Abstract
Hematopoietic stem cell transplantation (HSCT) is effective therapy for patients with chronic myelogenous leukemia (CML) but is now mostly indicated for patients who develop resistance to tyrosine kinase inhibitors (TKIs), which can be associated with point mutations in BCR-ABL1. We reviewed the outcomes of imatinib-resistant CML patients (chronic phase, n = 34; accelerated phase [AP], n = 9; and blast phase [BP], n = 4) who underwent HSCT and had BCR-ABL1 sequencing. Mutations were found in 19 patients (40%); 15 of 19 had advanced CML (AP + BP + second chronic phase). Patients with mutations were more likely to transform to AP/BP at time of imatinib failure (69% vs 35%, P = .03). Forty-two patients (89%) responded to HSCT: 32 (68%) had at least a major molecular response. The 2-year event-free survival was 36% and 58% (P = .05) for the mutant and nonmutant groups, respectively; and the 2-year overall survival was 44% and 76% (P = .02), respectively. HSCT is an important salvage option for TKI-resistant patients with or without BCR-ABL1 mutations. Patients with mutations were more likely to develop advanced disease and had worse outcomes after HSCT. HSCT should be considered early for patients deemed to have a low probability of responding to second-generation TKI.
Introduction
Tyrosine kinase inhibitors (TKIs) are now considered standard therapy for patients with chronic myelogenous leukemia (CML).1 The use of imatinib in frontline therapy for chronic phase (CP) CML leads to a major cytogenetic response (MCyR) rate of 89%, and an overall survival of 86% at 7 years.2 Despite these results, patients may develop resistance to imatinib. Data from the randomized International Randomized Study of Interferon Versus STI571 trial in CP patients receiving imatinib reveal that secondary resistance develops at a rate of 4% per year.3 Patients with advanced phase disease (accelerated phase [AP] and blastic phase [BP]) have an even worse outcome, with resistance developing in 70% of AP patients and 90% of BP at 4 years.4-6 The second-generation TKIs (dasatinib and nilotinib) have been developed as a treatment option for patients with imatinib-resistant CML.7 Their efficacy has been proven in all phases of CML, but development of secondary resistance also continues to be a problem, especially in more advanced phases.8-10
Mutations of the BCR-ABL1 kinase domain (KD) are virtually the only known molecular events associated with resistance to TKI. They are described in 42% to 90% of the patients with TKI resistance.11 Although most mutations that appear with imatinib are susceptible to therapy with the second-generation TKI, some mutations are resistant to all known TKIs (eg, T315I mutation).12 For patients who develop highly resistant mutations, there are few therapeutic strategies at the moment.
Hematopoietic stem cell transplantation (HSCT) is an effective and potentially curative therapy for patients with CML.13 The number of HSCTs for CML has declined over the past 10 years with the development of imatinib.14 Most patients who are transplanted for CML now are in advanced phases and have received previous therapy with imatinib and other TKIs.14 We previously reported on 10 patients with CML with BCR-ABL1 KD mutations that underwent HSCT, and suggested that allogeneic HSCT was associated with long-term disease control in a significant fraction of patients.15 Less is known, however, about TKI-resistant patients who had no evidence of mutations by BCR-ABL1 sequencing. Therefore, we sought to compare outcomes of resistant patients with and without a BCR-ABL1 KD mutation.
Methods
Patients and conditioning regimens
We investigated adult patients with a diagnosis of CML (all phases) or Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia who received a related or unrelated HSCT after developing resistance to TKI (imatinib, dasatinib, nilotinib, and/or bosutinib) and had BCR-ABL1 KD sequencing. Studies were approved by the M. D. Anderson Cancer Center Institutional Review Board and conducted in accordance with the Declaration of Helsinki. Informed consent was obtained in all patients.
Patients were classified into BP/Ph+ acute lymphoblastic leukemia (presence of ≥ 30% blasts in the peripheral blood or bone marrow or extramedullary disease), AP (peripheral or marrow blasts ≥ 15%; peripheral or marrow basophils ≥ 20%; thrombocytopenia < 100 × 109/L unrelated to therapy) and CP (not meeting criteria for BP or AP).1 Patients with CP could be in first CP (no prior progression to AP/BP) or second CP (prior progression to AP/BP). Advanced CML was defined as patients classified as second CP, AP, or BP.
Resistance to imatinib and other TKI was defined as (1) loss of cytogenetic response or complete hematologic response (CHR) while on therapy; (2) failure to achieve a CHR after 3 months of therapy (for CP patients) or any hematologic response (for AP and BP patients); (3) no cytogenetic response after 6 months of therapy or no MCyR after 12 months of therapy; and (4) transformation into AP (for CP patients) or BP (for CP and AP patients) while on therapy.1,16,17
Conditioning regimens were classified as myeloablative or reduced intensity conditioning (RIC). Briefly, myeloablative regimens included: (1) BuCy, intravenous busulfan 0.8 mg/kg every 6 hours for 16 doses and cyclophosphamide 60 mg/kg for 2 days; (2) BuFluClo, intravenous busulfan 130 mg/m2 for 4 days, fludarabine 10 to 30 mg/m2 for 4 days, and clofarabine 10 to 40 mg/m2 for 4 days; (3) CyTBIRituxan, cyclophosphamide 60 mg/kg for 2 days, total body irradiation 300 cGy for 4 days (total 1200 cGy), and rituximab 375 mg/m2 for 3 doses. RIC regimens included: (1) FluMel, fludarabine 30 mg/m2 for 4 days and melphalan 140 mg/m2; (2) FluBu, intravenous busulfan 130 mg/m2 for 2 days and fludarabine 40 mg/m2 for 4 days; (3) FluBuGleevec, intravenous busulfan 130 mg/m2 for 2 days, fludarabine 40 mg/m2 for 4 days, and imatinib 400 mg orally twice daily for 9 days; (4) gemtuzumab ozogamycin 2 mg/m2, fludarabine 30 mg/m2 for 4 days and melphalan 140 mg/m2; (5) FluMelRituxan, fludarabine 25 mg/m2 for 5 days and melphalan 70 mg/m2 for 2 days and rituximab 375 mg/m2 for 3 doses; and (6) FluMelThiotepa, fludarabine 40 mg/m2 for 4 days, thiotepa 10 mg/kg and melphalan 140 mg/m2. Graft-versus-host disease (GVHD) prophylaxis regimens consisted of (1) tacrolimus and methotrexate 5 mg/m2 on days 1, 3, 6, and 11 after HSCT, (2) tacrolimus, methotrexate (same dose), and pentostatin; (3) tacrolimus and mycophenolate mofetil 15 mg/kg twice daily (maximum 1 g per dose). Antithymocyte globulin (2.5 mg/kg per day for 3 days) was given to recipients of unrelated donor transplants. All patients received granulocyte colony-stimulating factor 5 μg/kg daily from day 7 until neutrophil engraftment. No treatment with TKI after HSCT was given unless patients presented with clear signs of disease relapse.
Response criteria and mutation analysis
Standard criteria were used to score CML response.1 Briefly, a CHR required normalization, for at least 4 weeks, of the bone marrow (< 5% blasts) and peripheral blood with a white blood cell count less than 10 × 109/L, without blasts, promyelocytes, or myelocytes, a platelet count less than 450 × 109/L, in addition to the disappearance of all signs and symptoms of CML. Patients who achieved a CHR were categorized by cytogenetic responses, defined by the percentage of Ph+ metaphases in an analysis of at least 20 metaphases from a bone marrow aspirate sample (fluorescence in situ hybridization if cytogenetic analysis not informative; eg, insufficient metaphases): complete cytogenetic response (CCyR), 0% Ph+ metaphases; partial cytogenetic response, 1% to 35% Ph+ metaphases; minor cytogenetic response, 36% to 65% Ph+ metaphases; minimal cytogenetic response, 66% to 95% Ph+ metaphases; and no response, 96% to 100% Ph+ metaphases. MCyR was defined as CCyR plus partial cytogenetic response (< 36% Ph+ metaphases).
Molecular responses were defined by the ratio of BCR-ABL1 gene transcripts to ABL gene transcripts on a real-time quantitative polymerase chain reaction (PCR) analysis of a peripheral blood sample, and negative results were confirmed by nested PCR as previously described.18 A complete molecular response was defined as absence of BCR-ABL1 transcripts. A major molecular response was defined as BCR-ABL1/ABL1 ratio less than .05%. For mutational analysis, the entire KD of the BCR-ABL1 fusion transcript was sequenced using a nested PCR method, as previously described.15,19
Hematopoietic chimerism was evaluated on peripheral blood or bone marrow by multiplex PCR-based DNA microsatellite polymorphism analysis using PCR D6S264, D3S1282, D18S62, and D3S1300 fluorescence-labeled primer sets, followed by separation by capillary electrophoresis and analysis using GeneScan software (Applied Biosystems).15 Mixed chimerism was defined as the presence of any detectable (1%) recipient DNA.
Toxicity was graded according to National Cancer Institute criteria, Version 2.0. GVHD was graded according to standard criteria.
Definition of EFS and OS
Event-free survival (EFS) was measured as the time from HSCT to the occurrence of an event (death in complete remission or disease relapse). Patients alive and who did not have an event were censored at last follow-up. Overall survival (OS) was measured from the time of HSCT until death from any cause. Patients alive at last follow-up were censored.
Statistical analysis
Categorical and continuous variables were compared using the χ2/Fisher exact test and Mann-Whitney U test, respectively. Survival curves and probabilities were estimated by the Kaplan-Meier method. The log-rank test was used to compare time to event outcomes. Calculations were done in Statistica Version 6.1 (StatSoft).
Results
Patient's clinical features and transplantation characteristics
From March 2004 until November 2007, there were 47 patients with CML resistant to TKI who received an HSCT. Patients and transplant characteristics stratified by mutant or no-mutant BCR-ABL1 are summarized in Table 1. The median age was 44 years (range, 19-64 years). All patients received imatinib, and the median time on imatinib was 15 months (range, 1-57 months). The best response obtained on imatinib was a CHR only in 20 patients (43%), MCyR in 17 patients (36%), CCyR in 15 (32%), and major molecular response in one patient (2%). The most common reason for imatinib failure was transformation to AP/BP, which occurred in 23 patients (49%). Patients with a BCR-ABL1 mutation were more likely to have disease transformation (AP/BP) at the time of TKI failure (69% vs 35%, P = .03). Twenty-nine patients (62%) received a second TKI (nilotinib, n = 13; dasatinib, n = 13; bosutinib, n = 3). A greater proportion of patients with BCR-ABL1 mutation received a second TKI (84% vs 46%, P = .01). Of those 29 patients, 25 (86%) developed resistance to the second TKI, the reason being no response in 8 patients (28%), loss of CHR in 7 (24%), loss of cytogenetic response in 3 (10%), and progression to BP in 7 (24%) patients. Five patients (11%) received a third TKI (dasatinib, n = 3; nilotinib, n = 1; bafetinib [INNO406], n = 1). Of those, 2 were refractory, one achieved a partial hematologic response (after bafetinib) one achieved a CHR and the other achieved a CCyR (both after dasatinib). All patients had KD sequencing, and 19 patients (40%) developed 21 different mutations (2 patient developed 2 mutations). Mutations are detailed in Table 2. The most common mutations were T315I (N = 4, 19%) and E255K/V (N = 4, 19%). P-loop mutations were detected in 9 cases (45%). Mutations were detected after imatinib in 15 cases (75%), after nilotinib in 4 cases (F359C, F359C, E255V/K, E255K), and after dasatinib in 2 cases (F317L, E255K).
Patients and transplantation characteristics
| Characteristic . | All (n = 47) . | Mutated BCR-ABL1 (n = 19) . | Nonmutated BCR-ABL1 (n = 28) . | P . |
|---|---|---|---|---|
| Median age, y (range) | 44 (19-64) | 46 (19-63) | 43 (22-64) | .87 |
| Male sex, % | 27 (57) | 12 (63) | 15 (54) | .56 |
| Previous IFN-α, % | 14 (30) | 7 (37) | 7 (25) | .51 |
| Median time on imatinib, mo (range) | 15 (1-57) | 12 (1-49) | 18 (3-57) | .16 |
| Best response to imatinib | .53 | |||
| CHR, % | 20 (43) | 10 (53) | 10 (36) | |
| MCyR, % | 17 (36) | 6 (32) | 11 (39) | |
| CCyR, % | 15 (32) | 5 (26) | 10 (36) | |
| Reason for TKI failure, % | .07 | |||
| Intolerance | 1 (2) | 1 (5) | 0 (0) | |
| Hematologic resistance | 2 (4) | 1 (5) | 1 (4) | |
| Loss of CHR | 7 (15) | 3 (16) | 4 (14) | |
| Cytogenetic resistance | 6 (13) | 0 (0) | 6 (21) | |
| Loss of cytogenetic response | 8 (17) | 1 (5) | 7 (26) | |
| Transformation to AP | 7 (15) | 3 (16) | 4 (14) | |
| Transformation to BP | 16 (34) | 10 (53) | 6 (21) | |
| Received second TKI, % | 29 (62) | 16 (84) | 13 (46) | .01 |
| Nilotinib, % | 13 (28) | 8 (42) | 5 (18) | |
| Dasatinib, % | 13 (28) | 7 (37) | 6 (21) | |
| Bosutinib, % | 3 (6) | 1 (5) | 2 (7) | |
| Best response to second TKI | .91 | |||
| CHR, % | 4 (14) | 3 (18) | 1 (8) | |
| MCyR, % | 8 (28) | 5 (31) | 3 (23) | |
| CCyR, % | 6 (21) | 4 (25) | 2 (15) | |
| Received third TKI, % | 4 (8.5) | 2 (11) | 2 (7) | > .999 |
| Median time from diagnosis to HSCT, mo (range) | 25 (6-168) | 24 (6-114) | 28 (6-168) | .39 |
| Stage at HSCT | .18 | |||
| First CP, % | 16 (34) | 4 (21) | 12 (43) | |
| AP, % | 12 (26) | 4 (21) | 8 (28) | |
| BP, % | 9 (19) | 6 (32) | 3 (11) | |
| Second CP, % | 10 (21) | 5 (26) | 5 (18) | |
| Matched-related HSCT, % | 23 (49) | 11 (58) | 12 (43) | .67 |
| RIC regimen, % | 32 (68) | 10 (53) | 22 (78) | .10 |
| Characteristic . | All (n = 47) . | Mutated BCR-ABL1 (n = 19) . | Nonmutated BCR-ABL1 (n = 28) . | P . |
|---|---|---|---|---|
| Median age, y (range) | 44 (19-64) | 46 (19-63) | 43 (22-64) | .87 |
| Male sex, % | 27 (57) | 12 (63) | 15 (54) | .56 |
| Previous IFN-α, % | 14 (30) | 7 (37) | 7 (25) | .51 |
| Median time on imatinib, mo (range) | 15 (1-57) | 12 (1-49) | 18 (3-57) | .16 |
| Best response to imatinib | .53 | |||
| CHR, % | 20 (43) | 10 (53) | 10 (36) | |
| MCyR, % | 17 (36) | 6 (32) | 11 (39) | |
| CCyR, % | 15 (32) | 5 (26) | 10 (36) | |
| Reason for TKI failure, % | .07 | |||
| Intolerance | 1 (2) | 1 (5) | 0 (0) | |
| Hematologic resistance | 2 (4) | 1 (5) | 1 (4) | |
| Loss of CHR | 7 (15) | 3 (16) | 4 (14) | |
| Cytogenetic resistance | 6 (13) | 0 (0) | 6 (21) | |
| Loss of cytogenetic response | 8 (17) | 1 (5) | 7 (26) | |
| Transformation to AP | 7 (15) | 3 (16) | 4 (14) | |
| Transformation to BP | 16 (34) | 10 (53) | 6 (21) | |
| Received second TKI, % | 29 (62) | 16 (84) | 13 (46) | .01 |
| Nilotinib, % | 13 (28) | 8 (42) | 5 (18) | |
| Dasatinib, % | 13 (28) | 7 (37) | 6 (21) | |
| Bosutinib, % | 3 (6) | 1 (5) | 2 (7) | |
| Best response to second TKI | .91 | |||
| CHR, % | 4 (14) | 3 (18) | 1 (8) | |
| MCyR, % | 8 (28) | 5 (31) | 3 (23) | |
| CCyR, % | 6 (21) | 4 (25) | 2 (15) | |
| Received third TKI, % | 4 (8.5) | 2 (11) | 2 (7) | > .999 |
| Median time from diagnosis to HSCT, mo (range) | 25 (6-168) | 24 (6-114) | 28 (6-168) | .39 |
| Stage at HSCT | .18 | |||
| First CP, % | 16 (34) | 4 (21) | 12 (43) | |
| AP, % | 12 (26) | 4 (21) | 8 (28) | |
| BP, % | 9 (19) | 6 (32) | 3 (11) | |
| Second CP, % | 10 (21) | 5 (26) | 5 (18) | |
| Matched-related HSCT, % | 23 (49) | 11 (58) | 12 (43) | .67 |
| RIC regimen, % | 32 (68) | 10 (53) | 22 (78) | .10 |
IFN indicates interferon-α.
Patients with BCR-ABL1 mutations
| Patient no. . | Mutation . | Disease stage . | TKI in use at time of mutation detection . |
|---|---|---|---|
| 1 | T315I | AP | Imatinib |
| 2 | M244V | AP | Imatinib |
| 3 | E459K | AP | Imatinib |
| 4 | A433T | AP | Imatinib |
| 5 | T315I | CP | Imatinib |
| 6 | F317L | AP | Imatinib |
| 7 | Y253H | BP | Imatinib |
| 8 | Q252H | BP | Imatinib |
| 9 | T315I | BP | Imatinib |
| 10 | E255V/K | BP | Nilotinib |
| 11 | Q252K | AP | Imatinib |
| 12 | E255K | BP | Nilotinib |
| 13 | F359C | BP | Nilotinib |
| 14 | F317L | BP | Dasatinib |
| 15 | Y253H | CP | Imatinib |
| 16 | E459K | BP | Imatinib |
| 17 | G250E | BP | Imatinib |
| 17 | F359C | BP | Nilotinib |
| 18 | E255K | BP | Imatinib |
| 19 | T315I | BP | Imatinib |
| 19 | E255K | BP | Dasatinib |
| Patient no. . | Mutation . | Disease stage . | TKI in use at time of mutation detection . |
|---|---|---|---|
| 1 | T315I | AP | Imatinib |
| 2 | M244V | AP | Imatinib |
| 3 | E459K | AP | Imatinib |
| 4 | A433T | AP | Imatinib |
| 5 | T315I | CP | Imatinib |
| 6 | F317L | AP | Imatinib |
| 7 | Y253H | BP | Imatinib |
| 8 | Q252H | BP | Imatinib |
| 9 | T315I | BP | Imatinib |
| 10 | E255V/K | BP | Nilotinib |
| 11 | Q252K | AP | Imatinib |
| 12 | E255K | BP | Nilotinib |
| 13 | F359C | BP | Nilotinib |
| 14 | F317L | BP | Dasatinib |
| 15 | Y253H | CP | Imatinib |
| 16 | E459K | BP | Imatinib |
| 17 | G250E | BP | Imatinib |
| 17 | F359C | BP | Nilotinib |
| 18 | E255K | BP | Imatinib |
| 19 | T315I | BP | Imatinib |
| 19 | E255K | BP | Dasatinib |
The median time to transplantation was 25 months (range, 6-168 months). At the time of transplantation, 16 patients (34%) were in first CP, 12 (26%) were in AP, 9 (19%) were in BP, and 10 were in second CP (21%). Thirty-one patients (66%) had advanced-phase CML at the time of transplantation. Among patients with BCR-ABL1 KD mutation, 79% were in advanced-phase CML at the time of HSCT, compared with 57% of patients without BCR-ABL1 KD mutation (P = .20). A total of 6 patients (32%) with BCR-ABL1 KD mutations were in BP at time of HSCT, versus 3 patients (11%) without BCR-ABL1 KD mutations (P = .12). The donor was a matched related sibling in 23 patients (49%), a matched unrelated donor in 21 patients (45%), an unrelated cord blood unit in 2 patients (4%), and a haploidentical parent in one case (2%). The conditioning regimen was myeloablative in 15 patients (32%) and RIC in 32 patients (68%). GVHD prophylaxis administered was tacrolimus + methotrexate in 38 patients (81%), tacrolimus + methotrexate + pentostatin in 4 (8.5%), tacrolimus + mycophenolate mofetil in 4 (8.5%), and one patient received T cell-depleted peripheral blood stem cells from a haploidentical donor, and no pharmacologic GVHD prophylaxis was used.
Engraftment, GVHD, and outcomes
Transplant characteristics are summarized in Table 3. Forty-five patients (96%) engrafted. The patients who did not engraft were the ones that received a cord blood unit. Median time to neutrophil engraftment was 12 days (range, 5-20 days) and median time to platelet engraftment was 15 days (range, 10-87 days). Chimerism studies done at day 30 after HSCT were 100% of donor type in 27 patients (60%) and mixed in 18 patients (40%). Twenty-eight patients (62%) developed acute GVHD: it was grade 2-IV in 15 (32%) and grade 3-IV in 8 (17%). Twenty-one patients (45%) developed chronic GVHD; it was extensive in 9 patients (24%). Patients with BCR-ABL1 mutations were more likely to develop chronic GVHD after HSCT (67% vs 33%, P = .03).
Engraftment and GVHD
| Characteristic . | All (n = 47) . | Mutated BCR-ABL1 (n = 19) . | Nonmutated BCR-ABL1 (n = 28) . | P . |
|---|---|---|---|---|
| Engraftment, no. (%) | 45 (96) | 19 (100) | 26 (93) | > .999 |
| Time to ANC > 0.5 × 109/L, d (range) | 12 (5-20) | 12 (5-20) | 12 (6-18) | .64 |
| Time to platelets > 20 × 109/L, d (range) | 15 (10-87) | 16 (11-87) | 14 (10-69) | .58 |
| Acute GVHD, no. (%) | 28 (60) | 14 (74) | 14 (50) | .13 |
| I-II | 20 (42) | 10 (53) | 10 (36) | |
| III-IV | 8 (17) | 4 (21) | 4 (14) | |
| Chronic GVHD, no. (%)* | 21 (46) | 12 (67) | 9 (33) | .03 |
| Limited | 12 (30) | 5 (28) | 7 (26) | |
| Extensive | 9 (24) | 7 (39) | 2 (7) |
| Characteristic . | All (n = 47) . | Mutated BCR-ABL1 (n = 19) . | Nonmutated BCR-ABL1 (n = 28) . | P . |
|---|---|---|---|---|
| Engraftment, no. (%) | 45 (96) | 19 (100) | 26 (93) | > .999 |
| Time to ANC > 0.5 × 109/L, d (range) | 12 (5-20) | 12 (5-20) | 12 (6-18) | .64 |
| Time to platelets > 20 × 109/L, d (range) | 15 (10-87) | 16 (11-87) | 14 (10-69) | .58 |
| Acute GVHD, no. (%) | 28 (60) | 14 (74) | 14 (50) | .13 |
| I-II | 20 (42) | 10 (53) | 10 (36) | |
| III-IV | 8 (17) | 4 (21) | 4 (14) | |
| Chronic GVHD, no. (%)* | 21 (46) | 12 (67) | 9 (33) | .03 |
| Limited | 12 (30) | 5 (28) | 7 (26) | |
| Extensive | 9 (24) | 7 (39) | 2 (7) |
ANC indicates absolute neutrophil count.
Available data on 18 patients with mutated BCR-ABL1 and 27 patients with unmutated BCR-ABL1.
Disease response is summarized in Table 4. Forty-three patients (91%) achieved a response after HSCT. Eleven patients (23%) achieved only a CCyR. Thirty-two (68%) patients achieved major molecular response, which was complete in 31 patients (66%). Four patients (6%) did not respond, one of them because of engraftment failure. The other 3 patients had E255K mutations. Two of these patients were in BP at the time of HSCT, and the other had progressed to BP but was in second CP at the time of HSCT after receiving dasatinib. After a median follow-up of 22 months (range, 5-53 months), 18 patients (38%) have relapsed, at a median of 6 months after HSCT (range, 0-44 months after HSCT). Among the 19 patients with a history of KD mutation, 8 (42%) relapsed compared with 10 (35%) of the 28 patients without a KD mutation (P = .65). Mutations previously detected in relapsing patients were either the gatekeeper T315I mutation (n = 2) or P-loop mutations (G250E, n = 1; Q252K, n = 1; Y253H, n = 1; E255K, n = 3). Two patients had a molecular relapse, 4 patients had a cytogenetic relapse, 3 patients relapsed as AP, 6 patients relapsed as BP, and 2 patients relapsed as BP with extramedullary disease (one with leptomeningeal disease and one with skin involvement).
Transplantation outcome
| . | All (n = 47) . | Mutated BCR-ABL1 (n = 19) . | Nonmutated BCR-ABL1 (n = 28) . | ||
|---|---|---|---|---|---|
| Stage at time of HSCT, no. (%) | — | CP, 4 (21) | Advanced phase,* 15 (79) | CP, 12 (43) | Advanced phase,* 16 (57) |
| Best response, no. (%) | |||||
| CMR | 31 (66) | 4 (100) | 7 (47) | 10 (83) | 10 (62) |
| MMR | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| CCyR | 11 (23) | 0 (0) | 5 (33) | 1 (8) | 5 (31) |
| Relapse, no. (%) | 18 (38) | 1 (25) | 7 (47) | 3 (25) | 7 (44) |
| Total deaths, no. (%) | 16 (34) | 2 (50) | 8 (53) | 2 (17) | 4 (25) |
| Deaths in CR, no. (%) | 7 (15) | 2 (50) | 3 (20) | 1 (8) | 1 (6) |
| Alive in CR, no. (%) | 22 (47) | 1 (25) | 5 (33) | 8 (67) | 8 (50) |
| . | All (n = 47) . | Mutated BCR-ABL1 (n = 19) . | Nonmutated BCR-ABL1 (n = 28) . | ||
|---|---|---|---|---|---|
| Stage at time of HSCT, no. (%) | — | CP, 4 (21) | Advanced phase,* 15 (79) | CP, 12 (43) | Advanced phase,* 16 (57) |
| Best response, no. (%) | |||||
| CMR | 31 (66) | 4 (100) | 7 (47) | 10 (83) | 10 (62) |
| MMR | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 1 (6) |
| CCyR | 11 (23) | 0 (0) | 5 (33) | 1 (8) | 5 (31) |
| Relapse, no. (%) | 18 (38) | 1 (25) | 7 (47) | 3 (25) | 7 (44) |
| Total deaths, no. (%) | 16 (34) | 2 (50) | 8 (53) | 2 (17) | 4 (25) |
| Deaths in CR, no. (%) | 7 (15) | 2 (50) | 3 (20) | 1 (8) | 1 (6) |
| Alive in CR, no. (%) | 22 (47) | 1 (25) | 5 (33) | 8 (67) | 8 (50) |
— indicates not applicable; CMR, complete molecular response; MMR, major molecular response; and CR, complete remission.
Advanced phase: AP, BP, and second CP.
Treatment for disease relapse after HSCT was dasatinib in 8 patients, imatinib alone in 3 patients, imatinib and donor lymphocyte infusion, and imatinib and homoharringtonine and KW-249 in 1 patient each. Two patients in BP did not receive any treatment and were transferred to palliative care, and one patient remained in observation with low levels of molecular disease.
Mutational analysis was done after relapse in 7 patients (4 who had a prior mutation and 3 with no history of BCR-ABL1 KD mutation). The 3 patients who did not have previous mutation did not acquire one; one patient who had both a G250E and F359C mutation relapsed without mutations; 2 patients relapsed with the same mutations as before HSCT (T315I in one patient and E255K in the other), and one patient who had a E255K mutation relapsed with a new clone with the Q250H mutation only.
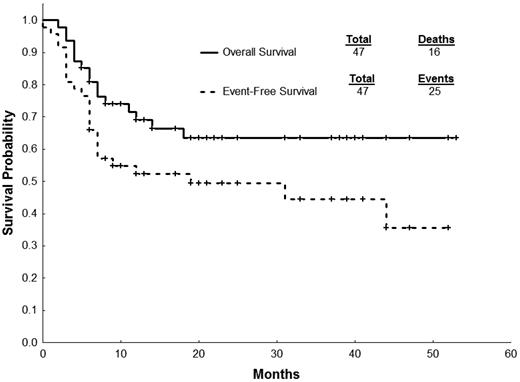
Currently, 31 patients (66%) are alive; 16 patients have died, and the causes of death were disease progression (N = 10), GVHD (N = 3), infections (N = 2), and unknown (N = 1). Treatment-related mortality was 13% at 2 years. Survival outcomes are summarized in Table 5. The estimated 2-year EFS and OS after HSCT for all patients was 49% and 63%, respectively (Figure 1). Patients who harbored BCR-ABL1 KD mutations had significantly inferior EFS and OS compared with patients without BCR-ABL1 mutation (Figure 2; Table 5). The same trend was not observed when patients where stratified by disease stage (CP vs advanced phase CML) at the time of HSCT (Figure 3; Table 5). Intensity of conditioning regimen did not impact 2-year EFS (67% [myeloablative] vs 35% [RIC]; P = .20) or 2-year OS (73% [myeloablative] vs 59% [RIC]; P = .38). Development of chronic GVHD also had no impact on outcomes (2-year EFS: 52% [yes] vs 47% [no], P = .47; 2-year OS: 61% [yes] vs 69% [no], P = .86)
Survival outcomes after HSCT
| Group . | n . | 2-y EFS, % (95% CI) . | 2-y OS, % (95% CI) . |
|---|---|---|---|
| All patients | 47 | 49 (35-64) | 63 (49-78) |
| BCR-ABL1 mutation | |||
| Yes | 19 | 36 (14-58) | 44 (20-67) |
| No | 28 | 58 (39-77) | 76 (59-93) |
| P | .05 | .02 | |
| Stage | |||
| CP | 16 | 62 (39-86) | 72 (49-96) |
| AP | 31 | 44 (25-61) | 59 (41-77) |
| P | .27 | .30 | |
| CP only | |||
| BCR-ABL1 mutation | 4 | 25 (0-67) | 33 (0-87) |
| No BCR-ABL1 mutation | 12 | 75 (50-99) | 81 (58-100) |
| P | .20 | .13 | |
| Advanced phase only | |||
| BCR-ABL1 mutation | 15 | 40 (15-65) | 46 (20-71) |
| No BCR-ABL1 mutation | 16 | 45 (18-72) | 72 (48-96) |
| P | .20 | .12 |
| Group . | n . | 2-y EFS, % (95% CI) . | 2-y OS, % (95% CI) . |
|---|---|---|---|
| All patients | 47 | 49 (35-64) | 63 (49-78) |
| BCR-ABL1 mutation | |||
| Yes | 19 | 36 (14-58) | 44 (20-67) |
| No | 28 | 58 (39-77) | 76 (59-93) |
| P | .05 | .02 | |
| Stage | |||
| CP | 16 | 62 (39-86) | 72 (49-96) |
| AP | 31 | 44 (25-61) | 59 (41-77) |
| P | .27 | .30 | |
| CP only | |||
| BCR-ABL1 mutation | 4 | 25 (0-67) | 33 (0-87) |
| No BCR-ABL1 mutation | 12 | 75 (50-99) | 81 (58-100) |
| P | .20 | .13 | |
| Advanced phase only | |||
| BCR-ABL1 mutation | 15 | 40 (15-65) | 46 (20-71) |
| No BCR-ABL1 mutation | 16 | 45 (18-72) | 72 (48-96) |
| P | .20 | .12 |
CI indicates confidence interval.
Patients with BCR-ABL1 KD mutations more commonly had presented with features of advanced disease (AP or BP) at some point during disease course, which might potentially confound the results obtained in survival analysis. We analyzed survival outcomes among patients with and without BCR-ABL1 mutations stratified by CML stage at time of HSCT (CP vs advanced phase; Table 5). There was no statistically significant difference in survival in all the comparisons made; however, patients with mutations always had a tendency to an inferior outcome, and the lack of statistical significance might be the result of the small number of patients. We also compared outcomes between patients with and without BCR-ABL1 mutations after excluding patients in BP, who have an intrinsic worse outcome after HSCT compared with other phases. We could no longer detect a statistically significant difference in EFS between patients with and without mutations (2-year: 45% [95% confidence interval, 17%-73%] vs 54% [95% confidence interval, 31%-76%]; P = .28), but patients with mutations still had a significantly worse OS (2-year: 49% [95% confidence interval, 20%-78%] vs 82% [95% confidence interval, 65%-98%]; P = .02).
Discussion
We analyzed a cohort of patients with CML who received a HSCT and had BCR-ABL1 KD sequencing. Even though many patients were in advanced stage (66%) at the time of HSCT, a high rate (66%) of complete molecular response was obtained. Treatment-related mortality was acceptable (13% at 2 years), especially considering that 51% of patients received stem cells from a source other than a matched related sibling. In our cohort, there was no statistical difference in OS and EFS between patients transplanted in CP and those transplanted with more advanced disease, but that could be a reflection of the small number of patients. However, we did find that patients who developed a mutation at the time of TKI failure and underwent HSCT had a worse outcome (EFS and OS) at 2 years compared with patients who did not develop mutations.
Despite the excellent results obtained with imatinib, it is clear that it does not eradicate the leukemic stem cell,20 as the majority of patients who stop imatinib will eventually relapse, and development of secondary resistance is an increasing problem.11 Mechanisms of resistance to imatinib and other TKI in CML are varied and include overexpression of the BCR-ABL1 gene, acquisition of secondary genetic abnormalities, activation of alternative signaling pathways for proliferation (such as Src kinases), and development of point mutations in the BCR-ABL1 gene.11 TKI resistance in CML is related to the genetic instability caused by the BCR-ABL oncoprotein, acquisition of mutations and chromosomal aberrations, and selection of resistant clones by the continuous use of TKI.21-25 Acquisition of BCR-ABL1 mutations seems to be an important phenomenon for progressing into advanced phases, particularly in lymphoid BP.26 This may suggest that BCR-ABL1 KD mutations are a marker for genetic instability and indicate the presence of clones that have a higher propensity for disease progression.27 In our cohort, patients with and without BCR-ABL1 KD mutations had similar baseline clinical features. However, patients who had BCR-ABL1 KD mutations at the time of resistance to imatinib were more likely to have transformed at some point during disease course to more advanced stages (AP, BP), as previously reported.28,29 In another report, patients who developed BCR-ABL1 KD mutations during CP without signs of disease progression had a worse outcome and a high probability of losing CCyR.30 Soverini et al reported that, among patients with primary cytogenetic resistance to imatinib, the finding of BCR-ABL1 mutations is associated with a higher chance of progressing to AP/BP and worse survival.29 However, in another study, the impact of BCR-ABL1 mutations on prognosis of patients with imatinib-resistant CML was limited, with stage at time of imatinib resistance being the most relevant factor.28 The prognosis of CML patients with resistance to imatinib is multifactorial and depends, among other factors, on disease stage, level of response to imatinib, and presence of BCR-ABL1 mutations.28,29,31 In our study, we could not assess whether the prognostic impact of BCR-ABL1 mutations was independent of disease stage, and the relatively small number of patients precluded us from doing a multivariate analysis. It is possible that the development of BCR-ABL1 KD mutations per se is not an independent prognostic factor for survival after HSCT in CML, but for that to be answered a study with a larger number of patients is needed.
Although it is clear that HSCT is an effective therapy for salvage of CML resistant to imatinib, it is still a matter of debate whether patients who develop resistance to imatinib should be sent to allograft or should receive a second-generation TKI. In CP patients, dasatinib and nilotinib are capable of inducing high rates of CCyR and responses are durable.32,33 In patients with AP/BP, outcomes with second-generation TKI are usually worse and responses are of short duration, especially in BP.10 HSCT is effective in all stages of CML, but its efficacy is also diminished in patients with advanced disease.34 Currently, the European LeukemiaNet consensus recommends allogeneic HSCT for all patients who progress to AP/BP (pretreatment with a TKI is recommended), for patients who develop the T315I mutation, and for patients who fail more than or equal to 2 TKIs.35 Early identification of patients with a low probability of response to second-generation TKI, such as patients with no cytogenetic response to imatinib therapy that harbor KD mutations with low sensitivity to second-generation TKIs, is useful, as such patients could be considered for early HSCT.
One study analyzed predictive factors for outcome with second-generation TKI after imatinib failure in CP CML.31 Two variables were associated with inferior EFS: (1) lack of prior cytogenetic response to imatinib and (2) performance status more than or equal to 1. Patients with both characteristics had 2-year EFS of only 20%. Importantly, failure to achieve MCyR at 12 months is also associated with inferior outcome in therapy with second-generation TKI, similar to what has been observed with imatinib.31,36 In the same study, lack of any cytogenetic response to imatinib was the only factor associated with failure to achieve MCyR by 12 months of therapy with second-generation TKI.31 Recently, the Hammersmith's group identified 4 factors as independently associated with the achievement of CCyR on second-generation TKI therapy: low Sokal risk score at diagnosis, best cytogenetic response obtained on imatinib, occurrence of neutropenia at any time during imatinib therapy that required imatinib dose reduction less than 400 mg/day despite growth factor support, and time from detection of imatinib failure to start of second TKI.37 Patients could be divided into 3 risk groups (low, intermediate, and high) with cumulative incidence of CCyR at 2.5 years of 100%, 52.5%, and 13.8%, respectively.37 Another report evaluated patients with CP CML treated solely with nilotinib, and the presence of basophilia, anemia, low-sensitive mutations, and lack of MCyR at 12 months were associated with inferior survival.38 Molecular responses, measured by quantitative reverse-transcriptase PCR, can also predict survival after second-generation TKI as early as 3 months after start of therapy.39 For those patients who present with mutations, the presence of intermediate-sensitivity BCR-ABL1 mutations (half-maximal inhibitory concentration ≥ 3nM for dasatinib and ≥ 150nM for nilotinib) is associated with inferior response rates.40,41 However, some authors have cast doubt on the utility of guiding therapy by the half-maximal inhibitory concentration value, as issues such as drug plasma concentration are not taken into consideration when analyzing solely the half-maximal inhibitory concentration value42 ; therefore, other factors should be considered, among them previous cytogenetic response to imatinib therapy. If a patient develops the T315I mutation, HSCT is the best therapeutic option, and experimental agents (eg, AP24534, omacetaxine) could be tried while a donor is procured. Patients who have failed 2 TKIs also have a suboptimal outcome with a third TKI (nilotinib or dasatinib; median failure-free survival, 20 months in CP).43 Thus, patients who present with features predictive of poor response and survival to second-generation TKI, such as high Sokal risk score, frequent neutropenia during therapy with imatinib, lack of cytogenetic response to imatinib, lack of MCyR after 12 months of therapy with second-generation TKI, and prior use of more than or equal to 2 TKIs, should be considered for alternative therapies, such as HSCT, especially if a matched donor can be rapidly secured and expected treatment-related mortality (ie, European Group for Blood and Marrow Transplantation score) is low. In addition, all patients should be screened for BCR-ABL1 mutations at the time of TKI failure to detect the presence of the T315I mutations and/or other mutations with intermediate sensitivity to specific second-generation TKI.44
In conclusion, HSCT remains an important salvage option for patients with or without BCR-ABL1 KD mutations who develop resistance to TKI therapy.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.J. and M.D.L. designed the study, analyzed data, treated patients, and wrote and approved the manuscript; J.C. treated patients and wrote and approved the manuscript; F.P.S.S. analyzed data and wrote and approved the manuscript; D.J. performed BCR-ABL sequencing and approved the manuscript; and S.O., G.R., U.P., S.G., H.K., P.K., and R.C. treated patients and approved the manuscript.
Conflict-of-interest disclosure: E.J. received honoraria from BMS and Novartis. H.K. and J.C. have received research grants from Novartis and BMS. The remaining authors declare no competing financial interests.
Correspondence: Marcos de Lima, Department of Stem Cell Transplantation and Cell Therapy, The University of Texas M. D. Anderson Cancer Center, 1515 Holcombe Blvd, Unit 0428, Houston, TX 77030; e-mail: mdelima@mdanderson.org.