The studies by Smith et al reported in this issue of Blood send us full circle, back to the starting model for the fibrinogen complex with plasma FXIII.1 These authors report that plasma factor XIII, called FXIII-A2B2, binds to the αC domain of fibrin(ogen) with high affinity, ∼ 30nM.
Factor XIII is a plasma- and platelet-derived protransglutaminase that is essential for hemostasis and wound healing.2 Thirty-year-old studies show fibrin(ogen) is a cofactor for the thrombin-catalyzed activation of FXIII-A2B2. In 1981, Lorand's group reported that fibrinogen, and specifically a fragment from the fibrinogen α chain, modulates the generation of catalytically active factor XIII, FXIIIa.3 In 1982, Greenberg and Shuman showed FXIII-A2B2 bound specifically to fibrinogen with an affinity of ∼ 10nM. Based on this affinity, they concluded that fibrinogen and FXIII-A2B2 circulate as a complex in plasma.4
Subsequent studies by Mosesson's group support the existence of this circulating complex.5 They found FXIII-A2B2 copurifies with fibrinogen, but selectively with a fibrinogen fraction called peak 2. Further characterization showed pure FXIII-A2B2 mixed with pure fibrinogen coeluted on ion exchange or gel filtration chromatography as long as the fibrinogen component was peak 2, and not peak 1. In contrast, platelet factor XIII, FXIII-A2, did not coelute with either fibrinogen. The most substantive difference between peak1 and peak 2 fibrinogens is the presence of the alternatively spiced variant fibrinogen chain known as γ′.6 This variant is 16 residues longer than the more common chain, called γA. Because fibrinogen is a dimer and the 2 chains are stochastically assembled, ∼ 15% of fibrinogen molecules are heterodimers with 1 copy of each variant. The authors concluded that interactions between γ′ fibrinogen and the B subunit of FXIII-A2B2 support the circulating complex. The model shown in panel A of the figure is based on these studies.
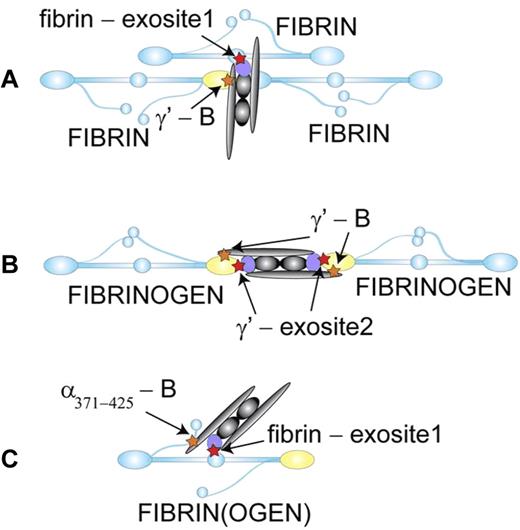
Fibrin(ogen) as a cofactor for the thrombin-catalyzed activation of FXIII-A2B2. (A) Thrombin (violet) binds (red star) through exosite 1 to the central domain of one fibrin molecule (blue) and FXIII-A2B2 (gray) binds (orange star) through the B subunit to the γ′ chain (yellow) of a second fibrin molecule.9 When fibrin polymerizes, enzyme and substrate are brought together to enhance the rate of activation. (B) FXIII-A2B2 binds through the B subunit to the γ′ chains of 2 fibrinogen molecules.7 When fibrin polymerizes, thrombin binds through exosite 2 to the γ′ chain to enhance the rate of substrate cleavage. In this model, the γ′ chain also enhances the calcium-dependent activation step. (C) Thrombin binds through exosite 1 to the central domain of fibrinogen and FXIII-A2B2 binds through the B subunit to α chain residues 371-425. Either fibrinogen or fibrin monomer could serve as the cofactor in this configuration where enzyme and substrate are in close proximity on one molecule. Because kinetic analyses indicate FXIII-A2B2 activation occurs subsequent to thrombin-catalyzed cleavage of fibrinopeptide A from fibrinogen, this model suggests that substrate specificity determines the sequential appearance of fibrin and FXIIIa. Illustration by Oleg V. Gorkun, PhD.
Fibrin(ogen) as a cofactor for the thrombin-catalyzed activation of FXIII-A2B2. (A) Thrombin (violet) binds (red star) through exosite 1 to the central domain of one fibrin molecule (blue) and FXIII-A2B2 (gray) binds (orange star) through the B subunit to the γ′ chain (yellow) of a second fibrin molecule.9 When fibrin polymerizes, enzyme and substrate are brought together to enhance the rate of activation. (B) FXIII-A2B2 binds through the B subunit to the γ′ chains of 2 fibrinogen molecules.7 When fibrin polymerizes, thrombin binds through exosite 2 to the γ′ chain to enhance the rate of substrate cleavage. In this model, the γ′ chain also enhances the calcium-dependent activation step. (C) Thrombin binds through exosite 1 to the central domain of fibrinogen and FXIII-A2B2 binds through the B subunit to α chain residues 371-425. Either fibrinogen or fibrin monomer could serve as the cofactor in this configuration where enzyme and substrate are in close proximity on one molecule. Because kinetic analyses indicate FXIII-A2B2 activation occurs subsequent to thrombin-catalyzed cleavage of fibrinopeptide A from fibrinogen, this model suggests that substrate specificity determines the sequential appearance of fibrin and FXIIIa. Illustration by Oleg V. Gorkun, PhD.
Follow-up studies from Farrell's group also suggested preferential binding to the γ′/γA heterodimer, but added 2 surprising findings.7 First, the difference in affinity between the 2 fibrinogen forms was only 20-fold; the heterodimer bound more tightly than the γA/γA homodimer. Because the concentrations of the 2 chains in fibrinogen differ by ∼ 15-fold, one would anticipate FXIII-A2B2 would distribute between the 2 forms and not be exclusively bound to peak 2 fibrinogen. Of note, the influence of calcium concentration differed between these 2 fibrinogen forms. Second, these studies showed the complex existed with a stoichiometry of 2:1, with 2 fibrinogen molecules for each FXIII-A2B2 molecule. As there are 2 B subunits, it appears reasonable that each FXIII-A2B2 molecule could bind 2 fibrinogen molecules. The model shown in panel B of the figure is based on these studies.
The current work characterized the interactions between factor XIII and fibrin(ogen), but focused on the fibrinogen αC residues 233-425. Using surface plasmon resonance to measure both kinetic and equilibrium parameters, they examined interactions between immobilized fibrinogen, fibrin, or recombinant fragments of the α chain and the 5 forms of factor XIII2 : (1) recombinant FXIII-A2, analogous to factor XIII found in platelets; (2) activated rFXIII-A2, (3) FXIII-A2B2 purified from plasma (4) FXIII-A2B2 cleaved with thrombin and assayed in the presence of EDTA, which limits subunit dissociation; and (5) activated FXIII-A2B2, cleaved with thrombin and assayed in the presence of calcium. No binding was detected with rFXIII-A2. After activation, rFXIII-A2 bound with moderate affinity to the fragment of α chain residues 371-425, Kd = 3μM. All 3 forms of FXIII-A2B2 bound to this α chain fragment with nanamolar affinity; Kd varied from 5nM (form 4) to 30nM (form 3). As the affinity for activated FXIII-A2B2 is markedly stronger than for activated FXIII-A2, these data indicate that the B subunit is the binding partner. Moreover, all 3 forms of FXIII-A2B2 bound to immobilized fibrinogen and fibrin with affinities similar to those found with the shorter α chain peptides. Indeed, the binding affinities were nanamolar (Kd = 3-35nM) for all 12 combinations of FXIII-A2B2 and fibrinogen, fibrin, or αC fragments. This similarity suggests that the interacting domain in fibrinogen is α chain 371-425 and the interacting subunit in FXIII-A2B2 is B. These data suggest the model shown in panel C of the figure.
As with all biochemistry experiments, each of these studies has its limitations. Greenberg and Shuman immobilized fibrinogen on acrylonitrile beads and measured binding of radiolabeled factor XIII.4 As surface-bound fibrinogen is different from solution fibrinogen, these studies may not be relevant to solution complexes in plasma. Of note, the concern for surface immobilization is also true of the current studies. Here however, they also completed competitive inhibition studies and showed the α chain peptide was a competitive inhibitor of activated FXIII-A2B2 (that is, subunit B) binding to fibrin. The Mosesson studies are limited by the nature of peak 2.5 Peak 2 fibrinogen clearly contains γ′/γA heterodimers, but this peak may have other coincident differences as well. Perhaps the γ′ chain favors a conformation that exposes the relevant α chain domain. Lastly, the studies of Farrell's group measured interactions by equilibrium analytical ultracentrifugation.7 This approach requires that FXIII-A2B2 and fibrinogen remain together at high concentrations for more than 24 hours. Under these conditions, Mosesson has found and we have confirmed, FXIII-A2B2 will catalyze the formation of γ dimers in fibrinogen. Thus, Farrell's studies may measure the interaction of FXIII-A2B2 with fibrinogen dimers, leading to the observed 2:1 stoichiometry. We have recently examined the binding of FXIII-A2B2 to recombinant fibrinogens γA/γA, γA/γ′, and γ′/γ′.8 All 3 fibrinogens bound with equal affinity, Kd = 41nM. These results suggest the γ′ chain is not critical for binding FXIII-A2B2. Nevertheless, because our studies were done with immobilized fibrinogens, the results may not be relevant to binding in solution.
In conclusion, the very clear and convincing data in the current studies support the original experiments that associated cofactor activity with the fibrinogen α chain. Nevertheless, further studies are needed to directly define the interactions in a solution complex between fibrinogen and factor XIII. Ongoing studies in my laboratory have not identified this complex in solution. It is notable that all of the published studies were completed in somewhat contrived conditions relative to circulating blood. Therefore, it remains an open question whether or not plasma fibrinogen is a carrier for FXIII-A2B2.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health