Abstract
Erdheim-Chester disease (ECD) is a rare form of non-Langerhans histiocytosis, with noncodified therapeutic management and high mortality. No treatment has yet been shown to improve survival in these patients. We conducted a multicenter prospective observational cohort study to assess whether extraskeletal manifestations and interferon-α treatment would influence survival in a large cohort of ECD patients. To achieve this goal, we thoroughly analyzed the clinical presentation of 53 patients with biopsy-proven ECD, and we performed a survival analysis using Cox proportional hazard model. Fifty-three patients (39 men and 14 women) with biopsy-proven ECD were followed up between November 1981 and November 2010. Forty-six patients (87%) received interferon-α and/or PEGylated interferon-α. Multivariate survival analysis using Cox proportional hazard model revealed that central nervous system involvement was an independent predictor of death (hazard ratio = 2.51; 95% confidence interval, 1.28-5.52; P = .006) in our cohort. Conversely, treatment with interferon-α was identified as an independent predictor of survival (hazard ratio = 0.32; 95% confidence interval, 0.14-0.70; P = .006). Although definitive confirmation would require a randomized controlled trial, these results suggest that interferon-α improves survival in ECD patients. This may be seen as a significant advance, as it is the first time a treatment is shown to improve survival in this multisystemic disease with high mortality.
Introduction
Erdheim-Chester disease (ECD) is a rare form of non-Langerhans histiocytosis first described by Jakob Erdheim and William Chester in 1930.1 By November 2010, less than 400 distinct cases have been reported in the medical literature. ECD is a systemic and heterogeneous disease mainly involving the bones, lungs, skin, retro-orbital tissues, central nervous system (CNS), pituitary gland, large vessels, kidneys, retroperitoneum, and heart.2,3 The clinical course of ECD is largely dependent on the extent and distribution of the disease, which may range from asymptomatic bone lesions to multisystemic, life-threatening forms with poor prognosis, especially in case of specific CNS or cardiovascular involvements.2,4 ECD diagnosis is currently based on clinical, radiologic, and typical pathologic features with the biopsy specimen displaying infiltration by CD68+CD1a− foamy histiocytes, which emphasizes the distinction from Langerhans cell histiocytosis. Treatments for ECD include steroids, cytotoxic agents such as cladribin,5 interferon-α-2a (interferon-α),6,7 recombinant human interleukin-1 receptor antagonist,8 thyrosine kinase inhibitors,9,10 biphosphonates,11 and autologous hematopoietic stem cell transplantation,12 but an optimal therapeutic strategy remains to be defined, mostly because these treatments were evaluated only in a limited number of patients. Although interferon-α has demonstrated valuable results in ECD,6,7 our group has also shown that its efficacy was variable by patient and site of disease involvement.7 Until now, no treatment has been shown to improve survival in ECD patients, which is truly a critical issue because the overall mortality of the disease is high.
The present study was undertaken to assess which extraskeletal manifestations would influence survival in a large cohort of ECD patients and whether treatment with interferon-α would improve survival in these patients. To achieve this goal, we thoroughly analyzed the clinical presentation of 53 patients with biopsy-proven ECD and performed a survival analysis using the Cox proportional hazard model.
Methods
Study design and setting
The present series is based on the prospective follow-up of a multicenter observational cohort of 58 patients with biopsy-proven ECD, who were referred to the internal medicine departments of either Pitié-Salpêtrière Hospital (Paris, France) or of Centre Hospitalier Universitaire of Nantes (Nantes, France), between November 1981 and November 2010. A single patient was referred in 1981 (and died in 1991). All other patients were referred after 1996. These patients were originally referred from: France (n = 45), Belgium (n = 3), Italy (n = 2), Spain (n = 2), Germany (n = 2), and from England, Israel, Norway, and Portugal (each n = 1).
Eligibility criteria and methods of follow-up
In all cases, ECD was diagnosed based on the presence of typical histologic findings (infiltration with foamy histiocytes nested among polymorphic granuloma and fibrosis or xanthogranulomatosis with CD68+ and CD1a− immunohistochemical staining). Five patients were excluded from further analyses because they had an overlap between ECD and another form of histiocytosis. Thus, the subsequent analyses included 53 patients.
Among these 53 patients, 46 (87%) received interferon-α and/or PEGylated interferon-α, and 7 did not receive any of these treatments, either because these patients had a contraindication to interferon-α or because the physician in charge of the patient did not consider this therapeutic option, given the lack of formal demonstration of efficacy at that time.
All patients but one were followed every 3 to 6 months, depending on disease activity (2 patients had limited follow-up but underwent complete evaluation as described hereafter). All patients were evaluated with a complete physical examination, and a standardized imaging protocol including a computed tomography (CT) scan or magnetic resonance imaging (MRI) of the CNS, a CT scan or an MRI of the whole aorta, a cardiac MRI, a CT scan of the chest, abdomen, and pelvis, and a transthoracic echocardiography. All patients but the one referred in 1981 (who died in 1991) underwent at least one MRI of the CNS and of the cardiovascular system at final evaluation. Cardiovascular imaging, high-resolution chest CT scans, and skull MRI and CT scans were, respectively, reviewed in consensus by 2 vascular radiologists, 2 chest radiologists, and 3 neuroradiologists. Because all patients but one were referred after 1996, the techniques used for CT scans and MRIs were consistent for all evaluations and among centers. All data recorded until November 1, 2010 were considered for the subsequent analyses. Patients' informed consent and approval by the CPP Île de France IV, Paris Institutional Review Board were obtained before the study began in accordance with the Declaration of Helsinki.
Assessment of variables of interest
Cardiac involvement was defined as the presence of a coronary involvement (presence of a pericoronary infiltration and/or stenosis of a coronary artery on cardiovascular MRI and/or CT scan) and/or ECD valvulopathy (defined echocardiographically) and/or pericardial thickening or effusion (on cardiovascular MRI and/or chest CT scan) and/or right atrial pseudotumor (presence of an abnormal infiltration of the right atrium and/or of the atrioventricular sulcus on cardiovascular MRI and/or CT scan).2,13 Large-vessel involvement was defined as the presence of a perivascular thickening around the aorta and/or its main branches (on thoracic, abdominal, and/or pelvic CT scan or on cardiovascular MRI).2,14 CNS involvement was defined radiologically as the presence of a diencephalic and/or meningeal and/or cerebellar infiltration (on brain MRI and/or CT scan).15,16 Hypophyseal involvement was defined as the presence of a pituitary infiltration on brain MRI and/or CT scan or as the lack of the normal T1 high signal intensity of the posterior pituitary lobe on brain MRI.16 Maxillary and sinus involvement were, respectively, defined as the presence of an osteosclerosis of the maxillary bone or of a sinus wall on CT scan and/or on skull MRI (bone thickening with low signal intensity on both T1- and T2-weighted images).16 Orbital involvement was defined as the presence of an orbital infiltration on CT scan and/or on skull MRI.16 Retroperitoneal and adrenal involvements were, respectively, defined as the infiltration of the perirenal fat (hairy kidney aspect) or of the adrenal gland on abdominal CT scan.2,17 Pulmonary involvement was defined radiologically (typical lesions on high-resolution chest CT scan), according to previously published criteria.18,19
Statistics
Survival curves were built according to the Kaplan-Meier method, considering the time from diagnosis of ECD to death or to last follow-up as the time to event. Comparison of Kaplan-Meier survival estimates was performed using the log-rank test. Multivariate Cox proportional hazard regression analysis was performed to identify independent predictors of survival. First, an age-adjusted model, including all clinical variables with P < .20 in univariate analyses using the likelihood ratio test, was built to identify clinical predictors of poor survival (age at compilation was expressed as quartiles of the distribution). Because we have previously shown that cardiovascular involvement was an important cause of death in ECD,2 this previous model was further adjusted for the presence of cardiovascular involvement. Second, we built a model including all clinical variables with P < .20 in univariate analyses as well as the use of interferon-α in these patients (as a dichotomous variable), adjusted for the age at compilation, and for use of corticosteroids and/or any other immunosuppressive drugs. For each variable, the proportional hazards assumption was tested visually using Kaplan-Meier curves and by examining a plot of -log(-log(survival time)) against the log(time). Differences between groups of patients were tested using the Mann-Whitney test for continuous data, and Fisher exact test or the χ2 test for categorical data. All tests were 2-sided, and a P value < .05 was considered statistically significant. Statistical analyses were performed using GraphPad Prism Version 5.0 software (GraphPad Software) and JMP8 (SAS institute).
Results
Patients characteristics and treatment
Data were obtained from 53 patients (39 men and 14 women). Median follow-up durations from ECD onset and ECD diagnosis were, respectively, of 89.3 months (range, 3.6-430.3 months) and 37.6 months (range, 0.7-188.7 months). Skeletal involvement was present in 51 (96%) patients, cardiovascular involvement in 77%, retroperitoneal infiltration in 68%, CNS involvement in 51%, and pulmonary involvement in 43%. At least one extraskeletal manifestation was present in 52 patients (98%). Comparison by sex revealed similar age at onset and at diagnosis (P = .27 and P = .28, respectively). Frequencies of visceral involvement were similar between the sexes. Forty-six patients (87%) received interferon-α and/or PEGylated interferon-α for a median duration of 18.8 months (range, 0.4-182.2 months). Maximum doses of interferon-α reached in these patients were: 3 million International Units [MIU] (n = 10), 4.5 MIU (n = 1), 6 MIU (n = 2), or 9 MIU (n = 14), 3 times a week; or PEGylated interferon-α at 135 μg (n = 5), 180 μg (n = 13), 200 μg (n = 1), once a week. We thus considered that 16 of 46 patients (35%) received regular doses of interferon-α (< 6MIU, 3 times a week) or of PEGylated interferon-α (< 180 μg/wk) and that 30 patients (65%) received high doses. The indication for these high doses was progressive disease despite regular doses of interferon-α and/or severe disease with cardiovascular or CNS involvements. Patients treated with and without interferon-α and/or PEGylated interferon-α had similar clinical characteristics (comparison of sex, age at onset and at compilation, diagnostic delay, duration of follow-up, and frequency of all clinical manifestations between patients treated with and without interferon: P > .05 in all). Detailed demographic data, frequency of skeletal and extraskeletal manifestations, and treatments for the whole cohort are shown in Table 1.
Characteristics of 53 patients with ECD
| Variable of interest . | Value . |
|---|---|
| Epidemiologic data | |
| Male sex, no. (%) | 39 (74) |
| Median age at onset, y (range) | 53 (6-76) |
| Median age at diagnosis, y (range) | 57 (16-80) |
| Median age at compilation, y (range) | 62 (22-81) |
| Median follow-up from disease onset, mo (range) | 89 (4-430) |
| Median follow-up from diagnosis, mo (range) | 38 (1-189) |
| Global mortality, no. (%) | 14 (26) |
| 1-year survival, % | 96 |
| 5-year survival, % | 68 |
| Skeletal involvement, no. (%) | 51 (96) |
| Extraskeletal involvement, no. (%) | 52 (98) |
| Cardiac involvement, no. (%) | 34 (64) |
| Coronary involvement | 22 (42) |
| Pericardial involvement | 22 (42) |
| Right atrial pseudotumor | 21 (40) |
| Valvulopathy | 9 (17) |
| Large-vessel involvement, no. (%) | 35 (66) |
| CNS involvement, no. (%) | 27 (51) |
| Diencephalic involvement | 23 (43) |
| Meningeal involvement | 9 (17) |
| Cerebellar involvement | 11 (21) |
| Other manifestations of the disease, no. (%) | |
| Paranasal sinus involvement | 33 (62) |
| Maxillary involvement | 19 (36) |
| Cutaneous involvement (xanthelasma) | 15 (28) |
| Orbital involvement | 13 (25) |
| Hypophyseal involvement | 12 (23) |
| Pulmonary involvement | 23 (43) |
| Retroperitoneal infiltration | 36 (68) |
| Adrenal gland infiltration | 12 (23) |
| Treatments, no. (%) | |
| Corticosteroids | 30 (57) |
| Interferon-α | 46 (87) |
| PEGylated-interferon-α | 18 (34) |
| Mesylate imatinib | 7 (13) |
| Sunitinib malate | 6 (11) |
| Vinblastine | 2 (4) |
| 2CDA (cladribine) | 2 (4) |
| Infliximab | 1 (2) |
| Etoposide | 1 (2) |
| Anakinra | 1 (2) |
| Methotrexate | 1 (2) |
| Cyclophosphamide | 1 (2) |
| Variable of interest . | Value . |
|---|---|
| Epidemiologic data | |
| Male sex, no. (%) | 39 (74) |
| Median age at onset, y (range) | 53 (6-76) |
| Median age at diagnosis, y (range) | 57 (16-80) |
| Median age at compilation, y (range) | 62 (22-81) |
| Median follow-up from disease onset, mo (range) | 89 (4-430) |
| Median follow-up from diagnosis, mo (range) | 38 (1-189) |
| Global mortality, no. (%) | 14 (26) |
| 1-year survival, % | 96 |
| 5-year survival, % | 68 |
| Skeletal involvement, no. (%) | 51 (96) |
| Extraskeletal involvement, no. (%) | 52 (98) |
| Cardiac involvement, no. (%) | 34 (64) |
| Coronary involvement | 22 (42) |
| Pericardial involvement | 22 (42) |
| Right atrial pseudotumor | 21 (40) |
| Valvulopathy | 9 (17) |
| Large-vessel involvement, no. (%) | 35 (66) |
| CNS involvement, no. (%) | 27 (51) |
| Diencephalic involvement | 23 (43) |
| Meningeal involvement | 9 (17) |
| Cerebellar involvement | 11 (21) |
| Other manifestations of the disease, no. (%) | |
| Paranasal sinus involvement | 33 (62) |
| Maxillary involvement | 19 (36) |
| Cutaneous involvement (xanthelasma) | 15 (28) |
| Orbital involvement | 13 (25) |
| Hypophyseal involvement | 12 (23) |
| Pulmonary involvement | 23 (43) |
| Retroperitoneal infiltration | 36 (68) |
| Adrenal gland infiltration | 12 (23) |
| Treatments, no. (%) | |
| Corticosteroids | 30 (57) |
| Interferon-α | 46 (87) |
| PEGylated-interferon-α | 18 (34) |
| Mesylate imatinib | 7 (13) |
| Sunitinib malate | 6 (11) |
| Vinblastine | 2 (4) |
| 2CDA (cladribine) | 2 (4) |
| Infliximab | 1 (2) |
| Etoposide | 1 (2) |
| Anakinra | 1 (2) |
| Methotrexate | 1 (2) |
| Cyclophosphamide | 1 (2) |
Mortality rate and causes of death
Fourteen patients (26%) died after a median follow-up of 55.9 months (range, 14.4-197.6 months) from disease onset, and 26.5 months (range, 0.7-165.3 months) from diagnosis. The causes of death were as follow: CNS involvement (n = 4; including 2 patients with fatal cerebral hypertension resulting from rapidly progressive cerebral involvement and 2 patients with slowly progressive CNS involvement), myocardial infarction (n = 2), digestive tract hemorrhage (n = 1), pulmonary infection under mechanical ventilation in the postoperative course of a mitral valve replacement complicated by a tamponade (n = 1), multisystemic severe ECD with cardiovascular involvement and end-stage colon cancer (n = 1), unexplained metabolic abnormalities after unsuccessful removal of a JJ ureteral stent (n = 1), sudden death in a patient with severe vascular involvement (n = 1), multiorgan failure in a patient with end-stage cardiac failure (n = 1), aortic dissection (n = 1), and unexplained malignant hypercalcemia and intracerebral hemorrhage (n = 1). The 1-year and 5-year survival rates were 96% and 68%, respectively.
Clinical predictors of survival
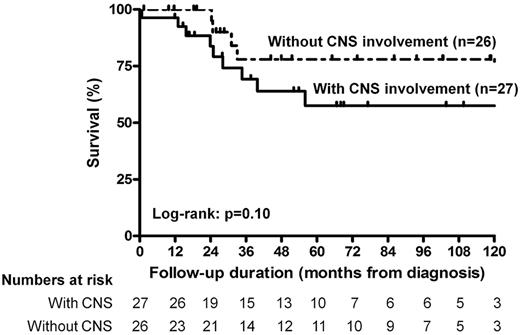
Although comparison of survival curves revealed a nonsignificant difference in survival (P = .10) between patients with and without CNS involvement (Figure 1), multivariate survival analysis using Cox proportional hazard model (Table 2) adjusted for the age at compilation revealed that CNS involvement was the sole independent clinical predictor of fatal outcome in our cohort (hazard ratio [HR] = 2.43; 95% confidence interval [CI], 1.33-4.94; P = .004). Because we have previously reported that cardiovascular involvement may be an important cause of death in ECD,2 we further adjusted this model for the presence of cardiovascular involvement, and CNS involvement remained a major independent predictor of death (HR = 2.74; 95% CI, 1.43-5.93; P = .002) among our patients.
Kaplan-Meier survival estimates of the survival function for the patients with ECD treated with (n = 27) or without (n = 26) involvement of the CNS. Hash marks indicate censored persons.
Kaplan-Meier survival estimates of the survival function for the patients with ECD treated with (n = 27) or without (n = 26) involvement of the CNS. Hash marks indicate censored persons.
Clinical predictors of poor survival in ECD (multivariate survival analysis using the Cox proportional hazard model)
| Variable . | Univariate analysis (P) . | Cox multivariate survival analysis* . | |
|---|---|---|---|
| HR (95% CI) . | P . | ||
| Sex | .55 | ||
| Cardiovascular involvement | .57 | ||
| CNS involvement | .10 | 2.43 (1.33-4.94) | .004 |
| Hypophyseal involvement | .93 | ||
| Paranasal sinus involvement | .54 | ||
| Maxillary involvement | .23 | ||
| Xanthelasma | .98 | ||
| Orbital involvement | .57 | ||
| Pulmonary involvement | .21 | ||
| Retroperitoneal involvement | .31 | ||
| Adrenal involvement | .53 | ||
| Variable . | Univariate analysis (P) . | Cox multivariate survival analysis* . | |
|---|---|---|---|
| HR (95% CI) . | P . | ||
| Sex | .55 | ||
| Cardiovascular involvement | .57 | ||
| CNS involvement | .10 | 2.43 (1.33-4.94) | .004 |
| Hypophyseal involvement | .93 | ||
| Paranasal sinus involvement | .54 | ||
| Maxillary involvement | .23 | ||
| Xanthelasma | .98 | ||
| Orbital involvement | .57 | ||
| Pulmonary involvement | .21 | ||
| Retroperitoneal involvement | .31 | ||
| Adrenal involvement | .53 | ||
P < .20 in univariate analysis were entered in the multivariate model.
Adjusted for the age at compilation (quartiles of the distribution).
Therapeutic predictors of survival
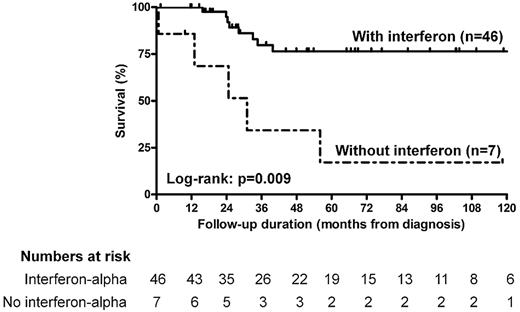
Comparison of survival curves revealed significantly improved survival in patients who received interferon-α and/or PEGylated interferon-α (log-rank test, P = .009) than in those who did not (Figure 2). Interestingly, patients who received regular doses of interferon had similar survival as those who received high doses (P = .68). Finally, we found no significant difference in survival between patients who received corticosteroids (P = .07) and other immunosuppressive agents (P = .13) than in those who did not.
Kaplan-Meier survival estimates of the survival function for the patients with ECD treated with (n = 46) or without (n = 7) interferon-α and/or PEGylated interferon-α. Comparison of patients treated with and without interferon-α and/or PEGylated interferon-α shows a significant difference in survival between those 2 groups (log-rank test, P = .004). Hash marks indicate censored persons.
Kaplan-Meier survival estimates of the survival function for the patients with ECD treated with (n = 46) or without (n = 7) interferon-α and/or PEGylated interferon-α. Comparison of patients treated with and without interferon-α and/or PEGylated interferon-α shows a significant difference in survival between those 2 groups (log-rank test, P = .004). Hash marks indicate censored persons.
To further decide whether treatment with interferon-α would be an independent predictor of survival among ECD patients, we built a multivariate survival Cox model, including the presence of CNS involvement and the use of interferon-α treatment. This analysis was adjusted for the age at compilation and the use of corticosteroids or of any other immunosuppressive drugs (Table 3). In this model, CNS involvement remained an independent predictor of fatal outcome (HR = 2.51; 95% CI, 1.28-5.52; P = .006). Furthermore, treatment with interferon-α was identified as a major independent predictor of survival among our patients (HR = 0.32; 95% CI, 0.14-0.70; P = .006), whereas the use of neither corticosteroids (P = .40) nor of any immunosuppressive drug (P = .13) was a significant contributor to this model. Similar results were also observed after further adjustment for the presence of cardiovascular involvement. Finally, because we thought that no relevant therapeutic effect was probably observed in patients treated with interferon-α and/or PEGylated interferon-α for less than 3 months, we repeated these analyses after excluding 3 such patients, and interferon-α remained a major independent predictor of survival.
Clinical and therapeutic predictors of survival in ECD (multivariate survival analysis using the Cox proportional hazard model)
| Variable . | Univariate analysis (P) . | Cox multivariate survival analysis* . | |
|---|---|---|---|
| HR (95% CI) . | P . | ||
| Sex | .55 | ||
| Cardiovascular involvement | .57 | ||
| CNS involvement | .10 | 2.51 (1.28-5.52) | .006 |
| Hypophyseal involvement | .93 | ||
| Paranasal sinus involvement | .54 | ||
| Maxillary involvement | .23 | ||
| Xanthelasma | .98 | ||
| Orbital involvement | .57 | ||
| Pulmonary involvement | .21 | ||
| Retroperitoneal involvement | .31 | ||
| Adrenal involvement | .53 | ||
| Treatment with interferons† | .03 | 0.32 (0.14-0.70) | .006 |
| Variable . | Univariate analysis (P) . | Cox multivariate survival analysis* . | |
|---|---|---|---|
| HR (95% CI) . | P . | ||
| Sex | .55 | ||
| Cardiovascular involvement | .57 | ||
| CNS involvement | .10 | 2.51 (1.28-5.52) | .006 |
| Hypophyseal involvement | .93 | ||
| Paranasal sinus involvement | .54 | ||
| Maxillary involvement | .23 | ||
| Xanthelasma | .98 | ||
| Orbital involvement | .57 | ||
| Pulmonary involvement | .21 | ||
| Retroperitoneal involvement | .31 | ||
| Adrenal involvement | .53 | ||
| Treatment with interferons† | .03 | 0.32 (0.14-0.70) | .006 |
P < .20 in univariate analysis were entered in the multivariate model.
Adjusted for the age at compilation (quartiles of the distribution) and the use of corticosteroids or of any other immunosuppressive drugs.
Treatment with interferon-α and/or PEGylated-interferon-α.
Discussion
Proper care of ECD patients is complicated by the wide clinical spectrum of the disease.3 Until now, no treatment has shown efficacy to improve survival in ECD patients, and clinical predictors of survival have not been clearly identified. In this study, we thoroughly analyzed the clinical presentation and survival data of a large cohort of 53 patients with biopsy-proven ECD, so that we were able to identify independent predictors of poor prognosis.
Among other extraskeletal lesions, ECD patients have frequent diencephalic, meningeal, and/or cerebellar involvements.16 Altogether, CNS lesions were found in 51% of patients and directly accounted for 29% of all deaths observed during the study. Interestingly, the presence of CNS involvement was identified as the sole independent clinical predictor of poor survival in the Cox model, with an HR of 2.43 (95% CI, 1.33-4.94; P = .004). Indirectly, these data underline the importance of performing a careful clinical and morphologic CNS evaluation at initial diagnosis and during the follow-up.
Both univariate and multivariate analyses using the Cox model revealed that treatment with interferon-α and/or PEGylated interferon-α was a major predictor of survival among our patients (HR = 0.32; 95% CI, 0.14-0.70; P = .006). Patients treated with and without interferon had similar epidemiologic and clinical characteristics, suggesting that these parameters cannot account for the differences observed in survival. Furthermore, treatment with interferon-α remained a strong independent predictor of survival after exclusion of the 3 patients with short treatment durations, suggesting robustness of results. Finally, comparison of survival curves between patients who received regular and high doses of interferon-α and/or of PEGylated interferon-α revealed no significant difference, either suggesting that survival improvement may be observed at regular doses or conversely that high doses are required to improve prognosis in the most severe patients, in which these high doses were used.
Among the limitations of this prospective observational study is the relatively small number of patients who did not receive interferon-α and/or PEGylated interferon-α. Indeed, 46 patients (87%) received interferon-α and/or PEGylated interferon-α and 7 did not, either because they had a contraindication to interferon-α or because the physician in charge of the patient did not consider this therapeutic option, given the lack of formal demonstration of efficacy at that time. Nevertheless, patients who received interferon-α and/or PEGylated interferon-α and those who did not had similar epidemiologic and clinical characteristics, allowing valid comparison between these 2 groups. Moreover, this study of 53 patients is the largest published to date and collates a sample of patients large enough to reveal a statistically significant association between treatment with interferon-α and survival in both univariate and Cox multivariate analyses. Most importantly, these analyses were adjusted for treatment with corticosteroids and/or other immunosuppressive agents so that we could account for the potential efficacy of theses drugs. Furthermore, these drugs were not identified as significant contributors to the multivariate survival model. Although 2 centers were involved in this study, data collection and interpretation were performed according to a standardized protocol, and thus were homogeneous.
Although definitive confirmation would require a randomized controlled trial, which would be quite difficult to set up in such a rare disease, the results of this prospective observational study suggest that interferon-α should be used as the first-line therapy in ECD because this treatment improves survival at a regular dose of 3 MIU, 3 times a week. Our data may thus be seen as a significant advance in the care of ECD patients, as it is the first time a treatment is shown to improve survival in this multisystemic disease with high mortality.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: L. Arnaud, Z.A., and J.H. designed research; L. Arnaud, B.H., A.N., M.A.H., J.-E.K., B.W., G.P.-P., B.B., J.-G.F., F.D., A.M., C.M., V.N., M.P., J.C., A.-B.B., N.C.-C., L. Aaron, J.S., C.G., P.C., V.D., C.D., P.B., C.H., G.G., Z.A., and J.H. collected data; L. Arnaud performed statistical analysis; L. Arnaud, B.H., A.N., Z.A., and J.H. analyzed and interpreted data; L. Arnaud, Z.A., and J.H. wrote the manuscript; and all authors approved the final manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Julien Haroche, Service de Médecine Interne 2, Groupe Hospitalier Pitié-Salpêtrière, 47-83 bd de l'Hôpital, 75013, Paris, France; e-mail: julien.haroche@psl.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal