed data collection is needed to confirm the characteristics of HTLV-1 proviral load levels by family histories among asymptomatic HTLV-1 carriers, as this is necessary to determine genetic determinants of HTLV-1–associated diseases.
HTLV-1 carriers have various comorbidities, such as infectious, autoimmune, and malignant diseases.4,25,35-38 In the present study, 45 participants had various infectious diseases at enrollment (Table 1). We found that the median proviral load of these participants was significantly higher than that of those with no comorbidity (Table 2). The results of the present study support previous reports indicating higher HTLV-1 proviral loads in HTLV-1 carriers with comorbid Strongyloides stercoralis or bladder and kidney infections than those without such infections.25,35,36 HTLV-1 carriers with rheumatoid arthritis or connective tissue disease and those with myelodysplastic syndromes carrying HLA-A26 were also reported to have higher HTLV-1 proviral loads compared with the median proviral load of those without such diseases.37,38 In the present study, however, the median proviral load was not significantly high in those with autoimmune and malignant diseases. Further studies are required to find other predisposing factors affecting the proviral load level in each person.
A high HTLV-1 proviral load is currently considered as one of the main indicators for the progression to ATL.20,28 In the present study, 14 participants of asymptomatic HTLV-1 carriers progressed to overt ATL as of 2009, all of whose baseline proviral load levels were high (range, 4.17-28.58 copies/100 PBMCs; Table 3). Therefore, we suggest that those with a high proviral load level (∼ > 4 copies/100 PBMCs) are in a high-risk group for developing ATL (this group accounted for ∼ 29% of the cohort). Multivariate Cox analyses confirmed that a higher proviral load level was a strong factor in the development of ATL (Table 5). This result strongly supports previous small-scale studies.20,28 However, the role of the high proviral load level still remains unclear because the majority of asymptomatic carriers with a high HTLV-1 proviral load level in the present study remain carrier status. In the present study, male gender was not a significant risk factor for ATL, even though the median proviral load was significantly higher in males than in females. A high HTLV-1 proviral load is also reported to be associated with HAM/TSP.20,21,27 These findings suggest that a high proviral load alone is not a unique predictive marker for ATL. In addition, the present study showed that the median proviral load level at enrollment was lower in those who developed aggressive types of ATL (5.1 copies/100 PBMCs) than that in those who developed smoldering types of ATL (11.4 copies/100 PBMCs; P = .02). This also suggests that a high proviral load alone is not a predictive marker for aggressive types of ATL.
In the present study, multivariate Cox analysis indicated that increased age, family history of ATL, and first opportunity to learn of HTLV-1 infection during treatment of other diseases were also independent risk factors for the development of ATL, after adjusting for proviral load (Table 5). This suggests that multiple risk factors (including unknown factors) are related to the progression from HTLV-1 carrier status to ATL. The reason why “opportunity to learn of HTLV-1 infection during treatment of other diseases” was an independent risk factor is unknown. The findings that more advanced states of HTLV-1 carriers (ie, an intermediate state6 and a preleukemic state13 ) tend to be complicated by various comorbid diseases and that HTLV-1 carriers with various comorbid diseases had higher HTLV-1 proviral loads25,35-38 could in part explain the reason.
Some prospective studies serially evaluated HTLV-1 proviral loads in HTLV-1 carriers and reported that their proviral load level was relatively stable over time with a certain level of fluctuations for persons.25,26,28 Taylor et al reported that proviral loads of 20 HTLV-1 carriers were stable over a mean of 27 months, even though 9 carriers with various comorbidities showed high proviral load levels.25 Meanwhile, an increasing proviral load was observed before progression to HAM/TSP and ATL.27,28 However, there remain more questions how much of the fluctuations in proviral load over time could predict disease progression over the natural fluctuations within persons. Factors other than the proviral load level might be influencing the development of HTLV-1–associated diseases. Future studies should perform serial evaluations of HTLV-1 proviral loads by considering risk factors that have been confirmed in the present study.
The present study has several limitations. The number of ATL events was very small to obtain a conclusive result. However, we have a confidence for our results because we used a robust variance estimate in the multivariate analysis and because 2 validity analyses confirmed the original results. Data collection was insufficient for some items in the questionnaire. To resolve this issue, we will need to administer the questionnaire repeatedly. Our study design did not include enough information for evaluating the development of HAM/TSP. The follow-up duration is too short with regard to the natural history of ATL that has a long latency. Further follow-up of this cohort and similar prospective investigations should provide data needed to support more detailed conclusions. We did not compare the proviral loads by place of enrollment because we realized that many HTLV-1 carriers have migrated from the southern area to the metropolitan area.39 The migration of HTLV-1 carriers has raised some public health issues in Japan. Screening for HTLV-1 in pregnant women and prevention programs for mother-to-child transmission of HTLV-1 are conducted in endemic areas40,41 but not in metropolitan areas, which could introduce a higher chance of new HTLV-1 infections in the metropolitan area. To date, there is no nationwide program for preventing new HTLV-1 infections in Japan. Further nationwide studies are needed to determine the precise numbers of HTLV-1 carriers and to prevent HTLV-1 infection.
In conclusion, the present cohort study of 1218 asymptomatic HTLV-1 carriers provided detailed distributions for HTLV-1 proviral loads regarding the host-specific characteristics and the associations with the development of ATL. We confirmed that a higher proviral load levels (especially ∼ > 4 copies/100 PBMCs), advanced age, family history of ATL, and having the first opportunity to learn of HTLV-1 infection during treatment of other diseases were independent risk factors for progression from carrier status to ATL. Further large-scale epidemiologic studies are needed to clearly identify the determinants of ATL for early detection and rapid cure for HTLV-1–associated diseases.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank staff members in all collaborating institutions and Mr Makoto Nakashima, Ms Takako Akashi, and other technical members in the central office of the JSPFAD for efforts in sample processing and biologic assays.
This work was supported by the Ministry of Education, Culture, Sports, Science and Technology of Japan (Grant-in-Aid for Scientific Research on Priority Areas 17015047).
Authorship
Contribution: M.I. managed the study database, analyzed data, and wrote the manuscript; T.W. organized the study and managed processing of the samples and measurement of proviral loads; A.U., A.O., K. Uchimaru, K.-R.K., M.O., H.K., K. Uozumi, M.M., K.T., Y. Saburi, M.Y., J.T., and Y.M. were responsible for participant enrollment and data collection; Y. Sagara managed the biomaterial bank; S.H. organized the study and managed the database; S.K. and K.Y. established the study; and all authors critically reviewed the article and approved the final version.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of JSPFAD participants is available online in the supplemental Appendix.
Correspondence: Masako Iwanaga, Department of Hematology and Molecular Medicine, Atomic Bomb Disease Institute, Nagasaki University Graduate School of Biomedical Sciences, 1-12-4 Sakamoto, Nagasaki, 852-8523, Japan; e-mail: masakoiwng@gmail.com.

![Figure 1. Distribution of baseline HTLV-1 proviral load levels among 1218 asymptomatic HTLV-1 carriers. (A) Scatter plot of raw data of proviral load (left) and the vertical box and whiskers plot (right): the box delineates 25th percentile (0.29 copies/100 periopheral blood mononuclear cells [PBMCs]), median (1.60 copies/100 PBMCs), and 75th percentiles (4.54 copies/100 PBMCs), and the whiskers delineate the minimum (0 copies/100 PBMCs) and maximum (55.8 copies/100 PBMCs). (B) Scatter plot of square-root transformed values of the raw proviral load (left) and the vertical box and whiskers plot (right): the box delineates 25th percentile (0.54 copies/100 PBMCs), median (1.26 copies/100 PBMCs), and 75th percentiles (2.13 copies/100 PBMCs), and the whiskers delineate the minimum (0 copies/100 PBMCs) and maximum (7.47 copies/100 PBMCs). (C) The frequency of participants in the quartile distributions of proviral load. Q1 indicates quartile 1 (< 25th percentile); Q2, quartile 2 (25th percentile to median); Q3, quartile 3 (median to 75th percentile); Q4: quartile 4 (> 75th percentile); Sqrt, square-root transformation; and N, number of participants.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/116/8/10.1182_blood-2009-12-257410/4/m_zh89991055450001.jpeg?Expires=1767726128&Signature=mEGJoTORZJ-6axssBTcOg~2rdlc0vvkdgW6ZA5q2LJK~Aj55nEtggGt4I6lqNe-Iir0tRDCBrp1VDdCJ8FgLcC8mxJ-aDBw5P96Oj99vV98urEMN3~qxNytCpB76KCfnKiLVXzPiPW0npGX6pmmmVifYJBNRkxUAb9q2WQyux8gS7M0zKXnnGXi5yklbPXLFP9IifoYYJhgmhLmS3-pm52V6IXUKZtYd4UeSQoKYarsv7q79KTTS1p4KJUuTU2u1~sLo0dFk3Ijh8nsWrWp7foVTUIXTpbBpKmRqMdjEBkEY3tupg3qGSrPSzYTQ4y9THu-q0k0xY~I0lhOpuaUorg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)