Abstract
Clofarabine (2-chloro-2′-fluoro-deoxy-9-β-D-arabinofuranosyladenine) is a second-generation nucleoside analog with activity in acute leukemias. As clofarabine is a potent inhibitor of ribonucleotide reductase (RnR), we hypothesized that clofarabine will modulate ara-c triphosphate accumulation and increase the antileukemic activity of cytarabine (ara-C). We conducted a phase 1-2 study of clofarabine plus ara-C in 32 patients with relapsed acute leukemia (25 acute myeloid leukemia [AML], 2 acute lymphoblastic leukemia [ALL]), 4 high-risk myelodysplastic syndrome (MDS), and 1 blast-phase chronic myeloid leukemia (CML).1 Clofarabine was given as a 1-hour intravenous infusion for 5 days (days 2 through 6) followed 4 hours later by ara-C at 1 g/m2 per day as a 2-hour intravenous infusion for 5 days (days 1 through 5). The phase 2 dose of clofarabine was 40 mg/m2 per day for 5 days. Among all patients, 7 (22%) achieved complete remission (CR), and 5 (16%) achieved CR with incomplete platelet recovery (CRp), for an overall response rate of 38%. No responses occurred in 3 patients with ALL and CML. One patient (3%) died during induction. Adverse events were mainly less than or equal to grade 2, including transient liver test abnormalities, nausea/vomiting, diarrhea, skin rashes, mucositis, and palmoplantar erythrodysesthesias. Plasma clofarabine levels generated clofarabine triphosphate accumulation, which resulted in an increase in ara-CTP in the leukemic blasts. The combination of clofarabine with ara-C is safe and active. Cellular pharmacology data support the biochemical modulation strategy.
Introduction
Since the introduction of nucleoside analogues in the 1960s, further progress has made this class of drugs into one of the most active components of therapy for patients with hematopoietic malignancies.1-4 Although several analogues such as fludarabine, cladribine, nelarabine, gemcitabine, and deoxycoformycin have been introduced to the clinic, cytarabine (ara-C) remains the only active nucleoside analog for treatment of adult acute myeloid leukemia (AML).5,6
Clofarabine (2-chloro-2′-fluoro-deoxy-9-β-D-arabinofuranosyladenine) was synthesized as a hybrid molecule to overcome the limitations of fludarabine and cladribine and to incorporate their best qualities.7 Halogenation at the 2 position of adenine and substitution of a fluorine group at the C-2′ position of the arabinofuranosyl moiety were essential for many of the characteristic features of clofarabine: (1) high resistance to phosphorolytic cleavage by bacterial purine nucleoside phosporylase; (2) increased acid stability stimulating the development of an oral compound; (3) resistance to deamination by adenosine deaminase; and (4) the DNA-directed activity of clofarabine.8-11 Because of its similarity to fludarabine and cladribine, it was postulated that clofarabine would be active in indolent leukemias rather than the adult acute leukemias. However, bypassing the dose-limiting neurotoxicity of other deoxyadenosine nucleoside analogues, through the modification in chemical structure, would allow exploration of higher dose schedules that demonstrate anti–acute leukemia activity but not neurotoxicity. This was the case in the single-agent phase 1 study, which escalated clofarabine to 20 times the solid tumor dose (from 2 mg/m2 per dose to 40 mg/m2 per dose) and demonstrated anti–acute leukemia activity without neurotoxicity.
In both phase 1 and phase 2 investigations of clofarabine as a single agent, impressive clinical activity without neurotoxicity was observed in patients with acute leukemias, especially those with AML. Among the 32 heavily pretreated patients in the phase 1 study of clofarabine, 5 patients responded to this therapy. The maximum tolerated dose (MTD) on a 5-day schedule was 40 mg/m2 per day for patients with acute leukemias with hepatotoxicity as the dose-limiting toxicity (DLT).12 In a subsequent phase 2 study, 62 patients with relapsed and refractory AML, acute lymphoblastic leukemia (ALL), high-risk myelodysplastic syndrome (MDS), and blast-phase chronic myeloid leukemia (CML) were treated at the MTD. The overall response rate was 48% including 20 patients (32%) achieving a complete response (CR). Response rates reached 87% in AML patients with longer first remission durations (≥ 12 months), suggesting significant activity of clofarabine in this group of patients.13 Hence, clofarabine appears to be the second nucleoside analog (after ara-C) with efficacy against adult AML.
Ara-C is a prodrug that has to be phosphorylated to its triphosphate form to elicit cytotoxicity. A strong relationship exists between cellular accumulation and retention of ara-CTP and response to ara-C therapy.14,15 The accumulation of ara-CTP is saturated in leukemia cells at the intermediate dose rate of ara-C.16 Because of this limitation, ara-C has been combined with either fludarabine or cladribine to biochemically modulate accumulation of ara-CTP in adult acute leukemias.17-19 Clofarabine possesses metabolic and mechanistic qualities similar to fludarabine and cladribine, including potent inhibition of ribonucleotide reductase (RnR) that leads to depletion of normal deoxynucleotides (dNTPs).10,11,20-22 We hypothesized that the combination of clofarabine and ara-C in a sequential manner could result in increased accumulation of ara-CTP and improvement of the clinical response rate because of both biochemical modulation of ara-CTP and clinical activity of clofarabine as a single agent. Additionally, because of lack of neurotoxicity with clofarabine (a chronic clinical issue with fludarabine and cladribine), this combination may be well tolerated by patients.
The current study was designed to test the above hypothesis, and to characterize the role of clofarabine in combination with ara-C in the treatment of patients with relapsed and refractory acute leukemias. As a potent inhibitor of RnR, clofarabine is ideally suited for biochemical modulation strategies with nucleoside analogues such as ara-C. In addition to demonstrating the safety and efficacy of the combination strategy, we also sought to show that combining clofarabine with ara-C leads to increased accumulation of ara-C triphosphate in leukemic cells. The antileukemic activity of clofarabine is thus complemented by a biochemical synergy between these agents that may produce better clinical efficacy.
Patients, materials, and methods
Study group
Adults with a diagnosis of acute leukemia or high-risk MDS were eligible after informed consent was obtained according to institutional guidelines. Diagnostic categories were as follows: (1) AML or ALL in first relapse or treatment as first salvage for primary refractory disease (up to 2 induction courses were permissible); (2) high-risk MDS as defined by more than or equal to 10% blood and/or marrow blasts with no more than one prior regimen of chemotherapy (use of hematopoietic growth factors, biologic or targeted therapies were allowed); and (3) CML in blast phase if treatment was received as frontline therapy or in first salvage. Patients were required to be off previous therapy for at least 2 weeks. Other eligibility criteria included (1) age at least 18 years; (2) performance status less than or equal to 2 (Eastern Cooperative Oncology Group [ECOG] scale); (3) adequate liver function (serum total bilirubin ≤ 2 mg/dL, alanine aminotransferase and aspartate aminotransferase ≤ 4× the upper limit of normal); and (4) adequate renal function (serum creatinine ≤ 2 mg/dL). Approval for the study was granted from the institutional review board (IRB) of the University of Texas M.D. Anderson Cancer Center. The study was conducted in accordance with the basic principles of the Declaration of Helsinki.
Treatment (doses and schedule)
The study was conducted in 2 parts: phase 1 followed by phase 2. For both parts of the study, clofarabine was administered as a 1-hour intravenous infusion daily for 5 consecutive days (days 2 through 6) followed 4 hours later by an intermediate dose of ara-C (1 g/m2 as a 2-hour constant-rate intravenous infusion) once daily for 5 consecutive days (days 1 through 5). On day 1, only ara-C was administered; on day 6, only clofarabine was administered. This ara-C infusion rate generated ara-C concentrations in plasma (15 μM-20 μM) that maximize the rate of ara-CTP accumulation in leukemic blasts.16 The infusion of ara-C was begun 4 hours after starting clofarabine on days 2 through 5, as previous studies demonstrated that this was the time of maximum clofarabine triphosphate accumulation in leukemia blasts.13 On the first day, ara-C was infused alone to characterize the ara-CTP pharmacokinetics in leukemia blasts for comparison with that on day 2, when ara-C was given after the first clofarabine infusion. A starting dose for clofarabine of 15 mg/m2 per day was chosen for the phase 1 part of the study. Patients were enrolled in cohorts of 3 patients at fixed clofarabine dose escalations of 15, 22.5, 30, and 40 mg/m2 per day (see “Statistical analyses”). No dose escalation of clofarabine beyond 40 mg/m2 per day, the established MTD for single-agent phase 2 studies, was considered. Cycles were repeated every 3 to 5 weeks as indicated by leukemia recurrence and recovery of normal hematopoiesis. Patients were allowed to receive a maximum of 2 cycles of induction therapy or until a CR, CR with incomplete platelet recovery (CRp), or partial response (PR) was achieved. Patients who did not achieve at least a PR after 2 induction courses were taken off the study. Responding patients could receive up to 6 additional cycles of maintenance therapy at 75% of the induction doses of both clofarabine and ara-C. Dose level reductions were implemented for grade 2 extramedullary toxicities (25%) and more than or equal to grade 3 extramedullary toxicities, including life-threatening infections (50%). Consolidation therapy and dose reductions due to toxicities could be modified at the discretion of the treating physician as judged to be in the best interest of the patient.
The pretreatment evaluation included history, physical examination, complete blood counts (CBCs) with differentials and platelet count, a complete chemistry survey, and marrow aspiration (with or without biopsy) with cytogenetic analysis. A marrow analysis was not required if the diagnosis of relapse could be made unequivocally from the peripheral blood, including immunophenotyping by flow cytometry. An echocardiogram or gated cardiac scan (MUGA) to evaluate left ventricular function was required. Follow-up studies included CBC, differential, and platelet count, as well as a chemistry profile at least weekly until response. Marrow aspiration was performed starting at day 21 and then every 2 weeks until remission or nonresponse. Marrow tests were repeated more frequently if considered indicated by the treating physician. Patients with marrow studies showing no evidence of leukemia, but with persistent cytopenia, were allowed time to recover their counts to document the best response. Cardiac assessments (echocardiogram or MUGA scan) were repeated every 4 cycles. Blood samples for pharmacokinetic, intracellular pharmacologic, and metabolic evaluation were collected at specified time points on days 1 through 6 during cycle 1. Supportive measures for optimal medical care were provided throughout the study as determined by the treating physician and the patient's medical need. Hematopoietic growth factors were allowed in patients with serious neutropenic complications (≥ grade 3 febrile neutropenia or obvious sepsis).
Response criteria
A CR required normalization of the marrow (≤ 5% blasts in a normocellular marrow) and peripheral counts with no circulating blast cells, a neutrophil count of more than or equal to 1 × 109/L and platelet counts more than or equal to 100 × 109/L. A partial response consisted of a blood count recovery as for CR, but with persistence of 5% to 25% marrow blasts. A CRp had criteria similar to a CR, but without recovery of platelets to more than or equal to 100 × 109/L.
A PR in MDS required improvement in at least 2 parameters: reduction of marrow blasts by more than or equal to 50% and to less than 10%; increase of platelet counts by 100% and to more than or equal to 100 × 109/L; increase of neutrophil count by 100% and to more than or equal to 0.5 × 109/L.
In blast-phase CML, a complete hematologic response (CHR) was similar to CR. A partial hematologic response (PHR) was the same as for a CHR but with the persistence of few immature peripheral cells (< 5% myelocytes and metamyelocytes) or with persistent but more than or equal to 50% reduced splenomegaly or thrombocytosis. A return to second chronic phase required disappearance of accelerated or blast-phase features. All other responses were considered treatment failures.
Clinical pharmacology
Blood samples (10 mL) were obtained from patients who agreed to blood draws for pharmacologic determinations. Samples were transferred to green stopper vacutainer tubes containing heparin and 1 μM deoxycoformycin (obtained from the National Cancer Institute [NCI], Bethesda, MD) as a precaution to inhibit deamination of clofarabine by adenosine deaminase. The tubes were immediately placed in an ice-water bath and transported to the laboratory. Control studies have demonstrated that normal and leukemia cells are stable under these conditions with respect to size and membrane integrity. The cellular nucleotide content is stable for at least 15 hours under these conditions.23 All patients gave written informed consent for plasma and cellular pharmacology investigations.
Plasma pharmacokinetics
Samples were obtained prior to therapy on day 1 for baseline values, prior to clofarabine infusion on day 2, at the end of the 1-hour infusion, and at 2, 3, 4, 5, 6, and 24 hours after the start of therapy. Additional samples (prior to end of infusion, and 6 hours) were obtained on days 3 to 5. On the sixth day when clofarabine was infused as a single agent, samples were taken as on day 1 to compare the influence of ara-C infusion on clofarabine pharmacokinetics. The plasma was removed after centrifugation and stored at –70°C until analyses were performed. Human plasma samples (100 mL) containing clofarabine were spiked with cladribine as the internal standard. Samples were precipitated, evaporated, and reconstituted with mobile phase. The samples were analyzed by reversed-phase high-performance liquid chromatography (HPLC) coupled to a tandem quadrupole mass spectrometer by a modified, previously described procedure by MicroConstants (San Diego, CA).24 Authentic clofarabine standard was used for assay validation, and for identification and quantitation of the nucleoside in plasma. The assay has a sensitivity range from 10 ng/mL to 5000 ng/mL. The coefficient of variation (CV) for precision was less than 7% and the accuracy was within plus or minus 7%.
Pharmacokinetic data were analyzed using compartmental models with nominal blood draw times as the independent variable. A 2-compartment model was fit to each individual patient's pharmacokinetic data using the Nelder-Mead algorithm with inverse predicted concentration used as the weights. All analyses were conducted using WinNonlin Professional, version 4.0 (Pharsight, Mountain View, CA).
Cellular pharmacology
For cellular pharmacology, samples were obtained on days 1 and 2. Samples were taken prior to therapy, at the end of clofarabine infusion, and 1, 2, or 3 hours after start of ara-C infusion. Cell pellets from blood samples were diluted with phosphate-buffered saline, and mononuclear cells were isolated using Ficoll-Hypaque density-gradient, step-gradient centrifugation procedures as described previously.25 A Coulter electronics channelizer (Coulter, Hialeah, FL) was used to determine the mean cell volume. After being washed with phosphate-buffered saline, cells were processed for nucleotide extraction. Normal nucleotides, ara-CTP, and clofarabine triphosphate were extracted from cells using standard procedures with HClO4. Triphosphates were separated on an anion-exchange Partisil-10 SAX column (Waters, Milford, MA) using HPLC as described in detail previously.25 The intracellular concentration was calculated and expressed as the quantity of nucleotides contained in the extract from a given number of cells of a determined mean volume. This calculation assumes that nucleotides are uniformly distributed in total cell water. In general, the lower limit of quantitation of this assay was approximately 1 pmol in an extract of 2 × 107 cells, corresponding to a cellular concentration of approximately 0.2 μM.
Statistical analyses
This was an open-label, phase 1-2 dose-escalation study with no randomization. The primary objective of phase 1 of the study was to determine the MTD and DLT of clofarabine when used in combination with ara-C. The dose escalation used the 3 + 3 design.12 All more than or equal to grade 3 drug-related toxicities were considered DLTs with the exception of nausea and vomiting (if manageable with supportive care measures), alopecia, drug-related fever, and lymphopenia. Secondary objectives were to evaluate the efficacy of this treatment regimen and to study the biochemical modulation of ara-CTP when given in combination with clofarabine. This is a single-arm study with objective tumor response as the primary efficacy end point of the phase 2 portion of the trial. Efficacy endpoints for the phase 2 portion included CR, CRp, overall response rates (CR plus CRp divided by the total number of eligible patients), duration of remission, and survival. Results are presented for all treated patients. Kaplan-Meier methodology is used to describe time-to-event outcomes.
Results
Study population
A total of 32 patients were treated. Their characteristics are summarized in Table 1. Twelve patients (3 per dose cohort) were enrolled in the phase 1 part of the study, whereas the remaining 20 patients participated in the phase 2 part of the study. The median age of the whole study population was 59 years (range, 18 to 84 years). There were 25 patients with AML, 4 with high-risk MDS as defined by more than or equal to 10% blood or marrow blasts, 1 with CML in myeloid blast phase (resistant to imatinib), and 2 patients with ALL. With the exception of the patient with CML who received the clofarabine/ara-C combination as frontline therapy for blast phase, all other patients were either primary refractory (n = 7) to the preceding induction regimen, or received the study treatment as first salvage following relapse (n = 24). Among the patients with AML and MDS, 16 had experienced a first remission duration of less than 12 months, and 6 patients were in remission for at least 12 months or longer. Cytogenetic analysis revealed abnormal karyotypes in 11 patients with AML.
Characteristics of the 32 patients studied
. | Phase 1 . | Phase 2 . |
|---|---|---|
| No. | 12 | 20 |
| Diagnosis, no. | ||
| AML | 9 | 16 |
| MDS | 1 | 3 |
| ALL | 2 | 0 |
| CML | 0 | 1 |
| Age, y, median (range) | 56 (23-74) | 63 (18-84) |
| Cytogenetics, no. | ||
| Diploid | 6 | 7 |
| -5/-7 | 1 | 4 |
| Other | 5 | 9 |
| Disease status, no. | ||
| First relapse | 11 | 13 |
| Primary refractory | 1 | 6 |
| CRD1, mo, median (range) | 7.6 (0-17.1) | 5.2 (0-18) |
. | Phase 1 . | Phase 2 . |
|---|---|---|
| No. | 12 | 20 |
| Diagnosis, no. | ||
| AML | 9 | 16 |
| MDS | 1 | 3 |
| ALL | 2 | 0 |
| CML | 0 | 1 |
| Age, y, median (range) | 56 (23-74) | 63 (18-84) |
| Cytogenetics, no. | ||
| Diploid | 6 | 7 |
| -5/-7 | 1 | 4 |
| Other | 5 | 9 |
| Disease status, no. | ||
| First relapse | 11 | 13 |
| Primary refractory | 1 | 6 |
| CRD1, mo, median (range) | 7.6 (0-17.1) | 5.2 (0-18) |
CRD1 indicates duration of first complete remission; AML, acute myeloid leukemia; MDS, myelodsyplastic syndrome; ALL, acute lymphoblastic leukemia; CML, chronic myeloid leukemia; -5/-7, monosomy of chromosomes 5 and 7.
Response and outcome
Response rates are shown in Table 2. Among all patients, 7 (22%) achieved CR, and 5 (16%) achieved CRp, for an overall response rate of 38%. No responses occurred in 3 patients with ALL and CML. Among the 29 patients with AML/MDS, 7 patients (24%) achieved CR. Another 5 patients (17%) had hematologic improvement (CRp) for an overall response rate of 41% in this group. In AML, 7 (28%) of 25 patients attained CR, 3 (12%) CRp, whereas 15 patients (60%) had no response. Of the 4 patients with MDS, 2 (50%) achieved CRp. No responses were seen in 2 patients with ALL and in one patient with CML in myeloid blast phase. Twenty-three patients with a diagnosis of AML and high-risk MDS were either primary refractory to their initial induction regimen or had a first remission duration of less than one year. Among them, 5 (22%) achieved CR and 3 (13%) achieved CRp. Among 6 patients with longer first remission durations (≥ 12 months), 2 (33%) had CR, and 2 (33%) had CRp, suggesting no major difference in outcome in this subset analysis. Characteristics of responding patients (CR and CRp) are shown in Table 3. Among all 32 patients, 20 received one course only, 10 received a second course, and 2 patients received 5 courses. Patient 3 achieved a CR at the lowest clofarabine dose level (15 mg/m2 per day). However, this patient received induction therapy with gemtuzumab plus interleukin-11 only and achieved a first remission duration of more than one year. It remains impossible to ascertain whether the CR following clofarabine plus ara-C is due to the combination of ara-C with clofarabine or whether ara-C alone or any other ara-C–containing regimen would not have had the same effect. Furthermore, 2 of the 5 patients with CRp achieved responses during the phase 1 portion of the study at doses below the level that had been established as the phase 2 dose. Patient 18 with primary refractory AML required 2 courses to achieve remission. All others achieved a CR or CRp following one course. Patient 18 has now completed another 3 courses of clofarabine/ara-C maintenance and remains in remission at more than 6 months. For patients who achieved CR (n = 7), the median time to platelet recovery more than or equal to 100 × 109/L was 42 days (range, 22 to 62 days) and the median time to neutrophil recovery more than or equal to 1 × 109/L was 33 days (range, 18 to 59 days). Prolonged myelosuppression (≥ 42 days from previous course) was typically not observed during consolidation courses. Patient 27 died aplastic in CR from septic shock with multisystem organ failure during the first consolidation course.
Overall response in 29 patients with AML and high-risk MDS
Response . | No. (%) . |
|---|---|
| Complete response, CR | 7 (24) |
| First complete remission duration | |
| Less than 12 mo | 5 (23) |
| 12 mo or more | 2 (29) |
| Diagnosis | |
| AML | 7 (28) |
| MDS | 0* |
| Hematologic improvement, CRp | 5 (17) |
| AML | 3 (60) |
| MDS | 2 (40) |
| Overall response, CR and CRp | 12 (41) |
| Induction death, within 4 wk | 1 (3) |
| Resistance | 16 (55) |
Response . | No. (%) . |
|---|---|
| Complete response, CR | 7 (24) |
| First complete remission duration | |
| Less than 12 mo | 5 (23) |
| 12 mo or more | 2 (29) |
| Diagnosis | |
| AML | 7 (28) |
| MDS | 0* |
| Hematologic improvement, CRp | 5 (17) |
| AML | 3 (60) |
| MDS | 2 (40) |
| Overall response, CR and CRp | 12 (41) |
| Induction death, within 4 wk | 1 (3) |
| Resistance | 16 (55) |
Two of 4 patients with MDS achieved CRp.
Characteristics of responding patients (CR and CRp)
Patient no. . | Age, y/sex . | Diagnosis . | Karyotype . | Prior therapy . | CRD1, mos . | Response (dose, mg/m2per day*)/duration, mos . | No. of courses to response . |
|---|---|---|---|---|---|---|---|
| 3 | 74/M | AML | Diploid | GO + IL-11 | 13.8 | CR (15)/7.4 | 1 |
| 4 | 53/F | AML | Diploid | FA + GO + CSA | 14.8 | CRp (22.5)/1.7 | 1 |
| 7 | 62/F | AML | Diploid | I + HDAC | 12.4 | CRp (30)/1.9 | 1 |
| 11 | 59/M | AML | Diploid | I + HDAC | 9.5 | CR (40)/2.9 | 1 |
| 17 | 71/M | AML | Tetraploidy | IA | 4.8 | CR (40)/5+ | 1 |
| 18 | 47/M | AML | Diploid | IA | 0 | CR (40)/6+ | 2 |
| 20 | 39/M | AML | Inv(16) | FLAG | 17.4 | CR (40)/5+ | 1 |
| 24 | 72/M | RAEB | Inv(3),del(13) | IA | 9.3 | CRp (40)/3+ | 1 |
| 25 | 66/F | AML | Diploid | IA | 10.5 | CRp (40)/5+ | 1 |
| 27 | 65/F | AML | -5/-7 | IA | 0 | CR (40)/0.5 (died in CR) | 1 |
| 29 | 74/M | AML | -5/-7 | IA | 7.6 | CR (40)/3+ | 1 |
| 30 | 56/M | RAEBT | Del(20) | IA + IL-11 | 6.5 | CRp (40)/3 | 1 |
Patient no. . | Age, y/sex . | Diagnosis . | Karyotype . | Prior therapy . | CRD1, mos . | Response (dose, mg/m2per day*)/duration, mos . | No. of courses to response . |
|---|---|---|---|---|---|---|---|
| 3 | 74/M | AML | Diploid | GO + IL-11 | 13.8 | CR (15)/7.4 | 1 |
| 4 | 53/F | AML | Diploid | FA + GO + CSA | 14.8 | CRp (22.5)/1.7 | 1 |
| 7 | 62/F | AML | Diploid | I + HDAC | 12.4 | CRp (30)/1.9 | 1 |
| 11 | 59/M | AML | Diploid | I + HDAC | 9.5 | CR (40)/2.9 | 1 |
| 17 | 71/M | AML | Tetraploidy | IA | 4.8 | CR (40)/5+ | 1 |
| 18 | 47/M | AML | Diploid | IA | 0 | CR (40)/6+ | 2 |
| 20 | 39/M | AML | Inv(16) | FLAG | 17.4 | CR (40)/5+ | 1 |
| 24 | 72/M | RAEB | Inv(3),del(13) | IA | 9.3 | CRp (40)/3+ | 1 |
| 25 | 66/F | AML | Diploid | IA | 10.5 | CRp (40)/5+ | 1 |
| 27 | 65/F | AML | -5/-7 | IA | 0 | CR (40)/0.5 (died in CR) | 1 |
| 29 | 74/M | AML | -5/-7 | IA | 7.6 | CR (40)/3+ | 1 |
| 30 | 56/M | RAEBT | Del(20) | IA + IL-11 | 6.5 | CRp (40)/3 | 1 |
CRD1 indicates first remission duration (0 indicates primary refractory to induction therapy); GO, gemtuzumab ozogamicin (mylotarg); IL-11, interleukin-11; FA, fludarabine, ara-C; CSA, cyclosporine A; I, idarubicin; HDAC, high-dose ara-C; IA, idarubicin and ara-C; FLAG, fludarabine, HDAC, G-CSF; inv(16), inversion of chromosome 16; inv(3), inversion of chromosome 3; del, deletion; -5/-7, monosomy of chromosomes 5 and 7; CR, complete remission; CRp, complete remission without platelet recovery ≥ 100 × 109/L.
Dose of clofarabine.
With a median follow up of 14.2 months (range, 5.5-15.2 months), the median remission duration (CR and CRp) is 3.2 months (0.5-14 months). Median overall survival of all patients is 5.5 months (0.2 to 15.2 months or longer) with a median overall survival of responding patients of 7.9 months (1.7 to 8.3 months or longer).
Side effects
Common side effects of the clofarabine/ara-C combinations included nausea and vomiting, diarrhea, skin rashes including hand-foot syndrome, and mucositis (Table 4). During the phase 1 portion of the study, no dose-limiting toxicities (DLTs) were observed during each of the 2-week observation periods necessary to establish further dose escalation. Facial flushing and headaches were noted during chemotherapy administration and typically resolved upon completion of the infusion. The side effect profile of the phase 1 and phase 2 populations was similar, although a higher incidence of hand-foot syndrome (palmoplantar erythrodysesthesia) was observed during phase 2 at the higher doses of clofarabine (40 mg/m2 per day). Transient liver dysfunctions were common. In the phase 1 portion, less than or equal to grade 2 hyperbilirubinemia was observed in 8 patients (67%) and elevations of transaminases in 5 patients (42%). These occurred anywhere between days 7 to 28 of cycle 1, but were most commonly clustered around days 7 to 11. Likewise, hyperbilirubinemia was seen in 16 patients (80%) of the phase 2 part of the study and elevations of transaminases in 13 (65%). Only 2 patients (10%) experienced grade 3 hyperbilirubinemia.
Nonhematologic side effects
. | No. (%) . | . | |
|---|---|---|---|
| Side effect . | Grade 1 or 2 . | Grade 3 or higher . | |
| Phase 1 | |||
| Nausea/vomiting | 11 (92) | — | |
| Diarrhea | 8 (67) | 1 (8)* | |
| Impaired liver function† | 8 (67) | — | |
| Skin rash | 6 (50) | 1 (8)* | |
| Mucositis | 5 (42) | — | |
| Facial flushing | 5 (42) | — | |
| Fatigue | 3 (25) | — | |
| Headaches | 2 (17) | — | |
| Palpitations/arrhythmias | 1 (8) | — | |
| Phase 2 | |||
| Impaired liver function† | 16 (80) | 2 (10) | |
| Nausea/vomiting | 14 (70) | — | |
| Skin rash | 12 (60) | 2 (10) | |
| Diarrhea | 10 (50) | 4 (20) | |
| Hand-foot syndrome | 9 (45) | 1 (5) | |
| Mucositis | 7 (35) | — | |
| Headaches | 7 (35) | — | |
| Facial flushing | 3 (15) | — | |
| Fatigue | 2 (10) | — | |
| Palpitations/arrhythmias | 1 (5) | 1 (5) | |
| Drug fever | 1 (5) | — | |
. | No. (%) . | . | |
|---|---|---|---|
| Side effect . | Grade 1 or 2 . | Grade 3 or higher . | |
| Phase 1 | |||
| Nausea/vomiting | 11 (92) | — | |
| Diarrhea | 8 (67) | 1 (8)* | |
| Impaired liver function† | 8 (67) | — | |
| Skin rash | 6 (50) | 1 (8)* | |
| Mucositis | 5 (42) | — | |
| Facial flushing | 5 (42) | — | |
| Fatigue | 3 (25) | — | |
| Headaches | 2 (17) | — | |
| Palpitations/arrhythmias | 1 (8) | — | |
| Phase 2 | |||
| Impaired liver function† | 16 (80) | 2 (10) | |
| Nausea/vomiting | 14 (70) | — | |
| Skin rash | 12 (60) | 2 (10) | |
| Diarrhea | 10 (50) | 4 (20) | |
| Hand-foot syndrome | 9 (45) | 1 (5) | |
| Mucositis | 7 (35) | — | |
| Headaches | 7 (35) | — | |
| Facial flushing | 3 (15) | — | |
| Fatigue | 2 (10) | — | |
| Palpitations/arrhythmias | 1 (5) | 1 (5) | |
| Drug fever | 1 (5) | — | |
There were 12 patients in phase 1 and 20 patients in phase 2. — indicates that there were no grade 3 or higher toxicities.
Grade 3 toxicity occurred after 2-week observation period necessary to establish dose escalation.
Includes hyperbilirubinemia and elevation of transaminases.
In the combined phase 1 and 2 studies, myelosuppression-associated complications included at least one febrile episode in 20 of 32 patients (63%; Table 5). Fever of unknown origin occurred in 10 patients (31%). Documented infections included 20 episodes of bacterial infections (predominantly catheter-related infections due to coagulase-negative Staphylococcus) and 3 episodes of fungal infections (at least one fungal pneumonia). One early death occurred at day 11 due to myelosuppression-associated complications (bacterial sepsis) and was not considered study drug–related.
Hematologic side effects in the 32 patients studied
Characteristics . | Value . |
|---|---|
| Median time to recovery for patients in CR* | |
| Platelet count at or above 100 × 109/L, d, mean (range) | 42 (22-62) |
| Neutrophil count at or above 1 × 109/L, d, mean (range) | 33 (18-59) |
| Febrile episodes, no. (%) | 20 (63) |
| Fever of unknown origin, no. (%) | 10 (31) |
| Documented infections, no. (%) | |
| Bacterial/sepsis | 14 (44) |
| Fungal | 3 (9) |
| Other/no identified source | 5 (16) |
Characteristics . | Value . |
|---|---|
| Median time to recovery for patients in CR* | |
| Platelet count at or above 100 × 109/L, d, mean (range) | 42 (22-62) |
| Neutrophil count at or above 1 × 109/L, d, mean (range) | 33 (18-59) |
| Febrile episodes, no. (%) | 20 (63) |
| Fever of unknown origin, no. (%) | 10 (31) |
| Documented infections, no. (%) | |
| Bacterial/sepsis | 14 (44) |
| Fungal | 3 (9) |
| Other/no identified source | 5 (16) |
n = 7.
Plasma pharmacokinetics
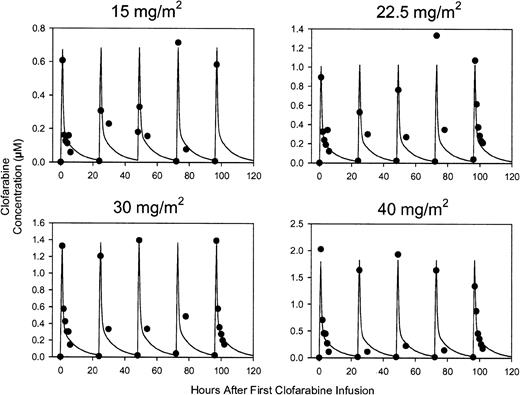
Each subject's pharmacokinetic results are presented in Table 6. A 2-compartment model appeared to adequately characterize the plasma pharmacokinetics of clofarabine. No systematic trend in the residuals was observed over time for any patient. The residuals also appeared to be normally distributed when pooled across patients. Figure 1 presents the median concentration-time profiles for each dose group and the model predicted concentrations. Concentrations increased with increasing dose. No evidence of nonlinearity was observed in the data, although the number of subjects at each dose level was too small to detect nonlinear pharmacokinetics.
Individual and mean plasma clofarabine pharmacokinetic parameters
Patient no. . | Clofarabine, mg/m2 . | Clearance, L/h/m2 . | Volume of central compartment, L/m2 . | Volume of distribution at steady-state, L/m2 . | α half-life, h . | β half-life, h . | Cmax, μM . |
|---|---|---|---|---|---|---|---|
| 1 | 15 | 12.1 | 51.3 | 294.4 | 0.41 | 18.6 | 0.48 |
| 5 | 22.5 | 17.4 | 45.7 | 128.7 | 0.19 | 14.1 | 0.89 |
| 6 | 22.5 | 12.7 | 44.4 | 209.1 | 0.29 | 5.8 | 1.05 |
| 7 | 30 | 11.3 | 15.6 | 90.7 | 0.37 | 6.9 | 2.65 |
| 8 | 30 | 16.2 | 10.9 | 90.8 | 0.19 | 7.4 | 2.95 |
| 9 | 30 | 22.1 | 45.3 | 126.5 | 0.17 | 5.1 | 1.29 |
| 10 | 40 | 27.0 | 43.1 | 109.5 | 0.15 | 5.1 | 1.96 |
| 11 | 40 | 20.3 | 39.1 | 110.4 | 0.21 | 3.4 | 2.08 |
| 12 | 40 | 33.1 | 41.4 | 122.8 | 0.19 | 5.3 | 1.52 |
| Mean | NA | 19.1 | 37.4 | 142.5 | 0.24 | 6.7 | ND |
| SD | NA | 7.3 | 14.1 | 66.7 | 0.09 | 3.1 | ND |
Patient no. . | Clofarabine, mg/m2 . | Clearance, L/h/m2 . | Volume of central compartment, L/m2 . | Volume of distribution at steady-state, L/m2 . | α half-life, h . | β half-life, h . | Cmax, μM . |
|---|---|---|---|---|---|---|---|
| 1 | 15 | 12.1 | 51.3 | 294.4 | 0.41 | 18.6 | 0.48 |
| 5 | 22.5 | 17.4 | 45.7 | 128.7 | 0.19 | 14.1 | 0.89 |
| 6 | 22.5 | 12.7 | 44.4 | 209.1 | 0.29 | 5.8 | 1.05 |
| 7 | 30 | 11.3 | 15.6 | 90.7 | 0.37 | 6.9 | 2.65 |
| 8 | 30 | 16.2 | 10.9 | 90.8 | 0.19 | 7.4 | 2.95 |
| 9 | 30 | 22.1 | 45.3 | 126.5 | 0.17 | 5.1 | 1.29 |
| 10 | 40 | 27.0 | 43.1 | 109.5 | 0.15 | 5.1 | 1.96 |
| 11 | 40 | 20.3 | 39.1 | 110.4 | 0.21 | 3.4 | 2.08 |
| 12 | 40 | 33.1 | 41.4 | 122.8 | 0.19 | 5.3 | 1.52 |
| Mean | NA | 19.1 | 37.4 | 142.5 | 0.24 | 6.7 | ND |
| SD | NA | 7.3 | 14.1 | 66.7 | 0.09 | 3.1 | ND |
NA indicates not applicable; ND, not done.
Observed median clofarabine concentrations versus time overlaid with model predicted concentrations for each dose group. Predicted concentrations were based on the average of the pharmacokinetic parameters pooled across all patients. One outlier in the 30 mg/m2 dose group at the end-of-infusion after the dose on day 4 (1134 ng/mL) is not shown. Clofarabine concentrations, (•); model predicted concentrations, solid line.
Observed median clofarabine concentrations versus time overlaid with model predicted concentrations for each dose group. Predicted concentrations were based on the average of the pharmacokinetic parameters pooled across all patients. One outlier in the 30 mg/m2 dose group at the end-of-infusion after the dose on day 4 (1134 ng/mL) is not shown. Clofarabine concentrations, (•); model predicted concentrations, solid line.
To determine if clofarabine plasma pharmacology was affected by ara-C infusion, clofarabine levels on day 2 were compared with those on day 6. On day 1 ara-C was infused alone; on day 2 ara-C was infused 4 hours after clofarabine administration. On day 6, clofarabine was infused alone (Table 7). The levels of clofarabine at the end of a 1-hour infusion were similar on days 2 and 6 (P = .694). Also, the plasma concentration of clofarabine at 6 hours after start of infusion was similar in patients on day 2 and day 6 (P = .12), suggesting that ara-C infusion did not significantly affect the clofarabine plasma levels.
Effect of ara-C infusion on clofarabine plasma pharmacokinetics
. | . | Clofarabine, μM; end of infusion . | . | Clofarabine, μM; 6-h sample . | . | ||
|---|---|---|---|---|---|---|---|
| Patient no. . | Clofarabine, mg/m2 per d . | Day 2 . | Day 6 . | Day 2 . | Day 6 . | ||
| 1 | 15 | 0.61 | 0.58 | 0.06 | ND | ||
| 5 | 22.5 | 0.89 | 0.86 | 0.14 | 0.21 | ||
| 6 | 22.5 | 0.90 | 1.28 | 0.10 | 0.21 | ||
| 7 | 30 | 1.34 | 2.01 | 0.31 | 0.31 | ||
| 8 | 30 | ND | 1.39 | 0.13† | 0.17* | ||
| 9 | 30 | 1.33 | 1.35 | 0.14 | 0.13 | ||
| 10 | 40 | 2.03 | 1.32 | 0.08 | 0.18 | ||
| 11 | 40 | 1.28 | 2.55 | 0.23 | 0.20 | ||
| 12 | 40 | 2.12 | 1.34 | 0.11 | 0.10 | ||
. | . | Clofarabine, μM; end of infusion . | . | Clofarabine, μM; 6-h sample . | . | ||
|---|---|---|---|---|---|---|---|
| Patient no. . | Clofarabine, mg/m2 per d . | Day 2 . | Day 6 . | Day 2 . | Day 6 . | ||
| 1 | 15 | 0.61 | 0.58 | 0.06 | ND | ||
| 5 | 22.5 | 0.89 | 0.86 | 0.14 | 0.21 | ||
| 6 | 22.5 | 0.90 | 1.28 | 0.10 | 0.21 | ||
| 7 | 30 | 1.34 | 2.01 | 0.31 | 0.31 | ||
| 8 | 30 | ND | 1.39 | 0.13† | 0.17* | ||
| 9 | 30 | 1.33 | 1.35 | 0.14 | 0.13 | ||
| 10 | 40 | 2.03 | 1.32 | 0.08 | 0.18 | ||
| 11 | 40 | 1.28 | 2.55 | 0.23 | 0.20 | ||
| 12 | 40 | 2.12 | 1.34 | 0.11 | 0.10 | ||
ND indicates not determined.
A 5-hour sample was used instead of a 6-hour sample.
Cellular pharmacokinetics
The objective of the cellular pharmacokinetics investigations was to determine if clofarabine triphosphate accumulation increases ara-CTP accumulation in circulating leukemia cells. Limited pharmacokinetics investigations were done in 8 patients due to relatively low number of circulating leukemia cells in this patient population (Table 8). Our previous studies suggested that a 20% variation in ara-CTP concentration is expected.18,19 Hence, values below 0.8 or above 1.2 were considered significant for a decline or increase in ara-CTP value when intracellular concentrations of ara-CTP were compared on day 1 versus day 2. With this cutoff point, 5 of 8 patients had an increase in ara-CTP accumulation after clofarabine infusion.
Cellular pharmacokinetics of ara-CTP and clofarabine triphosphate
. | . | . | Ara-CTP, μM (h) . | . | . | . | . | |
|---|---|---|---|---|---|---|---|---|
| Patient no. . | Diagnosis . | Clofarabine, mg/m2 per day . | Day 1 . | Day 2 . | Ara-CTP, ratio of day 2 to day 1 . | CTP, μM . | Response . | |
| 1 | AML | 15 | 374 (3) | 315 (3) | 0.8 | BLQ | Fail | |
| 6 | ALL | 22.5 | 337 (2) | 434 (2) | 1.3 | 13 | Fail | |
| 7 | AML | 30 | 159 (2) | 328 (2) | 2.1 | 19 | CRp | |
| 8 | ALL | 30 | 199 (2) | 191 (2) | 1.0 | 19 | Fail | |
| 9 | AML | 30 | 118 (2) | 101 (2) | 0.9 | BLQ | Fail | |
| 16 | CML MBP | 40 | 108 (1) | 142 (1) | 1.3 | 9 | Fail | |
| 17 | AML | 40 | 105 (1) | 152 (1) | 1.5 | 4 | CR | |
| 26 | RAEB-T | 40 | 168 (1) | 240 (1) | 1.4 | 10 | Fail | |
. | . | . | Ara-CTP, μM (h) . | . | . | . | . | |
|---|---|---|---|---|---|---|---|---|
| Patient no. . | Diagnosis . | Clofarabine, mg/m2 per day . | Day 1 . | Day 2 . | Ara-CTP, ratio of day 2 to day 1 . | CTP, μM . | Response . | |
| 1 | AML | 15 | 374 (3) | 315 (3) | 0.8 | BLQ | Fail | |
| 6 | ALL | 22.5 | 337 (2) | 434 (2) | 1.3 | 13 | Fail | |
| 7 | AML | 30 | 159 (2) | 328 (2) | 2.1 | 19 | CRp | |
| 8 | ALL | 30 | 199 (2) | 191 (2) | 1.0 | 19 | Fail | |
| 9 | AML | 30 | 118 (2) | 101 (2) | 0.9 | BLQ | Fail | |
| 16 | CML MBP | 40 | 108 (1) | 142 (1) | 1.3 | 9 | Fail | |
| 17 | AML | 40 | 105 (1) | 152 (1) | 1.5 | 4 | CR | |
| 26 | RAEB-T | 40 | 168 (1) | 240 (1) | 1.4 | 10 | Fail | |
BLQ indicates below limit of quantitation; CTP, clofarabine triphosphate; CML MBP, CML in myeloid blast phase.
Discussion
In this phase 1-2 study, we combined clofarabine, a novel nucleoside analog with antileukemic activity, with ara-C for patients with relapsed and refractory acute leukemias, high-risk MDS, and blast-phase CML. This study represents an important extension of the previous single-agent clofarabine trials based on clinical experience and laboratory results. The postulate that the sequential combination of clofarabine with ara-C may improve clinical response rates in patients with acute leukemias is supported for the following reasons. First, ara-C has been established as the single most active agent in this disease.5 Second, clofarabine by itself has produced encouraging responses in patients with acute leukemias.12,13 Third, studies in myeloid cell lines have demonstrated that clofarabine pretreatment increases ara-CTP accumulation.26 Fourth, on the daily-for-5-days schedule, both clofarabine and ara-C have nonoverlapping toxicities, making it feasible to combine these agents at the MTD of the single agents. Finally, previous studies have established the feasibility of biochemical modulation of ara-CTP accumulation in AML blasts by combination with nucleoside analogues.18,19,26-28 Such regimens are now generally used for therapy of relapsed AML, particularly for the elderly population.29-34
Single-agent clofarabine is active in acute leukemias. The phase 1 adult study included 32 patients with acute leukemias, 5 of whom (16%) responded (2 CR and 3 CRp).12,35 Higher responses were demonstrated in a phase 1 pediatric study. Of 22 evaluable children, 8 (36%) had an objective response including 5 children (23%) who achieved CR, according to the investigator.36 The adult phase 1 study defined a clofarabine dose of 40 mg/m2 daily for 5 days per course as the dose for phase 2 studies, the DLT being hepatotoxicity. A phase 2 study of single-agent clofarabine in patients with refractory and relapsed acute leukemias has recently been completed.13 Significant antileukemic activity was observed in AML, MDS, and blast-phase CML. Of 62 patients, 30 (48%) achieved a response (CR, CRp, or PR). In AML and high-risk MDS, the response rates were 42% and 25%, respectively. Response rates in AML were as high as 87% in patients with long first remission durations (> 12 months) and 67% for those treated with clofarabine as second or subsequent salvage therapy.
In consideration of mechanistic rationales for use of clofarabine with other agents, it is clear that clofarabine exhibits many of the characteristics of fludarabine that would contribute to increasing the metabolism of ara-C in leukemia blasts. The rate-limiting step in the synthesis of ara-CTP is catalyzed by dCyd kinase. The activity of dCyd kinase in turn is regulated by the intracellular level of dNTPs. Clofarabine, like fludarabine, is a potent inhibitor of RnR and is therefore able to decrease levels of dNTP.10,11 This leads to a decrease in the feedback inhibition of dCyd kinase, thus allowing increased production and accumulation of ara-CTP. That there can, indeed, be biochemical synergy between ara-CTP and clofarabine has been demonstrated in K562 cells in vitro.26 In this model system, a 3-hour pretreatment with clofarabine, followed by incubation with ara-C, led to a 2-fold increase in the concentration of ara-CTP relative to cells incubated with ara-C alone. Such biochemical modulation strategies for increasing intracellular nucleotide analog concentrations has been demonstrated clinically in combination strategies of ara-C with either fludarabine or cladribine in adult acute leukemias18,19,29-34,37,38 and in pediatric acute leukemias.39,40
In the present study of patients with relapsed and refractory AML and MDS, we report a CR rate of 24% and an overall response rate (including patients with CRp) of 41%. Only one patient died during induction (within 4 weeks of therapy start), constituting an induction mortality of 3%. The CR rate of 24% in AML and high-risk MDS compares favorably with that of standard therapy in this poor-prognosis group, but is lower than the 42% CR rate in relapsed AML reported in the single-agent clofarabine phase 2 study.13 This may be due to 2 factors. First, the combination study included a dose-finding phase 1 portion in which 9 patients received doses of clofarabine below the single-agent MTD dose of 40 mg/m2 per day for 5 days. Second, patient selection may have played a role. Whereas in the combination study 28% patients with AML had primary refractory disease to initial induction, and an additional 44% had a first remission duration of less than 12 months, only 35% of the AML population in the single-agent phase 2 study had a first remission duration of less than 12 months or had primary refractory disease.
The combination regimen was well tolerated, and both agents could be given at full doses. With single-agent intermittent infusion of ara-C, the DLTs were neurotoxicity and pulmonary toxicity.41 Neurotoxicity has been the major problem with high-dose ara-C.42 In contrast, the single-agent clofarabine DLT was hepatic in nature.12,13 The nonoverlapping toxicity profiles of these 2 agents allowed us to administer the full dose of each in this combination regimen, without untoward effects. The major toxicities of the combination regimen were gastrointestinal including transient liver test abnormalities, nausea and vomiting, diarrhea, and mucositis. Although skin rashes were frequently observed, they rarely exceeded grade 2 by NCI toxicity criteria. Central nervous system toxicity was not observed. This was in contrast to the fludarabine and ara-C combination treatment, where 8 (3.7%) of 219 patients suffered from neurotoxicity.43 This positive attribute of the clofarabine and ara-C regimen indicates that this combination may be suitable for elderly patients with AML.
Because of the sampling design it was not possible to statistically determine whether coadministration with ara-C resulted in a pharmacokinetic drug interaction. In a previous study in 13 adults with refractory or relapsed AML dosed once daily for 5 days with 40 mg/m2 clofarabine by 1-hour intravenous infusion, the observed clearance, volume of distribution at steady-state, and β half-life was estimated at 30.6 L/hour, 294 L, and 6.2 hours, respectively (V.G., unpublished data, April 2004). The plasma pharmacokinetic parameters observed in this study were consistent with those in patients with refractory or relapsed AML dosed with single-agent clofarabine, suggesting that no gross pharmacokinetic interaction was occurring between ara-C and clofarabine.
Cellular pharmacology of ara-CTP and clofarabine triphosphate suggested that clofarabine triphosphate accumulation resulted in an increase in ara-CTP accumulation in the leukemia blasts, validating the biochemical modulation strategy. However, the increase in ara-CTP accumulation was observed in only 5 of 8 patients, suggesting that there is heterogeneity among patients regarding augmentation of ara-CTP. This could be due to low intracellular levels of clofarabine triphosphate; indeed circulating leukemia blasts of patients 1 and 9, whose blasts had an undetectable level of clofarabine triphosphate, did not increase ara-CTP accumulation on day 2. On the other hand, blasts from patient 17 had a 1.5-fold increase in ara-CTP even with 4 μM clofarabine triphosphate. Because the sampling time is only once daily and the data are limited to 8 patients, the biochemical modulation effect of clofarabine needs to be fully investigated and then compared with that previously reported for fludarabine or cladribine. With the limited experience of clofarabine and ara-C to date, it appears that clofarabine does not result in higher levels of intracellular ara-CTP accumulation than has previously been reported with fludarabine.
In summary, clofarabine is one of the few nucleoside analogues (besides ara-C) that has shown activity in adult AML as a single agent and at well-tolerated doses. Clofarabine triphosphate is accumulated and retained in leukemia blasts, resulting in a decline of leukemia cells, and making it an ideal candidate for biochemical modulation strategies. We have shown that clofarabine can be safely combined with ara-C, and that the combination has clinical activity. Evaluation of this combination strategy in older patients with newly diagnosed AML and high-risk MDS is currently ongoing. Further mechanism-based combinations of clofarabine with other active agents (anthracyclines, alkylators) are planned.
Prepublished online as Blood First Edition Paper, October 14, 2004; DOI 10.1182/blood-2004-05-1933.
Supported in part by grants CA57629, CA55164, and CA101354 from the National Cancer Institute, Department of Health and Human Services, Bethesda, MD.
P.B. is employed by ILEX Oncology and has declared a financial interest in ILEX Oncology. ILEX Oncology produces clofarabine, which was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal