Abstract
Thpo/Mpl signaling plays an important role in the maintenance of hematopoietic stem cells (HSCs) in addition to its role in megakaryopoiesis. Patients with inactivating mutations in Mpl develop thrombocytopenia and aplastic anemia because of progressive loss of HSCs. Yet, it is unknown whether this loss of HSCs is an irreversible process. In this study, we used the Mpl knockout (Mpl−/−) mouse model and expressed Mpl from newly developed lentiviral vectors specifically in the physiologic Mpl target populations, namely, HSCs and megakaryocytes. After validating lineage-specific expression in vivo using lentiviral eGFP reporter vectors, we performed bone marrow transplantation of transduced Mpl−/− bone marrow cells into Mpl−/− mice. We show that restoration of Mpl expression from transcriptionally targeted vectors prevents lethal adverse reactions of ectopic Mpl expression, replenishes the HSC pool, restores stem cell properties, and corrects platelet production. In some mice, megakaryocyte counts were atypically high, accompanied by bone neo-formation and marrow fibrosis. Gene-corrected Mpl−/− cells had increased long-term repopulating potential, with a marked increase in lineage−Sca1+cKit+ cells and early progenitor populations in reconstituted mice. Transcriptome analysis of lineage−Sca1+cKit+ cells in Mpl-corrected mice showed functional adjustment of genes involved in HSC self-renewal.
Introduction
The cellular homolog of the myeloproliferative leukemia virus oncogene (Mpl) encodes a hematopoietic cytokine receptor. Mpl activation by its ligand thrombopoietin (Thpo) mediates context-dependent signals, mainly via the RAS/MAPK, JAK/STAT, or PI3K/AKT pathways. Thpo/Mpl signaling is not only essential for megakaryopoiesis and platelet activation1 but also for the maintenance and posttransplantation expansion of hematopoietic stem cells (HSCs).2-4
Loss of MPL function results in aplastic anemia and thrombocytopenia, a disease termed congenital amegakaryocytic thrombocytopenia (CAMT).5 Untreated CAMT patients die early in childhood, and the only curative therapy available today is bone marrow transplantation (BMT). However, suitable donors will not be available for every patient, and allogeneic BMT still involves a significant morbidity and mortality. Gene therapy may correct the patients' own HSCs by the addition of a functional MPL gene copy. Such gene addition approaches have been shown to be successful in patients with severe combined immunodeficiency syndromes (adenosine deaminase-severe combined immunodeficiency syndrome, X-linked severe combined immunodeficiency syndrome) or Wiskott-Aldrich syndrome.6-8
Similar to defects in DNA repair pathways (eg, Fanconi anemia), MPL deficiency is of special interest for HSC biology because it causes a defect in HSCs themselves. An Mpl-deficient (Mpl−/−) mouse model partially reproduces the phenotype of CAMT patients. Mpl−/− mice display thrombocytopenia, approximately 7-fold reduced numbers of HSCs, and an overall 50% reduction of multilineage progenitors.2,3 HSC defects become more obvious after BMT, indicating a reduced competitive fitness of Mpl−/− bone marrow (BM) cells.3 Recent approaches to decipher the underlying mechanism have shown maintenance of HSC quiescence by Thpo/Mpl signaling and have identified long-term repopulating stem cells as Mpl positive, regardless of their CD34 expression.9,10 Inhibition of Mpl signaling by blocking antibodies in vivo or lack of Thpo in Thpo−/− mice not only resulted in the down-regulation of HSC regulators HoxB4, HoxA9, and HoxA1011-13 but also triggered down-regulation of cyclin-dependent kinase inhibitors and up-regulation of c-Myc, with the consequence of cell cycle progression and HSC exhaustion.9,10,14,15
Thpo−/− recipients poorly support engraftment of wild-type (wt) BM cells,9 and administration of Mpl blocking antibodies in wt mice allows HSC engraftment without further conditioning.10 Experiments by Abkowitz and Chen,16 however, report equal engraftment potential for Mpl−/− and wt BM cells in Thpo−/− recipients, indicating a regulatory function of Thpo on posttransplantation expansion rather than an intrinsic defect of Mpl−/− cells. A correction of Mpl-deficient HSCs by reestablishment of Thpo responsiveness may therefore be possible.
Ectopic expression of Mpl by retroviral vectors in C57Bl/6 wt mice caused severe adverse reactions.17-19 Increased Mpl signaling induced a chronic myeloproliferative disorder (MPD), similar to MPD induced by constitutive active Mpl (MPL W515L/K) in patients.20 The chronic MPD was not lethal but progressed to pancytopenia with loss of HSCs. The delicate regulation of Thpo levels by the amount of its receptor Mpl was probably disturbed.21 We hypothesized that ectopic Mpl expression depleted Thpo, thereby abrogating Mpl signaling in target cells.17 Mpl gene therapy thus requires the restriction of Mpl expression to the correct target cells with physiologic expression levels. Accordingly, lineage-restricted Mpl expression from a 2-kb murine Mpl promoter fragment22 did not produce MPD or any other severe adverse reactions in C57Bl/6 wt mice.17
The same 2-kb promoter fragment22 was recently used in transgenic approaches to express Mpl in Mpl−/− mice.23,24 Partial correction of competitive repopulation abilities of HSCs, but thombocytosis resulting from low levels of expression during late megakaryopoiesis, was observed.24 However, these models reflect the expression from a single genomic locus that may be subject to epigenetic regulation. The same promoter fragments used in retroviral vectors may provide different results because of polyclonal repopulation with semirandom distribution of insertion sites. Furthermore, in contrast to the transgenic approaches, gene therapy requires the correction of an already depleted population of definitive HSCs.
In the present study, we overcame transplantation complications in Mpl−/− mice by optimizing transduction and transplantation conditions. We established a murine model for CAMT gene therapy based on the transplantation of in vitro corrected Mpl−/− hematopoietic cells into Mpl−/− recipient mice. To achieve physiologic expression of Mpl, tissue specific promoters of the murine Mpl or the human GPIba gene were used. GPIba encodes the platelet glycoprotein Ibα and expression from the GPIba promoter was expected to be high during megakaryopoiesis.25,26 We also developed novel lentiviral vectors equipped with a fragment of the human MPL promoter, which was only characterized in vitro so far.27 We demonstrate the correction of the stem cell defect, as well as partial correction of the defective megakaryopoiesis of Mpl−/− mice after Mpl gene transfer, and show the readjusted expression of genes involved in HSC regeneration.
Methods
Animals
C57BL/6 (B6.Ly5.2) and C57BL/6 PeP3b (B6 SJL/Ly5.1) mice were obtained from The Jackson Laboratory. Mpl knockout (Mpl−/−) mice were kindly provided by Warren Alexander (Walter and Eliza Hall Institute of Medical Research, Parkville, Australia).2 All mice were bred and kept in the specified pathogen-free animal facilities of the Hannover Medical School, Germany. Animal experiments were approved by the Hannover Medical School ethical committee and performed according to their guidelines.
Lentiviral vectors and vector production
The lentiviral backbone is the RRL self-inactivating lentiviral vector originally described by Dull et al with minor modifications.28,29 A fragment of the murine Mpl promoter was kindly provided by Radek Skoda.22 The 795-bp human Mpl promoter and 595-bp human GPIba promoter have been described.25,27 For details please refer to supplemental data (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
BM cell purification and transduction
Briefly, lineage-marker depleted (lin−) cells were isolated from complete BM by magnetic sorting using lineage-specific antibodies (Gr1, CD11b, CD45R/B220, CD3e, TER-119; Miltenyi Biotec). Before viral transduction, lin− BM cells were prestimulated for 18 hours in StemSpan SFEM medium (CellSystems), containing 10 ng/mL murine stem cell factor, 20 ng/mL murine Thpo, 10 ng/mL recombinant human fibroblast growth factor-1, 20 ng/mL murine insulin-like growth factor-2, 1% penicillin/streptomycin, and 2mM glutamine,30 plated in wells precoated with 10 μg/cm2 Retronectin (TaKaRa). For transduction, concentrated viral supernatant was added.
Mouse analysis and flow cytometry
The online data supplement contains details. Cell-surface staining for long-term (LT) lineage−Sca1+cKit+ (LSK), short-term (ST)–LSK, multipotent progenitor cells (MPPs), common myeloid progenitors (CMPs), granulocytic/monocytic progenitors (GMPs), and megakaryocytic/erythroid progenitors (MEPs) is given in supplemental Figure 1.
Megakaryocyte (MK) differentiation
Lin− cells from Mpl−/− or C57BL/6 mice were transduced with multiplicity of infection 12.5 and cultivated in the same medium for another 48 hours before transfer to Iscove modified Dulbecco medium containing 10% fetal calf serum, 2mM glutamine, 1% penicillin/streptomycin, 10 ng/mL murine stem cell factor, 50 ng/mL mThpo, 20 ng/mL murine interleukin-6. Cells were analyzed by flow cytometry after 72 hours for expression of CD41 and CD42.
Quantitative PCR and vector copy number
LSK cells were sorted by flow cytometry into RLT lysis buffer and stored at −80°C until processing with the RNeasy Micro Kit (QIAGEN), according to the manufacturer's instructions. cDNA synthesis was performed using the QuantiTect reverse transcription kit (QIAGEN). Vector copy number was determined by quantitative polymerase chain reaction (PCR) in genomic DNA from peripheral blood as described.17
Thpo quantification
Plasma was separated by centrifugation of whole blood at 400g for 30 to 45 minutes and stored at −20°C. Quantification of Thpo in mouse plasma was performed using a Thpo ELISA (R&D Systems).
Microarray gene expression measurements
Microarray analysis was performed on LSK cells RNA sorted by flow cytometry. The resulting material was hybridized to Affymetrix Mouse 430, Version 2.0. The data have been deposited into the GEO database with the accession number GSE26403.
For more information, see supplemental data.
Results
Lentiviral vectors with lineage-specific promoters restrict expression to murine HSCs and platelets
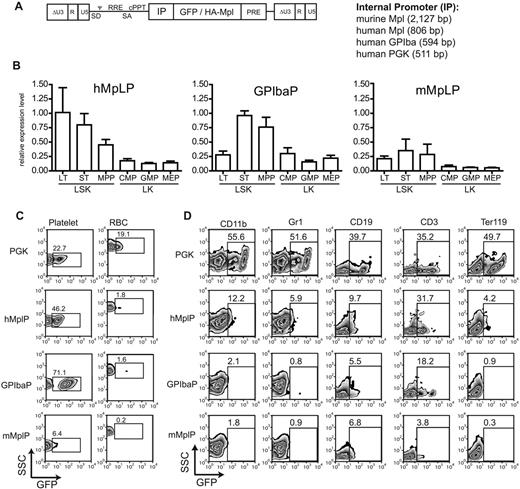
To achieve physiologic Mpl expression, we constructed lentiviral vectors with 3 selected cellular promoters because of their predicted specificity for expression in HSCs and during megakaryopoiesis: a 2127-bp fragment of the murine Mpl promoter,22 a 795-bp fragment of the human MPL promoter,27 and a 595-bp fragment of the human GPIba promoter.25 All 3 promoters were active in the human erythroleukemia cell line HEL, which expresses endogenous MPL (supplemental Figure 2). To some extent, the promoters were also active in the megakaryocytic cell line M07e, there was no expression in Jurkat cells (T-cell line) and U937 cells (macrophage cell line) compared with the expression from the ubiquitously active human phosphoglycerate kinase (PGK) promoter using a transient expression system. These data indicated that the selected promoters may restrict expression to the megakaryocytic lineage (supplemental Figure 2).
To assess putative HSC-restricted expression, we constructed lentiviral reporter vectors expressing eGFP from these promoters (Figure 1A). Transduced Lin− wt BM cells (multiplicity of infection = 12.5) were transplanted into lethally irradiated C57Bl/6 mice. Three mice each were analyzed that had a gene marking of 0.3 to 2 copies per cell (supplemental Figure 3). Expression of eGFP was analyzed in the peripheral blood to test for platelet specificity, and in the BM to detect the expression in HSCs, identifying the different subpopulations by cell-surface marker staining (supplemental Figure 1): mean fluorescence intensity of eGFP expression was analyzed in LT and ST HSCs, MPPs, CMPs, GMPs, and MEPs. All lineage-specific promoters showed higher expression levels in the LSK cells compared with the LK cells (Figure 1B). Transgene expression from the mMplP was lowest, whereas expression from the hMplP was stronger with a gradual decline during HSC differentiation (LT-HSCs > ST-HSCs > MPP). Interestingly, the GPIbaP was also active in ST-HSCs and MPP, with incipient expression in LT-HSCs (Figure 1B).
Lineage-specific promoters express specifically in MK and HSCs in vivo. (A) Self-inactivating (SIN) lentiviral vectors harboring lineage-specific internal promoter fragments (IP) for expression of eGFP or HA-Mpl. SD indicates splice donor; SA, splice acceptor; ψ, packaging signal; RRE, rev responsive element; and PPT, polypurine tract. (B) Relative expression of lineage-specific promoters in different immature and committed BM progenitor populations compared with the ubiquitously expressing PGK promoter. Mean fluorescence intensity of eGFP-positive cells was divided by the mean fluorescence intensity of the PGK promoter. (C) Representative eGFP expression from lineage-specific promoters in platelets and red blood cells (RBC). Percentage of eGFP-positive cells indicated. (D) Expression of lineage-specific promoters in other mature lineages of the BM. CD11b/Gr1 indicates granulocytes and monocytes; CD19, B cells; CD3, T cells; and Ter119, erythroid progenitors. Percentage of positive cells indicated.
Lineage-specific promoters express specifically in MK and HSCs in vivo. (A) Self-inactivating (SIN) lentiviral vectors harboring lineage-specific internal promoter fragments (IP) for expression of eGFP or HA-Mpl. SD indicates splice donor; SA, splice acceptor; ψ, packaging signal; RRE, rev responsive element; and PPT, polypurine tract. (B) Relative expression of lineage-specific promoters in different immature and committed BM progenitor populations compared with the ubiquitously expressing PGK promoter. Mean fluorescence intensity of eGFP-positive cells was divided by the mean fluorescence intensity of the PGK promoter. (C) Representative eGFP expression from lineage-specific promoters in platelets and red blood cells (RBC). Percentage of eGFP-positive cells indicated. (D) Expression of lineage-specific promoters in other mature lineages of the BM. CD11b/Gr1 indicates granulocytes and monocytes; CD19, B cells; CD3, T cells; and Ter119, erythroid progenitors. Percentage of positive cells indicated.
All 3 promoters mediated eGFP expression in platelets, with GPIbaP being the strongest (Figure 1C). Background activity in other lineages was low, with some residual activity in the lymphoid lineage, which contrasted with our in vitro experiments (Figure 1D). Lentiviral vectors equipped with the murine and human Mpl, or the human GPIba promoter thus showed largely specific expression in HSCs and platelets with low background activity in other lineages (Figure 1D).
Lineage specific lentiviral vectors correct defective megakaryopoiesis in vitro
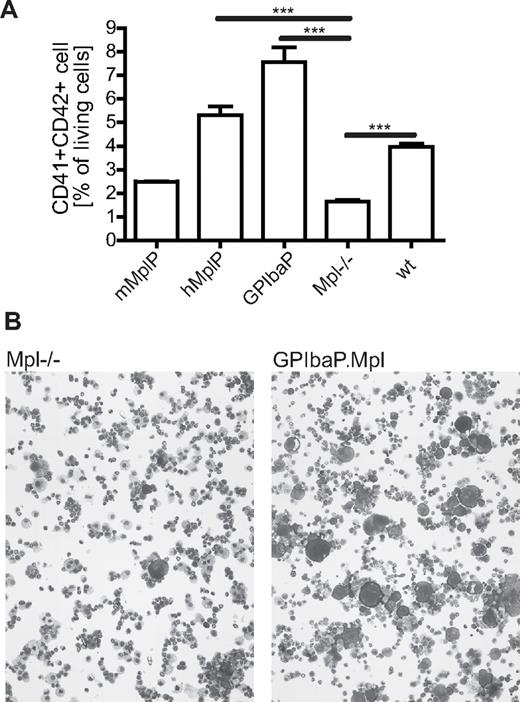
Next, we investigated the potential of the lineage-specific lentiviral vectors to correct the defective megakaryopoiesis of Mpl−/− cells in vitro by expressing Mpl by any of the 3 lineage-specific lentiviral vectors (Figure 1A). Lin− BM cells from Mpl−/− mice were transduced with multiplicity of infection 12.5 and transferred into megakaryocytic differentiation medium. MK differentiation was determined after 3 days by CD41/CD42 flow cytometry and cell morphology on cytospins. wt BM cells developed approximately 4% MK; the same levels were reached in Mpl−/− BM cells transduced with RRL.PPT.hMplP.Mpl.pre or RRL.PPT.PGK.Mpl.pre (Figure 2). Even higher levels (> 7% MK) were found in Mpl−/− cultures that expressed Mpl from the GPIba promoter. The rescue of megakaryopoiesis by Mpl expression from the mMplP was not as efficient, but numbers still increased (∼ 2.5% MK) compared with untransduced Mpl−/− BM (∼ 1.5% MK).
Mpl expression from lineage-specific promoters in lentiviral vectors corrects defective megakaryopoiesis of Mpl−/− cells in vitro. (A) Lin−Mpl−/− BM cells were transduced with SIN lentiviral vectors expressing Mpl from indicated promoters. After differentiation in medium containing Thpo, stem cell factor, and interleukin-6, MK numbers were determined by flow cytometry as CD41+CD42+ cells. C57Bl/6 (wt) cells were used as positive control (n = 3, mean ± SEM). ***P < .001 (Student t test, 2-tailed, unpaired). (B) Cytospins of in vitro cultured Mpl−/− cells (left) and Mpl corrected cells (GPIba.Mpl, right). May-Grün-Wald/Giemsa staining; original magnification, ×100.
Mpl expression from lineage-specific promoters in lentiviral vectors corrects defective megakaryopoiesis of Mpl−/− cells in vitro. (A) Lin−Mpl−/− BM cells were transduced with SIN lentiviral vectors expressing Mpl from indicated promoters. After differentiation in medium containing Thpo, stem cell factor, and interleukin-6, MK numbers were determined by flow cytometry as CD41+CD42+ cells. C57Bl/6 (wt) cells were used as positive control (n = 3, mean ± SEM). ***P < .001 (Student t test, 2-tailed, unpaired). (B) Cytospins of in vitro cultured Mpl−/− cells (left) and Mpl corrected cells (GPIba.Mpl, right). May-Grün-Wald/Giemsa staining; original magnification, ×100.
Lineage-restricted Mpl expression in Mpl−/− mice confers long-term survival and prevents lethal adverse reactions
To test the potential of our vectors to correct Mpl deficiency in vivo, we next transduced lin− BM cells from Mpl−/− mice with the lentiviral vectors RRL.PPT.mMplP.Mpl.pre, RRL.PPT.hMplP.Mpl.pre, and RRL.PPT.GPIbaP.Mpl.pre and transplanted the cells into Mpl−/− recipient mice. In previous experiments, we and others had failed to successfully transplant Mpl−/− mice because of their high sensitivity to irradiation conditioning. Because of their HSC defect, one would assume that engraftment of wt BM in unconditioned Mpl−/− mice may be possible. In our experiments, unconditioned Mpl−/− mice transplanted with 3 × 107 wt BM cells showed short-term engraftment of up to 15% but very low levels of long-term engraftment (∼ 1% at 4 months after BMT; supplemental Figure 4A-B), consistent with previous reports.16,31 We therefore examined irradiation conditions and identified 8 Gy as the lethal dose for Mpl−/− mice. Transplantation of 1 × 106 CD45.1+ wt BM cells into 8 Gy irradiated Mpl−/− mice gave a long-term chimerism of 85.5% plus or minus 1.2% (supplemental Figure 4A).
As negative control, Mpl−/− cells were transduced with a vector expressing eGFP from the PGK promoter (RRL.PPT.PGK.eGFP.pre) and transplanted into Mpl−/− mice. A positive control group of Mpl−/− mice was transplanted with syngeneic wt BM cells resembling fully matched BMT.
To test the effect of constitutive ectopic expression of Mpl in the Mpl−/− model, one group of mice was transplanted with Mpl−/− cells transduced with a vector expressing Mpl from the ubiquitously expressing PGK promoter (RRL.PPT.PGK.Mpl.pre). In C57Bl/6 mice, Mpl expression from the PGK promoter induced only mild adverse reaction compared with expression from a strong viral promoter.17 We transplanted 7 to 13 mice per vector in 3 independent experiments (Table 1).
Summary of the experimental groups
| Experiment no. . | Vector . | No. of mice (n = 52) . | Average copy no. 6 wks after treatment . | Average copy no. at final analysis . | No. of cells transduced per mouse, × 105, day 0 cells . | Secondary transplantation (n = 54) . |
|---|---|---|---|---|---|---|
| 1 | PGK.Mpl | 3 | NA | NA | 6.3 | 0 |
| 1 | mMplP.Mpl | 3 | 0.6 ± 0.2 | 0.6 ± 0.1 | 6.3 | 4 |
| 1 | hMplP.Mpl | 3 | 3.9 ± 4.1 | 3.6 ± 0.1 | 6.3 | 4 |
| 1 | GPIbaP.Mpl | 5 | 1 ± 0.8 | 1.9 ± 1.1 | 6.3 | 10 |
| 1 | PGK.GFP | 3 | 0.4 ± 0.2 | 0.9 ± n.a | 6.3 | 2 |
| 1 | WT cells | 3 | NA | NA | 5.3 | 6 |
| 2 | PGK.Mpl | 4 | NA | NA | 2.1 | 0 |
| 2 | mMplP.Mpl | 4 | 1.1 ± 0.5 | 2.3 ± 1.5 | 2.1 | 8 |
| 2 | hMplP.Mpl | 4 | 2.3 ± 2.3 | 3.3 ± 3.6 | 2.1 | 6 |
| 2 | GPIbaP.Mpl | 4 | 5.6 ± 2 | 6.6 ± 4.1 | 2.1 | 6 |
| 2 | PGK.GFP | 4 | 1.9 ± 1.3 | 2.4 ± 1.3 | 2.1 | 4 |
| 2 | WT cells | 2 | NA | NA | 2 | 4 |
| 3 | GPIbaP.Mpl | 4 | 2.97 ± 1.44 | 3.74 ± 2.11 | 4.0 | ND |
| 3 | PGK.GFP | 4 | 0.95 ± 0.22 | 0.72 ± 0.52 | 4.0 | ND |
| 3 | WT cells | 2 | NA | NA | 5.0 | ND |
| Experiment no. . | Vector . | No. of mice (n = 52) . | Average copy no. 6 wks after treatment . | Average copy no. at final analysis . | No. of cells transduced per mouse, × 105, day 0 cells . | Secondary transplantation (n = 54) . |
|---|---|---|---|---|---|---|
| 1 | PGK.Mpl | 3 | NA | NA | 6.3 | 0 |
| 1 | mMplP.Mpl | 3 | 0.6 ± 0.2 | 0.6 ± 0.1 | 6.3 | 4 |
| 1 | hMplP.Mpl | 3 | 3.9 ± 4.1 | 3.6 ± 0.1 | 6.3 | 4 |
| 1 | GPIbaP.Mpl | 5 | 1 ± 0.8 | 1.9 ± 1.1 | 6.3 | 10 |
| 1 | PGK.GFP | 3 | 0.4 ± 0.2 | 0.9 ± n.a | 6.3 | 2 |
| 1 | WT cells | 3 | NA | NA | 5.3 | 6 |
| 2 | PGK.Mpl | 4 | NA | NA | 2.1 | 0 |
| 2 | mMplP.Mpl | 4 | 1.1 ± 0.5 | 2.3 ± 1.5 | 2.1 | 8 |
| 2 | hMplP.Mpl | 4 | 2.3 ± 2.3 | 3.3 ± 3.6 | 2.1 | 6 |
| 2 | GPIbaP.Mpl | 4 | 5.6 ± 2 | 6.6 ± 4.1 | 2.1 | 6 |
| 2 | PGK.GFP | 4 | 1.9 ± 1.3 | 2.4 ± 1.3 | 2.1 | 4 |
| 2 | WT cells | 2 | NA | NA | 2 | 4 |
| 3 | GPIbaP.Mpl | 4 | 2.97 ± 1.44 | 3.74 ± 2.11 | 4.0 | ND |
| 3 | PGK.GFP | 4 | 0.95 ± 0.22 | 0.72 ± 0.52 | 4.0 | ND |
| 3 | WT cells | 2 | NA | NA | 5.0 | ND |
NA indicates not applicable; and ND, not done.
All Mpl−/− mice that received wt BM cells survived long-term, as expected. Mice transplanted with BM cells expressing Mpl from the PGK promoter died within 5 days, and one mouse after 3 weeks (Figure 3A). Although we expected ubiquitous ectopic Mpl expression to cause adverse effects, the severity and rapidity were unexpected. These mice presented with enlarged spleens that consisted of erythroid progenitor cells (Figure 3B-C) and bleedings into the peritoneal cavity. Macroscopic and microscopic analysis revealed splenic ruptures with large necrotic areas beneath the spleen capsule because of the massive proliferation of erythroid blasts as cause for the sudden death (Figure 3D). Early death was not observed in a C57Bl/6 BMT model overexpressing Mpl from the PGK promoter.17 Mpl−/− mice have 5- to 6-fold elevated Thpo levels throughout life compared with wt mice.23 Therefore, Thpo stimulation of Mpl overexpressing cells in Mpl−/− mice may be much more rapid, resulting in the fast hyperproliferation.
Analysis of the erythroblastosis in mice expressing Mpl from the PGK promoter. (A) Kaplan-Meier survival curves of mice transplanted with lin−Mpl−/− BM cells transduced with SIN lentiviral vectors expressing Mpl from indicated promoters, or GFP from the PGK promoter. Untransduced C57Bl/6 lin− BM was transplanted as positive control (wt). Ticks indicate mice removed from the experiment either because of final analysis at 25 or 31 weeks or because of death of unrelated cause (n = 2). (B) Spleen weight of Mpl−/− mice transplanted with lin−Mpl−/− BM expressing Mpl from the PGK promoter (n = 4) when killed or found dead after 5 days. Spleen weight of untransplanted reference C57Bl/6 mice for comparison (n = 7). P < .0001 (Student t test, 2-tailed, unpaired). (C) Flow cytometric analysis of spleen cells from Mpl−/− mice transplanted with lin−Mpl−/− BM expressing Mpl from the PGK promoter. Cells were identified as early or late erythroid progenitors based on expression of CD71 alone or together with Ter119, respectively. Only minor amounts of granulocytic/monocytic cells (CD11b/Gr1) or lymphoid cells (CD3/CD19) were detectable. Spleen cells expressed high levels of Mpl as detected with anti-HA antibody. Percentages for each quadrant or gate are given in the lower right of each flow cytometry plot. (D) Spleen histopathology from Mpl−/− mice transplanted with lin−Mpl−/− BM expressing Mpl from the PGK promoter. (i) Black arrows indicate bleedings resulting from splenic rupture; and white arrow, necrotic area (hematoxylin and eosin staining, original magnification ×200). (ii) Close-up of a necrotic area with erythroid blasts (hematoxylin and eosin staining, original magnification ×400).
Analysis of the erythroblastosis in mice expressing Mpl from the PGK promoter. (A) Kaplan-Meier survival curves of mice transplanted with lin−Mpl−/− BM cells transduced with SIN lentiviral vectors expressing Mpl from indicated promoters, or GFP from the PGK promoter. Untransduced C57Bl/6 lin− BM was transplanted as positive control (wt). Ticks indicate mice removed from the experiment either because of final analysis at 25 or 31 weeks or because of death of unrelated cause (n = 2). (B) Spleen weight of Mpl−/− mice transplanted with lin−Mpl−/− BM expressing Mpl from the PGK promoter (n = 4) when killed or found dead after 5 days. Spleen weight of untransplanted reference C57Bl/6 mice for comparison (n = 7). P < .0001 (Student t test, 2-tailed, unpaired). (C) Flow cytometric analysis of spleen cells from Mpl−/− mice transplanted with lin−Mpl−/− BM expressing Mpl from the PGK promoter. Cells were identified as early or late erythroid progenitors based on expression of CD71 alone or together with Ter119, respectively. Only minor amounts of granulocytic/monocytic cells (CD11b/Gr1) or lymphoid cells (CD3/CD19) were detectable. Spleen cells expressed high levels of Mpl as detected with anti-HA antibody. Percentages for each quadrant or gate are given in the lower right of each flow cytometry plot. (D) Spleen histopathology from Mpl−/− mice transplanted with lin−Mpl−/− BM expressing Mpl from the PGK promoter. (i) Black arrows indicate bleedings resulting from splenic rupture; and white arrow, necrotic area (hematoxylin and eosin staining, original magnification ×200). (ii) Close-up of a necrotic area with erythroid blasts (hematoxylin and eosin staining, original magnification ×400).
Remarkably, in contrast to ubiquitous expression from the PGK promoter, Mpl expression from the 3 lineage-specific lentiviral vectors in Mpl−/− BM cells conferred long-term survival of Mpl−/− recipients after BMT (Figure 3A). With a few exceptions of temporary mildly increased white blood cell or red blood cell counts, no uncontrolled cell expansion was observed (supplemental Figure 5). Careful analysis of peripheral blood cell parameters in mice expressing Mpl from any of the lineage-specific promoters did not show the signs of pancytopenia that were described in our earlier study in C57Bl/6 mice after transplantation of BM cells that expressed Mpl from ubiquitous promoters17 (supplemental Figure 5).
In contrast to the long-term survival of mice treated with lineage-specific vectors, mice that received Mpl−/− BM cells expressing eGFP showed an increased mortality resulting from graft failure beginning at 6 weeks after BMT. These observations indicate that the lineage-restricted expression of Mpl neither caused an imbalance of the Thpo/Mpl system nor induced uncontrolled cell expansion. In addition, the long-term survival of treated mice compared with graft failure in negative controls shows therapeutic efficacy of our vectors. Thus, all immediate and delayed severe adverse reactions observed here and in former studies could be prevented by the use of vectors with lineage-restricted expression.
Mpl gene transfer corrects the thrombocytopenia of Mpl−/− mice
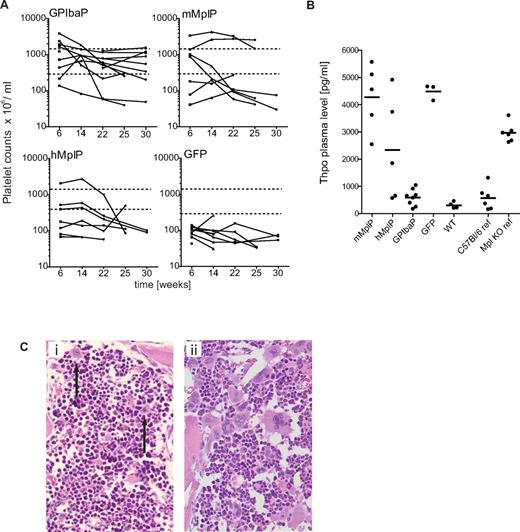
Severe thrombocytopenia is the immediate symptom of Mpl deficiency. Therefore, an increase in platelet counts was a major indicator for therapeutic response in our study. The platelet levels in mice transplanted with wt BM cells were much improved (824 ± 251 × 106/mL; platelet counts in wt C57Bl/6 mice: 960 ± 250 × 106/mL) compared with Mpl−/− mice transplanted with eGFP transduced Mpl−/− BM (94 ± 52 × 106/mL; Figure 4A). Robust increase of platelet counts (> 300 × 106/mL,> 2-fold over eGFP platelet counts) was achieved in 11 of 13 mice expressing Mpl from the GPIbaP. The levels persisted long-term and remained in the normal range in 8 of 13 mice indicating stable therapeutic success (Figure 4A). In contrast, only 3 of 7 mice engrafted with cells corrected with RRL.PPT.hMplP.Mpl.pre and 4 of 7 corrected with RRL.PPT.mMplP.Mpl.pre showed an elevation of platelet counts more than 300 × 106/mL with decline after week 14 (Figure 4A). Nevertheless, vector copy numbers of 0.5 to 7.3 were detected in those mice on final analysis (Table 1), proving long-term engraftment of gene modified donor cells.
Rescue of thrombocytopenia in Mpl−/− mice after Mpl gene therapy. Mpl−/− mice were transplanted with Lin−Mpl−/− BM cells transduced with SIN lentiviral vectors expressing Mpl from lineage-specific promoters as indicated, or eGFP from the PGK promoter (GFP). Lin− BM from C57Bl/6J was transplanted as positive control (wt). (A) Peripheral blood thrombocyte counts of transplanted Mpl−/− mice were monitored every 6 weeks in 3 independent experiments up to 25 or 31 weeks, respectively. Hatched lines represent the window of therapeutic success (> 2-fold more than mean eGFP platelet counts and lower as 1500 × 106/mL). (B) Thrombopoietin plasma levels as determined by ELISA from plasma taken on final analysis (25 or 31 weeks). Untreated Mpl−/− mice (Mpl−/− ref) show 5- to 6-fold increased Thpo levels compared with untreated C57Bl/6 (C57Bl/6 ref) mice (GPIbaP vs GFP, P < .0001; hMplP vs GFP, P = .11; GPIbaP vs WT, P = .11; WT vs GFP, P < .0001; mean ± SEM; Student t test, 2-tailed, unpaired). (C) Representative histologic analysis of the BM from a control Mpl−/− mouse, which expressed eGFP by the PGK promoter (i) and a mouse that expressed Mpl by the GPIba promoter (ii). Advanced megakaryocyte maturation was found in treated mice compared with micro-megakaryocytes (arrows) in GFP control animals. Hematoxylin and eosin staining; original magnification, ×200.
Rescue of thrombocytopenia in Mpl−/− mice after Mpl gene therapy. Mpl−/− mice were transplanted with Lin−Mpl−/− BM cells transduced with SIN lentiviral vectors expressing Mpl from lineage-specific promoters as indicated, or eGFP from the PGK promoter (GFP). Lin− BM from C57Bl/6J was transplanted as positive control (wt). (A) Peripheral blood thrombocyte counts of transplanted Mpl−/− mice were monitored every 6 weeks in 3 independent experiments up to 25 or 31 weeks, respectively. Hatched lines represent the window of therapeutic success (> 2-fold more than mean eGFP platelet counts and lower as 1500 × 106/mL). (B) Thrombopoietin plasma levels as determined by ELISA from plasma taken on final analysis (25 or 31 weeks). Untreated Mpl−/− mice (Mpl−/− ref) show 5- to 6-fold increased Thpo levels compared with untreated C57Bl/6 (C57Bl/6 ref) mice (GPIbaP vs GFP, P < .0001; hMplP vs GFP, P = .11; GPIbaP vs WT, P = .11; WT vs GFP, P < .0001; mean ± SEM; Student t test, 2-tailed, unpaired). (C) Representative histologic analysis of the BM from a control Mpl−/− mouse, which expressed eGFP by the PGK promoter (i) and a mouse that expressed Mpl by the GPIba promoter (ii). Advanced megakaryocyte maturation was found in treated mice compared with micro-megakaryocytes (arrows) in GFP control animals. Hematoxylin and eosin staining; original magnification, ×200.
Atypically high platelet counts were observed early after transplantation in some of the mice but persisted in only 2 mice expressing Mpl from the mMplP, which was also accompanied by increased numbers of megakaryocytes (Figure 4A). This observation was consistent with the earlier described transgenic approach in which insufficient promoter activity of the mMplP in late megakaryopoiesis was demonstrated.23,24 Mice that expressed Mpl from the GPIbaP had the most stable correction of platelet counts, which persisted even in secondary recipients (supplemental Figure 6). Because of sufficiently high expression on platelets, these mice also presented with decreased Thpo plasma levels not significantly differing from wt levels (Figure 4B).
Histopathology revealed reduced numbers of MK, which were of smaller size and lacked polyploidization as seen in eGFP control mice (Figure 4Ci). Polyploid MK was found in the majority of the analyzed mice treated with vectors containing GPIbaP (8 of 9), and also in hMplP (5 of 7) and mMplP (3 of 7) (mice of experiments 1 and 2; Figure 4Cii). However, 5 of the mice that expressed Mpl from the GPIbaP and hMplP showed atypical giant-sized MK. In these mice, megakaryopoiesis was abnormal both in size and number of MK and accompanied by incipient marrow fibrosis in 2 (supplemental Figure 7) and abnormal neo-formation of bone (osteosclerosis) in 5 mice. Because of the close topographic relationship between abnormal giant MK and fibrotic or osteosclerotic BM regions, fibrosis and osteosclerosis appeared to be induced by the abnormal megakaryopoiesis as shown by others (reviewed by Kacena et al32 ).
HSCs numbers in Mpl−/− increase after Mpl gene therapy
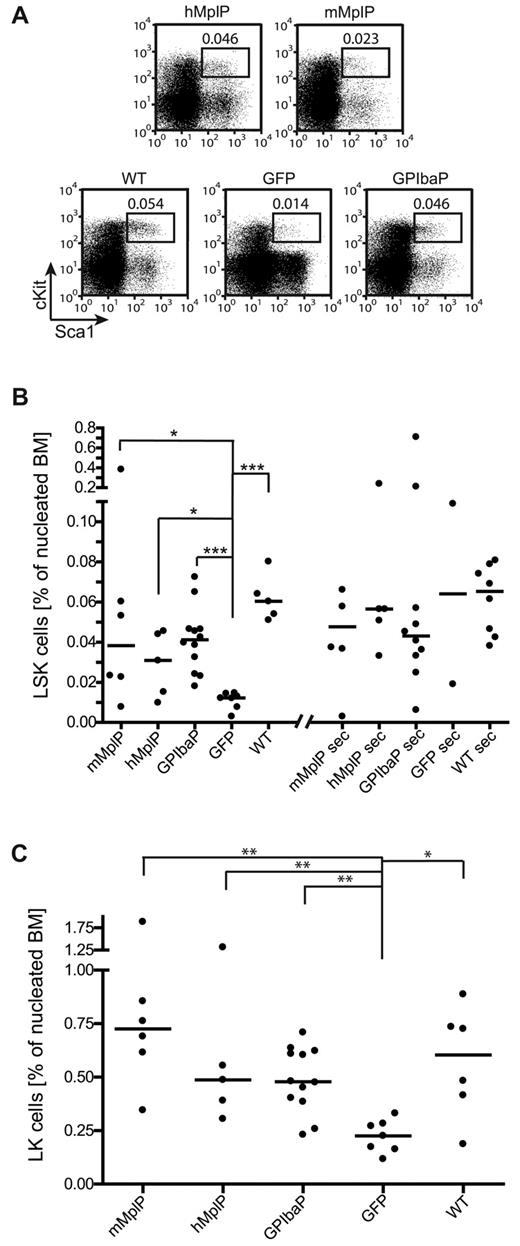
Most of the mice (24 of 27, 89%) that were transplanted with BM expressing Mpl from any of the 3 lineage-specific vectors survived long-term (> 20 weeks), whereas 4 of 9 (44%) mice that received uncorrected Mpl−/− BM died because of graft failures indicated by low peripheral blood cell counts and hypoplastic to aplastic BM (supplemental Figure 8). This observation gave first evidence for a correction of HSCs function by restored Mpl expression. We therefore determined whether the number of HSCs and primitive progenitors was increased, quantifying the percentage of LSK cells in the BM of gene-corrected mice 6 to 7 months after transplantation by flow cytometry.
Mice that received Mpl−/− cells transduced with any of the 3 Mpl vectors had increased LSK cell numbers, and in 4 animals these were similar to mice transplanted with wt cells (median percentage LSK cells of the nucleated BM mMplP = 0.038 ± 0.145, hMplP = 0.031 ± 0.016, GPIbaP = 0.041 ± 0.016, eGFP =0.012 ± 0.004; WT = 0.60 ± 0.011; Figure 5A-B). A significant increase was observed in mice that expressed Mpl from the GPIbaP (GPIbaP vs GFP, P = .0005) followed by the mMplP (mMplP vs GFP, P = .022) and the hMplP (hMplP vs GFP, P = .048, Mann-Whitney test). Importantly, flow cytometry revealed a normal proportion of the most primitive LT-HSCs (n = 9, supplemental Figure 9; 5%–10% of LSK cells in nucleated BM fraction as in healthy WT mice). Finally, we noted a significant increase in LK cells in Mpl expressing mice compared with GFP control mice (Figure 5C), indicating restored formation of multilineage progenitors in Mpl−/− mice.
Mpl gene therapy regenerates HSCs in primary and secondary Mpl−/− recipient mice. Mpl−/− mice were transplanted with Lin−Mpl−/− BM cells transduced with SIN lentiviral vectors expressing Mpl from lineage-specific promoters as indicated, or eGFP from the PGK promoter (GFP). Lin− BM from C57Bl/6 was transplanted as positive control (wt). (A) Representative flow cytometry plots of LSK cells from primary mice shown in panels B and C. Percentages of LSK cells indicated. (B) Percentage of LSK cells as determined by flow cytometry in primary and secondary Mpl−/− recipients on final analysis. Each primary recipient was transplanted into 2 secondary Mpl−/− mice with 5 × 106 BM cells (median ± SD). *P < .05 (Mann-Whitney test). ***P < .001 (Mann-Whitney test). (C) Percentage of LK cells as determined by flow cytometry in primary recipients on final analysis (median ± SD). *P < .05, **P < .01 (Mann-Whitney test).
Mpl gene therapy regenerates HSCs in primary and secondary Mpl−/− recipient mice. Mpl−/− mice were transplanted with Lin−Mpl−/− BM cells transduced with SIN lentiviral vectors expressing Mpl from lineage-specific promoters as indicated, or eGFP from the PGK promoter (GFP). Lin− BM from C57Bl/6 was transplanted as positive control (wt). (A) Representative flow cytometry plots of LSK cells from primary mice shown in panels B and C. Percentages of LSK cells indicated. (B) Percentage of LSK cells as determined by flow cytometry in primary and secondary Mpl−/− recipients on final analysis. Each primary recipient was transplanted into 2 secondary Mpl−/− mice with 5 × 106 BM cells (median ± SD). *P < .05 (Mann-Whitney test). ***P < .001 (Mann-Whitney test). (C) Percentage of LK cells as determined by flow cytometry in primary recipients on final analysis (median ± SD). *P < .05, **P < .01 (Mann-Whitney test).
To address the regeneration of HSC function, we performed secondary transplantations (2 secondary recipients per primary donor, n = 54). More than 66% of the secondary recipients survived long-term (mMplP = 6 of 11, hMplP = 7 of 9, GPIbaP = 11 of 16; supplemental Figure 10). Thirty percent (2 of 6) of the secondary recipients from the eGFP mice survived beyond 20 weeks, but only one of the surviving mice showed eGFP expression beyond 16 weeks as determined by flow cytometry (supplemental Figure 11). Our reduced irradiation conditioning of Mpl−/− mice probably enabled long-term survival from their endogenous hematopoietic system when short-term hematopoiesis was supported by BMT.
To further confirm that secondary recipients also showed a reconstitution of the HSC compartment, we analyzed the LSK cells by flow cytometry. Except for 4 mice with very high LSK numbers, the increase in LSK cell numbers was equal to the situation in primary donor mice (Figure 5B) but did not further increase, indicating physiologic homeostasis in most of the mice. Secondary recipients also presented with approximately 10% of LT-HSCs in the BM (supplemental Figure 9).
Gene-corrected Mpl−/− HSCs adjust the expression of essential self-renewal genes
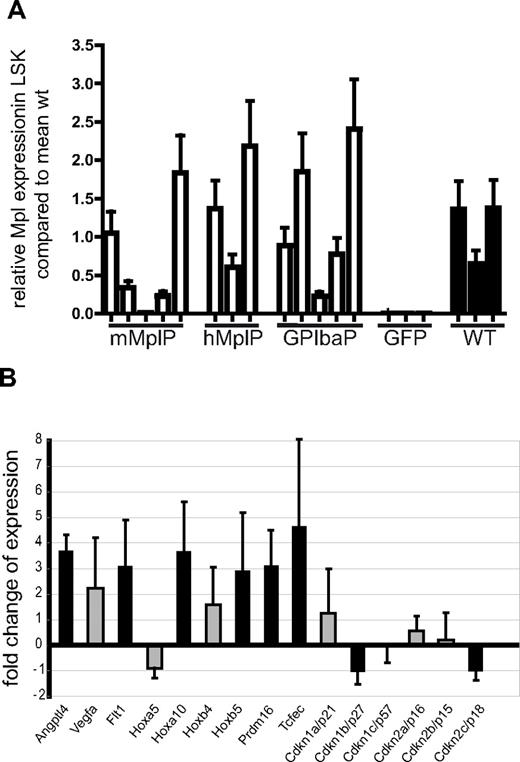
To investigate whether the lineage-specific lentiviral vectors indeed conferred physiologic Mpl expression levels in LSK cells, we sorted LSK cells by flow cytometry and determined Mpl expression by quantitative reverse-transcribed (RT)-PCR (Figure 6A). With some interindividual variability, Mpl mRNA was increased in the LSK cells of all mice that received cells transduced with any of the lineage-specific lentiviral vectors. Expression levels matched those of Mpl expression in wt BM LSK cells transplanted in Mpl−/− mice.
Expression analysis in LSK cells of gene-corrected mice. (A) Average Mpl expression in LSK cells sorted from Mpl−/− mice, which were transplanted with Mpl−/− cells transduced with the vector as indicated, determined by quantitative RT-PCR (mean ± SEM). LSK cells from 3 Mpl−/− mice transplanted with wt lin− BM were used as reference. (B) Fold difference of expression of genes as indicated in LSK cells of Mpl-corrected Mpl−/− mice compared with eGFP expressing Mpl−/− LSK cells. RNA was isolated form 3 Mpl-corrected mice and 3 eGFP control mice. Expression was determined by microarray analysis. Black bars represent the genes that were regulated with P < .05 (t test with Bayesian shrinkage). Array results and results of validating quantitative RT-PCR are shown in supplemental Figures 9 and 10.
Expression analysis in LSK cells of gene-corrected mice. (A) Average Mpl expression in LSK cells sorted from Mpl−/− mice, which were transplanted with Mpl−/− cells transduced with the vector as indicated, determined by quantitative RT-PCR (mean ± SEM). LSK cells from 3 Mpl−/− mice transplanted with wt lin− BM were used as reference. (B) Fold difference of expression of genes as indicated in LSK cells of Mpl-corrected Mpl−/− mice compared with eGFP expressing Mpl−/− LSK cells. RNA was isolated form 3 Mpl-corrected mice and 3 eGFP control mice. Expression was determined by microarray analysis. Black bars represent the genes that were regulated with P < .05 (t test with Bayesian shrinkage). Array results and results of validating quantitative RT-PCR are shown in supplemental Figures 9 and 10.
If lentiviral expression of Mpl would correct the HSC defect, we would assume reconstituted Mpl signaling to correct necessary stem cell pathways. To address this hypothesis, we performed microarray analysis with the limited material available from sorted LSK cells.
The gene-corrected mice needed increased HSC self-renewal to refill the stem cell pool. Consistent with this, we found the cyclin-dependent kinase inhibitor (Cdkn) Cdkn2c (p18) to be 50% down-regulated (Figure 6B; supplemental Figure 12). Absence of p18 leads to increased self-renewal divisions,33 in contrast to stem cell exhaustion caused by the lack of Cdkn1a (p21).14,34 Cdkn1a (p21) expression was high in LSK cells of all mice except one GFP control mouse. In Mpl gene-corrected LSK cells, we found a 50% reduction in expression of Cdkn1b (p27kip), a Cdkn that was also reported to increase HSC frequency when deleted.35 In contrast, there was no difference in expression of Cdkn1c (p57) between gene-corrected and GFP control LSK cells (Figure 6B), although an increase may have been expected because of its reported low expression levels in Thpo−/− mice.9 This suggests important functional differences between regenerating hematopoiesis and the situation found in adapted knockout mice.
Furthermore, Vegfa, Hoxb4, Hoxa5, or Hoxa10 are suggested candidates for Thpo/Mpl-induced HSC self-renewal,36,37 and we found those to be up-regulated in LSK cells harvested from Mpl gene–corrected mice (Figure 6B). We also found Angptl4, implicated to expand cultured HSCs,38 to be expressed at higher levels and 2 genes (Prdm16 and Tcfec) that were recently shown to increase HSC activity in a mouse model when retrovirally overexpressed in the BM.39 In addition to the array analysis, we confirmed the expression of 3 of the genes by quantitative RT-PCR (Vegfa, Hoxa10, and Tcfec; supplemental Figure 13). Taken together, expression analysis also confirmed the correction of Mpl expression with adjustment of genes involved in HSC regeneration.
Discussion
In our study, we show, for the first time, the correction of Mpl deficiency by gene therapy. High toxicity of ectopic Mpl expression17-19 (current report), the strong susceptibility to the toxic effects of pretransplantation conditioning of Mpl−/− recipients, depletion of the HSC pool before the therapeutic intervention, and poor in vitro stimulation of Mpl−/− HSCs made attempts to develop a gene therapy for Mpl deficiency demanding. We overcame these problems using lentiviral vectors, which can transduce nondividing cells, and introduced lineage-specific cellular promoters that restrict Mpl expression to its physiologic target cells and levels. Short transduction protocols with stem cell supportive cytokines30 were used to prevent further stem cell loss in vitro. Moreover, we established reduced irradiation conditions that supported HSC engraftment with high chimerism and low toxicity in Mpl−/− recipient mice.
The lentiviral vectors with lineage-specific promoters (GPIbaP, hMplP, and mMplP) showed high specificity of expression during megakaryopoiesis, in line with earlier studies in transgenic mice,22-24 in BMT models,25,26 and in vitro.27 Accordingly, the vectors restored megakaryopoiesis in Mpl−/− cells, both in vitro and in vivo, where approximately 60% (18 of 27) of mice receiving Mpl-corrected cells had increased platelet counts at 6 weeks after transplantation. Importantly, we also demonstrated activity of these promoters in HSCs (LT- and ST-HSCs) in vivo. Long-term survival of transplanted Mpl−/− mice after gene therapy and increased LSK cell numbers in 24 of 27 treated mice convincingly demonstrated the correction of the stem cell defect in Mpl-deficient mice.
All 3 lineage-specific lentiviral vectors prevented adverse reactions in transplanted Mpl−/− mice. In remarkable contrast, Mpl expression from the PGK promoter induced rapid death of transplanted Mpl−/− recipients because of erythroblastosis-induced splenic rupture. In wt C57Bl/6 mice, Mpl expression from the PGK promoter induced no acute adverse event but a progressive myelodysplastic syndrome-like disease.17 The fatal acute erythroblastosis encountered in Mpl−/− mice that received cells ectopically expressing Mpl is therefore a specific risk factor in gene therapy of Mpl deficiency, probably because of increased endogenous Thpo levels. Interestingly, splenic rupture is also described as a rare complication in patients treated with G-CSF for stem cell mobilization or myelodysplastic syndrome.40,41
As another adverse reaction, we observed thrombocytosis in 2 of our mice that expressed Mpl by the murine MplP. A similar phenotype was reported in transgenic mice that expressed Mpl by the same murine MplP fragment. Insufficient Mpl expression from mMplP in late megakaryopoiesis has been demonstrated at levels that are unable to reduce Thpo levels but maintaining Thpo responsiveness, thus inducing thrombocytosis. In our study, the mMplP presented as the most specific but also the weakest promoter of the lineage-specific promoters. Our observation of thrombocytosis in 2 of our mice therefore confirmed studies in transgenic mice.23,24 However, the greater variability of the phenotype observed in our study, which used lentiviral gene transfer into HSCs, also reveals important differences in somatic versus germline transgenesis. Interanimal variability after lentiviral gene transfer into HSCs may be related to clonal restriction over time with variable expression levels of Mpl depending on the lentiviral integration site.
The GPIbaP mediated the best correction of platelet counts and Thpo levels. In the BM of 5 mice of the GPIbaP and hMplP groups, however, MK were of size and ploidy exceeding physiologic levels, but enlarged MK did not correlate with increased platelet counts. This observation suggests inefficient contribution of these MK to platelet production. Mpl was shown not only to be unnecessary for platelet formation but to favor endomitosis over platelet production in MK.42,43 Increased endomitosis resulting from high Mpl expression might have induced the atypically large MK combined with low platelet counts. In some of the mice with high numbers of MK, we also found marrow fibrosis and bone neo-formation. High numbers of MK in the BM, induced by extensive Mpl signaling, may inhibit the activity of osteoclasts by osteoprotegerin expression and support osteoblast function by secretion of osteopontin, osteocalcin, osteonectin, bone sialoproteins, and bone morphogenetic proteins (reviewed by Kacena et al32 ). Marrow fibrosis was also found in various mouse models that overexpress Thpo44 and in mouse models that lack Gata-1 or NF-E2, which are also associated with inhibited MK maturation,45 and accumulation of higher MK numbers causing myelofibrosis and gain of bone mass.32 Similarly, myelofibrosis in patients is often accompanied by increased numbers of morphologic atypical MK,46 and constitutive activation of MPL by mutations (eg, MPL W515L/K) has been found to cause myelofibrosis in patients with MPD.20,47 Therefore, increased Mpl signaling may actively induce myelofibrosis.
Mpl gene therapy aims to restore a signal transduction pathway crucial to HSCs. Ectopic expression of most stem cell regulating genes results in (pre-)leukemic states, which is also true for Mpl.12 Therefore, HSC regeneration and subsequent homeostasis were the most challenging task of our therapeutic approach. Because of the progressive loss of HSCs in Mpl-deficient mice and CAMT patients, it was also unclear whether their regeneration was generally possible. Importantly, we observed a controlled increase of LSK cells in all groups of mice that were transplanted with Mpl-transduced cells. Secondary transplantation demonstrated engraftment by regenerated LT-HSCs with normal frequencies of maturation toward ST-HSCs and MPP. We could thereby show that reintroduction of Mpl into Mpl-deficient cells not only reconstituted progenitor proliferation but also regenerated long-term reconstituting HSCs. This demonstrated that the Mpl-deficient situation did not lead to irreversible defects in self-renewal potential. This is a very important finding with implications for CAMT therapy and other aplastic anemias because progressive stem cell loss is responsible for the mortality of these patients.
We made use of this unique scenario of HSC regeneration to investigate the underlying gene expression profile. In contrast, previous studies of HSC transcriptomes were largely based on knockout models or gain-of-function studies with a (pre-)leukemic outcome. Various Mpl targets have been described, and many of them were implicated in Mpl's role of preserving the HSC phenotype. Among them, we saw an up-regulation of Hoxa10, Hoxb5, and Vegfa and down-regulation of Cdkn2c (p18) and Cdkn1 (p27kip). We also found the Vegfr1/Flt1 to be up-regulated, which may be more relevant than cell-autonomous expression of its ligand Vegfa. Thpo signaling may directly up-regulate hypoxia-responsive genes by stabilization of Hif1α37,48 ; and interestingly, expression of Vegfa and Flt1 is regulated by hypoxia.
Deneault et al performed a comprehensive study to find positive effectors of HSC activity.39 Prdm16 and Tcfec, both identified in their experiments, were also up-regulated in the LSK cells of Mpl-treated mice. Regenerating, Mpl-corrected HSCs thus adjusted the expression of many genes with a known function in HSC self-renewal.
Mpl signaling is implicated in the induction of HSC quiescence in the stem cell niche.9,10,49 Several cell cycle-associated target genes of Mpl have been described (Cdkn1c/p57, Cdkn2d/p19, c-Myc, and Tie-2); however, we found the expression of none of those genes to be significantly altered compared with uncorrected eGFP-transduced Mpl−/− LSK cells. In contrast to studies that characterized the expression profile in knockout versus wt mice, or shortly after the application of Mpl inhibiting factors (AMM2 antibodies), the mice in our model underwent BMT with long-term regeneration of the HSC pool. Therefore, HSCs in our model expanded on the basis of restored Mpl signaling, and the genes identified in our model are probably directly linked to HSC regeneration.
In conclusion, the new vectors and complex findings described here not only represent a major step toward development of gene therapy for Mpl-deficient aplastic anemia but may also allow refined studies of gene function in regenerating HSCs, which are of generic interest to develop advanced therapies for inherited or acquired disorders of hematopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sabine Knoess, Johanna Krause, and Rena-Mareike Struss for excellent technical assistance, Thomas Neumann for performing microarray experiments, Bernhard Schiedlmeier for his help with flow cytometry, and Joerg Fruehauf from the irradiation facility (all Hannover Medical School, Hannover, Germany). The murine Mpl promoter was kindly provided by Radek Skoda, Basel, Switzerland.
This work was supported by Deutsche Forschungsgemeinschaft (SFB 566, and Excellence Cluster REBIRTH). D.H. was supported by a Hannover Biomedical Research School stipend.
Authorship
Contribution: D.H. performed research, collected, analyzed, and interpreted data, and wrote the manuscript; D.C.W., J.M., and A.S. performed research; M.H.B. performed array analysis and analyzed the data; G.B. analyzed the histopathology; M.B. analyzed data; C.B. designed research, interpreted data, and wrote the manuscript; and U.M. designed and performed research, collected, analyzed, and interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of D.C.W. is Hannover Clinical Trial Center GmbH, Hannover, Germany.
Correspondence: Ute Modlich, Department of Experimental Hematology, OE6960, Hannover Medical School, Carl-Neuberg-Str 1, 30625 Hannover, Germany; e-mail: modlich.ute@mh-hannover.de.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal