Abstract
T-cell development is critically dependent on the activities of the Src-family kinases p56lck and p59fyn. While Lck plays a dominant role in the initiation of T-cell receptor (TCR) signaling and in thymocyte differentiation, Fyn plays a more subtle regulatory role. We sought to determine the role of intracellular localization in the differing functions of Lck and Fyn in T cells. By generating transgenic mice that express chimeric Lck-Fyn proteins, we showed that the N-terminal unique domain determines the intracellular localization and function of Lck in pre-TCR and mature αβTCR signaling in vivo. Furthermore, coexpression of a “domain-swap” Lck protein containing the Fyn unique domain with an inducible Lck transgene resulted in the development of thymomas. In contrast to previous reports of Lck-driven thymomas, tumor development was dependent on either pre-TCR or mature TCR signals, and was completely ablated when mice were crossed to a recombination activating gene 1 (Rag1)–deficient background. These data provide a mechanistic basis for the differing roles of Lck and Fyn in T-cell development, and show that intracellular localization as determined by the N-terminal unique domains is critical for Src-family kinase function in vivo.
Introduction
Thymocyte differentiation is regulated at 2 key checkpoints. The first acknowledges successful rearrangement and expression of a T-cell receptor β (TCRβ) chain, which, in combination with the pre-Tα chain, permits ligand-independent signaling. As a result, CD4−CD8− double-negative 3 (DN3) thymocytes enforce allelic exclusion of the TCRβ locus, proliferate, and express CD4 and CD8 coreceptors. The second checkpoint occurs upon expression of a rearranged TCRα chain by CD4+CD8+ double-positive (DP) thymocytes, which facilitates ligand-dependent signals transduced by the αβTCR interacting with the major histocompatibility complex (MHC) and results in 3 distinct cell fates: (1) “death by neglect” in the absence of signal; (2) negative selection, in the case of high-avidity interactions; and (3) positive selection from low-avidity interactions. Src-family protein tyrosine kinases (SFKs), particularly Lck, are critical for transition through these checkpoints.
Studies have shown that thymocyte development is substantially blocked at the DN3 stage in the absence of Lck, and is completely blocked in the absence of both Lck and Fyn.1 Furthermore, positive selection of DP thymocytes is greatly impaired and few mature T cells are present in the periphery of Lck−/− mice. Loss of Fyn alone does not impair thymocyte development, and peripheral T cells are present in normal numbers in Fyn−/− mice.2 Therefore, although SFKs have partially redundant functions in thymopoiesis, Lck is critical for normal thymocyte development and Fyn plays a minor compensatory role.
Several studies have focused on the role of functional domains within Lck and Fyn. Both proteins have N-terminal sites of myristoylation and palmitoylation critical for membrane association, which share little homology and are called “unique” domains. In addition, they have highly homologous Src homology 3 (SH3) and SH2 domains, a catalytic domain, and a C-terminal-negative regulatory tyrosine residue (Tyr505 for Lck, Tyr528 for Fyn).3,4 This unique domain is critical for the association of Lck with CD4 and CD8,5-7 and is required for recruitment of Fyn to the CD3 complex.5 Furthermore, the unique domain might be important in the association of Lck with phosphatases and dephosphorylation of Tyr505 by CD45,8,9 whereas studies using truncated versions of Lck have shown that the unique domain may also affect substrate specificity.10
Given that the unique domains are key in determining the cellular localization of Lck and Fyn, it seems likely that these regions are fundamental determinants of the specific roles of these kinases in vivo. We sought to test this hypothesis by generating transgenic mice on an Lck−/− background that express a “domain-swap” version of Lck in which the unique domain has been replaced with that of Fyn; these are called Fyn-unique Lck (FU-Lck). T-cell development in these mice was compared with control transgenic mice expressing similar levels of wild-type (WT) Lck. These experiments showed that the Lck unique domain was important for both DN-DP transition and selection of single-positive (SP) cells during thymocyte development. Interestingly, when domain-swap FU-Lck transgenic mice, but not WT Lck transgenic mice, were crossed to an Lck-inducible transgenic background, thymomas developed in 100% of individuals. This process differed from previous reports of thymomas caused by overexpression of dysregulated Lck in that tumor development was dependent on the expression of TCR components. These data indicate a critical role for the unique domain of Lck in the normal development of T cells, and provide a mechanistic basis for the differing roles of Lck and Fyn in T cells.
Methods
Mice
The FU-Lck transgene was constructed by swapping the Lck unique domain (a.ac. 1-61) for the Fyn unique domain (a.ac 1-85) and adding a C-terminal V5 epitope tag. FU-Lck cDNA was sequenced and cloned into the VA expression vector containing the human CD2 control regions.11 Control Lck cDNA containing a V5 tag was subcloned into the VA vector. Transgenic mice were generated by pro-nuclear injection into (CBAxC57Bl/10)F2 fertilized oocytes. Mice positive for transgene expression were identified by slot-blot and polymerase chain reaction (PCR) analysis and bred to an Lck−/− background.12 Where indicated, mice were crossed with Lck-inducible (Lckind) Lck−/−13 and LckindLck−/−Rag1−/− F5 TCR-transgenic mice (Rag1 stands for recombination activating gene 1).14 Lckind mice were fed doxycycline in their food (1 mg/g) from birth. C57BL/6 mice served as WT controls. Mice were maintained under local and United Kingdom Home Office guidelines.
Flow cytometry
Antibodies were from eBioscience, BD Pharmingen, or Cell Signaling Technology. For intracellular staining, cells were permeabilized with 0.5% saponin or fixed in 2% formaldehyde and permeabilized in 90% ice-cold methanol. Samples were collected on a flow cytometer (FACSCalibur, BD BioSciences) and analyzed using FlowJo Version 8.8.6 software (TreeStar).
For analysis of Ca2+ mobilization, thymocytes were loaded with indo-1-AM calcium sensor dye (Sigma-Aldrich) and stained with biotinylated TCR (H57-597) and CD4 (RM4.5) monoclonal antibodies (mAbs). CD8α-PE (53-6.7) and CD4-FITC (YTA3.1) Abs were used to identify thymus cell populations. Biotinylated Abs were cross-linked using streptavidin-allophycocyanin conjugates, and samples were collected on a flow cytometer (LSR II; BD BioSciences).
PCR analysis of TCR gene rearrangements
DNA was prepared from thymocytes after lysis in 100mM Tris (pH 8), 5mM ethylenediaminetetraacetic acid (EDTA), 0.2% sodium dodecyl sulfate (SDS), 200mM NaCl, and 100 μg/mL proteinase K. The primers used were (Dβ2.1) GTAGGCACCTGTGGGGAAGAAACT and (Jβ2.7) TGAGAGCTGTCTCCTA C TATCCATT.15
Western blotting and immunoprecipitation
Cells were lysed in 1% Triton X-100, 0.5% n-dodecyl-b-D-maltoside, 50mM Tris-HCl (pH 7.5), 150mM NaCl, 20mM EDTA, 1mM NaF, and 1mM sodium orthovanadate containing protease inhibitors, and samples were separated by SDS–polyacrylamide gel electrophoresis (SDS-PAGE). For immunoprecipitations, lysates were precleared with 10 μL of protein A-Sepharose® (Sigma-Aldrich) slurry before immunoprecipitation with 2 μg of anti-CD3ϵ (2C11), anti-CD4 (YTA3.1), or anti-CD8β (YTS-156) coupled to Sepharose beads. Immunoprecipitates were washed with lysis buffer, and proteins were transferred to polyvinylidene difluoride membranes (Millipore). Membranes were incubated in blocking reagent (LI-COR Biosciences) before immunoblotting with anti-V5, anti-extracellular signal–regulated kinase 2 (anti-ERK2; Santa Cruz Biotechnology), anti-phosphotyrosine (anti-pTyr; 4G10), anti–ζ-chain-associated protein kinase 70 (anti-ZAP70), anti-ZAP70 pY319, anti-Lck, anti-Lck pY505, anti-Src pY416, and anti-caspase 3 (Cell Signaling Technology). Proteins were detected with secondary Abs and visualized using an infrared imaging system (Odyssey; LI-COR Biosciences).
Results
Defects in T-cell development in domain-swap FU-Lcktg mice
To study the role of the unique domain of Lck, we generated transgenic mice expressing WT Lck (Lcktg) or a domain-swapped version of Lck in which the unique domain was replaced with that of Fyn (FU-Lcktg) (Figure 1A). Western blot analysis using a V5 mAb specific for an introduced epitope tag showed that line 1 of the Lcktg mice and line 3 of the FU-Lcktg mice expressed similar levels of protein (Figure 1B). In both cases, the level of expression of transgenic Lck was low (< 5% of WT levels; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). These mice allowed us to determine the impact of expression of low levels of WT Lck on T-cell development, as well as the function of the Lck unique domain in this process.
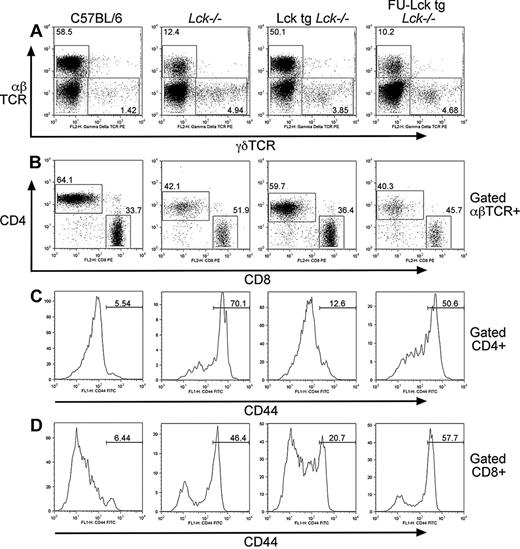
The Lck unique domain is required for T-cell development. (A) Schematic showing structural domains of WT (top) and FU-Lck (bottom) proteins. (B) Western blot analysis showing relative expression levels of Lck and FU-Lck proteins in thymocyte lysates from mice from lines 1 (L1) and 2 (L2) of Lcktg mice and lines 1 and 3 (L3) of FU-Lcktg mice. A V5 mAb was used to detect transgenic Lck, which was tagged with the V5 epitope, while ERK reprobes served as loading controls. (C) Dot plots showing distribution of T-cell subsets in thymi from 7-week-old WT, Lck−/−, Lcktg/Lck−/−, and FU-Lcktg/Lck−/− mice as defined by surface CD4 and CD8 expression and FACS analysis. Values within dot plots represent proportions of cells within CD4 SP, DP, and CD8 SP gates. Dot plots represent staining from 1 of at least 8 mice of each genotype. (D) FACS dot plots showing distribution of DN populations in mice thymi. γδTCR−CD4−CD8− cells were subdivided into DN1 to DN4 populations by expression of CD44 and CD25: DN1 = CD44+CD25−; DN2 = CD44+CD25+; DN3 = CD44−CD25+; and DN4 = CD44−CD25−. Values represent the proportion of cells in each DN population, and dot plots represent analysis of 1 of at least 5 mice of each genotype. (E) Histograms showing FACS analysis of γδTCR expression in CD4−CD8− DN populations from mouse thymi. Values represent proportions of γδTCR-positive and TCR-negative cells as defined by the respective gates. Data are representative of 1 of 5 mice analyzed of each genotype. (F) Histograms showing DP thymocyte expression of surface αβTCR (left side) and CD5 (right side). Filled histograms represent levels of expression of αβTCR or CD5 in WT DP cells; fine black line overlays represent Lck−/− levels; bold black overlays represent Lcktg/Lck−/− levels; and bold gray lines represent FU-Lcktg/Lck−/− levels. Data are representative of at least 5 mice of each genotype.
The Lck unique domain is required for T-cell development. (A) Schematic showing structural domains of WT (top) and FU-Lck (bottom) proteins. (B) Western blot analysis showing relative expression levels of Lck and FU-Lck proteins in thymocyte lysates from mice from lines 1 (L1) and 2 (L2) of Lcktg mice and lines 1 and 3 (L3) of FU-Lcktg mice. A V5 mAb was used to detect transgenic Lck, which was tagged with the V5 epitope, while ERK reprobes served as loading controls. (C) Dot plots showing distribution of T-cell subsets in thymi from 7-week-old WT, Lck−/−, Lcktg/Lck−/−, and FU-Lcktg/Lck−/− mice as defined by surface CD4 and CD8 expression and FACS analysis. Values within dot plots represent proportions of cells within CD4 SP, DP, and CD8 SP gates. Dot plots represent staining from 1 of at least 8 mice of each genotype. (D) FACS dot plots showing distribution of DN populations in mice thymi. γδTCR−CD4−CD8− cells were subdivided into DN1 to DN4 populations by expression of CD44 and CD25: DN1 = CD44+CD25−; DN2 = CD44+CD25+; DN3 = CD44−CD25+; and DN4 = CD44−CD25−. Values represent the proportion of cells in each DN population, and dot plots represent analysis of 1 of at least 5 mice of each genotype. (E) Histograms showing FACS analysis of γδTCR expression in CD4−CD8− DN populations from mouse thymi. Values represent proportions of γδTCR-positive and TCR-negative cells as defined by the respective gates. Data are representative of 1 of 5 mice analyzed of each genotype. (F) Histograms showing DP thymocyte expression of surface αβTCR (left side) and CD5 (right side). Filled histograms represent levels of expression of αβTCR or CD5 in WT DP cells; fine black line overlays represent Lck−/− levels; bold black overlays represent Lcktg/Lck−/− levels; and bold gray lines represent FU-Lcktg/Lck−/− levels. Data are representative of at least 5 mice of each genotype.
Expression of either transgene had no effect on T-cell development in Lck+/+ backgrounds (data not shown), so analysis was done on an Lck−/− background. T-cell development was assessed by fluorescence-activated cell sorting (FACS) analysis. As previously reported for Lck−/− mice,12 the proportions, but not the numbers, of DN cells were increased, whereas both the proportions and numbers of DP and CD4 SP thymocytes were decreased compared with WT controls (Figure 1C, Table 1). Despite the low expression levels, Lcktg substantially rescued DP and SP cell numbers and proportions in Lck−/− mice (Figure 1C, Table 1), although these did not reach WT levels. Expression of FU-Lcktg resulted in an intermediate phenotype between that of the Lcktg mice and that of the Lck−/− mice. Thus, the numbers and proportions of DP and SP thymocytes in FU-Lcktg mice were elevated compared with Lck−/− mice, but were reduced by approximately 50% and approximately 85%, respectively, compared with Lcktg mice (Figure 1C, Table 1). The majority of the CD8 SP thymocytes in both Lck−/− and FU-Lcktg mice were immature, TCR-negative cells (Table 1), indicative of a late block in progression to the DP stage.
Absolute thymocyte cell numbers in 7- to 9-week-old mice
| . | Total . | DN . | DP . | CD4 SP . | CD8 SP . | CD8 SP TCR+ . |
|---|---|---|---|---|---|---|
| B6 (n = 8) | 125.4 ± 35.7 | 4.4 ± 1.4 | 106.5 ± 21 | 8.4 ± 2.1 | 3.0 ± 0.9 | 2.0 ± 0.7 |
| Lck−/− (n = 8) | 18.7 ± 5.1 | 6.7 ± 1.6 | 10.1 ± 3.9 | 0.15 ± 0.06 | 1.1 ± 0.5 | 0.25 ± 0.12 |
| Lcktg (n = 12) | 76 ± 20 | 5.7 ± 1.4 | 62.3 ± 17.2 | 3.3 ± 1.1 | 2.5 ± 0.9 | 1.6 ± 0.5 |
| FU-Lcktg (n = 8) | 40 ± 10.8 | 4.3 ± 1.4 | 32.1 ± 8.9 | 0.45 ± 0.16 | 1.84 ± 0.69 | 0.47 ± 0.29 |
| . | Total . | DN . | DP . | CD4 SP . | CD8 SP . | CD8 SP TCR+ . |
|---|---|---|---|---|---|---|
| B6 (n = 8) | 125.4 ± 35.7 | 4.4 ± 1.4 | 106.5 ± 21 | 8.4 ± 2.1 | 3.0 ± 0.9 | 2.0 ± 0.7 |
| Lck−/− (n = 8) | 18.7 ± 5.1 | 6.7 ± 1.6 | 10.1 ± 3.9 | 0.15 ± 0.06 | 1.1 ± 0.5 | 0.25 ± 0.12 |
| Lcktg (n = 12) | 76 ± 20 | 5.7 ± 1.4 | 62.3 ± 17.2 | 3.3 ± 1.1 | 2.5 ± 0.9 | 1.6 ± 0.5 |
| FU-Lcktg (n = 8) | 40 ± 10.8 | 4.3 ± 1.4 | 32.1 ± 8.9 | 0.45 ± 0.16 | 1.84 ± 0.69 | 0.47 ± 0.29 |
Cell numbers reported are ×10−6.
Further analyses were performed to compare DN populations. An accumulation of CD44−CD25+ DN3 cells was apparent in Lck−/− thymi (Figure 1D). Expression of the Lcktg partially rescued this phenotype, although more DN3 cells were present compared with WT thymi. Expression of FU-Lcktg resulted in a DN phenotype intermediate between that of Lcktg mice and that of the Lck−/− controls (Figure 1D). By contrast, proportions of γδTCR+ cells were similar in all groups of mice (Figure 1E).
Levels of TCRβ and CD5 expression on DP thymocytes were assessed. TCRβ expression on Lck−/− DP thymocytes was elevated above that of WT cells (Figure 1F), reflecting the requirement for Lck in the down-regulation of surface TCR expression.16 TCR expression on FU-Lcktg DP thymocytes was similar to Lck−/− levels (Figure 1F), whereas expression on Lcktg cells was intermediate between WT and Lck−/−. Thymocyte CD5 expression is developmentally regulated and controlled by TCR signals and avidity.17 As expected, our results showed that few Lck−/− DP thymocytes expressed high levels of CD5 (Figure 1F). More DP cells from FU-Lcktg thymi expressed high levels of CD5 compared with Lck−/− cells (34% and 14%, respectively). These data indicate that the FU-Lck protein can transduce TCR signals, resulting in the up-regulation of CD5 in DP thymocytes. Lcktg DP thymocytes expressed levels of CD5 that were consistently above WT levels (Figure 1F), which may reflect increased expression of TCR on the former relative to WT DP cells.17
The ability of FU-Lcktg and Lcktg to facilitate positive selection of F5 TCR-transgenic thymocytes was addressed. In Rag1−/−F5 TCR-transgenic mice, Lck expression is essential for the development of DP and CD8SP cells. Expression of either FU-Lcktg or Lcktg rescued DN-DP progression to a variable degree in Rag1−/−Lck−/− F5 mice (data not shown). However, conversion of DP to mature CD8 SP thymocytes was profoundly defective in mice expressing FU-Lcktg compared with WT Lck or Lcktg, which was also reflected in the reduced numbers of CD8SP cells (supplemental Figure 2A-B), confirming the importance of the Lck unique domain in positive selection.
These data show that the unique domain is critical for Lck function in T-cell development. Expression of low levels of WT Lck relieved the DN3 block in Lck−/− mice to a greater extent than expression of similar levels of domain-swap FU-Lck, leading to differentiation of more DP cells. Similarly, DP-SP progression was impaired in FU-Lcktg thymi because fewer cells up-regulated CD5 and progressed to mature SP cells than in Lcktg animals. However, the fact that FU-Lcktg expression partially rescues DP and SP cell numbers in Lck−/− mice suggests that possession of the specific unique domain is not an absolute prerequisite for Lck function in αβ T-cell development. In contrast to development of αβ lineage T cells, numbers of thymic γδTCR+ cells were previously shown to be unaffected by Lck deficiency,18 and similar numbers of γδTCR+ cells were present in the thymi of all groups of mice examined in the present study. In contrast, thymic natural killer (NK) T-cell development was profoundly blocked in Lck−/−, FU-Lcktg, and Lcktg mice, suggesting that the development of this T-cell subset was dependent on higher Lck expression levels than either αβ or γδ T cells (supplemental Figure 2B). This precludes analysis of the role of the Lck unique domain in invariant NKT (iNKT)-cell development in the current work.
Reduced αβ but elevated γδ T-cell numbers in domain-swap FU-Lcktg mice
Examination of peripheral T cells in Lcktg- and FU-Lcktg-transgenic mice showed reduced proportions of αβ T cells in lymph nodes from Lck−/− and FU-Lcktg mice compared with either WT or Lcktg mice (Figure 2A), which is consistent with the observed thymus defects. Furthermore, the ratio of CD4:CD8 T cells was approximately 1.5:1 in WT and Lcktg mice, whereas in Lck−/− and FU-Lcktg mice, this ratio was reversed (Figure 2B).
Reduced numbers of lymph node αβ T cells but not γδT cells in FU-Lcktg mice. (A) FACS dot plots showing proportions of αβ and γδ T cells in lymph nodes from 7-week-old WT, Lck−/−, Lcktg/Lck−/−, and FU-Lcktg/Lck−/− mice. Values within dot plots represent the percentage of lymphocyte-gated αβ and γδTCR-positive cells. (B) Distribution of CD4+ and CD8+ T cells in lymph nodes. Values represent proportions of αβTCR+ cells within CD4+ and CD8+ gates. Histograms showing CD44 expression on gated lymph node αβTCR+CD4+ T cells (C) or CD8+ T cells (D) from WT, Lck−/−, Lcktg/Lck−/−, and FU-Lcktg/Lck−/− mice. Values in histograms represent percentages of cells falling within the illustrated CD44 “high” gate. In all cases, data are representative of at least 11 mice of each genotype.
Reduced numbers of lymph node αβ T cells but not γδT cells in FU-Lcktg mice. (A) FACS dot plots showing proportions of αβ and γδ T cells in lymph nodes from 7-week-old WT, Lck−/−, Lcktg/Lck−/−, and FU-Lcktg/Lck−/− mice. Values within dot plots represent the percentage of lymphocyte-gated αβ and γδTCR-positive cells. (B) Distribution of CD4+ and CD8+ T cells in lymph nodes. Values represent proportions of αβTCR+ cells within CD4+ and CD8+ gates. Histograms showing CD44 expression on gated lymph node αβTCR+CD4+ T cells (C) or CD8+ T cells (D) from WT, Lck−/−, Lcktg/Lck−/−, and FU-Lcktg/Lck−/− mice. Values in histograms represent percentages of cells falling within the illustrated CD44 “high” gate. In all cases, data are representative of at least 11 mice of each genotype.
Quantification of CD4 and CD8 T-cell numbers showed that these were reduced in lymph nodes from Lck−/−, Lcktg, and FU-Lcktg mice compared with WT mice, which is in agreement with the analysis of thymocyte development (Table 2). Lcktg mice had 3.5-fold more CD4 T cells and 2-fold more CD8 T cells than FU-Lcktg mice (Table 2). Nonetheless, numbers of CD4 T cells, but not CD8 T cells, were significantly higher in FU-Lcktg mice compared with Lck−/− mice (Table 2).
Absolute T-cell numbers in lymph nodes from 7- to 9-week-old mice
| . | αβ CD4 . | αβ CD8 . | γδ . |
|---|---|---|---|
| B6 (n = 11) | 19.8 ± 6.7 | 15.2 ± 5.7 | 0.70 ± 0.19 |
| Lck−/− (n = 11) | 1.6 ± 0.9 | 2.9 ± 1.7 | 0.75 ± 0.23 |
| Lcktg (n = 15) | 11.9 ± 4.6 | 8.8 ± 2.9 | 1.6 ± 0.28 |
| FU-Lcktg (n = 11) | 3.7 ± 0.8 | 4.4 ± 1.8 | 2.5 ± 0.5 |
| . | αβ CD4 . | αβ CD8 . | γδ . |
|---|---|---|---|
| B6 (n = 11) | 19.8 ± 6.7 | 15.2 ± 5.7 | 0.70 ± 0.19 |
| Lck−/− (n = 11) | 1.6 ± 0.9 | 2.9 ± 1.7 | 0.75 ± 0.23 |
| Lcktg (n = 15) | 11.9 ± 4.6 | 8.8 ± 2.9 | 1.6 ± 0.28 |
| FU-Lcktg (n = 11) | 3.7 ± 0.8 | 4.4 ± 1.8 | 2.5 ± 0.5 |
Cell numbers reported are ×10−6.
Interestingly, the proportions of γδ T cells were elevated in Lck−/−, Lcktg, and FU-Lcktg compared with WT lymph nodes (Figure 2A). Furthermore, although absolute numbers of γδ T cells were similar in WT and Lck−/− mice, they were approximately 2- and 4-fold higher in Lcktg and FU-Lcktg mice, respectively (Table 2). γδ T cells can develop thymically and extrathymically.19 It is unclear whether the greater numbers of γδ T cells found in Lcktg and FU-Lcktg mice resulted from expansion of thymic-derived γδ T cells in lymph nodes or through increased differentiation of extrathymic γδ T cells.
Levels of surface CD44 expression on αβ T cells are used as an indicator of prior antigen experience; high expression of CD44 is maintained on activated or memory cells. However, T cells generated in lymphopenic conditions also up-regulate CD44 as a result of homeostatic expansion.20 Although the majority of WT CD4 and CD8 T cells expressed low levels of CD44 (Figure 2C-D), Lck−/− and FU-Lcktg CD4 and CD8 T cells expressed high levels of CD44 (Figure 2C-D). Most CD4 T cells from Lcktg mice expressed low levels of CD44, although some expansion of CD44hi CD8 T cells was apparent. In summary, these data indicate that defects in T-cell development in Lck−/− and FU-Lcktg mice result in the presence of reduced numbers of peripheral αβ CD4 and CD8 T cells that expand in the periphery, becoming CD44hi. Interestingly, γδ T-cell numbers were not affected in Lck−/− mice and were elevated in Lcktg and FU-Lcktg mice.
Coexpression of inducible Lck and domain-swap FU-Lck transgenes results in the development of thymic tumors
To circumvent the block in T-cell development in FU-Lcktg mice, we introduced an inducible Lck transgene. In Lckind mice, Lck expression is controlled by the induction of a tetracycline-responsive transgene by adding doxycycline to the diet.13 When crossed to the Lck−/− background, expression of the Lckind transgene bypasses defects in T-cell development and allows the accumulation of peripheral T cells. After doxycycline is removed from the diet, thymus involution occurs and peripheral T cells lose Lck protein expression.
Analysis of Lcktg/Lckind mice maintained on a doxycycline diet showed that coexpression of these transgenes resulted in normal proportions of thymocyte populations (Figure 3A). In contrast, thymi from FU-Lcktg/Lckind mice maintained on a doxycycline diet were grossly enlarged and necessitated euthanasia of mice > 12 wks old (supplemental Figure 3A). FACS analysis demonstrated the presence of increased proportions of CD8 SP cells (Figure 3A). Analysis of forward light scatter showed that DP and CD8 SP cells from FU-Lcktg/Lckind mice were substantially larger than their counterparts in Lcktg/Lckind mice (Figure 3B). Strikingly, FU-Lcktg/Lckind DP and CD8 SP cells did not express detectable levels of surface αβTCR (Figure 3C). Furthermore, both DP and CD8 SP cells from FU-Lcktg/Lckind mice expressed low levels of CD5 and high levels of CD24 compared with control cells (Figure 3D-E), indicative of an immature phenotype. The presence of enlarged thymi was apparent in 100% of FU-Lcktg/Lckind mice analyzed at > 10 weeks of age (n = 17), and although the proportions of DN, DP, and SP cells varied between individual mice, in all cases CD4−CD8+TCR− cells were present in increased numbers (supplemental Figures 3B-D). This phenotype was not simply a consequence of increased Lck levels, because the WT Lcktg together with the Lckind transgene led to normal maturation and surface expression of TCR.
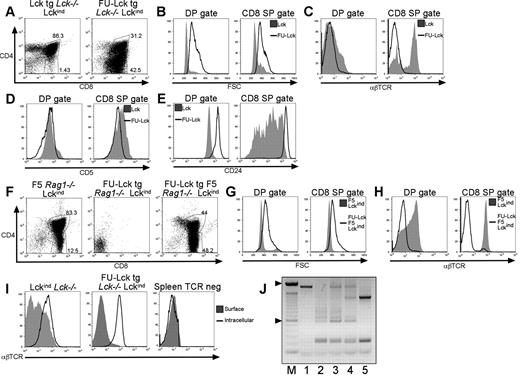
Thymic lymphoproliferation in FU-Lcktg/Lckind mice. (A) Dot plots showing distribution of T-cell subsets in thymi from 12-week-old Lcktg/Lck−/−/Lckind and FU-Lcktg/Lck−/−/Lckind mice defined by surface CD4 and CD8 expression and FACS analysis. Values within dot plots represent proportions of cells with DP and CD8 SP gates. Dot plots represent staining from 1 of at least 5 mice of each genotype. FACS histograms showing cell size (B) as assessed by analysis of forward scatter (FSC) and surface expression of αβTCR (C), CD5 (D), and CD24 (E) on gated CD4+CD8+ DP cells and CD8+ SP cells from thymi of Lcktg/Lck−/−/Lckind and FU-Lcktg/Lck−/−/Lckind mice. Filled histograms represent Lcktg/Lck−/−/Lckind mice; black line overlays represent FU-Lcktg/Lck−/−/Lckind mice. Data represent 1 of at least 5 mice of each genotype. (F) FACS dot plots showing distribution of T-cell subsets in thymi from 12-week-old F5 Rag1−/−/Lck−/−/Lckind, FU-Lcktg/Rag1−/−/Lck−/−/Lckind, and FU-Lcktg/F5/Rag1−/−/Lck−/−/Lckind mice. Values represent the proportions of cells within DP and CD8 SP gates as indicated. Data represent 1 of at least 7 mice of each genotype. Cell size (G) and surface expression of αβTCR (H) on DP and CD8 SP cells. Filled histograms represent F5 Rag1−/−/Lck−/−/Lckind mice; black line overlays represent FU-Lcktg/F5/Rag1−/−/Lck−/−/Lckind mice. (I) Comparison of cell surface and intracellular αβTCR FACS staining of thymocytes from Lck−/−/Lckind and FU-Lcktg/Lck−/−/Lckind mice and CD19+ splenocytes from FU-Lcktg/Lck−/−/Lckind mice. For intracellular staining, cells were permeabilized in 0.5% saponin before staining with αβTCR mAb. Filled histograms represent levels of surface αβTCR; black line overlays represent levels of staining after cell permeabilization. (J) PCR analysis of TCR Dβ2.1-Jβ2.7 rearrangements. The figure represents a 1.2% agarose gel of PCR products from B6 ear,1 B6 thymus,2 and 3 individual 10-week-old FU-Lcktg/Lck−/−/Lckind thymi.3-5 The 100-bp markers are also shown (M).
Thymic lymphoproliferation in FU-Lcktg/Lckind mice. (A) Dot plots showing distribution of T-cell subsets in thymi from 12-week-old Lcktg/Lck−/−/Lckind and FU-Lcktg/Lck−/−/Lckind mice defined by surface CD4 and CD8 expression and FACS analysis. Values within dot plots represent proportions of cells with DP and CD8 SP gates. Dot plots represent staining from 1 of at least 5 mice of each genotype. FACS histograms showing cell size (B) as assessed by analysis of forward scatter (FSC) and surface expression of αβTCR (C), CD5 (D), and CD24 (E) on gated CD4+CD8+ DP cells and CD8+ SP cells from thymi of Lcktg/Lck−/−/Lckind and FU-Lcktg/Lck−/−/Lckind mice. Filled histograms represent Lcktg/Lck−/−/Lckind mice; black line overlays represent FU-Lcktg/Lck−/−/Lckind mice. Data represent 1 of at least 5 mice of each genotype. (F) FACS dot plots showing distribution of T-cell subsets in thymi from 12-week-old F5 Rag1−/−/Lck−/−/Lckind, FU-Lcktg/Rag1−/−/Lck−/−/Lckind, and FU-Lcktg/F5/Rag1−/−/Lck−/−/Lckind mice. Values represent the proportions of cells within DP and CD8 SP gates as indicated. Data represent 1 of at least 7 mice of each genotype. Cell size (G) and surface expression of αβTCR (H) on DP and CD8 SP cells. Filled histograms represent F5 Rag1−/−/Lck−/−/Lckind mice; black line overlays represent FU-Lcktg/F5/Rag1−/−/Lck−/−/Lckind mice. (I) Comparison of cell surface and intracellular αβTCR FACS staining of thymocytes from Lck−/−/Lckind and FU-Lcktg/Lck−/−/Lckind mice and CD19+ splenocytes from FU-Lcktg/Lck−/−/Lckind mice. For intracellular staining, cells were permeabilized in 0.5% saponin before staining with αβTCR mAb. Filled histograms represent levels of surface αβTCR; black line overlays represent levels of staining after cell permeabilization. (J) PCR analysis of TCR Dβ2.1-Jβ2.7 rearrangements. The figure represents a 1.2% agarose gel of PCR products from B6 ear,1 B6 thymus,2 and 3 individual 10-week-old FU-Lcktg/Lck−/−/Lckind thymi.3-5 The 100-bp markers are also shown (M).
Previous data showed that the expression of an “activated” Lck protein in which the regulatory Tyr505 residue had been mutated to phenylalanine (LckY505F) resulted in thymomas in mice.21,22 In LckY505F transgenic mice, transformation occurred at the DN stage and was independent of recombinase activity, because tumors developed in a Rag1−/− background.21,22 To determine whether the development of thymomas in FU-Lcktg/Lckind mice occurred in an analogous manner, mice were crossed to a Rag1−/− background. Surprisingly, the development of thymic abnormalities was completely ablated in FU-Lcktg/Lckind/Rag1−/− mice (Figure 3F). When an F5 TCR transgene was introduced to FU-Lcktg/Lckind/Rag1−/− mice, thymi were again grossly enlarged compared with littermate controls. As described for FU-Lcktg/Lckind mice, an increased proportion of FSChighCD4−CD8+TCR− cells was apparent in the thymi of FU-Lcktg/LckindRag1−/− F5 mice (Figure 3F-H).
Development of thymomas in FU-Lcktg/Lckind mice required either recombinase activity or the expression of a TCR transgene, yet cells from these thymi did not express surface TCR. However, intracellular staining revealed the presence of abundant intracellular TCRβ in FU-Lcktg/Lckind and FU-Lcktg/Lckind/Rag1−/− F5 thymocytes (Figure 3I and data not shown). PCR analysis of TCR Dβ2.1-Jβ2.7 gene rearrangements was used to assess clonality of individual tumors,15 and the data suggested that tumors were predominantly oligoclonal (Figure 3J).
To determine whether tumors persisted in the absence of Lckind transgene expression, FU-Lcktg/Lckind mice were maintained on doxycycline until 12 weeks of age, and the development of a typical thymoma was confirmed in one individual (supplemental Figure 4). Littermates were removed from doxycycline for a further 4 weeks. At the time of culling, all mice were healthy and thymi were greatly reduced in size compared with the tumor from the mouse analyzed at 12 weeks (data not shown). FACS analysis demonstrated variable expression of CD4 and CD8, with 2 of 3 thymi retaining a high proportion of CD4−CD8+ cells (supplemental Figure 4). By contrast, surface TCRβ expression was detected on thymocytes from all FU-Lck/Lckind OFF-dox mice. These data suggest that the growth of thymomas in FU-Lck/Lckind mice and the internalization of TCRβ by tumor cells requires continuous expression of the Lckind transgene.
Lymphoproliferation in FU-Lcktg/Lckind mice is associated with dysregulated Lck expression and phosphorylation and elevated ZAP70 activation
Western blot analyses were performed to assess the biochemical basis for the thymic phenotype of FU-Lcktg/Lckind mice. Levels of pTyr in lysates of control Lcktg/Lckind thymocytes were low (Figure 4A). Higher levels of pTyr were apparent in lysates from FU-Lcktg/Lckind mice, with bands running at approximately 55 kDa and 70 to 75 kDa being particularly hyperphosphorylated (Figure 4A). Lck activates the downstream kinase ZAP70, and autophosphorylation on residue Tyr319 is critical for ZAP70 function.23 Using a specific antibody, we confirmed that ZAP70 Tyr319 was hyperphosphorylated in FU-Lcktg/Lckind cell lysates (Figure 4B).
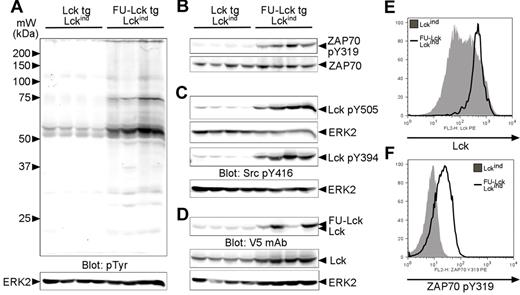
Dysregulated Lck expression and phosphorylation and elevated ZAP70 activation in FU-Lck Lckind thymocytes. (A) Western blot analysis showing levels of pTyr in whole thymus lysates taken directly ex vivo from 4 12-week-old Lcktg/Lck−/−/Lckind and 4 age-matched FU-Lcktg/Lck−/−/Lckind mice. Arrows represent molecular mass (kDa) markers. The blot was stripped and reprobed with anti-ERK2 to monitor protein loading. (B) Levels of ZAP70 Tyr319 phosphorylation were assessed; loading control = total ZAP70 expression. (C) Phosphorylation of Lck was assessed using Lck pTyr505 and Src pTyr416 Abs. The Src pTyr416 Ab cross-reacted with Lck pTyr394 in T cells. The ERK2 western blot serves as a loading control. (D) Expression of Lck and FU-Lck transgenes in thymocyte lysates was determined by Western blot analysis using a V5 mAb. Parallel blots using an Lck mAb monitored total Lck expression, while ERK blots functioned as loading controls. For all blots, images were obtained using an infrared imaging system (LI-COR). (E) FACS histogram showing intracellular levels of Lck in FU-Lcktg/Lckind and Lckind thymocytes. Cells were permeabilized with 0.5% saponin before Ab staining. (F) Levels of intracellular ZAP70 pY319 in gated DP thymocytes from FU-Lcktg/Lckind and Lckind mice. Cells were stained for surface CD4 and CD8 before fixation in 2% formaldehyde, permeabilization in 90% methanol, and ZAP70 pY319 Ab staining. Histograms represent 1 of 3 repeated experiments.
Dysregulated Lck expression and phosphorylation and elevated ZAP70 activation in FU-Lck Lckind thymocytes. (A) Western blot analysis showing levels of pTyr in whole thymus lysates taken directly ex vivo from 4 12-week-old Lcktg/Lck−/−/Lckind and 4 age-matched FU-Lcktg/Lck−/−/Lckind mice. Arrows represent molecular mass (kDa) markers. The blot was stripped and reprobed with anti-ERK2 to monitor protein loading. (B) Levels of ZAP70 Tyr319 phosphorylation were assessed; loading control = total ZAP70 expression. (C) Phosphorylation of Lck was assessed using Lck pTyr505 and Src pTyr416 Abs. The Src pTyr416 Ab cross-reacted with Lck pTyr394 in T cells. The ERK2 western blot serves as a loading control. (D) Expression of Lck and FU-Lck transgenes in thymocyte lysates was determined by Western blot analysis using a V5 mAb. Parallel blots using an Lck mAb monitored total Lck expression, while ERK blots functioned as loading controls. For all blots, images were obtained using an infrared imaging system (LI-COR). (E) FACS histogram showing intracellular levels of Lck in FU-Lcktg/Lckind and Lckind thymocytes. Cells were permeabilized with 0.5% saponin before Ab staining. (F) Levels of intracellular ZAP70 pY319 in gated DP thymocytes from FU-Lcktg/Lckind and Lckind mice. Cells were stained for surface CD4 and CD8 before fixation in 2% formaldehyde, permeabilization in 90% methanol, and ZAP70 pY319 Ab staining. Histograms represent 1 of 3 repeated experiments.
Experiments were undertaken to determine whether the hyperphosphorylated, approximately 55-kDa protein(s) represented Lck. Phosphospecific Abs showed that levels of Lck-inhibitory Tyr505 and activatory-Tyr394 phosphorylation were markedly increased in lysates from FU-Lcktg/Lckind mice compared with those from Lcktg/Lckind mice (Figure 4C). Additional analyses demonstrated that in some, but not all, thymomas, FU-Lcktg protein was expressed at higher levels compared with Lcktg protein in control mice (Figure 4D). Similarly, WT Lck protein levels were elevated approximately 2-fold in tumor cell lysates compared with control cell lysates (Figure 4D). Previous studies using intracellular staining indicated that Lck was expressed heterogeneously in thymocytes from Lckind-transgenic mice.13 The higher level of Lck in FU-Lcktg/Lckind thymus lysates compared with Lcktg/Lckind lysates could be a result of an outgrowth of cells expressing high levels of the Lckind transgene. Intracellular FACS staining demonstrated that this was indeed the case (Figure 4E and supplemental Figure 5).
Finally, it was possible that the elevated ZAP70 phosphorylation in tumor lysates was a result of the altered proportions of specific thymocyte subsets in FU-Lcktg/Lckind mice compared with Lcktg/Lckind mice. To address this possibility, intracellular FACS staining was performed, and the data indicated that levels of intracellular ZAP70 pTyr319 in gated DP cells were approximately 2- to 3-fold higher in cells from FU-Lcktg/Lckind mice compared with controls (Figure 4F).
Thymocytes from FU-Lcktg/Lckind mice are susceptible to DNA damage-induced apoptosis
Cell lines are derived readily from LckY505F thymomas by propagation in tissue-culture media containing serum without the addition of other growth factors.21,22 Moreover, cell lines and thymocytes taken directly from LckY505F tumors demonstrate resistance to apoptotic stimuli.22,24 To determine the susceptibility to apoptotic stimuli, thymocytes from FU-Lcktg/Lckind mice and control animals were subjected to γ-irradiation, cultured for 4 hours, and assessed for caspase activation by Western blot analysis. Blots showed that irradiation-induced activation of caspase-3, as judged by cleavage of the p32 zymogen and the appearance of the p17 active form, was comparable in all groups of mice (Figure 5). Furthermore, background levels of caspase-3 activation appeared somewhat higher in FU-Lcktg/Lckind cell lysates, because levels of p17 were higher in cells that had not received irradiation compared with cells from control mice cultured under the same conditions (Figure 5). These data suggest that thymocytes from FU-Lcktg/Lckind mice are sensitive to apoptotic stimuli and have elevated levels of spontaneous cell death compared with control thymocytes ex vivo.
Unimpaired caspase activation of FU-LcktgLck−/−Lckind thymocytes in response to DNA damage. Thymocytes from 12-week-old Lck−/−/Lckind, Lcktg/Lck−/−/Lckind, and FU-Lcktg/Lck−/−/Lckind mice were subjected to 1000 Rad in a γ-irradiator. Irradiated and nonirradiated control cells were cultured for 4 hours in complete culture medium and cell lysates prepared. Western blot analysis was performed to assess activation of caspase 3 as judged by the appearance of the p17 active form and the reduction in the intensity of the p32 pro-form of the enzyme. Reprobes for ERK2 acted as a loading control. The data shown represent data from 1 of 3 FU-Lcktg/Lck−/−/Lckind mice.
Unimpaired caspase activation of FU-LcktgLck−/−Lckind thymocytes in response to DNA damage. Thymocytes from 12-week-old Lck−/−/Lckind, Lcktg/Lck−/−/Lckind, and FU-Lcktg/Lck−/−/Lckind mice were subjected to 1000 Rad in a γ-irradiator. Irradiated and nonirradiated control cells were cultured for 4 hours in complete culture medium and cell lysates prepared. Western blot analysis was performed to assess activation of caspase 3 as judged by the appearance of the p17 active form and the reduction in the intensity of the p32 pro-form of the enzyme. Reprobes for ERK2 acted as a loading control. The data shown represent data from 1 of 3 FU-Lcktg/Lck−/−/Lckind mice.
Altered intracellular localization underlies the effects of domain-swap FU-Lck in thymocytes
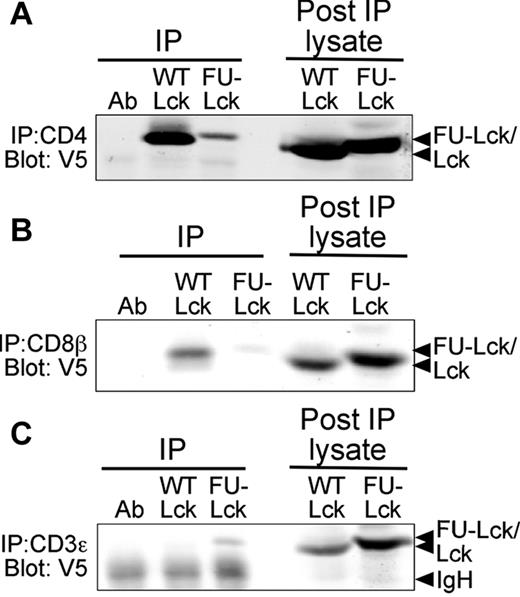
The unique domain of Lck regulates association with CD4 and CD8,6 and is also important for the localization of Lck in the absence of coreceptor expression.25 It was therefore useful to determine the intracellular localization of Lcktg and FU-Lcktg proteins in thymocytes. Immunoprecipitation of CD4 or CD8β followed by Western blot analysis showed that a large proportion of Lcktg expressed in thymocytes of LcktgLck−/− mice was coreceptor-associated (Figure 6A-B). Although a very small amount of FU-Lcktg and Fyn (data not shown) coprecipitated with anti-CD4 and anti-CD8, this only occurred in the Lck−/− background, presumably because WT Lck associates much better with coreceptors and displaces FU-domain–containing proteins. Nonetheless, the majority of FU-Lcktg protein failed to coimmunoprecipitate with either CD4 or CD8. Furthermore, a small proportion of FU-Lcktg but not Lcktg was associated directly with the TCR complex, as shown by CD3ϵ immunoprecipitation (Figure 6). These data confirm that WT Lcktg and FU-Lcktg are differentially localized in thymocytes.
Altered intracellular localization of the FU-Lck protein. Coimmunoprecipitation experiments were undertaken to determine the association of transgenic Lck proteins with CD4, CD8, and CD3ϵ. CD4 (A), CD8β (B), or CD3ϵ (C) were immunoprecipitated from thymocyte lysates using mAbs and protein-A Sepharose. Association of Lcktg or FU-Lcktg proteins was assessed by Western blot analysis using the V5 mAb. Data shown represent 1 of 3 repeated experiments.
Altered intracellular localization of the FU-Lck protein. Coimmunoprecipitation experiments were undertaken to determine the association of transgenic Lck proteins with CD4, CD8, and CD3ϵ. CD4 (A), CD8β (B), or CD3ϵ (C) were immunoprecipitated from thymocyte lysates using mAbs and protein-A Sepharose. Association of Lcktg or FU-Lcktg proteins was assessed by Western blot analysis using the V5 mAb. Data shown represent 1 of 3 repeated experiments.
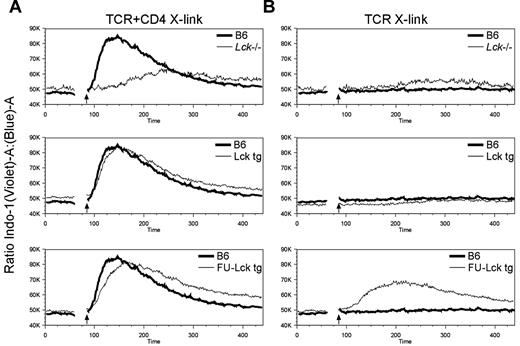
To ascertain the effect of altered localization of FU-Lcktg on signal transduction directly, we analyzed antibody-induced Ca2+ flux in DP thymocytes. Indo-1-loaded cells were labeled with biotin-conjugated TCRβ ± CD4 Abs, and Ca2+ flux was monitored after cross-linking with streptavidin. Cross-linking TCR+CD4, but not TCR alone, induced a robust peak of intracellular Ca2+ flux in WT DP thymocytes (Figure 7A-B). In the absence of Lck, TCR + CD4 cross-linking induced only a small Ca2+ flux that was substantially delayed, as has been reported previously,14 and may reflect low-level Fyn association with CD4. Expression of either WT Lcktg or FU-Lcktg restored the ability of TCR+CD4 cross-linking to induce Ca2+ flux, although this was delayed in FU-Lcktg compared with WT Lcktg DP cells (Figure 7A). Interestingly, TCR cross-linking alone consistently induced a substantial Ca2+ response in FU-Lcktg but not Lcktg or WT DP thymocytes (Figure 7B). This response was independent of the levels of TCR, because gates were set on cells with equivalent surface TCR expression. Therefore, relocalization of FU-Lck to the TCR-CD3 complex has direct effects on signal transduction in DP thymocytes.
Elevated TCR-induced Ca2+ response of FU-Lck transgenic thymocytes. Thymocytes were labeled with 2μM indo-1, and then stained with biotin-conjugated anti-TCRβ ± CD4 (RM4.5) Abs. Additional fluorescently conjugated CD8 and CD4 (clone YTA3.1 does not compete with clone RM4.5) Abs were used to identify thymocyte populations, and Ca2+ fluxes were monitored by flow cytometry. Baseline levels were determined for 60 seconds, and TCR ± CD4 cross-linking was achieved by the addition of streptavidin-allophycocyanin conjugates. Arrows on histograms indicate time of addition of streptavidin. Ca2+ traces in gated DP populations after cross-linking of TCR + CD4 are represented in the histograms shown in panel A, while those obtained after cross-linking of TCR alone are represented in panel B. In all cases, data represent 1 of 3 repeated experiments.
Elevated TCR-induced Ca2+ response of FU-Lck transgenic thymocytes. Thymocytes were labeled with 2μM indo-1, and then stained with biotin-conjugated anti-TCRβ ± CD4 (RM4.5) Abs. Additional fluorescently conjugated CD8 and CD4 (clone YTA3.1 does not compete with clone RM4.5) Abs were used to identify thymocyte populations, and Ca2+ fluxes were monitored by flow cytometry. Baseline levels were determined for 60 seconds, and TCR ± CD4 cross-linking was achieved by the addition of streptavidin-allophycocyanin conjugates. Arrows on histograms indicate time of addition of streptavidin. Ca2+ traces in gated DP populations after cross-linking of TCR + CD4 are represented in the histograms shown in panel A, while those obtained after cross-linking of TCR alone are represented in panel B. In all cases, data represent 1 of 3 repeated experiments.
Discussion
T-cell development and mature T-cell function are critically dependent on the activities of the SFKs Lck and Fyn. Lck is required for the initiation of TCR-signaling pathways, whereas Fyn contributes to these pathways but is also involved in the negative regulation of T-cell responses.1,12-14,26,27 In the current study, we generated transgenic mice to address the role of intracellular localization and the function of the N-terminal unique domains of Lck. These experiments demonstrated that the unique domain is important for the role of Lck in T-cell development. Furthermore, coexpression of very low levels of mislocalized FU-Lck with an Lck-inducible transgene led to the aberrant proliferation of immature thymocytes, and ultimately in thymic tumorigenesis.
Previous studies addressed the function of the Lck unique domain through transfection of cell lines with mutant and truncated forms of the kinase. These studies showed that the unique domain regulates membrane25 and coreceptor association, and might influence substrate specificity10 and interactions with regulatory phosphatases.8 However, the significance of these findings for the role of Lck in T-cell development had not been addressed previously. In an attempt to understand the molecular basis for the differing roles of Lck and Fyn, Abraham et al generated transgenic mice that expressed chimeric Lck-Fyn proteins in the absence of endogenous Lck.28 In their study, substitution of the Lck catalytic domain for that of Fyn had little effect on DP-cell numbers but impaired positive selection. By contrast, substitution of the Lck N-terminal-unique SH3 and SH2 domains for those of Fyn severely impaired the production of DP cells in transgenic mice.28 These data suggested that specific Lck catalytic activity was important for positive selection, whereas the N-terminal regulatory domains were critical for Lck function in DN cells. However, the individual requirements for the Lck SH3, SH2, and unique domains in DN cells were not clear from these experiments. A recent study demonstrated that a “knockin” mutation in the Lck SH3 domain resulted in a 50% reduction in thymocyte cell numbers in homozygous mice, due to a partial block in DN3 progression and in positive selection.29 Our data indicate that replacement of the unique domain of Lck for that of Fyn also results in impaired DN3 progression. It remains possible that the low level of expression of FU-Lcktg protein exacerbates the defect in DN thymocytes. Nonetheless, it is apparent that at a similar or even lower level of expression, WT Lck is superior in promoting DN3 progression. These data are consistent with data showing that the unique domain is important for Lck localization in coreceptor-negative cell lines.25
Previous reports indicate that recruitment of Lck is important for the coreceptor function of CD4 and CD8,30,31 and we found that DP maturation and positive selection were impaired in FU-Lcktg mice. Furthermore, analysis of Ca2+ flux in DP thymocytes indicated that relocalization of Lck altered responses to stimulation with TCR ± CD4 cross-linking. Specifically, FU-Lcktg DP cells were hyporesponsive to TCR+CD4 cross-linking but hyperresponsive to TCR cross-linking alone. While Ab-induced signals are likely to be markedly different from those required for selection processes in vivo, these experiments suggest that altered localization of Lck could fundamentally affect the types of signals transduced in primary thymocytes. While FU-Lcktg expression resulted in an altered CD4:CD8 T-cell ratio, it should be noted that the defects in positive selection predominantly resulted in a quantitative reduction in SP cell numbers rather than in an overt, qualitative change in lineage selection. In this regard, both CD4 and CD8 SP cell numbers were dramatically reduced.
Expression of both the Lcktg and the FU-Lcktg transgenes was much lower than that of endogenous Lck, but despite this, the Lcktg transgene substantially rescued thymus differentiation in Lck−/− mice and was as efficient as WT at positive selection. Similarly, transgenic mice expressing only 3% of WT CD45 activity were shown to have normal numbers of thymocyte subsets and peripheral T cells.32 If only low levels of Lck are required for T-cell development, why are higher levels expressed in WT animals? One possibility is that higher levels of expression are required for full responsiveness of peripheral T cells. However, CD4 T cells from Lcktg mice up-regulate activation markers and undergo proliferation in response to CD3/CD28 stimulation in a similar manner to WT cells (data not shown). Another possibility is that while low levels of Lck expression permit thymocyte selection processes, the T-cell repertoire in these mice may be skewed. Although this may be the case, we did not see any evidence of the development of autoimmune disease, which can occur with muted signaling during differentiation.33
During β-selection, coreceptors are not expressed, and it is unclear how Lck associates with the pre-TCR to facilitate signaling. Despite the ability of the FU-Lcktg to associate directly with CD3 chains, it was much poorer than Lcktg, which showed no such association, at promoting β-selection in an Lck−/− background. Therefore, it was surprising that when coexpressed with an Lckind transgene, Fu-Lcktg mice developed thymomas. Overexpression of LckY505F was previously shown to lead to thymoma development, but this occurred independently of pre-TCR signaling.22 In the current study, some aspect of overexpression was contributory to tumor formation because FU-Lcktg mice on a WT Lck+/+ background were tumor-free. However, control Lcktg/Lckind cells, which express as much Lck as FU-Lcktg/Lckind tumor cells, never developed into tumors. Therefore, some unique aspect of coexpression of FU-Lcktg but not Lcktg precipitated uncontrolled growth of this population. Development of thymic lymphoproliferation was dependent on expression of either Rag1 or a TCR transgene, and was mainly represented in an immature stage, indicating that pre-TCR signals can drive the phenotype, as might αβTCR signals. Signals through pre-TCR drive proliferation rather than differentiation and are ligand independent.34 It is possible that mislocalization of FU-Lck to the CD3 chains initiates a signaling cascade that, in the presence of excessive levels of Lck, results in unregulated proliferation. A further, not mutually exclusive, explanation is that FU-Lck may disrupt the function of Fyn. It is established that Fyn can negatively regulate T-cell activation26,35 and that FU-Lck might prevent Fyn-dependent feedback inhibition pathways; however, Fyn−/− mice do not develop tumors. Nevertheless, it is clear that coexpression of FU-Lck results in dysregulation of WT Lck, because it shows hyperphosphorylation at both the activatory Y394 and the inhibitory Y505 residues.
The ability of signaling proteins to localize to specific cellular compartments is critical for TCR signal transduction. Intracellular localization is regulated dynamically through interactions mediated via specific functional domains. Mislocalization of signaling proteins can dramatically affect the outcome of TCR signaling. Thus, constitutive targeting of CD45 or SLP76 to plasma membrane lipid microdomains results in defective TCR signaling.36,37 Lck has long been known to associate with the CD4 and CD8 coreceptors, and deletion of the Lck unique domain critical for coreceptor association results in a failure to induce interleukin-2 promoter activity in Jurkat cells.10 In the present study, we show that the FU domain cannot substitute for that of Lck in transgenic mice, and that a mislocalized FU-Lck protein is greatly impaired in mediating signals at all stages of T-cell development. Furthermore, aberrant signaling as a consequence of coexpression of FU-Lck and WT Lck results in thymomas. These data show that the regulation of intracellular localization by unique domains is critical for SFK function in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants 0251 and 05096 from Leukemia & Lymphoma Research UK (to R.J.S., A.F., and R.Z.) and from the Medical Research Council UK (to R.Z., N.P., and A.I.M.).
Authorship
Contribution: R.J.S. designed and performed experiments, analyzed data, and cowrote the paper; A.F. and N.P. performed experiments; A.I.M. designed experiments; and R.Z. designed the study and cowrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.F. is Flow Cytometry Facility, London Research Institute, Cancer Research UK, London, United Kingdom.
Correspondence: Rose Zamoyska, Institute of Immunology and Infection Research, University of Edinburgh, West Mains Rd, Edinburgh EH9 3JT,United Kingdom; e-mail: Rose.Zamoyska@ed.ac.uk.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal