FLT3 inhibition has emerged as an attractive concept to overcome the dismal prognosis of FLT3 mutated AML patients. Sato and colleagues have identified that high plasma levels of FLT3 ligand could impede bioactivity of FLT3 inhibitors in these patients.1 Along this line, Levis and colleagues present lessons learned regarding bioactivity of the FLT3 inhibitor lestaurtinib investigated within a clinical trial.2
FLT3, a growth factor receptor tyrosine kinase is mutated in ∼ 30% of acute myeloid leukemia (AML) patients. FLT3 mutations represent one of the most frequently identified genetic alterations in AML. The presence of an FLT3–internal tandem duplication (ITD) mutation significantly correlates with an increased risk of relapse and dismal overall survival.3,4 Therefore, aberrantly activated FLT3-ITD kinase is considered to represent an attractive therapeutic target in AML. Several small molecule FLT3 inhibitors have been developed and examined in AML patients as single agents or in combination with chemotherapy.5 However, in recent years we have learned that a genetically complex malignancy such as AML present a multitude of challenges when trying to achieve therapeutic inhibition of FLT3. One of the major questions in this field is why there are clinically meaningful responses in some patients, no responses in others, minor and short-lived in the rest.
In the article by Sato et al, the authors performed a systematic analysis on whether plasma FLT3 ligand (FL) levels could influence bioactivity of a panel of FLT3 inhibitors.1 They found that after intensive chemotherapy FL rose to high levels in plasma of these patients: the mean for baseline levels in newly diagnosed patients was 3 pg/mL, whereas for samples obtained on day 15 of induction chemotherapy the mean was 488 pg/mL. Interestingly, in relapsed patients the mean for samples obtained on day 15 of induction chemotherapy was significantly higher and reached 1148 pg/mL. FL levels were shown to continue to rise with successive courses of chemotherapy to a mean of 3251 pg/mL after the fourth course. The authors then performed in vitro studies using a panel of FLT3 inhibitors and showed that exogenous FL at concentrations similar to that observed in patients inhibited FLT3 phosphorylation and cytotoxicity in cell lines and in primary AML samples. Sato and colleagues reason that the dramatic increase in FL levels after chemotherapy documented in this study may interfere with bioactivity of FLT3 inhibitors in the clinical setting. This is an interesting and potentially important aspect when applying therapeutic FLT3 inhibition. However, it remains to be shown whether elevations in FL levels observed are indeed clinically meaningful. This issue needs to be addressed in upcoming clinical trials investigating FLT3 inhibition.
Another interesting aspect of this paper relates to the mechanism by which FL impairs activity of FLT3 inhibitors, which is unclear at this time. However, it has been shown that ligand activation of a receptor may significantly change affinity for a kinase inhibitor.6 This may be because of an influence on the equilibrium of active or inactive conformation of the receptor.
In the second article, Levis et al present eagerly awaited results from a randomized trial of salvage chemotherapy followed by lestaurtinib for patients with FLT3 mutant AML in first relapse.2 This is the first full report on a clinical trial investigating efficacy and toxicity of FLT3 inhibition in a randomized fashion in this cohort of patients. Two hundred twenty-four patients received chemotherapy alone or followed by 80 mg of the FLT3 inhibitor lestaurtinib twice daily. By intention-to-treat analysis, there was no statistically significant improvement in remission rate or overall survival for patients receiving lestaurtinib. Obviously, this is a discouraging result for the field of therapeutic FLT3 inhibition in AML. Do results obtained challenge the concept of therapeutic FLT3 inhibition?
A major strength of the study by Levis and colleagues is that bioactivity of lestaurtinib was carefully monitored using a plasma inhibitory activity (PIA) assay. In addition, lestaurtinib plasma concentrations, FLT3 ligand levels, and AGP levels were also determined. From previous in vitro and in vivo studies the authors had estimated that FLT3 needed to be suppressed to less than 15% of its baseline activity to induce a cytotoxic effect. However, at aplasia assessment, only 46 of 79 patients (58%) analyzed actually achieved this goal and only 21 of these achieved this degree of inhibition later on at the day of outcome assessment. Why did lestaurtinib fail to induce meaningful bioactivity? The authors provide some important suggestions: (1) Decreasing plasma levels of lestaurtinib over the course of treatment were observed and could have contributed. (2) Elevated FL levels after chemotherapy as described by Sato et al were observed and thus may have also impeded bioactivity of lestaurtinib. (3) In addition to FL, α-1 acid glycoprotein (AGP) rose from baseline by day 15 of induction chemotherapy, and remained elevated by day 42. In human plasma, lestaurtinib binds with high affinity to AGP. Thus, higher levels of AGP in plasma result in lower levels of free lestaurtinib. (4) In some patients, very high levels of lestaurtinib had been noted that appeared to predict toxicity and yet did not predict in vivo FLT3 inhibition. Thus, the complexities of the pharmacokinetics resulting in toxic off-target effects may have also contributed to limited efficacy of lestaurtinib in this trial.
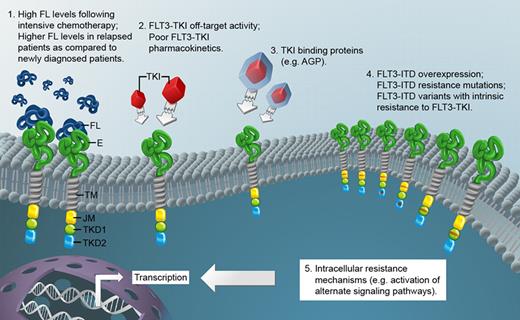
Another reason that lestaurtinib failed to provide benefit to patients may relate to the population of AML patients being studied. It is conceivable that a number of resistance mechanisms are already operating in leukemic cells of relapsed patients. This may include activation of alternate pathways, resistance mutations, and FLT3 up-regulation. The latter mechanisms would not have been detected by the PIA assay used. The accompanying figure summarizes molecular mechanisms potentially impeding bioactivity of FLT3 tyrosine kinase inhibitors (FLT3-TKI) as suggested by Sato et al and by the results of the trial presented by Levis et al.
FL indicates FLT3 ligand; E, extracellular domain of FLT3; TM, transmembrane domain; JM, juxtamembrane domain; TKD1, tyrosine kinase domain 1; TKD2, tyrosine kinase domain 2; TKI, tyrosine kinase inhibitor; AGP, alpha-1 acid glycoprotein; and FLT3-ITD, FLT3 receptor with internal tandem duplication mutation.
FL indicates FLT3 ligand; E, extracellular domain of FLT3; TM, transmembrane domain; JM, juxtamembrane domain; TKD1, tyrosine kinase domain 1; TKD2, tyrosine kinase domain 2; TKI, tyrosine kinase inhibitor; AGP, alpha-1 acid glycoprotein; and FLT3-ITD, FLT3 receptor with internal tandem duplication mutation.
One encouraging aspect of the study by Levis et al, however, was that in vivo FLT3 inhibition correlated very highly with remission rate. This suggests that FLT3 inhibition as a therapeutic modality is still very promising once we are able to apply inhibitors with improved pharmacokinetic and pharmacodynamic properties and once we are able to overcome bioactivity issues identified in the 2 papers.
Conflict of interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal