Abstract
Flavopiridol is a protein bound, cytotoxic, cyclin-dependent kinase inhibitor. Flavopiridol given by 1-hour bolus at 50 mg/m2 daily 3 times followed by cytosine arabinoside and mitoxantrone (FLAM) is active in adults with poor-risk acute leukemias. A pharmacologically derived “hybrid” schedule (30-minute bolus followed by 4-hour infusion) of flavopiridol was more effective than bolus administration in refractory chronic lymphocytic leukemia. Our phase 1 trial “hybrid FLAM” in 55 adults with relapsed/refractory acute leukemias began at a total flavopiridol dose of 50 mg/m2 per day 3 times (20-mg/m2 bolus, 30-mg/m2 infusion). Dose-limiting toxicity occurred at level 6 (30-mg/m2 bolus, 70-mg/m2 infusion) with tumor lysis, hyperbilirubinemia, and mucositis. Death occurred in 5 patients (9%). Complete remission occurred in 22 (40%) across all doses. Overall and disease-free survivals for complete remission patients are more than 60% at more than 2 years. Pharmacokinetics demonstrated a dose-response for total and unbound plasma flavopiridol unrelated to total protein, albumin, peripheral blast count, or toxicity. Pharmacodynamically, flavopiridol inhibited mRNAs of multiple cell cycle regulators, but with uniform increases in bcl-2. “Hybrid FLAM” is active in relapsed/refractory acute leukemias, with a recommended “hybrid” dose of bolus 30 mg/m2 followed by infusion of 60 mg/m2 daily for 3 days. This clinical trial is registered at www.clinicaltrials.gov as #NCT00470197.
Introduction
Flavopiridol is a serine-threonine kinase inhibitor that inhibits cell cycle progression by targeting multiple cyclin-dependent kinases, inducing checkpoint arrest, interrupting transcriptional elongation, and triggering cell death via multiple mechanisms.1-11 We have conducted longitudinal clinical-laboratory studies of flavopiridol followed in a timed sequential manner by the cell cycle–dependent, antileukemia drugs cytosine arabinoside (Ara-C) and mitoxantrone.12-14 The hypothesis-driven design of this regimen (“FLAM”) was generated in an in vitro model demonstrating that administration of flavopiridol to marrow leukemic blasts followed sequentially by Ara-C resulted in synergistic enhancement of Ara-C-related blast cell apoptosis.15,16 Serial phase 1 and 2 clinical trials of FLAM, conducted initially in relapsed and refractory adult acute leukemias12,13 and more recently in newly diagnosed adult acute myelogenous leukemia (AML) with poor-risk features,14 have documented reproducible and durable complete remissions (CRs) in a significant proportion of such patients, with a low rate of morbidity and mortality.
Early studies of flavopiridol's mechanisms and spectrum of activity conducted in fludarabine-resistant chronic lymphocytic leukemia (CLL) cells demonstrated that flavopiridol had major cytotoxic activity, even in cells with p53 deletions or mutations.17 Despite these findings in vitro, clinical trials of flavopiridol given as 24- or 72-hour infusions failed to demonstrate any activity, whereas flavopiridol given as a 1-hour bolus led to a response rate of 11% in refractory CLL patients.18,19 Byrd et al realized that the in vitro studies had been conducted in the presence of fetal bovine serum,17 which does not bind flavopiridol avidly. In contrast, flavopiridol is highly protein bound in human serum, making the lethal concentration 50% 4- to 5-fold greater in human serum than in fetal bovine serum.19,20 This discrepancy raised the possibility that cytotoxic levels were not being achieved in humans in vivo, particularly with the continuous infusion strategies.
In an attempt to overcome the avid human serum protein binding of flavopiridol, Byrd et al developed a pharmacologically modeled “hybrid” schedule of flavopiridol administration of a 30-minute bolus of approximately one-third to one-half the total dose followed by a 4-hour infusion of the remainder.21 Clinical data in high-risk CLL demonstrated that this administration schedule induced an acute tumor lysis syndrome (TLS) characterized by massive hyperkalemia, hyperphosphatemia, and increases in lactic dehydrogenase (LDH), particularly in the setting of marked hyperleukocytosis (white blood cell count > 200 000/mm3), with dramatic and durable clinical responses in 40% to 50% of refractory patients, including those with poor-risk genetic features and/or bulky disease.21-23 The TLS induced with the pharmacologically modeled, “hybrid” bolus-infusion schedule of flavopiridol administration is strikingly different from the pattern we detected using the 1-hour flavopiridol infusion.12-14 Similarly, studies of the hybrid bolus-infusion schedule of single-agent flavopiridol administration in refractory acute leukemias24 also appear to have a similar pattern of TLS, albeit less dramatic than the one seen in CLL patients.
On the basis of our phase 2 data using a 1-hour infusion of flavopiridol in the FLAM regimen and the encouraging results of the “hybrid” schedule of flavopiridol as a single agent in refractory CLL, we conducted a phase 1 trial of “Hybrid FLAM,” using the template of our previous combination therapy trial to explore the hybrid bolus-infusion flavopiridol schedule in a dose-escalation fashion. As a starting point, we selected a total daily flavopiridol dose that was 50% below the maximal tolerated dose (MTD) in the single-agent AML trial of “hybrid” flavopiridol24 and equal to the phase 2 total daily dose of flavopiridol used in combination with Ara-C and mitoxantrone.13,14 The purpose of our study was to determine the MTD of “hybrid” flavopiridol administration when used in timed sequence with Ara-C and mitoxantrone, as well as to explore the clinical responses, pharmacokinetics (PK), and pharmacodynamics of this pharmacologically derived schedule of flavopiridol administration.
Methods
Patient eligibility and selection
Patients 18 years of age and older with pathologically confirmed, relapsed, and refractory AML, acute lymphoblastic leukemia (ALL), or acute biphenotypic leukemias (ABLs) were eligible provided that they had Eastern Cooperative Oncology Group performance status 0 to 2, serum creatinine < 2.0 mg/dL, hepatic enzymes less than 5 times upper limit of normal, bilirubin < 2.0 mg/dL, and left ventricular ejection fraction > 45%. Patients were eligible if they had undergone no more than 4 courses of cytotoxic induction therapies. Patients who had undergone allogeneic or autologous stem cell transplantation (SCT) and had relapsed or were refractory thereafter were eligible for this study. Patients were not eligible if they had a peripheral blast count > 50 000/mm3 but were allowed to receive hydroxyurea for cytoreduction before beginning flavopiridol. Full criteria for eligibility and ineligibility were similar to those detailed for previous FLAM studies.12-14 All patients provided written informed consent according to the Johns Hopkins Medical Institutional Review Boards and guidelines.
Treatment schema
Flavopiridol was administered daily for 3 days (days 1-3) as a 30-minute intravenous bolus followed by a 4-hour infusion in a dose-escalating fashion over a total of 6 dose levels, as detailed in Table 1. The total starting dose of flavopiridol (20 mg/m2 bolus, 30 mg/m2 infusion) equaled the bolus dose administered daily for 3 days (50 mg/m2 per dose, days 1-3) in our phase 2 FLAM clinical trials.13,14 Using a traditional “3 + 3” design, the total daily dose of flavopiridol was escalated by 10 mg/m2 per day for each cohort, with the bolus being fixed after dose level 3 at 30 mg/m2 per day and the infusion being increased accordingly. As in our previous FLAM trials,12-14 a 72-hour continuous infusion of Ara-C 2 g/m2 (667 mg/m2 per 24 hours) began on day 6 and mitoxantrone 40 mg/m2 was administered as a single intravenous bolus over 60 to 120 minutes on day 9, 12 hours after completing the Ara-C infusion. The occurrence of any dose-limiting toxicity (DLT) in 33% of a patient cohort defined the MTD. Patients who achieved CR after cycle 1 were eligible to receive a second cycle of FLAM beginning 30 + 7 days after hospital discharge from the first cycle. Patients who achieved CR and had a suitable matched related or unrelated donor or a related haploidentical donor were eligible to undergo allogeneic BMT after the first or second cycle of FLAM.
Dose-escalation schema for flavopiridol
| Dose level . | Daily bolus dose, mg/m2 per day . | Daily infusion dose, mg/m2 per day . | Total daily dose, mg/m2 per day . |
|---|---|---|---|
| 1 | 20 | 30 | 50 |
| 2 | 25 | 35 | 60 |
| 3 | 30 | 40 | 70 |
| 4 | 30 | 50 | 80 |
| 5 | 30 | 60 | 90 |
| 6 | 30 | 70 | 100 |
| Dose level . | Daily bolus dose, mg/m2 per day . | Daily infusion dose, mg/m2 per day . | Total daily dose, mg/m2 per day . |
|---|---|---|---|
| 1 | 20 | 30 | 50 |
| 2 | 25 | 35 | 60 |
| 3 | 30 | 40 | 70 |
| 4 | 30 | 50 | 80 |
| 5 | 30 | 60 | 90 |
| 6 | 30 | 70 | 100 |
Supportive care
Prophylaxis measures against TLS, infection, and menstrual breakthrough were similar to those used in our previous FLAM studies.12-14 Nonetheless, dramatic TLS has been observed with the “hybrid” bolus-infusion regimen, predominantly in patients with CLL,21-23 but also in patients with AML and ALL, especially in the presence of high peripheral counts.14,24 TLS resulting from flavopiridol historically occurs between days 1 and 3, with onset within the first 4 to 6 hours after completion of the flavopiridol infusion. To decrease the risk for TLS-related hyperkalemia after the first dose of flavopiridol, serum potassium was measured within 4 hours of beginning the flavopiridol bolus, at the end of the flavopiridol bolus, at the end of the flavopiridol infusion, at 2 and 4 hours after the end of the infusion, and twice daily thereafter on days 2 and 3. A patient who developed a rise in serum potassium to more than 4.5 mg/dL would receive a 30-g dose of kayexalate immediately. Any patient who developed a rise in serum potassium to more than 5.0 mg/dL would receive a 10-unit dose of intravenous insulin and an ampule of 50% dextrose. Any patient who developed a rise in serum potassium to more than 5.5 mg/dL would be considered for emergency intermittent or continuous hemodialysis. Development of hyperkalemia more than 5.0 mg/dL would result in holding subsequent doses of flavopiridol until hyperkalemia resolved.
Response and toxicity evaluations
Bone marrow aspirates and biopsies were performed before treatment, on day 14, and at the time of hematologic recovery or when leukemia regrowth was suspected. Hematologic recovery was defined as absolute neutrophil count more than 500/mm3 and transfusion-independent platelet count of 50 000/mm3. The definitions of response were as follows25 : (1) CR required normal marrow aspirate with absence of identifiable leukemia, absolute neutrophil count more than 1000/mm3, platelet count more than 100 000/mm3, absence of blasts in peripheral blood, and absence of extramedullary disease; (2) CR with incomplete recovery required all CR criteria except for residual neutropenia or thrombocytopenia, as defined for CR; (3) cytogenetic CR required reversion to normal karyotype at the time of CR or incomplete recovery in cases with an abnormal karyotype pretreatment; (4) partial remission was defined as the presence of trilineage hematopoiesis in the marrow with normalization of peripheral counts but with 5% to 25% blasts in the marrow; and (5) no remission was defined as persistent leukemia in marrow and/or blood without significant decrease from pretreatment levels. Adverse events were described and graded based on National Cancer Institute Common Toxicity Criteria Version 3.0 and treating physician's assessment.
Laboratory correlates
Pharmacokinetics.
Blood samples were collected before the start of flavopiridol on each day of administration (days 1-3), after the bolus and before the start of the infusion on day 1 (∼ 30 minutes), and after the end of the infusion day 1 (∼ 4.5 hours from the start of the bolus infusion). Samples were processed within 30 minutes and stored until subsequent analysis using a validated liquid chromatography/tandem mass spectrometry method over the concentration range of 10 to 5000 ng/mL. Unbound drug concentrations were determined using a micro-equilibrium dialysis method that was optimized and validated for determining the fraction unbound (fu) flavopiridol in human plasma. Individual pharmacokinetic parameters were estimated by standard noncompartmental analysis using WinNonlin Version 5.3 (Pharsight).26 The a priori level of significance was set at P less than .05.
Pharmacodynamics.
To determine the in vivo effects of flavopiridol on the expression of selected target genes, we obtained peripheral blood samples from 12 patients treated at dose level 5 with pretreatment peripheral blood blast counts of at least 1000/mm3 before therapy and 2 hours after completion of day 1 flavopiridol. Ficoll-enriched blast populations were prepared for quantitative real-time polymerase chain reaction (RT-PCR) to assess mRNA levels for each of the target proteins. Total RNA from the blasts was isolated using RNeasy Mini Kit (QIAGEN), quantified on a Nanodrop ND-1000 spectrophotometer (NanoDrop Technologies), and converted to cDNA using a cDNA conversion kit (Applied Biosystems) as previously described.27 A negative control reaction without reverse transcriptase amplification was included in all experiments. Control CD34+ hematopoietic stem cells were obtained from a commercial source (www.allcells.com); mRNA levels from the hematopoietic stem cells were arbitrarily assigned a value of 1.0. Quantitative RT-PCR of cDNA was performed as previously described27 with the following modifications. For most genes, we used TaqMan PCR reagents (Applied Biosystems) and primers for E2F1, BCL-2, VEGF-A, the 2A subunit of RNA polymerase II (POLR2A), Cyclin D1, and Mcl-1. For comparative purposes, the housekeeping gene human phosphoprotein served as the control for HMGA1, E2F1, and Mcl-1 as previously described,27 and β-actin served as the control for Bcl-2, VEFG-A, and RNA Pol II 2A. Because the TaqMan primers do not distinguish STAT3 from STAT3β, we assessed STAT3 using SYBR Green master mix (Applied Biosystems) as previously described28 with β2 microglobulin as the control gene for sample loading. Reactions were performed in triplicate and repeated if the SEs were more than 30%. The results are expressed as the mean plus or minus SD. The mRNA level for each gene was compared with its expression in CD34+ hematopoietic stem cells. The quantitative RT-PCR results were analyzed using the software provided by the manufacturer (δδCt method).
Statistical considerations
Overall survival (OS) was calculated from day 1 of FLAM to date of last known follow-up or death. Disease-free survival (DFS) was calculated from achievement of CR to date of last known follow-up or relapse. Survival data were analyzed as of May 1, 2010. The Kaplan-Meier method was used to estimate OS and DFS for the whole cohort and stratified by groups of interest. Log-rank P values were used for descriptive comparison of survival outcomes among disease characteristics and treatments. Fold changes in bcl-2 expression were calculated to express the change in pre- and post-flavopiridol treatment values. Wilcoxon rank-sum tests were used to determine whether: (1) the fold changes were significantly different from 1.0 among all patients and by response; and (2) fold changes were different between patients who achieved CR and those who did not.
Results
Patient characteristics
Between May 2007 and January 2009, 55 adults were entered on study. A total of 36 patients were entered on the phase 1 dose-escalation part of the trial, and an additional 19 patients with AML were entered on the expansion phase at the MTD. Clinical demographics and biologic disease features for the entire group (49 AML, 3 ALL, and 3 T/myeloid ABL) are presented in Table 2. The majority (78%) of patients had refractory acute leukemia, and the remaining 22% had relapsed disease. For the relapsed cohort of 12 AML patients, 4 had at least 2 previous relapses and 9 had undergone allogeneic SCT 2 to 20 months before recurrent disease. The majority had adverse genetics (37 of 49 AML, 2 of 3 ALL, and 2 of 3 ABL adverse cytogenetics plus 7 primary refractory AML patients with FLT-3 internal tandem duplication).
Demographic and biologic characteristics of 55 adults with relapsed and refractory acute leukemia
| . | Dose escalation (n = 36) . | Expansion (n = 19) . | Total (n = 55) . |
|---|---|---|---|
| Sex | |||
| Male | 16 (44%) | 9 (47%) | 25 (45%) |
| Female | 20 (56%) | 10 (53%) | 30 (55%) |
| Median age, y (range) | 56 (20-72) | 51 (22-69) | 54 (20-72) |
| AML | 31 (86%) | 18 (95%) | 49 (89%) |
| Relapse | 7 | 5 | 12* |
| Primary refractory | 13 | 4 | 17 |
| Multirefractory | 10 | 6 | 16 |
| MPD/AML | 1 | 4 | 5 |
| ALL | 3 (8%) | 0 (0%) | 3 (5%) |
| Biphenotypic T/AML (ABL) | 2 (6%) | 1 (5%) | 3 (5%) |
| Prior regimens, median (range) | 2 (1-4) | 3 (1-4) | 2 (1-4) |
| Prior drug exposure, n (%) | |||
| Ara-C | 28 (77%) | 16 (84%) | 44 (80%) |
| HiDAc | 14 | 8 | 22 |
| 2 g/m2 per 72 hours | 11 | 5 | 16 |
| Prior allogeneic SCT | 8 | 1 | 9 |
| Adverse genetics | 29 (81%) | 12 (63%) | 41 (75%) |
| AML | 26 | 11 | 37 (67%) |
| Single cytogenetics | 11 | 3 | 14 |
| Complex cytogenetics | 11 | 6 | 17 |
| FLT3 mutation | 4 | 3 | 7 |
| ALL | 2 | 0 | 2 (66%) |
| ABL | 1 | 1 | 2 (66%) |
| . | Dose escalation (n = 36) . | Expansion (n = 19) . | Total (n = 55) . |
|---|---|---|---|
| Sex | |||
| Male | 16 (44%) | 9 (47%) | 25 (45%) |
| Female | 20 (56%) | 10 (53%) | 30 (55%) |
| Median age, y (range) | 56 (20-72) | 51 (22-69) | 54 (20-72) |
| AML | 31 (86%) | 18 (95%) | 49 (89%) |
| Relapse | 7 | 5 | 12* |
| Primary refractory | 13 | 4 | 17 |
| Multirefractory | 10 | 6 | 16 |
| MPD/AML | 1 | 4 | 5 |
| ALL | 3 (8%) | 0 (0%) | 3 (5%) |
| Biphenotypic T/AML (ABL) | 2 (6%) | 1 (5%) | 3 (5%) |
| Prior regimens, median (range) | 2 (1-4) | 3 (1-4) | 2 (1-4) |
| Prior drug exposure, n (%) | |||
| Ara-C | 28 (77%) | 16 (84%) | 44 (80%) |
| HiDAc | 14 | 8 | 22 |
| 2 g/m2 per 72 hours | 11 | 5 | 16 |
| Prior allogeneic SCT | 8 | 1 | 9 |
| Adverse genetics | 29 (81%) | 12 (63%) | 41 (75%) |
| AML | 26 | 11 | 37 (67%) |
| Single cytogenetics | 11 | 3 | 14 |
| Complex cytogenetics | 11 | 6 | 17 |
| FLT3 mutation | 4 | 3 | 7 |
| ALL | 2 | 0 | 2 (66%) |
| ABL | 1 | 1 | 2 (66%) |
HiDAc indicates high-dose Ara-C; and SCT, stem cell transplantation (all performed in AML patients).
Relapse: 7 CR1 (2 after BMT), median, 11 months (range, 7-18 months); 4 ≥ CR2 (3 after BMT), median 7 months (range, 3-12 months).
Toxicities
As presented in Table 3, tumor lysis occurred in 28 (51%), manifested by transient hyperphosphatemia (range, 4.9-7.2 mg/dL) with or without hyperuricemia in 20 (36%) and increases in LDH to more than 5 times upper limit of normal (> 1250 mg/dL) in 12 (22%). No patient experienced chemical or clinical coagulopathy. TLS with hyperkalemia after the first dose of flavopiridol was observed in 2 patients: one with T-cell ALL (potassium 6.4 mg/dL) accompanied by acute renal failure and requiring hemodialysis and one with refractory myeloproliferative disease (potassium 6.6 mg/dL), rapidly reversed without hemodialysis. One additional patient with grade 3 tumor lysis experienced transient creatinine elevation to 1.7 mg/dL without hyperkalemia and without needing dialysis. DLT was reached in the first 2 patients at dose level 6 (flavopiridol bolus 30 mg/m2, infusion 70 mg/m2) with grade 4 TLS, oral mucositis, hyperbilirubinemia, and overwhelming sepsis (grade 5). Based on these DLTs, we chose to expand our experience with Hybrid FLAM at dose level 5 (flavopiridol bolus 30 mg/m2, infusion 60 mg/m2). Time to hematologic recovery was similar to our previous trials of FLAM using bolus administration,12,13 with the median time to absolute neutrophil count > 500/mm3 being day 34 (range, days 27-45) and median time to platelets > 50 000/mm3 being day 35 (range, days 21-62). Five (9%) experienced cardiac toxicities, including 2 patients with decreases in left ventricular ejection fraction (grade 2 at level 1 and grade 3 at level 5), 2 patients with atrial fibrillation with rapid ventricular response (grade 2 at dose level 1 and grade 4 at dose level 2 in the setting of multiorgan failure), and 1 patient with grade 3 pericarditis at dose level 2. Death occurred in 5 patients (9%) as a result of overwhelming infection in 4 (dose levels 2 and 5) and multiorgan failure in 1 (dose level 2).
Nonhematologic toxicities for 55 adults with relapsed and refractory acute leukemia
| Dose level, mg/m2 (no. of patients) . | Tumor lysis . | Mucositis . | Increase in bilirubin . | Cardiac . | Documented infection . | Death* . | |
|---|---|---|---|---|---|---|---|
| Oral . | GI . | ||||||
| 1. 20/30 (6) | 1 | 3 (2 grade 3) | 0 (1 grade 3) | 4 | 2 | 5 | 0 |
| 2. 25/35 (10) | 4 (1 grade 5) | 1 | 2 (1 grade 3) | 3 (2 grade 3) | 2 (2 grade 3/4)† | 5 (1 grade 5) | 2 |
| 3. 30/40 (6) | 2 | 0 | 5 | 1 | 0 | 3 | 0 |
| 4. 30/50 (6) | 5 (1 grade 3) | 1 | 2 | 0 | 0 | 3 | 0 |
| 5. 30/60 (25)‡ | |||||||
| (6) | 5 (1 grade 3) | 1 | 2 | 2 (1 grade 3) | 0 | 3 | 0 |
| (19) | 9 (1 grade 3) | 2 (1 grade 3) | 5 | 3 (2 grade 3) | 1 (grade 3)§ | 7 (2 grade 5) | 2 |
| 6. 30/70 (2)‖ | 2 (1 grade 4) | 2 (1 grade 4) | 2 (1 grade 3) | 1 (1 grade 4) | 0 | 1 (1 grade 5) | 1 |
| Grade 3-5 toxicity | 5 (9%) | 4 (7%) | 2 (4%) | 6 (11%)¶ | 2 (4%) | 4 (7%) | 5 (9%)# |
| Total | 28 (51%) | 10 (18%) | 18 (33%) | 14 (25%) | 5 (9%) | 27 (49%) | 5 (9%) |
| Dose level, mg/m2 (no. of patients) . | Tumor lysis . | Mucositis . | Increase in bilirubin . | Cardiac . | Documented infection . | Death* . | |
|---|---|---|---|---|---|---|---|
| Oral . | GI . | ||||||
| 1. 20/30 (6) | 1 | 3 (2 grade 3) | 0 (1 grade 3) | 4 | 2 | 5 | 0 |
| 2. 25/35 (10) | 4 (1 grade 5) | 1 | 2 (1 grade 3) | 3 (2 grade 3) | 2 (2 grade 3/4)† | 5 (1 grade 5) | 2 |
| 3. 30/40 (6) | 2 | 0 | 5 | 1 | 0 | 3 | 0 |
| 4. 30/50 (6) | 5 (1 grade 3) | 1 | 2 | 0 | 0 | 3 | 0 |
| 5. 30/60 (25)‡ | |||||||
| (6) | 5 (1 grade 3) | 1 | 2 | 2 (1 grade 3) | 0 | 3 | 0 |
| (19) | 9 (1 grade 3) | 2 (1 grade 3) | 5 | 3 (2 grade 3) | 1 (grade 3)§ | 7 (2 grade 5) | 2 |
| 6. 30/70 (2)‖ | 2 (1 grade 4) | 2 (1 grade 4) | 2 (1 grade 3) | 1 (1 grade 4) | 0 | 1 (1 grade 5) | 1 |
| Grade 3-5 toxicity | 5 (9%) | 4 (7%) | 2 (4%) | 6 (11%)¶ | 2 (4%) | 4 (7%) | 5 (9%)# |
| Total | 28 (51%) | 10 (18%) | 18 (33%) | 14 (25%) | 5 (9%) | 27 (49%) | 5 (9%) |
Death within 60 days of the start of chemotherapy.
Gade 3 pericarditis, grade 4 atrial fibrillation with rapid ventricular response.
Dose level 5: 6 patients in dose-escalation phase, 19 patients in expansion phase.
Grade 3 LVEF decrease.
Both patients had grade 4 toxicity in one or more parameters (1 grade 5).
Hyperbilirubinemia: 4 tumor lysis, 2 overwhelming infection.
Four with infection and 1 multiorgan failure (tumor lysis).
Clinical outcome
The “hybrid” schedule resulted in a 50% or greater decrease in peripheral blood blast counts in 34 (77%) of the 44 patients who had peripheral blood blasts before treatment by median day 2 (range < 12 hours after first dose, day 3) and a > 80% decrease in 21 (48%) by day 3 of flavopiridol. There was a possible dose-response in terms of magnitude and timing of cytoreduction, with 3 of 13 (23%) at less than dose level 2, 2 of 4 at dose level 3, 3 of 4 at dose level 4, 14 of 21 (67%) at dose level 5, and 2 of 2 at dose level 6 exhibiting 50% or greater decreases in peripheral blasts 12 hours after the first dose of flavopiridol (day 1). Nonetheless, there was no clear relationship between either the magnitude or rate of blast cell decrease and clinical outcome. No patient had sustained increases in peripheral counts during flavopiridol.
As delineated in Table 4, 22 (40%) patients achieved CR and an additional 3 (5%) achieved partial responses. Of the 21 CR patients, one treated at dose level 1 for relapse after allogeneic SCT had incomplete recovery (with incomplete platelet recovery). Notably, the CR rate at the MTD dose level (30 mg/m2 bolus, 60 mg/m2 infusion) was 13 of 25 (52%). All CRs were associated with clearance of cytogenetic abnormalities. Responses occurred across all dose levels with the exception of dose level 6, where DLT precluded the ability to measure clinical response endpoints. Clinical outcome for the 49 patient AML cohort appeared to relate to selected features reflecting disease biology (Table 5), with CR in 92% of relapsed AML (including those with 2 relapses) and CR in 31% of primary refractory AML. Of 9 patients who had undergone previous allogeneic SCT 2 to 20 months previously, 4 (44%) achieved CR. For 16 heavily previously treated patients with multiply refractory disease, however, there was only one CR and 2 partial remissions (18%). Two of the 5 myeloproliferative disease/AML (40%) patients achieved CR. An overall response rate of 34% (29% CR + 5% partial remission) was observed for the 38 AML patients with adverse genetics.
Clinical outcome for 55 adults with relapsed or refractory acute leukemia according to “hybrid” bolus-infusion flavopiridol dose level
| Dose level, mg/m2/disease . | CR . | PR . | NR . | NE . |
|---|---|---|---|---|
| 1. 20/30 | ||||
| AML (5) | 2* | 0 | 3 | 0 |
| ALL (1) | 1 | 0 | 0 | 0 |
| 2. 25/35 | ||||
| AML (7) | 1 | 1 | 4 | 1 |
| ALL (2) | 0 | 0 | 1 | 1 |
| ABL (1) | 0 | 1 | 0 | 0 |
| 3. 30/40 | ||||
| AML (5) | 2 | 0 | 3 | 0 |
| ABL (1) | 1 | 0 | 0 | 0 |
| 4. 30/50 | ||||
| AML (6) | 2 | 1 | 3 | 0 |
| 5. 30/60† | ||||
| AML (24) | 12 | 0 | 10 | 2 |
| ABL (1) | 1 | 0 | 0 | 0 |
| 6. 30/70 | ||||
| AML (2) | 0 | 0 | 0 | 2 |
| Subtotal | ||||
| AML (49) | 19 (39%) | 2 (4%) | 23 (42%) | 5 (10%) |
| ALL (3) | 1 (33%) | 0 | 1 (33%) | 1 (33%) |
| ABL (3) | 2 (67%) | 1 (33%) | 0 | 0 |
| MTD† (25) | 13 (52%) | 0 | 10 (40%) | 2 (8%) |
| Total (55) | 22 (40%) | 3 (5%) | 24 (44%) | 6 (11%) |
| Dose level, mg/m2/disease . | CR . | PR . | NR . | NE . |
|---|---|---|---|---|
| 1. 20/30 | ||||
| AML (5) | 2* | 0 | 3 | 0 |
| ALL (1) | 1 | 0 | 0 | 0 |
| 2. 25/35 | ||||
| AML (7) | 1 | 1 | 4 | 1 |
| ALL (2) | 0 | 0 | 1 | 1 |
| ABL (1) | 0 | 1 | 0 | 0 |
| 3. 30/40 | ||||
| AML (5) | 2 | 0 | 3 | 0 |
| ABL (1) | 1 | 0 | 0 | 0 |
| 4. 30/50 | ||||
| AML (6) | 2 | 1 | 3 | 0 |
| 5. 30/60† | ||||
| AML (24) | 12 | 0 | 10 | 2 |
| ABL (1) | 1 | 0 | 0 | 0 |
| 6. 30/70 | ||||
| AML (2) | 0 | 0 | 0 | 2 |
| Subtotal | ||||
| AML (49) | 19 (39%) | 2 (4%) | 23 (42%) | 5 (10%) |
| ALL (3) | 1 (33%) | 0 | 1 (33%) | 1 (33%) |
| ABL (3) | 2 (67%) | 1 (33%) | 0 | 0 |
| MTD† (25) | 13 (52%) | 0 | 10 (40%) | 2 (8%) |
| Total (55) | 22 (40%) | 3 (5%) | 24 (44%) | 6 (11%) |
PR indicates partial remission; NR, no remission; and NE, not evaluable.
Includes 1 patient with incomplete platelet recovery.
All patients including the expansion cohort.
Clinical outcome for 49 AML patients according to disease biology
| . | CR, no. (%) . | PR, no. (%) . | NR, no. (%) . | NE, no. (%) . | Overall response, no. (%) . |
|---|---|---|---|---|---|
| Stage of disease | |||||
| Relapse (12) | 11 | 0 | 1 | 0 | 11 (92) |
| Prior allo-SCT (9) | 4 | 1 | 4 | 0 | 5 (55) |
| Primary refractory (16) | 5 | 0 | 10 | 1 | 5 (31) |
| Multirefractory (16) | 1 | 2 | 12 | 1 | 3 (19) |
| MPD/AML (5) | 2 | 0 | 2 | 1 | 2 (40) |
| Total (49) | 19 (39) | 2 (4) | 25 (51) | 3 (6) | 21 (43) |
| Genetics | |||||
| Nonadverse (11) | 8 | 0 | 3 | 0 | 8 (72) |
| Adverse | |||||
| Single (13) | 7 | 2 | 4 | 0 | 9 (50) |
| Complex (18) | 2 | 0 | 14 | 2 | 2 (11) |
| FLT3 mutation (7) | 2 | 0 | 5 | 0 | 2 (29) |
| Total adverse (38) | 11 (29) | 2 (5) | 23 (61) | 2 (5) | 13 (34) |
| . | CR, no. (%) . | PR, no. (%) . | NR, no. (%) . | NE, no. (%) . | Overall response, no. (%) . |
|---|---|---|---|---|---|
| Stage of disease | |||||
| Relapse (12) | 11 | 0 | 1 | 0 | 11 (92) |
| Prior allo-SCT (9) | 4 | 1 | 4 | 0 | 5 (55) |
| Primary refractory (16) | 5 | 0 | 10 | 1 | 5 (31) |
| Multirefractory (16) | 1 | 2 | 12 | 1 | 3 (19) |
| MPD/AML (5) | 2 | 0 | 2 | 1 | 2 (40) |
| Total (49) | 19 (39) | 2 (4) | 25 (51) | 3 (6) | 21 (43) |
| Genetics | |||||
| Nonadverse (11) | 8 | 0 | 3 | 0 | 8 (72) |
| Adverse | |||||
| Single (13) | 7 | 2 | 4 | 0 | 9 (50) |
| Complex (18) | 2 | 0 | 14 | 2 | 2 (11) |
| FLT3 mutation (7) | 2 | 0 | 5 | 0 | 2 (29) |
| Total adverse (38) | 11 (29) | 2 (5) | 23 (61) | 2 (5) | 13 (34) |
PR indicates partial remission; NR, no remission; NE, not evaluable; and MPD, myeloproliferative disease.
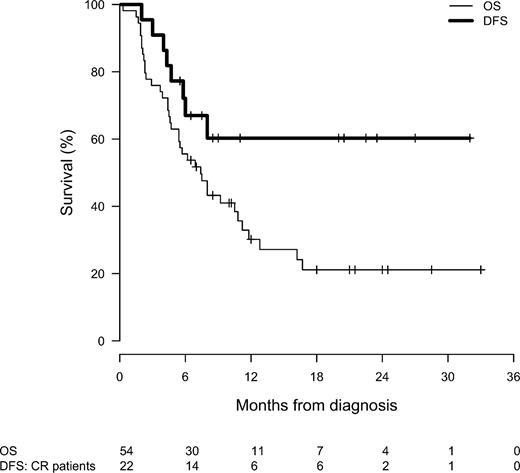
As shown in Figure 1, median OS for all 55 patients was 7.4 months (95% confidence interval, 5.4-11.2 months), with 22% alive at 2 years (95% confidence interval, 12%-41%). For the 22 patients achieving CR (19 AML, 1 ALL, and 2 ABL), median OS has not been reached and ranges from 3.7 to 31 months, with 15 patients still alive. One- and 2-year OS is dependent on achievement of CR (P < .0001), nonrefractory disease (P = .0148), and noncomplex cytogenetics (P = .0022). In contrast, there were no significant differences in OS with regard to dose level (P = .773), prior SCT (P = .811), or number of prior antileukemia therapies (P = .093). For the 22 CR patients, median DFS has not been reached and ranges from 1.8 to 30 months, with 15 remaining in CR at 6 to 30 months. The 1- and 2-year DFS for CR patients of 62% (95% confidence interval, 43%-89%) does not appear to be dependent on stage of disease (relapsed vs refractory, P = .759), cytogenetics (P = .233), prior SCT (P = .611), or number of prior therapies (P = .441). Sixteen (73%) of the 22 CR patients underwent allogeneic SCT, 2 who had undergone prior SCT received donor lymphocyte infusion in FLAM-induced CR, and 5 experienced progressive disease (CR 2-6 months) precluding SCT. Of 14 CR patients whose DFS can be assessed at one year to date, 8 (57%) remain in CR at 12 to 30 months.
OS for all 55 patients treated with hybrid FLAM (solid line) and DFS (bold line) for the 22 CR patients.
OS for all 55 patients treated with hybrid FLAM (solid line) and DFS (bold line) for the 22 CR patients.
PK
Because the impetus for the hybrid flavopiridol dosing schedule is to circumvent the extensive protein binding in human plasma, we measured the PK profile of both total flavopiridol and unbound flavopiridol levels at all dose levels (Table 6). As noted in previous PK studies of bolus,12,20 infusional,29 and hybrid (both bolus and infusional)21,23 flavopiridol, there was significant interindividual variation at all dose levels. Nonetheless, there was evidence of dose-response for total and unbound flavopiridol plasma concentrations measured on day 1 end of infusion and day 2 before drug administration (Table 5). The variation in PK is with both total and unbound drug concentration and does not appear to be correlated with total protein, albumin, or white blood cells. In addition, there was no correlation between PK and toxicity.6,8
Hybrid (bolus-infusion) flavopiridol PK
| Dose level . | Bolus-infusion, mg/m2 . | No. of patients . | Total/unbound . | Day 1 end of bolus, μM . | Day 1 end of infusion, μM . | Pretreatment day 2, μM . | Pretreatment day 3, μM . | Day 1 AUC last, μM/hr . |
|---|---|---|---|---|---|---|---|---|
| 1 | 20/30 | 5 | Total | 0.84 ± 0.09 | 0.56 ± 0.16 | 0.05* | 0.11* | 4.1 ± 2.3 |
| Unbound | 0.075 ± 0.020 | 0.053 ± 0.030 | 0.005* | 0.009* | 0.38 ± 0.26 | |||
| 2 | 25/35 | 9 | Total | 1.51 ± 0.41 | 0.71 ± 0.33 | 0.05 ± 0.03 | 0.04 ± 0.01 | 8.9 ± 5.1 |
| Unbound | 0.126 ± 0.026 | 0.086 ± 0.033 | 0.005 ± 0.003 | 0.005 ± 0.001 | 1.06 ± 0.61 | |||
| 3 | 30/40 | 6 | Total | 0.93 ± 0.16 | 0.59 ± 0.19 | 0.02, 0.03* | BLQ | 5.4 ± 2.4 |
| Unbound | 0.120 ± 0.051 | 0.069 ± 0.024 | 0.003, 0.003* | BLQ | 0.62 ± 0.24 | |||
| 4 | 30/50 | 4 | Total | 1.06 ± 0.31 | 0.79 ± 0.32 | 0.03, 0.06* | 0.04, 0.06* | 7.3 ± 3.5 |
| Unbound | 0.123 ± 0.065 | 0.079 ± 0.026 | 0.004, 0.007* | 0.005, 0.006* | 0.76 ± 0.34 | |||
| 5 | 30/60 | 20 | Total | 1.57 ± 0.46 | 1.48 ± 0.60 | 0.04 ± 0.02 | 0.07 ± 0.05 | 16.8 ± 8.3 |
| Unbound | 0.168 ± 0.056 | 0.141 ± 0.051 | 0.005 ± 0.002 | 0.009 ± 0.009 | 1.67 ± 0.82 | |||
| 6 | 30/70 | 2 | Total | 0.12, 1.08 | 1.45, 1.52 | 0.08, 1.45 | 0.04, N.S. | 19.4, 30.9 |
| Unbound | 0.012, 0.131 | 0.138, 0.155 | 0.007, 0.197 | 0.006, N.S. | 1.92, 3.60 |
| Dose level . | Bolus-infusion, mg/m2 . | No. of patients . | Total/unbound . | Day 1 end of bolus, μM . | Day 1 end of infusion, μM . | Pretreatment day 2, μM . | Pretreatment day 3, μM . | Day 1 AUC last, μM/hr . |
|---|---|---|---|---|---|---|---|---|
| 1 | 20/30 | 5 | Total | 0.84 ± 0.09 | 0.56 ± 0.16 | 0.05* | 0.11* | 4.1 ± 2.3 |
| Unbound | 0.075 ± 0.020 | 0.053 ± 0.030 | 0.005* | 0.009* | 0.38 ± 0.26 | |||
| 2 | 25/35 | 9 | Total | 1.51 ± 0.41 | 0.71 ± 0.33 | 0.05 ± 0.03 | 0.04 ± 0.01 | 8.9 ± 5.1 |
| Unbound | 0.126 ± 0.026 | 0.086 ± 0.033 | 0.005 ± 0.003 | 0.005 ± 0.001 | 1.06 ± 0.61 | |||
| 3 | 30/40 | 6 | Total | 0.93 ± 0.16 | 0.59 ± 0.19 | 0.02, 0.03* | BLQ | 5.4 ± 2.4 |
| Unbound | 0.120 ± 0.051 | 0.069 ± 0.024 | 0.003, 0.003* | BLQ | 0.62 ± 0.24 | |||
| 4 | 30/50 | 4 | Total | 1.06 ± 0.31 | 0.79 ± 0.32 | 0.03, 0.06* | 0.04, 0.06* | 7.3 ± 3.5 |
| Unbound | 0.123 ± 0.065 | 0.079 ± 0.026 | 0.004, 0.007* | 0.005, 0.006* | 0.76 ± 0.34 | |||
| 5 | 30/60 | 20 | Total | 1.57 ± 0.46 | 1.48 ± 0.60 | 0.04 ± 0.02 | 0.07 ± 0.05 | 16.8 ± 8.3 |
| Unbound | 0.168 ± 0.056 | 0.141 ± 0.051 | 0.005 ± 0.002 | 0.009 ± 0.009 | 1.67 ± 0.82 | |||
| 6 | 30/70 | 2 | Total | 0.12, 1.08 | 1.45, 1.52 | 0.08, 1.45 | 0.04, N.S. | 19.4, 30.9 |
| Unbound | 0.012, 0.131 | 0.138, 0.155 | 0.007, 0.197 | 0.006, N.S. | 1.92, 3.60 |
Data are mean ± SD. If n = 2, individual values are reported.
AUC indicates area under the curve.
Remaining samples were below limits of quantitation.
Pharmacodynamics
In our previous phase 1 study of FLAM of bolus flavopiridol,12 we examined paired marrow samples obtained before and on day 3 after flavopiridol administration, and detected variable degrees of down-regulation of one or more proteins targeted by flavopiridol, including POLR2A, Mcl-1, cyclin D1, phosphoSTAT-3, and Bcl-2 in response to flavopiridol exposure in vivo. In the current phase 1 study, we examined paired peripheral blood blast samples from 12 patients treated at dose level 5 (expansion cohort) before and on day 1 at the end of the flavopiridol infusion for changes in mRNA expression in POLR2A,4,7 MCL-1,6,8 VEGF-A,9,11,12 and bcl-2.5 We also studied the HMGA1 oncogene, which encodes a chromatin remodeling protein downstream of c-myc30-33 and which transcriptionally activates STAT328,33 and other genes that encode flavopiridol client proteins.3,8-10 As shown in Table 7, flavopiridol inhibited the expression of one or more of the 8 selected mRNAs in vivo in leukemic blasts from all 12 patients independent of clinical response. Blasts from all patients exhibited significant decreases in at least 3 mRNAs. Indeed, HMGA1 expression was down-regulated after flavopiridol administration in 10 of 12 (83%) patients. Likewise, STAT3, E2F1, POLR2A, and VEGF-A mRNAs were significantly down-regulated in blasts for the majority (67%-75%) of patients, whereas MCL-1 was down-regulated in only 2 of 12 (17%). The relative magnitude of change for each of these genes was similar for CR and no remission subgroups except for VEGF-A mRNA, where 7 of 8 (88%) CR patients evinced significant suppression in contrast to only 1 of 4 (25%) no remission patients. Unexpectedly, both cyclin D1 and bcl-2 mRNA were dramatically up-regulated in post-flavopiridol blasts from 10 of 12 (83%) and 12 of 12 (100%), respectively (median increase, 6.0- and 7.5-fold; range, 0.9- to 11.8-fold and 3.9- to 80-fold, respectively).
In vivo effects of flavopiridol on gene blast cell gene expression from 12 AML patients treated at dose level 5 (30 mg/m2 bolus, 60 mg/m2 infusion)
| . | HMGA1 . | STAT3 . | E2F1 . | POLR2A . | VEGFA . | MCL1 . | CyclinD1 . | BCL2 . |
|---|---|---|---|---|---|---|---|---|
| All (12) | ||||||||
| Median* | 0.65 | 0.7 | 0.5 | 0.75 | 0.4 | 1.14 | 6.0 | 7.5 |
| Range | 0.2-1.15 | 0.1-1.4 | 0.1-1.0 | 0.2-1.9 | 0.07-6.1 | 0.2-4.9 | 0.9-11.8 | 3.9-80 |
| Decrease† | 10 (83%) | 8 (67%) | 9 (75%) | 8 (67%) | 8 (67%) | 2 (17%) | 0 | 0 |
| Increase‡ | 0 | 1 (8%) | 0 | 1 (8%) | 4 (33%) | 5 (42%) | 10 (83%) | 12 (100%) |
| P < .05§ | 9 (75%) | 9 (75%) | 9 (75%) | 9 (75%) | 9 (75%) | 7 (58%) | 9 (75%) | 12 (100%) |
| CR (8) | ||||||||
| Median | 0.6 | 0.7 | 0.5 | 0.8 | 0.3 | 1.1 | 6.0 | 7.1 |
| Range | 0.2-1.15 | 0.3-1.0 | 0.1-1.0 | 0.1-1.9 | 0.07-2.5 | 0.2-4.9 | 0.9-11.6 | 3.9-11.7 |
| Decrease | 7 (88%) | 6 (75%) | 6 (75%) | 4 (50%) | 7 (88%) | 2 (25%) | 0 | 0 |
| Increase | 0 | 0 | 0 | 1 (13%) | 1 (13%) | 3 (38%) | 6 (75%) | 8 (100%) |
| P < .05 | 6 (75%) | 6 (75%) | 6 (75%) | 5 (63%) | 7 (88%) | 5 (63%) | 5 (63%) | 8 (100%) |
| NR (4) | ||||||||
| Median | 0.6 | 0.6 | 0.5 | 0.3 | 1.5 | 1.2 | 6.5 | 9.5 |
| Range | 0.5-0.7 | 0.1-1.4 | 0.4-1.0 | 0.2-0.5 | 0.14-6.1 | 1.0-1.7 | 2.1-11.8 | 7.4-80 |
| Decrease | 4 (100%) | 3 (75%) | 3 (75%) | 4 (100%) | 1 (25%) | 0 | 0 | 0 |
| Increase | 0 | 1 (25%) | 0 | 0 | 3 (75%) | 3 (75%) | 4 (100%) | 4 (100%) |
| P < .05 | 3 (75%) | 3 (75%) | 3 (75%) | 4 (100%) | 2 (50%) | 2 (50%) | 4 (100%) | 4 (100%) |
| . | HMGA1 . | STAT3 . | E2F1 . | POLR2A . | VEGFA . | MCL1 . | CyclinD1 . | BCL2 . |
|---|---|---|---|---|---|---|---|---|
| All (12) | ||||||||
| Median* | 0.65 | 0.7 | 0.5 | 0.75 | 0.4 | 1.14 | 6.0 | 7.5 |
| Range | 0.2-1.15 | 0.1-1.4 | 0.1-1.0 | 0.2-1.9 | 0.07-6.1 | 0.2-4.9 | 0.9-11.8 | 3.9-80 |
| Decrease† | 10 (83%) | 8 (67%) | 9 (75%) | 8 (67%) | 8 (67%) | 2 (17%) | 0 | 0 |
| Increase‡ | 0 | 1 (8%) | 0 | 1 (8%) | 4 (33%) | 5 (42%) | 10 (83%) | 12 (100%) |
| P < .05§ | 9 (75%) | 9 (75%) | 9 (75%) | 9 (75%) | 9 (75%) | 7 (58%) | 9 (75%) | 12 (100%) |
| CR (8) | ||||||||
| Median | 0.6 | 0.7 | 0.5 | 0.8 | 0.3 | 1.1 | 6.0 | 7.1 |
| Range | 0.2-1.15 | 0.3-1.0 | 0.1-1.0 | 0.1-1.9 | 0.07-2.5 | 0.2-4.9 | 0.9-11.6 | 3.9-11.7 |
| Decrease | 7 (88%) | 6 (75%) | 6 (75%) | 4 (50%) | 7 (88%) | 2 (25%) | 0 | 0 |
| Increase | 0 | 0 | 0 | 1 (13%) | 1 (13%) | 3 (38%) | 6 (75%) | 8 (100%) |
| P < .05 | 6 (75%) | 6 (75%) | 6 (75%) | 5 (63%) | 7 (88%) | 5 (63%) | 5 (63%) | 8 (100%) |
| NR (4) | ||||||||
| Median | 0.6 | 0.6 | 0.5 | 0.3 | 1.5 | 1.2 | 6.5 | 9.5 |
| Range | 0.5-0.7 | 0.1-1.4 | 0.4-1.0 | 0.2-0.5 | 0.14-6.1 | 1.0-1.7 | 2.1-11.8 | 7.4-80 |
| Decrease | 4 (100%) | 3 (75%) | 3 (75%) | 4 (100%) | 1 (25%) | 0 | 0 | 0 |
| Increase | 0 | 1 (25%) | 0 | 0 | 3 (75%) | 3 (75%) | 4 (100%) | 4 (100%) |
| P < .05 | 3 (75%) | 3 (75%) | 3 (75%) | 4 (100%) | 2 (50%) | 2 (50%) | 4 (100%) | 4 (100%) |
Fold change in mRNA expression in blasts obtained 2 hours after the end of the flavopiridol infusion relative to mRNA expression in pretreatment blasts.
No. (%) of patients' blast cell populations in which flavopiridol was associated with a 20% or greater decrease in target gene mRNA.
No. (%) of patients' blast cell populations in which flavopiridol was associated with a 20% or greater increase in target gene mRNA.
Two-tailed t test, signifying the number (percentage) of patients whose blasts evinced significant (P < .05) increase or decrease in mRNA expression for each gene tested.
Discussion
This phase 1 trial demonstrates that the pharmacologically derived hybrid bolus-infusion schedule of flavopiridol administration in combination with Ara-C and mitoxantrone (“hybrid FLAM”) is feasible, tolerable, and associated with clinical activity in adults with relapsed and/or refractory acute leukemias, including patients who have undergone previous allogeneic SCT. Relative to our prior FLAM studies using a bolus flavopiridol administration,12-14 there were no substantial differences in terms of toxicities or time to recovery with the “hybrid” flavopiridol schedule. Although both hybrid and bolus FLAM13 produced high CR rates in patients with relapsed AML (92% and 80%, respectively), the hybrid regimen may hold more promise than the bolus regimen for primary refractory AML (31% vs 15% CR), although small numbers preclude any definitive comparison. Response rates in the multiply refractory patients were poor for both regimens. Overall, the clinical outcome for this cohort of adults with relapsed and refractory acute leukemias treated with hybrid FLAM compares favorably with recently published combinations of high-dose Ara-C with mitoxantrone,34,35 clofarabine,36 or cloretazine (laromustine).37 Nonetheless, direct comparisons of efficacy between our current phase 1 study and studies of other regimens in comparable populations would require contemporaneously randomized phase 2 or 3 trials.
Our PK data are consistent with previous literature.12,20-24,29 Because the hypothesis for the hybrid dosing schedule was to alter the dosing administration to maximize the unbound drug concentrations, we have explored the unbound drug concentrations which, for the most part, had similar variability to the total drug concentrations. Therefore, it will be more informative to compare the unbound concentrations from bolus versus hybrid schedules. Although our dataset is small, there appears to be a nonlinear difference between day 1 PK at dose level 4 versus dose levels 5 and 6. This difference is consistent with previous literature that describes flavopiridol PK as being saturable.29 The variability in PK was not explained by simple clinical covariation (ie, albumin, total protein, and white blood cells) and did not correspond to toxicity. However, unlike Phelps et al,23 we did not explore the flavopiridol glucuronide PK.
Our pharmacodynamics studies demonstrate that the in vivo administration of flavopiridol in a hybrid fashion modulates the expression of diverse intracellular molecules that play pivotal roles in cell cycle progression, survival, and leukemogenesis. The finding that flavopiridol inhibits transcription of HMGA1 mRNA sheds new light on the mechanisms by which flavopiridol may exert its antileukemic effects, particularly at the level of a putative leukemia stem cell.32,38,39 HMGA1 encodes the HMGA1a and HMGA1b chromatin binding proteins, which are highly conserved transcriptional regulators first discovered in HeLa cervical cancer cells.30 HMGA1 expression is enriched in human embryonic stem cells,38 CD34+ cells isolated from normal human marrow,39 and a broad spectrum of high-grade epithelial and hematopoietic malignancies.27,28,30-33,40-42 HMGA1 proteins bind to the minor groove of AT-rich DNA sequences through basic repeats known as “AT hooks,” thereby inducing conformational changes in chromatin, displacing histones, and relieving histone-mediated transcriptional repression.32
In the current study, target modulation occurred to various degrees in the majority of the 12 patients' leukemia blast populations, with HMGA1, STAT-3, E2F-1, POLR2A, and VEGF-A mRNA down-regulated in 67% to 83% of patients. In contrast, MCL-1 expression was decreased in only 17% and increased in 42%, cyclin D1 expression was increased in 83% patients and bcl-2 expression was increased in 100%. Interestingly, in our original phase 1 trial of FLAM using bolus flavopiridol and measuring flavopiridol-induced changes in selected proteins in marrow blasts,12 the most consistently down-regulated proteins were phospho-RNA polymerase II and phosphoSTAT-3, whereas the effects on MCL-1, Cyclin D1, and BCL-2 were more variable. The current finding of consistent cyclin D1 and bcl-2 mRNA up-regulation raises the possibility that these may be compensatory responses to flavopiridol's ability to trigger multiple critical cycle-arresting and apoptotic pathways. Whether or not the magnitude of up-regulation of one or both of these gene products might relate to clinical outcome will require study in a larger group of patients. Nonetheless, on the basis of these findings, the combination of flavopiridol with agents that block this bcl-2 “rebound” expression, for example, bcl-2-directed antisense constructs43,44 or small molecule inhibitors,45 might afford synergistic enhancement of apoptosis, a hypothesis that could be tested clinically.
In conclusion, the “hybrid” administration of flavopiridol followed in timed sequence by Ara-C and mitoxantrone appears to have significant clinical activity in adults with relapsed and refractory acute leukemias, including subsets with traditionally poor outcomes. The recommended dose of flavopiridol for “hybrid” FLAM for relapsed and refractory leukemias is bolus 30 mg/m2 followed by infusion 60 mg/m2 daily for 3 days. This study serves as a springboard for continuing development of FLAM for poor-risk acute leukemias at diverse stages of disease and drug resistance. In particular, whether or not “hybrid” FLAM offers significant advantages over bolus FLAM in terms of flavopiridol cytotoxicity and net clinical outcome is being tested in a randomized phase 2 study for adults with newly diagnosed, poor-risk AML.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Johns Hopkins Sidney Kimmel Cancer Center nursing staff for superb medical care, and the patients and their families, without whose partnership we could never have conducted the trial and from whom we have learned critical information that will help us to improve the treatment of these diseases.
This work was supported by the National Institutes of Health (grant 2U01CA70095), the National Cancer Institute (Cooperative Agreement U01 CA70095) (J.E.K., M.A.R.), National Cancer Institute Cancer Center Support (grant 2P30 CA069773-45), National Center for Research (resources grant UL1 RR025005), the J.P. McCarthy Foundation (L.S.R., J.E.K.), and Dr Robert E. Fischell in memory of his late wife Marian (philanthropic funds) (J.E.K.).
National Institutes of Health
Authorship
Contribution: J.E.K., B.D.S., L.S.R., and M.A.R. designed and performed research, analyzed the data, and wrote the paper; M.J.L., S.D.G., H.C., and M.A.M. designed the protocol, performed research, analyzed the data, and wrote the paper; M.Z. performed pharmacokinetic studies and analyzed pharmacokinetic data; L.B. collected and analyzed pharmacokinetic data; J.M.G., K.A., K.M., and J.B. coordinated sample acquisition for laboratory studies, performed research, collected data, and analyzed data; D.M.-N. and B.J. conducted and analyzed pharmacodynamic studies; and A.B., L.A.D., and J.J.W. participated in protocol design and performed critical review of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Judith E. Karp, Johns Hopkins Sidney Kimmel Comprehensive Cancer Center, 1650 Orleans St, CRB 1, Rm 2M44, Baltimore, MD 21231-1000; e-mail: jkarp2@jhmi.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal