Abstract
Epidemiologic data show that the immune system may control or promote the emergence and growth of neoplastic lymphomatous clones. Conversely, systemic lymphomas, especially myeloma and chronic lymphocytic leukemia (CLL), are associated with clinical immunodeficiency. This prospective controlled study demonstrates substantially reduced circulating T helper cells, predominantly naive CD4+ cells, in patients with nonleukemic follicular lymphoma and extranodal marginal zone lymphoma, but not in monoclonal gammopathy and early CLL. These changes were correlated with a preactivated phenotype, hyperreactivity in vitro, presenescence, and a T helper 2 shift of peripheral T helper cells. No prominent alterations existed in the regulatory T-cell compartment. Gene expression profiling of in vitro–stimulated CD4+ cells revealed an independent second alteration of T helper cell physiology, which was most pronounced in early CLL but also detectable in follicular lymphoma/extranodal marginal zone lymphoma. This pattern consisted of down-regulation of T-cell receptor signaling cascades and globally reduced cytokine secretion. Both types of T-cell dysfunction may contribute to significant immunodeficiency in nonleukemic indolent B-cell lymphomas as demonstrated by unresponsiveness to hepatitis B vaccination. The precise definition of systemic T-cell dysfunction serves as the basis to study its prognostic impact, its relationship to the established influence of the lymphoma microenvironment, and its therapeutic manipulation.
Introduction
Numerous aspects of lymphoma epidemiology and pathophysiology indicate mutual interactions between the normal immune system and lymphoma as its “autochthonous” malignancy. These interactions may either promote or control lymphomagenesis and the clinical behavior of established lymphomas. The severity of acquired or iatrogenic immunosuppression correlates with the risk of lymphoma,1,2 and restoration of immune function is generally sought for immunodeficiency-associated lymphomas. The graft-versus-lymphoma effect of allogeneic stem cell transplantation demonstrates the efficacy of antilymphoma immune activity.3 Induction of antilymphoma immune responses by active specific immunotherapy is consistently associated with superior progression-free survival in follicular lymphoma (FL).4-7
However, immune activation may also promote the development and progression of lymphoma. Specifically, extranodal marginal zone lymphomas (eMZLs) originate at the site of chronic inflammation under the influence of T-cell help.8-10 Biased antigen receptor repertoires in B-cell lymphoma and autoantigen recognition by chronic lymphocytic leukemia (CLL)–derived immunoglobulin suggest a role for antigen receptor signaling in lymphomagenesis.11,12 Several lymphoma entities are associated with systemic autoimmune diseases.13
There is also evidence for reciprocal and predominantly inhibitory relationships between overt B-cell neoplasia and the immune system: CLL and multiple myeloma patients have a susceptibility to bacterial and opportunistic infections.14,15 In myeloma, this predisposition is commonly attributed to hypogammaglobulinemia. In CLL and FL, neoplastic B cells inhibit normal T lymphocytes through the impairment of the formation of the immunologic synapse in a contact-dependent manner.16-18 Because of the leukemic nature of CLL, such inhibiting cell contacts can occur permanently and ubiquitously. The resulting state of cellular immunodeficiency offers a plausible explanation for the frequent infections and secondary cancers seen in CLL.19 In addition, a relative increase of circulating regulatory T cells (Tregs) has been observed in myeloma, CLL, and other B-cell lymphomas.20-22 FL cells appear to induce the expression of the Treg master regulator FoxP3 in conventional T cells by direct cell contact.23
We have previously described a significant reduction in peripheral T helper cells in patients with untreated FL and eMZL.24 To delineate these observations with respect to CD4+ subpopulations, to explore their functional consequences, and to possibly identify underlying mechanisms, we performed a prospective global characterization of the cellular immune system in untreated patients with indolent B-cell lymphoma compared with age-matched healthy controls.
Methods
Patients and samples
Ethylenediaminetetraacetic acid-anticoagulated venous blood was obtained from patients with bioptically confirmed FL and eMZL, and from patients with B-CLL or monoclonal gammopathy of unknown significance (MGUS) according to standard diagnostic criteria.25 Patients with prior antineoplastic therapy, including corticosteroids, and patients with evidence for preexisting autoimmunity or immunodeficiency were excluded from this study. Age-matched healthy probands were recruited from the Volunteer Force of University Medical Center Freiburg. All patients and healthy probands gave informed consent in accordance with the Declaration of Helsinki. The study population encompassed all persons previously investigated for perturbations of their T-cell receptor (TCR) repertoire.24 The study was approved by the University of Freiburg institutional ethics committee. All samples were processed for immunophenotyping and in vitro stimulation assays within 2 hours after collection without cryopreservation of cells.
Quantitative immunophenotyping of peripheral blood mononuclear cells
Whole blood was subjected to erythrocyte lysis with NH4Cl (PharmLyse; BD Biosciences) in tubes containing a defined number of fluorescent beads (Trucount tubes; BD Biosciences). Leukocytes were stained at room temperature with antibody combinations to distinguish defined leukocyte subpopulations (Table 1). Antibodies were purchased from BD Biosciences, except anti-CD8-Pacific blue (Dako). Stained lymphocytes were analyzed by flow cytometry (CyAn ADP; Beckman Coulter) in a side scatter/CD45 gate with Summit Version 4.0 (Beckman Coulter) and FlowJo Version 6.4.3 (TreeStar) software. Staining for Ki-67 (Ki-67-phycoerythrin staining kit; BD Biosciences) and FoxP3 (FoxP3-allophycocyanin staining kit; eBioscience) was performed on peripheral blood mononuclear cells isolated by density gradient centrifugation.
Antibody staining combinations for quantitative flow cytometry and distinct cell populations
| Leukocyte subpopulation . | Immunophenotype . | Fluorescence label . | |||||
|---|---|---|---|---|---|---|---|
| PaB . | FITC . | PE . | PerCP . | APC . | APC-Cy7 . | ||
| Monocytes | CD14+ CD45+ | CD3 | CD8 | CD45 | CD14 | ||
| Lymphocytes | SSClow CD45high | ||||||
| B lymphocytes | CD45+ CD19+ | CD3 | CD16/56 | CD45 | CD19 | ||
| NK lymphocytes | CD45+ CD16/56+ | ||||||
| NK-T lymphocytes | CD45+ CD16/56+ CD3+ | ||||||
| T lymphocytes | CD45+ CD3+ or SSClow CD3+ | ||||||
| CD4+ T lymphocytes | CD3+ CD4+ | CD4 | CD69 | CD3 | HLA-DR | ||
| Recently activated CD4+ T lymphocytes | CD3+ CD4+ CD69+ | ||||||
| Remotely activated CD4+ T lymphocytes | CD3+ CD4+ HLA-DR+ | ||||||
| Naive CD4+ T lymphocytes (TN) | CD3+ CD4+ CD45RA+ CD27+ | CD45RA | CD27 | CD3 | CD4 | ||
| Central memory CD4+ T lymphocytes (TCM) | CD3+ CD4+ CD45RA− CD27+ | ||||||
| Peripheral memory CD4+ T cells (TEM) | CD3+ CD4+ CD45RA− CD27− | ||||||
| Terminally differentiated effector CD4+ T cells (TEMRA) | CD3+ CD4+ CD45RA+ CD27− | ||||||
| Proliferating CD4+ T lymphocytes | Ki-67+ CD4+ | Ki-67 | CD4 | ||||
| Senescent CD4+ lymphocytes | CD3+ CD4+ CD57+ | CD8 | CD57 | CD27 | CD3 | CD45RA | CD4 |
| Senescent CD4+ lymphocytes | CD3+ CD4+ CD28− | CD8 | CD45RA | CD27 | CD3 | CD28 | CD4 |
| Regulatory T cells (“natural”) | CD3+ CD4low CD25high | CD4 | CD25 | CD3 | CD62L | ||
| Regulatory T cells (“natural”) | CD3+ CD4low FoxP3+ | CD8 | CD4 | CD25 | CD3 | FoxP3 | |
| CD8+ T lymphocytes | CD3+ CD8+ | CD8 | CD69 | CD3 | HLA-DR | ||
| Recently activated CD8+ T lymphocytes | CD3+ CD8+ CD69+ | ||||||
| Remotely activated CD8+ T lymphocytes | CD3+ CD8+ HLA-DR+ | ||||||
| Naive CD8+ T lymphocytes (TN) | CD3+ CD8+ CD45RA+ CD27+ | CD45RA | CD27 | CD3 | CD8 | ||
| Central memory CD8+ T lymphocytes (TCM) | CD3+ CD8+ CD45RA− CD27+ | ||||||
| Peripheral memory CD8+ T lymphocytes (TEM) | CD3+ CD8+ CD45RA− CD27− | ||||||
| Terminally differentiated effector CD8+ T cells (TEMRA) | CD3+ CD8+ CD45RA+ CD27− | ||||||
| Proliferating CD8+ T lymphocytes | Ki-67+ CD8+ | Ki-67 | CD8 | ||||
| Senescent CD8+ lymphocytes | CD3+ CD8+ CD57+ | CD8 | CD57 | CD27 | CD3 | CD45RA | CD4 |
| Senescent CD8+ lymphocytes | CD3+ CD8+ CD28− | CD8 | CD45RA | CD27 | CD3 | CD28 | CD4 |
| Leukocyte subpopulation . | Immunophenotype . | Fluorescence label . | |||||
|---|---|---|---|---|---|---|---|
| PaB . | FITC . | PE . | PerCP . | APC . | APC-Cy7 . | ||
| Monocytes | CD14+ CD45+ | CD3 | CD8 | CD45 | CD14 | ||
| Lymphocytes | SSClow CD45high | ||||||
| B lymphocytes | CD45+ CD19+ | CD3 | CD16/56 | CD45 | CD19 | ||
| NK lymphocytes | CD45+ CD16/56+ | ||||||
| NK-T lymphocytes | CD45+ CD16/56+ CD3+ | ||||||
| T lymphocytes | CD45+ CD3+ or SSClow CD3+ | ||||||
| CD4+ T lymphocytes | CD3+ CD4+ | CD4 | CD69 | CD3 | HLA-DR | ||
| Recently activated CD4+ T lymphocytes | CD3+ CD4+ CD69+ | ||||||
| Remotely activated CD4+ T lymphocytes | CD3+ CD4+ HLA-DR+ | ||||||
| Naive CD4+ T lymphocytes (TN) | CD3+ CD4+ CD45RA+ CD27+ | CD45RA | CD27 | CD3 | CD4 | ||
| Central memory CD4+ T lymphocytes (TCM) | CD3+ CD4+ CD45RA− CD27+ | ||||||
| Peripheral memory CD4+ T cells (TEM) | CD3+ CD4+ CD45RA− CD27− | ||||||
| Terminally differentiated effector CD4+ T cells (TEMRA) | CD3+ CD4+ CD45RA+ CD27− | ||||||
| Proliferating CD4+ T lymphocytes | Ki-67+ CD4+ | Ki-67 | CD4 | ||||
| Senescent CD4+ lymphocytes | CD3+ CD4+ CD57+ | CD8 | CD57 | CD27 | CD3 | CD45RA | CD4 |
| Senescent CD4+ lymphocytes | CD3+ CD4+ CD28− | CD8 | CD45RA | CD27 | CD3 | CD28 | CD4 |
| Regulatory T cells (“natural”) | CD3+ CD4low CD25high | CD4 | CD25 | CD3 | CD62L | ||
| Regulatory T cells (“natural”) | CD3+ CD4low FoxP3+ | CD8 | CD4 | CD25 | CD3 | FoxP3 | |
| CD8+ T lymphocytes | CD3+ CD8+ | CD8 | CD69 | CD3 | HLA-DR | ||
| Recently activated CD8+ T lymphocytes | CD3+ CD8+ CD69+ | ||||||
| Remotely activated CD8+ T lymphocytes | CD3+ CD8+ HLA-DR+ | ||||||
| Naive CD8+ T lymphocytes (TN) | CD3+ CD8+ CD45RA+ CD27+ | CD45RA | CD27 | CD3 | CD8 | ||
| Central memory CD8+ T lymphocytes (TCM) | CD3+ CD8+ CD45RA− CD27+ | ||||||
| Peripheral memory CD8+ T lymphocytes (TEM) | CD3+ CD8+ CD45RA− CD27− | ||||||
| Terminally differentiated effector CD8+ T cells (TEMRA) | CD3+ CD8+ CD45RA+ CD27− | ||||||
| Proliferating CD8+ T lymphocytes | Ki-67+ CD8+ | Ki-67 | CD8 | ||||
| Senescent CD8+ lymphocytes | CD3+ CD8+ CD57+ | CD8 | CD57 | CD27 | CD3 | CD45RA | CD4 |
| Senescent CD8+ lymphocytes | CD3+ CD8+ CD28− | CD8 | CD45RA | CD27 | CD3 | CD28 | CD4 |
T helper cell isolation and in vitro stimulation
CD4+ T cells were enriched from heparinized blood after density gradient centrifugation to more than 95% purity (CD4+ T-cell isolation kit; Miltenyi Biotec) as verified by flow cytometry. In the case of CLL samples, B cells were depleted with microbead-conjugated CD19 antibodies (Miltenyi Biotec) before T-cell purification.
For gene expression profiling and activation immunophenotyping, 105 purified CD4+ T cells were stimulated (106 cells/mL) on CD3-coated plates (BD Biosciences) with 3 μg/mL anti-CD28 (BD Biosciences) in AIM-V medium (Invitrogen) under serum-free conditions. After 39 hours, cells were harvested and stained for flow cytometry with 7-amino-actinomycin D, anti-CD4-fluorescein isothiocyanate, anti-CD45RA-allophycocyanin, and phycoerythrin-labeled antibodies to CD69, CD25, CD40L, ICOS, CD95, or annexin V (BD Biosciences). Measurements were standardized with fluorescent calibration beads (6-Peak; Spherotech). After 40 hours, supernatants from parallel stimulation cultures were frozen for cytokine measurements, and cells were preserved in RNAlater (Ambion) for gene expression profiling. To assess proliferative responses, 5 × 104 cells were stimulated with 3 anti-CD3/anti-CD28 coated beads (Invitrogen) per cell in round-bottom plates. At 48 hours, quadruplicate cultures were pulsed with 1 μCi methyl-3H-thymidine. 3H incorporation was measured 16 hours later with a Packard TopCount LSC scintillation counter (GMI). The DNA content of stimulated cells was measured after ethanol fixation and RNase A digestion by propidium iodide staining and flow cytometry. The cell-cycle status was deduced from the DNA content with FlowJo software.
Quantification of TREC
DNA was isolated from purified CD4+ cells, including RNase treatment (Qiamp DNA Mini Kit; QIAGEN). A total of 10 μL DNA was amplified by real-time polymerase chain reaction (PCR) in a 50-μL reaction (TaqMan Universal PCR Master Mix; Applied Biosystems) with 0.3μM forward (5′-CGTGAGAACGGTGAATGAAGAGCAGACA-3′) and reverse (5′-CATCCCTTTCAACCATGC TGACACCTCT-3′) primers in the presence of 0.1μM probe (5′-VIC-TTTTTGTAAAGGTGCCCACTCCTGTGCACGGTGA-TAMRA-3′). Thermocycling conditions were 50°C for 2 minutes, 95°C for 10 minutes in the first cycle, followed by 45 cycles of 95°C for 30 seconds and 60°C for 45 seconds.
For absolute quantification of TCR-δ excision circles (TREC), serial dilutions of 1 × 108 to 1 × 101 copies of a Not I-linearized pCR-TOPO vector (Invitrogen) carrying an insert containing the signal joint region of TRECs (a kind gift from Jerome Ritz, Dana-Farber Cancer Institute, Boston, MA) were amplified in parallel. PCR signal intensities were recorded in real-time (ABI Prism 7700; Applied Biosystems) and processed with SDS Version 1.9 software (Applied Biosystems). All PCR products were verified by 2% agarose gel electrophoresis.
Cytokine measurements
The concentrations of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), interleukin-2 (IL-2), IL-4, IL-5, and IL-10 in simultaneously thawed supernatant aliquots were measured by cytometric bead array (Th1-Th2 differentiation kit; BD Biosciences).
Gene expression profiling
Total RNA was extracted from cryopreserved cell lysates (RNeasy Mini Kit, QIAGEN), precipitated with ethanol, and reconstituted at 0.5 μg/μL. RNA quality was confirmed by a median RNA integrity number of 10 (range, 9.3-10; Agilent 2100 Bioanalyzer; Agilent). Biotin-labeled cRNA was synthesized from 5 μg total RNA with the “One-Cycle kit” (Affymetrix) and fragmented according to the manufacturer's instructions. A total of 10 μg of cRNA per sample was hybridized for 16 hours at 45°C to U133Plus2.0 oligonucleotide microarrays (Affymetrix). Microarrays were washed and stained in a Fluidics Station 400 and scanned with a GeneChip Scanner 3000 (Affymetrix). The quality of raw data was verified by a typical GAPDH 3′/5′ signal ratio of less than 1.2 as assessed with Quality Reporter software (Affymetrix) after MAS5 condensation with GCOS software Version 1.4. The data are accessible at Gene Expression Omnibus (National Center for Biotechnology Information; www.ncbi.nlm.nih.gov/geo/) under accession number GSE23293.
Statistical analysis
Absolute numbers of defined cell subpopulations were compared between patients and probands with the 2-sided Wilcoxon matched sample test. Comparisons between 2 and 3 groups of lymphoma patients defined by clinical stage were performed with the Mann-Whitney test or the Kruskal-Wallis test with Dunn posttest for comparison of selected groups, respectively (Prism Version 4.0; GraphPad Software). Spearman correlation coefficient was computed for correlations of continuous variables.
Microarray data were analyzed with Expressionist Version 5.3.3 software (Genedata). Gene expression values for subsequent analyses were calculated from raw data (.CEL files) with GC-RMA condensation after quantile normalization. Groups of differentially regulated genes were determined by an unpaired t test, including estimation of false-positive results by Benjamini-Hochberg Q values. Separation of genes into up- and down-regulated was performed based on their ratio of means with an “N-fold regulation” analysis. The gene expression profiles corresponding to external, nonexpression data, like the production of IFN-γ or IL-4 in FL/eMZL CD4+ cells, were determined through a “profile distance search” as a nonparametric correlation. Genes with significant correlations were separated into positively and negatively correlating genes based on the values of the corresponding correlation coefficients. The statistical significance of overrepresentation of members of specific pathways among various groups of deregulated genes was tested with Fisher exact test after import of the KEGG pathway annotations included in the current Bioconductor package, Version 2.5 (annotation package hgu133plus2.db Version 2.3.5) based on KEGG release 52 (September 2009), which includes 358 human pathways (www.bioconductor.org).
Results
Circulating T-cell subpopulations in patients with chronic B-cell malignancies
The characteristics of the patients recruited into this noninterventional study are shown in Table 2. With regard to CLL, only patients with very early disease, defined as Rai stage 0 and leukocyte counts less than 28 000/μL, were enrolled.
Patients and age-matched healthy donors
| Characteristic . | Diagnosis . | Healthy donors . | |||
|---|---|---|---|---|---|
| FL . | eMZL . | CLL . | MGUS . | ||
| No. | 11 | 7 | 9 | 6 | 18 |
| Median age, y (range) | 65 (37-68) | 65 (27-77) | 66 (42-80) | 53 (43-66) | 62.5 (28-76) |
| Sex, male/female | 8/3 | 4/3 | 8/1 | 3/3 | 5/13 |
| Stage* | |||||
| 0 | NA | NA | 9 | ||
| I | 1 | 2 | 0 | ||
| II | 1 | 3 | 0 | ||
| III | 7 | 0 | 0 | ||
| IV | 2 | 2 | 0 | ||
| B symptoms present | 4 | 0 | 0 | 0 | NA |
| IPI risk | |||||
| 0/5 | 1 | 1 | NA | NA | NA |
| 1/5 | 2 | 4 | NA | NA | NA |
| 2/5 | 6 | 2 | NA | NA | NA |
| 3/5 | 1 | 0 | NA | NA | NA |
| 4/5 | 1 | 0 | NA | NA | NA |
| Comments | 3 H pylori+ | Leukocytes < 28/nL | |||
| Characteristic . | Diagnosis . | Healthy donors . | |||
|---|---|---|---|---|---|
| FL . | eMZL . | CLL . | MGUS . | ||
| No. | 11 | 7 | 9 | 6 | 18 |
| Median age, y (range) | 65 (37-68) | 65 (27-77) | 66 (42-80) | 53 (43-66) | 62.5 (28-76) |
| Sex, male/female | 8/3 | 4/3 | 8/1 | 3/3 | 5/13 |
| Stage* | |||||
| 0 | NA | NA | 9 | ||
| I | 1 | 2 | 0 | ||
| II | 1 | 3 | 0 | ||
| III | 7 | 0 | 0 | ||
| IV | 2 | 2 | 0 | ||
| B symptoms present | 4 | 0 | 0 | 0 | NA |
| IPI risk | |||||
| 0/5 | 1 | 1 | NA | NA | NA |
| 1/5 | 2 | 4 | NA | NA | NA |
| 2/5 | 6 | 2 | NA | NA | NA |
| 3/5 | 1 | 0 | NA | NA | NA |
| 4/5 | 1 | 0 | NA | NA | NA |
| Comments | 3 H pylori+ | Leukocytes < 28/nL | |||
NA indicates not applicable.
Staging for FL and eMZL was by Ann Arbor staging, and for CLL, by Rai stage.
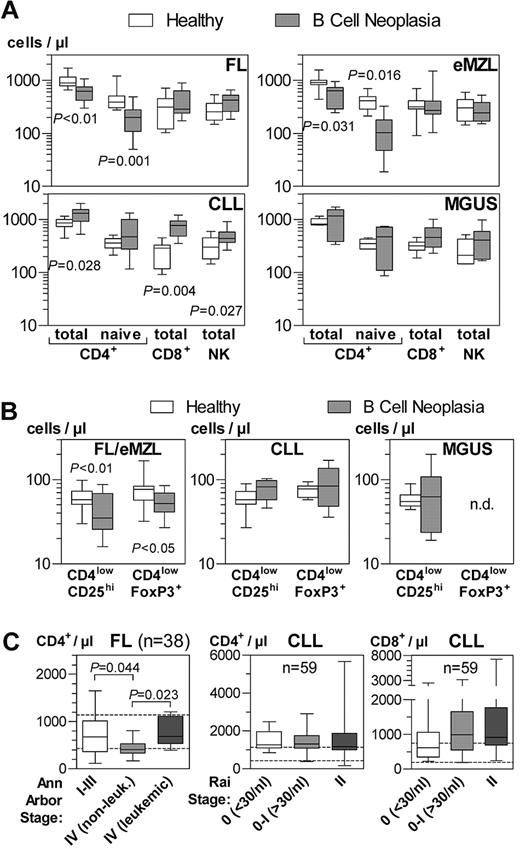
Calibrated quantitative immunophenotyping of peripheral blood cells confirmed the dominant decrease in CD4+ T cells in FL and eMZL (Figure 1A).24 This observation was mostly attributable to a reduction of naive (CD4+CD45RA+CD27+) T helper cells to 51% in FL and 25% in eMZL compared with age-matched controls. No significant changes were noted for central (CD4+CD45RA−CD27+) and peripheral (CD4+CD45RA−CD27−) T helper memory cells (data not shown). Global numbers of NK cells and naive/memory subsets of CD8+ T cells and NKT cells (Figure 1A and data not shown) were normal.
Absolute numbers of circulating lymphocyte subpopulations in patients with indolent B-cell lymphomas and healthy donors. Whole blood samples were stained and analyzed by flow cytometry within 2 hours after phlebotomy. Horizontal bars, boxes, and whiskers represent median, 25%/75% quartiles, and range, respectively. (A) Bead-calibrated absolute cell numbers of CD4+, CD8+, and NK lymphocyte subsets. FL, n = 11; eMZL, n = 7; CLL, n = 9; MGUS, n = 6 patients. (B) Bead-calibrated absolute cell numbers of regulatory T cells. FL/eMZL, n = 13; CLL, n = 8; MGUS, n = 6 patients. (C) Retrospective analysis of data from the diagnostic immunophenotyping laboratory of the Freiburg Department of Hematology/Oncology. T-cell counts were calculated from the percentage of T-cell subsets as measured by flow cytometry and the absolute lymphocyte count provided by the central clinical laboratory. T-cell subsets were compared between clinical stages as documented in the patients' records. Patient numbers are indicated in the graphs.
Absolute numbers of circulating lymphocyte subpopulations in patients with indolent B-cell lymphomas and healthy donors. Whole blood samples were stained and analyzed by flow cytometry within 2 hours after phlebotomy. Horizontal bars, boxes, and whiskers represent median, 25%/75% quartiles, and range, respectively. (A) Bead-calibrated absolute cell numbers of CD4+, CD8+, and NK lymphocyte subsets. FL, n = 11; eMZL, n = 7; CLL, n = 9; MGUS, n = 6 patients. (B) Bead-calibrated absolute cell numbers of regulatory T cells. FL/eMZL, n = 13; CLL, n = 8; MGUS, n = 6 patients. (C) Retrospective analysis of data from the diagnostic immunophenotyping laboratory of the Freiburg Department of Hematology/Oncology. T-cell counts were calculated from the percentage of T-cell subsets as measured by flow cytometry and the absolute lymphocyte count provided by the central clinical laboratory. T-cell subsets were compared between clinical stages as documented in the patients' records. Patient numbers are indicated in the graphs.
In contrast to FL and eMZL, increased numbers of circulating helper and cytotoxic T cells, as well as NK and NKT cells were observed in very early CLL (Figure 1A and data not shown). Naive and memory T cells were not significantly altered in CLL. No significant abnormalities of cellular immunity were detected in MGUS patients. Given the common pattern of alterations in FL and eMZL, these indolent lymphomas were analyzed together in subsequent assays and contrasted with CLL.
The numbers of circulating Treg, defined as CD4lowCD25hi in absolute bead-calibrated measurements or as CD4lowFoxP3+ for density gradient-isolated cells, were also reduced in indolent lymphomas (Figure 1B). However, because of the larger reduction of total and naive CD4+ cells, there was a tendency for a relative increase of Treg within the CD4+ compartment (P = .055) as observed by others.22 In CLL and MGUS, absolute and relative numbers (Figure 1B and data not shown) of Tregs were not detectably altered.
To assess whether the T-cell perturbations in FL and CLL were correlated with the gross tumor burden, we retrospectively analyzed all available immunophenotyping data from untreated patients from our diagnostic laboratory (Figure 1C). There was a more pronounced decrease in CD4+ T cells in nonleukemic stage 4 FL patients compared with earlier stages. In leukemic FL, however, CD4+ T-cell counts were higher than in nonleukemic stage 4 cases. In contrast, there was no progressive increase in CD4+ or CD8+ T cells from very early to stage 2 CLL.
Functional immunophenotype of circulating T-cell subpopulations
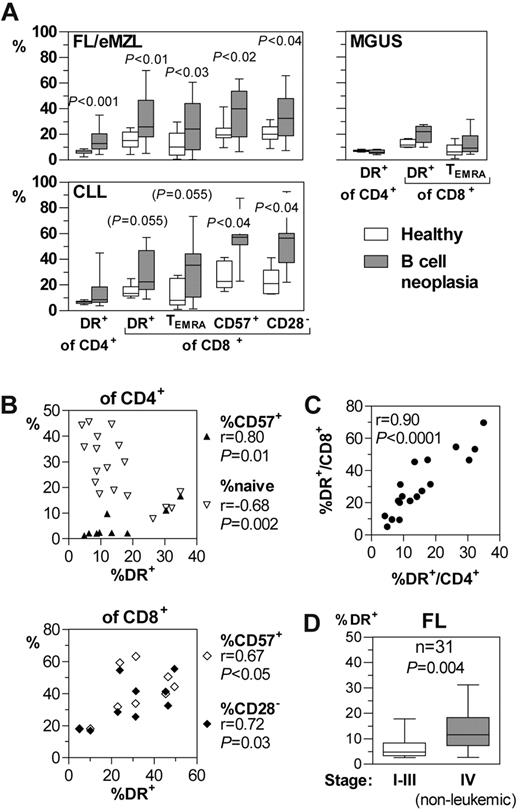
The percentages of activated (HLA-DR+) T helper and cytotoxic T cells were increased in FL and eMZL patients compared with healthy donors (Figure 2A). Recently activated CD69+ and OX40+ T cells were completely absent from the peripheral blood of these patients (data not shown), suggesting that the T-cell activation takes place outside the circulation. Terminally differentiated CD45RA+CD27− effector memory (TEMRA) and senescent (CD57+ or CD28−) cells were increased within the circulating CD8+ compartment. Because of the absence of the corresponding subpopulations within the CD4+ compartment in the majority of healthy probands and lymphoma patients, their numbers could not be meaningfully analyzed.
Functional immunophenotype of circulating T-cell subpopulations in patients with indolent B-cell malignancies. Whole blood samples were stained and analyzed by flow cytometry within 2 hours after phlebotomy. (A) Selected subpopulations of activated and senescent T cells compared with healthy donors. Horizontal bars, boxes, and whiskers represent median, 25%/75% quartiles, and range, respectively. FL/eMZL, n = 18; CLL, n = 9; MGUS, n = 6 patients. (B) Quantitative correlations between HLA-DR expression as activation marker with naive cells and senescence markers in CD4+ and CD8+ T cells of patients with FL/eMZL. (C) Quantitative correlation of activated HLA-DR+ cells between CD4+ and CD8+ T-cell compartments. (D) Retrospective analysis of data from the diagnostic immunophenotyping laboratory of the Freiburg Department of Hematology/Oncology. The fractions of activated HLA-DR+ T cells in 31 FL patients were compared between clinical stages as documented in the patients' records.
Functional immunophenotype of circulating T-cell subpopulations in patients with indolent B-cell malignancies. Whole blood samples were stained and analyzed by flow cytometry within 2 hours after phlebotomy. (A) Selected subpopulations of activated and senescent T cells compared with healthy donors. Horizontal bars, boxes, and whiskers represent median, 25%/75% quartiles, and range, respectively. FL/eMZL, n = 18; CLL, n = 9; MGUS, n = 6 patients. (B) Quantitative correlations between HLA-DR expression as activation marker with naive cells and senescence markers in CD4+ and CD8+ T cells of patients with FL/eMZL. (C) Quantitative correlation of activated HLA-DR+ cells between CD4+ and CD8+ T-cell compartments. (D) Retrospective analysis of data from the diagnostic immunophenotyping laboratory of the Freiburg Department of Hematology/Oncology. The fractions of activated HLA-DR+ T cells in 31 FL patients were compared between clinical stages as documented in the patients' records.
In indolent lymphoma patients, T-cell activation was quantitatively correlated to conversion into TEMRA and senescent cells (Figure 2B). Furthermore, the degree of T-cell activation was tightly correlated between the CD4+ and CD8+ subpopulations (Figure 2C). Like the absolute CD4+ T-lymphocytopenia (Figure 1C), immune activation as indicated by the fraction of HLA-DR+ T helper cells increased with stage in FL in a retrospective analysis from routine diagnostic data (Figure 2D).
In contrast to the indolent lymphomas, immune activation in very early CLL patients was confined to the CD8+ compartment (Figure 2A). No significant changes of these subpopulations were detectable in MGUS.
T-helper cell turnover in patients with chronic B-cell malignancies
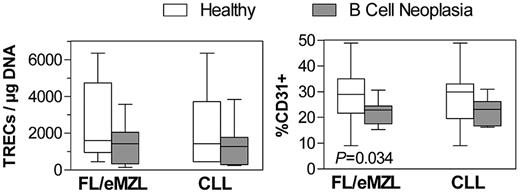
To explain the abnormal circulating CD4+ cell numbers in indolent lymphomas and very early CLL (Figure 1A), we assessed the thymic output through measurement of the content of TRECs by quantitative PCR and by expression of CD31 in purified CD4+ cells. The median TREC content was only slightly reduced in lymphoma patients compared with healthy donors (Figure 3). The fraction of CD31+CD4+ recent thymic emigrants as measured by flow cytometry was significantly reduced in indolent lymphomas but not in CLL. The percentage of proliferating, Ki-67-expressing CD4+ cells was unaltered in any of the analyzed diagnoses (data not shown).
Circulating recent thymic emigrant T cells in patients with indolent B-cell malignancies compared with healthy donors. Left panel: Content of TCR excision circles in DNA extracted from isolated CD4+ T cells as measured by calibrated quantitative PCR. Right panel: Fraction of CD31+ cells within the circulating CD4+ T-cell compartment as measured by flow cytometry. FL/eMZL, n = 13; CLL, n = 8 patients.
Circulating recent thymic emigrant T cells in patients with indolent B-cell malignancies compared with healthy donors. Left panel: Content of TCR excision circles in DNA extracted from isolated CD4+ T cells as measured by calibrated quantitative PCR. Right panel: Fraction of CD31+ cells within the circulating CD4+ T-cell compartment as measured by flow cytometry. FL/eMZL, n = 13; CLL, n = 8 patients.
Aberrant reactivity to immune receptor stimulation in circulating T helper cells
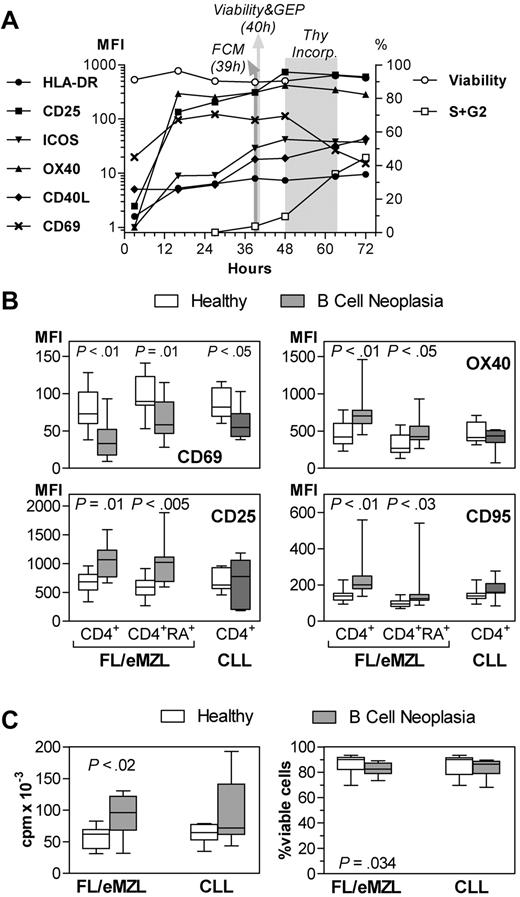
We next characterized the functional consequences of the immunophenotypic alterations of T lymphocytes of patients with chronic B-cell malignancies by investigating their ability to respond to immune receptor-mediated activation signals. Isolated CD4+ T cells were stimulated in vitro by binding of CD3 and CD28. The induced expression of activation markers was measured by flow cytometry after 39 hours. At this time point, the expression of activation markers, except for HLA-DR, had reached a plateau phase in the normal T-cell cultures, but the cells had not yet divided (Figure 4A). The stimulation-induced expression of CD25, CD95, and OX40 was significantly higher in indolent lymphomas than in healthy donors (Figure 4B). In accordance with this hyperactivated phenotype, T cells from lymphoma patients underwent a more vigorous proliferation in vitro (Figure 4C). In contrast, the early activation marker CD69 was significantly underexpressed in stimulated T cells from lymphoma patients. The expression of HLA-DR, which is typically up-regulated after 3 days, of CD40L, and of ICOS did not differ between patients and controls (data not shown). The hyperreactivity was accompanied by a marginally decreased viability of the cells after 40 hours of culture (Figure 4C). Except for a reduction in expression of CD69, T helper cells from patients with early CLL showed a normal response to in vitro stimulation with respect to up-regulation of activation markers, proliferation, and viability.
Reactivity of circulating CD4+ T cells of patients with indolent B-cell malignancies to in vitro stimulation. MFI indicates mean fluorescence intensity. FL/eMZL, n = 13; CLL, n = 8 patients. (A) Kinetics of activation marker expression, viability, and cell cycle of isolated CD4+ T cells after in vitro stimulation with anti-CD3 and anti-CD28. Results from one representative of a series of pilot experiments with T cells from healthy donors. FCM indicates flow cytometry; GEP, gene expression profiling; and Thy, thymidine. (B) Expression of activation markers in total and naive (CD45RA+) subsets of CD4+ T cells from nonleukemic lymphoma and CLL patients compared with healthy donors 39 hours after stimulation. (C) Proliferative response as determined by thymidine incorporation between 48 and 64 hours (left panel) and viability at 40 hours after stimulation as determined by annexin V staining and flow cytometry. Horizontal bars, boxes, and whiskers represent median, 25%/75% quartiles, and range, respectively.
Reactivity of circulating CD4+ T cells of patients with indolent B-cell malignancies to in vitro stimulation. MFI indicates mean fluorescence intensity. FL/eMZL, n = 13; CLL, n = 8 patients. (A) Kinetics of activation marker expression, viability, and cell cycle of isolated CD4+ T cells after in vitro stimulation with anti-CD3 and anti-CD28. Results from one representative of a series of pilot experiments with T cells from healthy donors. FCM indicates flow cytometry; GEP, gene expression profiling; and Thy, thymidine. (B) Expression of activation markers in total and naive (CD45RA+) subsets of CD4+ T cells from nonleukemic lymphoma and CLL patients compared with healthy donors 39 hours after stimulation. (C) Proliferative response as determined by thymidine incorporation between 48 and 64 hours (left panel) and viability at 40 hours after stimulation as determined by annexin V staining and flow cytometry. Horizontal bars, boxes, and whiskers represent median, 25%/75% quartiles, and range, respectively.
Activation-associated transcriptome alterations in circulating T helper cells
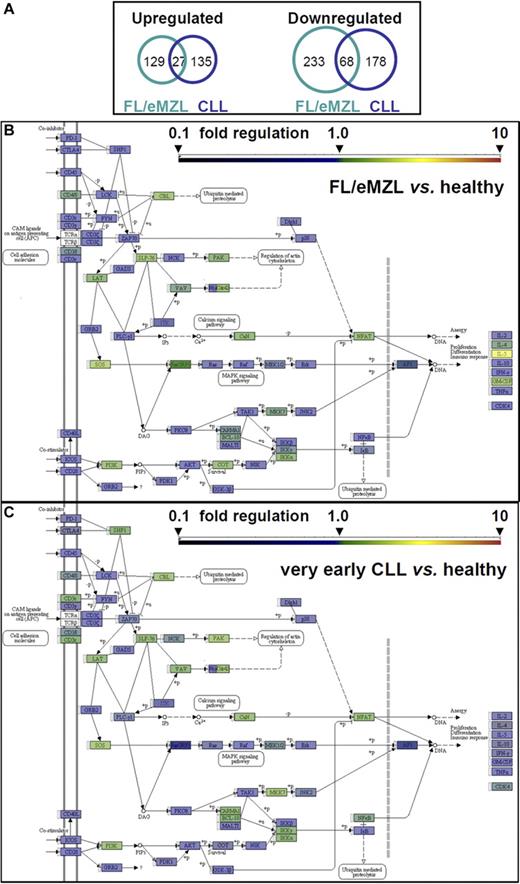
Gene expression profiling of in vitro–stimulated CD4+ cells was performed to further define the lymphoma-associated aberrant stimulation response of T helper cells at the transcriptomic level. A similar overall number of genes was significantly dysregulated in T cells of indolent lymphoma and CLL patients (457 and 408, respectively) with a low likelihood of false positivity (P < .001; Q < 0.05; supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Aberrantly down-regulated genes outnumbered up-regulated genes in both diagnostic groups (Figure 5A). Despite this similarity, few dysregulated genes were common to both lymphoma groups (Figure 5A; supplemental Table 1). No significant gene expression abnormalities were noted in CD4+ cells from MGUS patients (not shown).
Gene expression profiling of CD4+ T cells from patients with indolent B-cell malignancies compared with healthy donors 40 hours after in vitro stimulation. (A) Proportional Venn diagram of transcript numbers with highly significant (P < .001, Q < 0.05) up- or down-regulation in stimulated T cells. (B-C) Schematic diagram of the KEGG TCR signaling pathway (KEGG #4660). Fold changes in transcript levels between T cells from B-cell malignancies and age-matched healthy donors are color-coded according to the bar scale. NFAT box represents expression value of NFATc2; and RAS box, KRAS. For numerical values and statistical significance, see supplemental Table 2. (B) TCR signaling in FL/eMZL (n = 13) versus healthy donors. (C) TCR signaling in CLL (n = 8) versus healthy donors.
Gene expression profiling of CD4+ T cells from patients with indolent B-cell malignancies compared with healthy donors 40 hours after in vitro stimulation. (A) Proportional Venn diagram of transcript numbers with highly significant (P < .001, Q < 0.05) up- or down-regulation in stimulated T cells. (B-C) Schematic diagram of the KEGG TCR signaling pathway (KEGG #4660). Fold changes in transcript levels between T cells from B-cell malignancies and age-matched healthy donors are color-coded according to the bar scale. NFAT box represents expression value of NFATc2; and RAS box, KRAS. For numerical values and statistical significance, see supplemental Table 2. (B) TCR signaling in FL/eMZL (n = 13) versus healthy donors. (C) TCR signaling in CLL (n = 8) versus healthy donors.
Pathway analyses revealed a significant transcriptional dysregulation of the TCR signaling cascade with dominance of down-regulated over up-regulated genes in T helper cells from indolent lymphoma patients (Table 3; Figure 5B). Transcriptional down-regulation was prominent in proximal (CD3G, CD28, FYN, GRB2, and ITK) and intermediate TCR signaling factors (KRAS/MAPK, PDK1, RHOA, and MALT1; supplemental Table 2). An even more severe down-regulation of TCR signaling existed in CD4+ cells from early CLL patients as indicated by a larger number of repressed genes and a higher statistical significance (Table 3; Figure 5C). In CLL, the list of down-regulated genes also extended to other pathways of high importance for immune function, cell adhesion and cytokine signaling. Another difference between indolent lymphomas and early CLL was an up-regulation of the cytokine IL-5 and, to a lesser degree, of IL-4 and granulocyte-macrophage colony-stimulating factor, in FL/eMZL T cells. In contrast, all effector cytokines, including IL-5, were underexpressed in CLL T cells. These findings point to a T helper 2 (Th2) shift in indolent lymphomas that was supported further through overexpression of GATA-3 (mean expression ratio, 1.6; P = .0014; Q = 0.052).26
Down-regulated transcriptional pathways in CD4+ cells from patients with indolent lymphoma (n = 13) or very early CLL (n = 8) after αCD3/αCD28 stimulation
| Group/pathway . | KEGG no. . | P . | Q . |
|---|---|---|---|
| FL/eMZL | |||
| TCR signaling | 4660 | < 10−5 | < 0.001 |
| CLL | |||
| TCR signaling | 4660 | < 10−8 | < 10−6 |
| Cell adhesion molecules | 4514 | < .001 | 0.034 |
| Cytokines | 4060 | < .001 | 0.042 |
| Group/pathway . | KEGG no. . | P . | Q . |
|---|---|---|---|
| FL/eMZL | |||
| TCR signaling | 4660 | < 10−5 | < 0.001 |
| CLL | |||
| TCR signaling | 4660 | < 10−8 | < 10−6 |
| Cell adhesion molecules | 4514 | < .001 | 0.034 |
| Cytokines | 4060 | < .001 | 0.042 |
Each probe set group was analyzed with Fisher exact test against all probe sets of the HGU133Plus2.0 Affymetrix Chip and assigned to the current KEGG pathway annotation (KEGG release 52, September 2009, 358 pathways).
Cytokine production of stimulated T helper cells
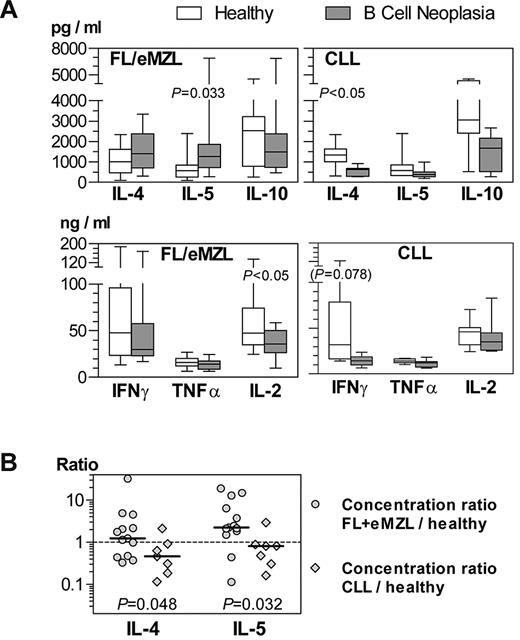
Consistent with the gene expression profiling results, CD4+ cells from patients with indolent lymphomas produced higher amounts of IL-5 on in vitro stimulation than cells from healthy donors (Figure 6A). In contrast, IL-4 was significantly underproduced by CLL T cells. Th1 cytokines had a tendency to be undersecreted compared with normal T cells in both indolent lymphomas and CLL. Calculation of IL-4 and IL-5 production as expression ratios between lymphoma patient and the respective healthy donor confirmed that the Th2 shift in FL and eMZL T cells was significant compared with CLL (Figure 6B).
Cytokine profiles of stimulated CD4+ T cells from patients with indolent B-cell malignancies compared with healthy donors. Culture supernatants collected at 40 hours after anti-CD3/anti-CD28 stimulation were analyzed by cytometric bead arrays. (A) Cytokine concentrations in supernatants. Horizontal bars, boxes, and whiskers represent median, 25%/75% quartiles, and range, respectively. FL/eMZL, n = 13; CLL, n = 8 patients. (B) Calculated concentration ratios of IL-4 and IL-5 between culture supernatants of T cells from indolent lymphoma patients and their respective healthy donor. Comparison of concentration ratios between FL/eMZL and CLL patients.
Cytokine profiles of stimulated CD4+ T cells from patients with indolent B-cell malignancies compared with healthy donors. Culture supernatants collected at 40 hours after anti-CD3/anti-CD28 stimulation were analyzed by cytometric bead arrays. (A) Cytokine concentrations in supernatants. Horizontal bars, boxes, and whiskers represent median, 25%/75% quartiles, and range, respectively. FL/eMZL, n = 13; CLL, n = 8 patients. (B) Calculated concentration ratios of IL-4 and IL-5 between culture supernatants of T cells from indolent lymphoma patients and their respective healthy donor. Comparison of concentration ratios between FL/eMZL and CLL patients.
Patterns of aberrant T-cell physiology in patients with chronic B-cell malignancies
Quantitative immunophenotyping and functional studies had revealed both common and differing aberrancies between CD4+ T cells from patients with indolent lymphomas and very early CLL: A prominent depletion of naive CD4+ T cells, an activated phenotype of circulating T cells in vivo, a hyperreactivity to in vitro stimulation, and a functional Th2 shift was observed predominantly in the indolent lymphomas. In contrast, a transcriptional down-regulation of TCR signaling with impaired expression of Th1 cytokines was common to all analyzed B-cell malignancies but more pronounced in early CLL.
To explore whether these observations could be assembled into distinct general patterns of T-cell dysfunction, we performed comprehensive correlative analyses of the aberrant parameters of CD4+ T-cell physiology. In the indolent lymphomas, numerical aberrancies in circulating absolute subpopulations (total and naive CD4+), steady-state immune activation (% HLA-DR+), hyperreactivity in vitro (MFI CD95), and Th2 shift (IL-5) formed a distinctive cluster of significantly (P < .05) correlated quantitative parameters (Table 4; supplemental Table 2). In contrast, IL-5 and parameters of cell activation and hyperreactivity had no correlation to the transcriptional disturbance of the TCR signaling cascade. Correlations of activation markers as measured by flow cytometry with the transcriptional pathways of cytokines, cell adhesion, apoptosis, DNA synthesis, and various metabolic processes were predicted from the functional studies and provided independent validation for the gene expression profiling results at the protein expression level.
Correlation between aberrant parameters of the CD4+ cellular compartment of FL/eMZL patients (n = 13)
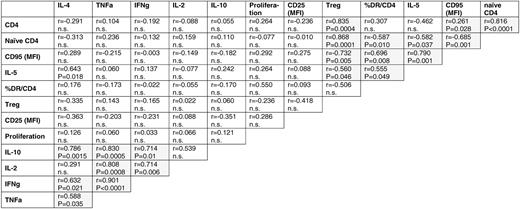
Overrepresentation of KEGG pathways (left column) among probe set groups correlating with parameters of aberrant T-cell physiology (upper row) in CD4+ cells from FL/eMZL patients (n = 13)
| KEGG pathway no., title . | IFNγ . | TNFα . | IL-4 . | IL-10 . | IL-5 . | % DR+ . | CD95 (MFI) . | OX40 (MFI) . | CD69 (MFI) . |
|---|---|---|---|---|---|---|---|---|---|
| 4660, TCR signaling | < 10−6 | < 10−5 | < 10−9 | < 10−5 | NS | NS | NS | NS | NS |
| 4060, Cytokines | NS | NS | NS | NS | NS | NS | < 10−11 | < 10−6 | NS |
| 4514, Cell adhesion molecules | NS | NS | NS | NS | NS | NS | < 10−10 | < 10−7 | < 0.0001* |
| 3030, DNA replication | NS | NS | NS | NS | NS | < 10−10 | NS | NS | < 0.001* |
| 0230, Purine metabolism | NS | NS | NS | NS | NS | < 10−5 | NS | NS | NS |
| 0240, Pyrimidine metabolism | NS | NS | NS | NS | NS | < 10−5 | NS | NS | < 0.0001* |
| 1100, Metabolic pathways | NS | NS | NS | NS | NS | NS | NS | NS | < 10−5* |
| 0970, Aminoacyl-tRNA biosynthesis | NS | NS | NS | NS | NS | NS | NS | NS | < 10−5* |
| 4210, Apoptosis | NS | NS | NS | NS | NS | NS | < 10−7 | NS | NS |
| KEGG pathway no., title . | IFNγ . | TNFα . | IL-4 . | IL-10 . | IL-5 . | % DR+ . | CD95 (MFI) . | OX40 (MFI) . | CD69 (MFI) . |
|---|---|---|---|---|---|---|---|---|---|
| 4660, TCR signaling | < 10−6 | < 10−5 | < 10−9 | < 10−5 | NS | NS | NS | NS | NS |
| 4060, Cytokines | NS | NS | NS | NS | NS | NS | < 10−11 | < 10−6 | NS |
| 4514, Cell adhesion molecules | NS | NS | NS | NS | NS | NS | < 10−10 | < 10−7 | < 0.0001* |
| 3030, DNA replication | NS | NS | NS | NS | NS | < 10−10 | NS | NS | < 0.001* |
| 0230, Purine metabolism | NS | NS | NS | NS | NS | < 10−5 | NS | NS | NS |
| 0240, Pyrimidine metabolism | NS | NS | NS | NS | NS | < 10−5 | NS | NS | < 0.0001* |
| 1100, Metabolic pathways | NS | NS | NS | NS | NS | NS | NS | NS | < 10−5* |
| 0970, Aminoacyl-tRNA biosynthesis | NS | NS | NS | NS | NS | NS | NS | NS | < 10−5* |
| 4210, Apoptosis | NS | NS | NS | NS | NS | NS | < 10−7 | NS | NS |
Data are P values. Probe set groups correlating (P < .05) with the parameters of the upper row (cytokine concentrations in supernatants or surface marker MFI) were obtained through a rank profile distance search of expression levels for all probe sets. Overrepresentations of KEGG pathways were considered significant according to Bonferroni adjustment for multiple testing (n = 358 KEGG pathways) when P values were < 1.4 × 10−4. Only selected pathways of immunologic interest are shown.
NS indicates not significant.
Inverse correlation.
Discussion
Prompted by the observations of reduced T-cell counts24 and the failure of most patients with untreated indolent B-cell lymphomas to respond to hepatitis B vaccination,27 we present here the results of an unbiased, prospective study to identify and characterize numerical and functional defects in circulating lymphocytes from patients with untreated chronic B-cell malignancies. We used calibrated methodologies for accurate measurements of absolute cell counts rather than relative frequencies as in most published studies.20-22 The principle of a matched-pair comparison with age-matched healthy donors and the parallel analysis of patients with very early CLL and MGUS permitted reliable statistical comparisons. Our principal result is the identification of 2 distinct and complex patterns of dysregulation of the T helper cell compartment in chronic B-cell malignancies.
First pattern of T helper cell dysfunction: chronic immune activation with Th2 shift, in vitro hyperreactivity, and T-cell senescence with propensity to undergo apoptosis
The first, hitherto unrecognized T-cell aberration exists to a similar extent in FL and eMZL. The hallmark of this pattern is a prominent reduction of naive T helper cells and recent thymic emigrants. In addition, the pattern composes an activated T helper phenotype in vivo, a Th2 shift, a hyperreactivity to TCR and CD28 costimulation in vitro, and an increased expression of CD95, which indicates a propensity to an accelerated activation-induced cell death. This complex type of T-cell dysfunction was not associated with transcriptional alterations of the TCR signaling cascades on in vitro stimulation.
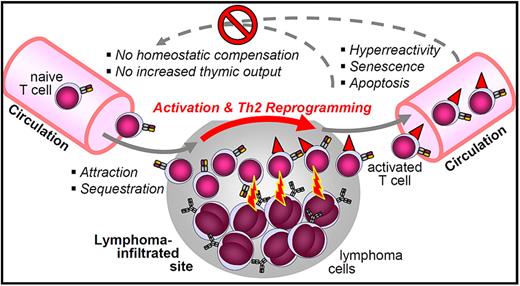
The systemic nature of this T helper cell aberration in nonleukemic cases points to its induction at anatomic lymphoma sites in a “local action–global effects” manner (Figure 7). Naive T cells appear to be attracted to and to be sequestered at lymphoma sites. This redistribution could be mediated by chemokines, such as CCL17 and CCL22.23 In the lymphoma microenvironment, these T cells presumably become activated, lose their naive phenotype, and are reprogrammed toward a lasting Th2 differentiation. Secretion of the Th2 cytokine IL-4 by intratumoral T cells appears to play an important role in the FL microenvironment.28 Recently activated, CD69+ or OX40+ T helper cells were absent from the blood of our patients, whereas they abound at indolent lymphoma sites.29-31 Some of these aberrantly activated T cells reenter the circulation, where they respond faster to immune receptor stimulation. On the other hand, these cells are already presenescent and prone to undergo apoptosis, facilitated by transcriptional up-regulation of the apoptosis cascade.
A model of the “local action–global consequences” T-cell dysfunction in nonleukemic indolent lymphomas. This hypothetical model integrates the correlated parameters of naive T-cell depletion, activated and presenescent phenotype, hyperreactivity to TCR/CD28 costimulation, Th2 shift, and lack of compensation of circulating T-cell counts.
A model of the “local action–global consequences” T-cell dysfunction in nonleukemic indolent lymphomas. This hypothetical model integrates the correlated parameters of naive T-cell depletion, activated and presenescent phenotype, hyperreactivity to TCR/CD28 costimulation, Th2 shift, and lack of compensation of circulating T-cell counts.
Taken together, the CD4+ cytopenia of nonleukemic indolent lymphomas could therefore result from a redistribution to the tumor site and also from an increased rate of activation-induced apoptosis. Because circulating total and naive CD4+ cell counts correlate inversely with immune activation (Table 5), redistribution and activation-induced apoptosis probably act in concert to decrease the circulating CD4+ cell counts in indolent lymphomas. The magnitude of these effects appears to increase with overall tumor burden as indicated by more pronounced T helper cell activation and worsening CD4+ lymphocytopenia in nonleukemic stage 4 disease compared with earlier stages (Figures 1C, 2D).
This pattern was not seen in very early CLL. However, T-cell activation and changes of cytokine secretion consistent with a Th2 shift have been described for advanced CLL.32-34 Furthermore, the redistribution of T helper cells toward tumor cells would not contribute to CD4+ lymphocytopenia in CLL because the circulating blood is itself an important tumor “site.” This effect may also explain why CD4+ cell counts in leukemic FL were higher than in nonleukemic stage 4 disease (Figure 1C).
Leaving the modulating effects of the anatomic distribution of malignant B cells (leukemic vs nodal/extranodal) aside, the observed differences in the extent of the T helper cell aberrancies indicate major pathophysiologic differences between indolent lymphomas and CLL: The tumor burden of even very early CLL, where this type of immune dysregulation was undetectable, corresponds to an advanced, leukemic stage 4 FL. However, even in early-stage indolent lymphomas, this T-cell dysregulation pattern was prominent. Therefore, it can be concluded that specific properties of FL and eMZL cells induce this aberrant T helper cell physiology much more potently than CLL cells.
Finally, evidence for similar immunologic aberrations has also been described in multiple myeloma,35,36 in patients with autoimmune diseases (eg, systemic lupus and rheumatoid arthritis),37-39 and even in healthy but immunosenescent persons.40 Chronic B-cell malignancies and chronic inflammatory conditions may therefore share a common fundamental pathology of the T-cell compartment that is not necessarily accompanied by serologic parameters of inflammation.
Second pattern of T helper cell dysfunction: down-regulation of TCR signaling and cytokine secretion
The second pattern of aberrant T-cell physiology is characterized by reduced expression of numerous genes involved in the TCR signaling cascade on TCR and CD28 costimulation. At the protein level, this pattern results in a reduced secretion of cytokines. Among the list of down-regulated genes are members of the nuclear factor-κB pathway and PI3KCB, which have also been identified as repressed by gene expression profiling of unstimulated CD4+ T cells from CLL patients at early and advanced stages.16 Furthermore, the transcriptional pattern of unstimulated T helper cells of CLL patients was predicted to mediate a reduction in Th1 differentiation,16 which was indeed seen in our gene expression profiling of stimulated CD4+ cells. Overall, the similarities between our results and the findings by Gorgun et al16 suggest largely identical transcriptome alterations. However, our data show that the detectable transcriptomic differences between healthy and CLL T cells are more pronounced after in vitro stimulation, with respect to both the number of dysregulated genes and their level of statistical significance.
A consequence of this type of T-cell dysfunction is a severely impaired formation of the immunologic synapse, which was described not only in CLL but also in leukemic cases of FL.17,18 Similar changes can be experimentally induced in T cells from healthy donors by direct cell contact with either CLL or FL cells.17,18 The results from our stimulation cultures, in contrast, demonstrate that transcriptional impairment of TCR signaling in circulating T helper cells is not only present in very early CLL, but also in patients with nonleukemic indolent lymphomas. Therefore, the aberrant transcriptional program of circulating T cells that is induced by cell-to-cell contact apparently persists after reentry of T cells into the circulation.
Both dysregulation patterns of T cells (the activation/Th2 shift and down-regulation of TCR signaling) appear to operate independent of each other. However, because they occur simultaneously, particularly in leukemic lymphomas, some aspects of both patterns may be counterbalanced or enhanced. For example, local T-cell activation may enhance the down-regulation of the TCR signaling cascade as seen in healthy T cells after in vitro stimulation.41 As another example, the globally reduced cytokine production secondary to down-regulation of the TCR signaling cascade may explain why the secretion of IL-4 in the context of the Th2 shift in indolent lymphomas was less evident than the production of IL-5 (Figure 6A).
The potential role of Treg in the pathophysiology of indolent B-cell lymphoma
Our study does not provide evidence for a significant role for Tregs early in the pathogenesis of indolent lymphomas. Studies performed in B-NHL, CLL, and MGUS/myeloma have suggested a relative increase of Treg in these diseases.20-22 In contrast, our calibrated measurements of absolute cell numbers reveal that circulating Tregs are indeed reduced compared with healthy donors. An apparent relative increase of Tregs was caused by the dominant decrease of naive T cells. Furthermore, Treg numbers were not correlated with any of the 2 principal patterns of immune aberration. Finally, the hyperreactivity of stimulated CD4+ T cells from FL/eMZL patients would fit better with a reduced rather than with an enhanced Treg activity.
However, our analyses pertain only to circulating lymphocytes and hence to the systemic immune status. Therefore, our study does not address frequency, distribution pattern, or function of Tregs in the lymphoma microenvironment, parameters that have been implicated in the pathogenesis and prognosis of FL.42-44
Clinical implications of T-cell dysfunction in indolent B-cell lymphoma
An improved understanding of an impaired immunity in indolent lymphomas may be clinically important for several reasons. First, the failure of hepatitis B vaccination in the majority patients with untreated indolent lymphomas indicates an impaired immune defense against infections.27 A restoration of immune function could not only permit efficacious vaccination of lymphoma patients but could potentially improve outcome in view of the well-established association of immunodeficiency with lymphomagenesis and prognosis.1,2
The unresponsiveness to vaccination may be caused by either pattern of T-cell dysregulation as defined in our study. A high proportion of senescent CD8+CD28− T cells, as observed in chronic immune activation and inflammation,45 predicts failure to influenza vaccination.46 A contracted naive T-cell pool might reduce the frequency of T cells expressing a TCR that recognizes a foreign neoantigen, such as HBs. Finally, transcriptional down-regulation of TCR signaling may also impair T-cell responsiveness to antigenic stimuli. Although we cannot attribute the T-cell deficiency of indolent lymphoma patients with certainty to either of the 2 distinct dysregulation patterns, the dominance of the immune activation/Th2 shift in indolent lymphomas suggests this as the more relevant pattern.
Beyond the aspects of immune defense against infections and malignancy, a reversion of the Th2 shift in indolent lymphomas may be important because Th2-polarized murine T cells that directly interact with normal B cells cause the development of frank B-cell lymphoma.10 In addition, the immune activation/Th2 dysregulation pattern may represent a molecular link between autoimmunity and indolent B-cell lymphoma and offer potential clues to the epidemiologic association of both conditions.37-39 For example, an increased expression of OX40 and a reduced expression of CD69, which we describe as systemic consequence of indolent lymphomas, are features known to contribute to the breakdown of T-cell tolerance.47,48
Finally, the influence of the tumor microenvironment on the prognosis of FL is being increasingly recognized.42,49 Existing evidence suggests that the signature of the immune microenvironment could correlate with the extent of systemic T-cell dysregulation, especially T-cell redistribution with immune activation (immune activation/Th2 pattern).29,42,50 In-depth studies of such interactions could establish a prognostic relevance of the systemic immune perturbations and provide insight into the mechanisms as to how anatomically localized interactions between T cells and B-lymphoma cells can affect global immunity.
In conclusion, we systematically characterize the systemic immune status in groups of patients with chronic B-cell malignancies that have not been studied prospectively with respect to global immune function: Patients with indolent germinal center-derived, nonleukemic B-cell lymphomas, and CLL patients in the earliest stage with a normal life expectancy. We delineate 2 distinct patterns of T-cell dysregulation in relationship to tumor burden and anatomic distribution, a chronic activation/Th2 reprogramming pattern and a pattern dominated by down-regulation of the TCR signaling cascade. An improved understanding of the mutual influences between the immune system and lymphoma is a prerequisite to develop successful strategies for the manipulation of the lymphoma-promoting or -controlling immune activity. This study provides the basis to assess the effects of such interventions with the appropriate assays. Reversal of lymphoma-associated immune dysfunction may offer novel opportunities to protect lymphoma patients better from infectious complications and to reprogram the immune system from lymphoma-promoting effects toward adverse conditions for the malignant B cells. A restored immune system may also offer improved immunologic efficacy of active antilymphoma immunotherapy, such as idiotype vaccination, adoptive cell transfer, and allogeneic stem cell transplantation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by the Research Commission of the Faculty of Medicine at Freiburg University.
Authorship
Contribution: P.C., P.F., M.F., D.P., and H.V. designed the study; P.C. and H.V. wrote the manuscript; P.C. recruited patients and donors and performed experiments; D.P., K.B., and J.T. assisted in statistical analyses of gene expression profiling; and M.F. and P.F. assisted in cell sorting and immunophenotyping.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hendrik Veelken, Leiden University Medical Center, Department of Hematology, Postbus 9600, Postzone C2-R-140, 2300 RC Leiden, The Netherlands; e-mail: j.h.veelken@lumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal