Abstract
Emergency mobilization of neutrophil granulocytes (neutrophils) from the bone marrow (BM) is a key event of early cellular immunity. The hematopoietic cytokine granulocyte-colony stimulating factor (G-CSF) stimulates this process, but it is unknown how individual neutrophils respond in situ. We show by intravital 2-photon microscopy that a systemic dose of human clinical-grade G-CSF rapidly induces the motility and entry of neutrophils into blood vessels within the tibial BM of mice. Simultaneously, the neutrophil-attracting chemokine KC (Cxcl1) spikes in the blood. In mice lacking the KC receptor Cxcr2, G-CSF fails to mobilize neutrophils and antibody blockade of Cxcr2 inhibits the mobilization and induction of neutrophil motility in the BM. KC is expressed by megakaryocytes and endothelial cells in situ and is released in vitro by megakaryocytes isolated directly from BM. This production of KC is strongly increased by thrombopoietin (TPO). Systemic G-CSF rapidly induces the increased production of TPO in BM. Accordingly, a single injection of TPO mobilizes neutrophils with kinetics similar to G-CSF, and mice lacking the TPO receptor show impaired neutrophil mobilization after short-term G-CSF administration. Thus, a network of signaling molecules, chemokines, and cells controls neutrophil release from the BM, and their mobilization involves rapidly induced Cxcr2-mediated motility controlled by TPO as a pacemaker.
Introduction
Neutrophils are the most abundant and, arguably, the most important leukocyte in the vertebrate immune system. Under normal conditions, human bone marrow (BM) produces ∼ 1011 neutrophils per day.1 In “danger situations” such as peripheral infections, the constant release of neutrophils can be dramatically increased within hours, a process termed danger or stress mobilization.2 The hematopoietic cytokine granulocyte-colony stimulating factor (G-CSF) is central to the danger mobilization of neutrophils in both humans and mice.3 However, although recombinant G-CSF has been used in clinical hematology for 20 years, the molecular mechanisms by which it mobilizes neutrophils are still not well understood.
Neutrophils are restrained in the BM by the binding of their chemokine receptor Cxcr4 to the chemokine Cxcl12, which is expressed in a membrane-associated fashion by BM stromal cells.4 There is evidence that G-CSF breaks the Cxcr4-Cxcl12 bond by activating neutrophil proteases,5 thereby releasing neutrophils from the BM into the bloodstream.3,6 However, several findings cannot be explained by the Cxcr4-Cxcl12 breakage concept alone. First, G-CSF can mobilize neutrophils in protease-deficient mice, arguing against the need for protease activation for this process.7,8 Second, because neutrophil-specific deletion of Cxcr4 in mice results in much higher numbers of circulating neutrophils compared with wild-type animals, factors other than Cxcr4 must be involved in steering neutrophils into the BM blood sinuses (unless the process is passive). Finally, the specific Cxcr4-antagonist AMD3100 acts highly synergistically with G-CSF to mobilize hematopoietic stem cells or neutrophils,9 constituting a regimen that is a U.S. Food and Drug Administration (FDA)–approved protocol for stem-cell mobilization.10 Because the Cxcr4-Cxcl12 bond is already disrupted by AMD3100 when this combination of agents is used, the synergism with G-CSF suggests that the cytokine induces an additional trigger to release neutrophils from the BM. To clarify the nature of this stimulus, we investigated G-CSF–mediated stress mobilization using a combination of intravital 2-photon microscopy in BM and immunohistology and cell culture of BM cells. We present evidence that an early signal generated by G-CSF centers on the stimulation of megakaryocytes and other responsive cells by newly released thrombopoietin (TPO), which causes a local and vascular spike of neutro-phil-attracting chemokines and a mobilization of neutrophils via Cxcr2.
Methods
Mice
C57BL/6J mice (8-12 weeks of age) were from Harlan-Winkelmann or the Laboratory Cancer Research United Kingdom Research Institute Animal Facility and housed in specific pathogen–free animal facilities. Animals expressing enhanced green fluorescent protein (EGFP) under the lysozyme promoter11 were provided by T. Graf and bred at Otto von Guericke University (Magdeburg, Germany). Mpl−/− mice12 were bred at the specific pathogen–free facilities of Charité University Medicine (Berlin, Germany). All animal experiments were approved by the Landesverwaltungsamt Sachsen-Anhalt (file number 203.h-42 502-2-879 Uni MD) and by the United Kingdom Home Office. Cxcr2−/− mice13 were originally backcrossed to C57BL/6 mice for 11 generations, and the Cxcr2−/− and control Cxcr2+/+ mice used for experimental purposes were obtained from heterozygous breeder pairs. All mice were genotyped as described previously.14 The Cxcr2 experiments were approved by the University of California-Irvine Institutional Animal Care and Use Committee.
Cells and cell lines
The human megakaryocytic cell line MEG-01 (ATCC, CRL 2021) was maintained in RPMI 1640 (GIBCO) plus 10% fetal calf serum. MEG-01 cells were added at 5 × 105 cells/mL to 24-well plates (Falcon; BD Biosciences) and stimulated with 20 or 50 μg/mL of human G-CSF (hG-CSF; NEUPOGEN; Amgen), 25 ng/mL of TPO (R&D Systems), or medium alone at 37°C. Culture supernatants were analyzed by enzyme-linked immunosorbent assay (ELISA) for interleukin-8 (IL-8; R&D Systems).
BM cells isolated from the femur and tibia were fractionated using a continuous Percoll gradient (Amersham Biosciences). Three major bands were collected at 1.035 g/mL (CD62-P+ megakaryocytes), 1.074 g/mL (F4/80-positive macrophages), and 1.088 g/mL (Gr-1+ neutrophils). Cells were stimulated with 50 μg/mL of hG-CSF (NEUPOGEN), 50 or 100 ng/mL of mouse TPO (R&D Systems), or medium alone at 37°C. Culture supernatants were analyzed by ELISA for keratinocyte-derived chemokine (KC) or macrophage inflammatory protein-2 (MIP-2; R&D Systems). Neutrophils were also obtained by positive selection from mouse BM, as described previously.15 BM cells were isolated as above after intravenous injection for 2 hours with either hG-CSF or phosphate-buffered saline (PBS) as below and then cell tested for TPO production in the supernatant by ELISA (R&D Systems) without further incubation.
hG-CSF and TPO stimulation of mice and various treatment protocols
hG-CSF (100 μL of 25 μg/mL) in PBS or an equivalent volume of endotoxin-free PBS was injected intravenously for various lengths of time. The same treatment protocol was used for intravital imaging under control (PBS injection) or hG-CSF–induced conditions. In some animals, imaging was started 6, 12, or 16 hours after G-CSF injection to demonstrate the physiologic stability of the approach (supplemental Figure 2, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Alternatively, 100 μL of 10 μg/mL murine TPO (R&D Systems) in PBS was injected intravenously, and 2 hours later 75 μL of peripheral blood was analyzed for neutrophil counts. Control animals received 100 μL of PBS intravenously.
Neutrophils.
To determine circulating neutrophil levels, 75 μL of peripheral blood were taken by retro-orbital puncture at 2 hours. After erythrocyte lysis, samples were stained with a phycoerythrin-labeled anti–Gr-1 antibody (clone RB6-8C5) and analyzed by fluorescence-activated cell sorting for the percentage of Gr-1+ cells. For experiments involving Cxcr2−/− mice, blood was taken by cardiac puncture and red cells were lysed by ammonium chloride-potassium chloride buffer. Quantification of absolute numbers of blood neutrophils using phycoerythrin-conjugated anti-Gr1 monoclonal antibody (mAb) compared with the rest of the blood leukocytes after G-CSF versus PBS treatment showed that it was only the neutrophil number that changed (data not shown). Therefore, in the experiments reported in this study, the percentage increase in neutrophil numbers was recorded.
Plasma.
For plasma collection, 200-300 μL of blood was collected by heart puncture over time and was separated by H-1077 Histopaque (Sigma). The plasma was collected and analyzed by ELISA for KC and MIP-2 (R&D Systems).
Cxcr2 blockade.
To block Cxcr2 in vivo, 100 μL of rabbit anti-Cxcr2 antiserum14 was injected intraperitoneally 48 hours before mobilization of cells by G-CSF. Control animals received equal amounts of normal rabbit serum (NRS).
Intravital 2-photon microscopy of cells in the BM of the tibia
Mice were prepared for intravital microscopy in long bones, as described previously16 (supplemental Figure 1). Two-photon microscopy on the tibia was performed using a Zeiss LSM-710 microscope with simultaneous detection via external nondescanned detectors and Zeiss ZEN software (2009 release). Illumination was performed at 800 or 850 nm using a MaiTai TiSa laser via a 20× water-dipping lens with 1.0 NA. Neutrophils were detected by EGFP, blood vessels by injection of rhodamine-B-isothiocyanate–labeled 40-kD dextran (Sigma), and bone by second harmonic generation signal. Images were recorded every 60 seconds for 60 minutes each at the indicated time points after injection of PBS or G-CSF. Raw data were reconstructed using Volocity software 4.0 (PerkinElmer/Improvision). Cell tracking was done manually, as described.15
Cell migration in 3-D collagen matrices
Neutrophil migration in 3-D collagen matrices was analyzed as described previously.15
Immunohistochemistry
BM tissue was harvested in one piece by flushing femurs from naive mice or mice injected intravenously with PBS or hG-CSF for 2 hours. Tissues were fixed for 1 hour at room temperature in 10% neutral buffered formalin and sectioned at 2-4 μm. Sections were stained with rat anti-KC mAb, rat anti–MIP-2 mAb, or biotinylated goat anti–mouse TPO or suitable isotype controls (all from R&D Systems) for 1 hour at room temperature. Biotinylated rabbit anti–rat antibody (Vector Laboratories) or biotinylated rabbit anti–goat antibody were applied for 45 minutes, followed by incubation with avidin-biotin complex solution (PK-6100; Vector Laboratories) for 30 minutes and visualized using diaminobenzidine chromogen solution (BioGenex). For immunofluorescence, murine BM from naive mice was stained with rat mAbs F4/80 (macrophages; eBioscience) and 2B10 (neutrophils; Laboratory Cancer Research United Kingdom, London Research Institute), and with rabbit antibody specific for the G-CSF receptor (M-20; Santa Cruz Biotechnology) for 1 hour at room temperature. Alexa Fluor 488 goat anti–rat or Alexa Fluor 555 goat anti–rabbit (Molecular Probes) antibodies were applied for 1 hour for detection. Slides were immersed in Sudan black (0.1% in 70% industrial methyl spirits) and mounted in hard-set Vectamount with 4′,6-diamidino-2-phenylindole, dihydrochloride (Vector Laboratories). Images were acquired on a 90i Nikon microscope using NIS Elements software Version 3.2.
qRT-PCR of BM
Two hours after systemic stimulation, BM cells were prepared by flushing the femur and tibia with PBS. Total RNA was extracted using the RNeasy MiniKit (QIAGEN), followed by cDNA synthesis using the QuantiTect quantitative reverse-transcription polymerase chain reaction (qRT-PCR) kit (QIAGEN). DNA (100 ng) was used for the qRT-PCR reaction (5 minutes of initial denaturation at 95°C and 40 cycles of 10 seconds/95°C and 30 seconds/60°C) with the QuantiFast SYBR-Green PCR kit (QIAGEN). Primers for β-actin (as an unregulated housekeeping control), Cxcl1-3, Cxcl5, and Cxcl7 were purchased from QIAGEN (https://www.qiagen.com/geneglobe). Data acquisition was performed on an ABI Prism 7000 system, and relative gene expression levels were calculated using the ΔΔCT method.
Statistical analysis
Data are shown as means ± SEM or median (in column scatter plots). Data were analyzed using GraphPad Prism Version 5.0 software using the unpaired Student t test or the 1-way analysis of variance (ANOVA). Differences were significant as follows: *P < .05; **P < .01; and ***P < .001.
Results
Neutrophil motility in long bones is rapidly increased by G-CSF
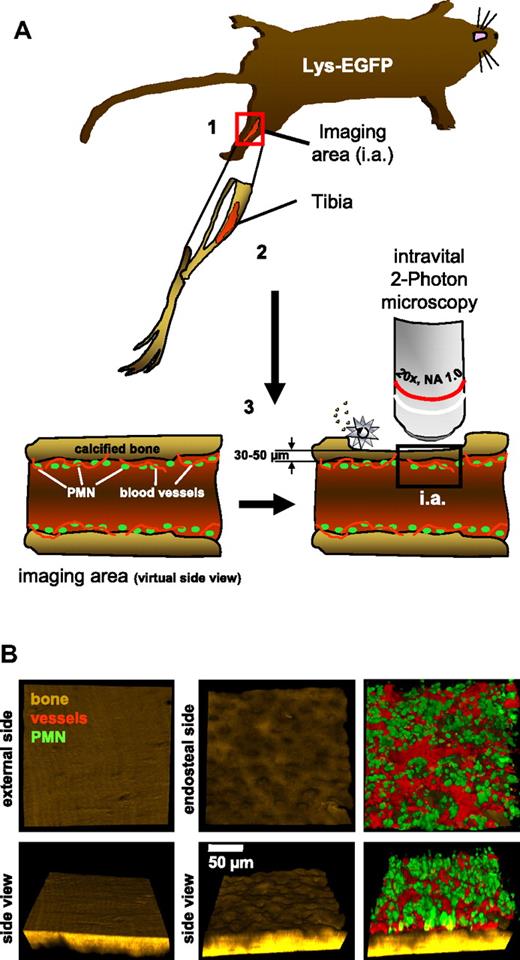
To investigate stress mobilization of neutrophils at the single-cell level in vivo, we established intravital 2-photon microscopy in the long bones of mice carrying a targeted insertion of EGFP in the lysozyme-M locus, therefore harboring mostly mature neutrophils in the brightly stained cell compartment11,17 (Figure 1A and supplemental Figure 1). Expanding on our recently developed novel approach,16 the technique used here allowed simultaneous visualization of the highly structured endosteal surface of the tibia, as well as blood flow in BM vessels and migration of neutrophils in live animals (Figure 1B and supplemental Video 1).
Principal setup for intravital 2-photon microscopy of neutrophils in the tibial BM. (A) (1) Schematic overview of the position of imaging in the experimental animal. The animal is anesthetized during the entire procedure. (2) Schematic drawing of the tibial bone in the respective orientation within the animal. (3) Virtual side view of the tibia to demonstrate the thinning approach using an electric drill (silver star). (B) Sample images taken from intravital experiments. Bone (second harmonic generation signal, brown) demonstrating the fine structure of the calcified tibial bone from the outside and inside. Note the appearance of numerous pits on the endosteal surface. Neutrophils (green) and blood vessels (red) of the same stack are shown in a top view as well as in a 60° rotation to demonstrate the thickness of a typical image stack. The respective video is provided as supplemental Video 1. The images are representative of > 20 animals.
Principal setup for intravital 2-photon microscopy of neutrophils in the tibial BM. (A) (1) Schematic overview of the position of imaging in the experimental animal. The animal is anesthetized during the entire procedure. (2) Schematic drawing of the tibial bone in the respective orientation within the animal. (3) Virtual side view of the tibia to demonstrate the thinning approach using an electric drill (silver star). (B) Sample images taken from intravital experiments. Bone (second harmonic generation signal, brown) demonstrating the fine structure of the calcified tibial bone from the outside and inside. Note the appearance of numerous pits on the endosteal surface. Neutrophils (green) and blood vessels (red) of the same stack are shown in a top view as well as in a 60° rotation to demonstrate the thickness of a typical image stack. The respective video is provided as supplemental Video 1. The images are representative of > 20 animals.
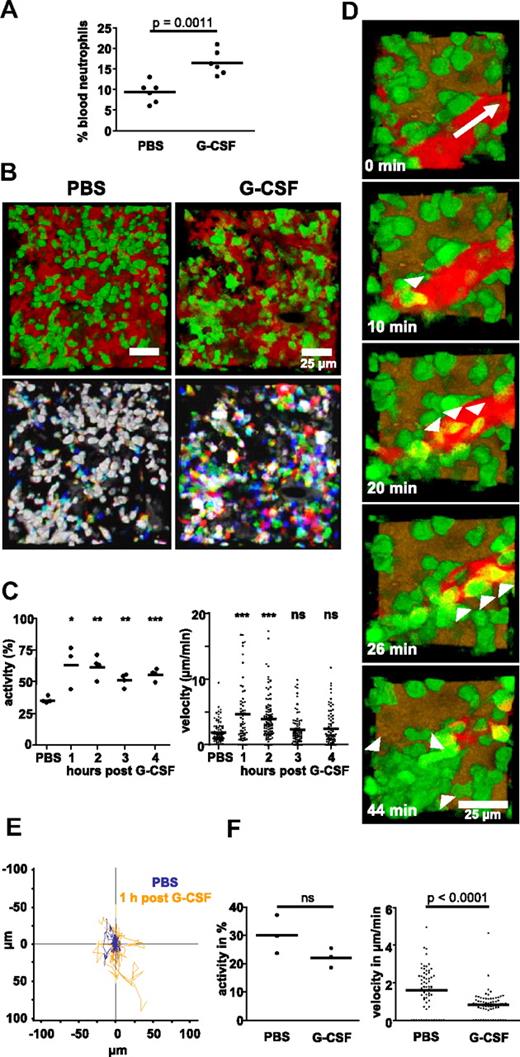
Injection of recombinant, clinical-grade human G-CSF induced a significant increase in the number of circulating neutrophils in the blood of C57/BL6 mice within 2 hours (Figure 2A). This rapid mobilization of neutrophils suggested that a detectable impact on the behavior of single cells should be observable within the BM in vivo. In nonmobilized animals, ∼ 30% neutrophils were motile with a velocity of 1.5 μm/min (Figure 2B-C and supplemental Video 2) and no obvious directionality (Figure 2E). However, within 1 hour after systemic G-CSF administration, we detected a large increase in overall neutrophil motility that recruited up to 75% cells into the mobile fraction and increased their velocity to ∼ 5 μm/min (Figure 2B, C, and E, supplemental Figure 2, and supplemental Video 3). This velocity is in the range measured previously for neutrophils migrating in the footpad18 but slower than their speed measured in skin.17 We also observed the exiting of individual neutrophils into blood vessels and their flushing away with the flow, providing the first direct visualization of neutrophil mobilization in vivo (Figure 2D and supplemental Video 4). This was not observable over a similar time period in nonmobilized mice (data not shown). The increased numbers of motile neutrophils were maintained for at least 16 hours (supplemental Figure 2), and the first peak of motility correlated well with the appearance of neutrophils in the peripheral blood. Cell velocity returned almost to baseline levels within 4 hours (Figure 2C).
G-CSF–induced mobilization of neutrophils to the blood and induction of cell motility in the BM. (A) Percentage of Gr-1–expressing cells in the blood of animals 2 hours after injection of G-CSF or PBS. Data are representative of 1 of 5 independently performed experiments with 3-6 mice per group. (B) Injection of G-CSF leads to a rapid induction of neutrophil motility in vivo, as visible from kinetic overlays. The green channels of 3 time frames, each 1 minute apart, were overlaid to create one RGB image. White cells have not moved within 3 minutes; colored cells have moved. The respective videos are provided as supplemental Videos 2 and 3. (C) Percentage of migrating cells (activity) and velocity of mobilized neutrophils over time after G-CSF or 1 hour after PBS injection. Data shown in panel B are representative of 4 independently analyzed animals, and data in panel C show the combined results of all 4 mice imaged for 4 hours each (the time point 1 hour after G-CSF was measured in 3 mice). (D) Emigration of several neutrophils into the bloodstream (white arrowheads) observed in a G-CSF–treated animal, white arrow depicts direction of blood flow. The accompanying video is provided as supplemental Video 4. Similar events were seen in 2 independently analyzed animals. (E) Tracks of 40 cells each migrating in either a nonmobilized (blue track color, tracked for 40 minutes) or a G-CSF–mobilized animal (orange track color, tracked for only 10 minutes.), illustrated with a common starting point. Tracks are 1 representative of 4 animals measured independently. (F) Migratory activity or velocity of green cells in CX3CR1-EGFP mice 1 hour after injection of PBS or G-CSF. Three mice were measured independently for each condition; ns = nonsignificant. In panels A, C, and F, the horizontal bar indicates the mean value.
G-CSF–induced mobilization of neutrophils to the blood and induction of cell motility in the BM. (A) Percentage of Gr-1–expressing cells in the blood of animals 2 hours after injection of G-CSF or PBS. Data are representative of 1 of 5 independently performed experiments with 3-6 mice per group. (B) Injection of G-CSF leads to a rapid induction of neutrophil motility in vivo, as visible from kinetic overlays. The green channels of 3 time frames, each 1 minute apart, were overlaid to create one RGB image. White cells have not moved within 3 minutes; colored cells have moved. The respective videos are provided as supplemental Videos 2 and 3. (C) Percentage of migrating cells (activity) and velocity of mobilized neutrophils over time after G-CSF or 1 hour after PBS injection. Data shown in panel B are representative of 4 independently analyzed animals, and data in panel C show the combined results of all 4 mice imaged for 4 hours each (the time point 1 hour after G-CSF was measured in 3 mice). (D) Emigration of several neutrophils into the bloodstream (white arrowheads) observed in a G-CSF–treated animal, white arrow depicts direction of blood flow. The accompanying video is provided as supplemental Video 4. Similar events were seen in 2 independently analyzed animals. (E) Tracks of 40 cells each migrating in either a nonmobilized (blue track color, tracked for 40 minutes) or a G-CSF–mobilized animal (orange track color, tracked for only 10 minutes.), illustrated with a common starting point. Tracks are 1 representative of 4 animals measured independently. (F) Migratory activity or velocity of green cells in CX3CR1-EGFP mice 1 hour after injection of PBS or G-CSF. Three mice were measured independently for each condition; ns = nonsignificant. In panels A, C, and F, the horizontal bar indicates the mean value.
The lysozyme promoter is not exclusively expressed in neutrophils; it is also expressed at lower levels in neutrophil precursors and in some dendritic cells and macrophages.11 Therefore, we further validated our focus on neutrophils by determining the effect of G-CSF on the motility of EGFP-positive cells in CX3CR1-EGFP transgenic mice harboring green BM dendritic cells and macrophages but not neutrophils.19 Whereas the activity of dendritic cells and macrophages in the BM of these mice was unaffected by G-CSF, their velocity was significantly reduced (Figure 2F).
G-CSF–triggered release of Cxcr2 ligands mediates mobilization
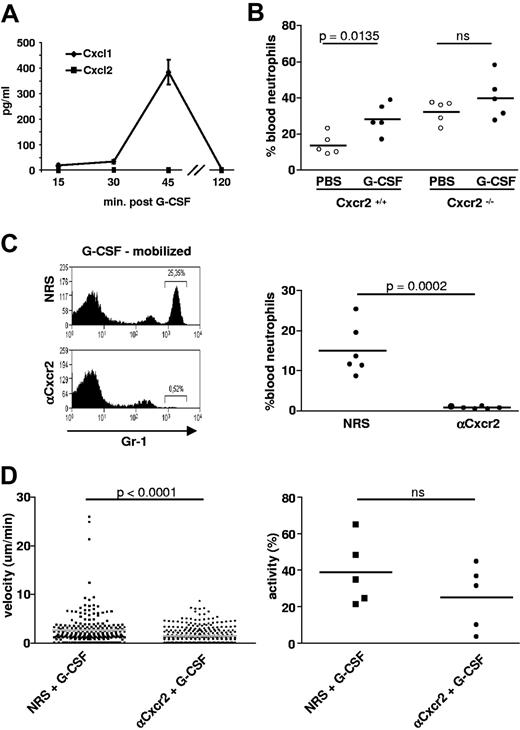
A key issue in this study was to define the molecular mechanisms behind the rapid mobilization of neutrophils in vivo. The induction of the chemokines KC (Cxcl1) and MIP-2 (Cxcl2) is a hallmark of inflammation and infection in peripheral tissues.3,20 Recruitment of neutrophils to inflamed sites is mediated by these chemokines via their receptor Cxcr2,3,20,21 and direct injection of KC/MIP-2 is capable of mobilizing neutrophils in vivo.3 In addition, a recent study in BM chimeras has shown that the expression of Cxcr2 on neutrophils is important for their mobilization.22 Shortly after injection of sterile hG-CSF, we detected a peak of free KC in the blood, suggesting a link between the 2 events and a role for the Cxcr2-binding chemokines in the mobilization of neutrophils from the BM (Figure 3A).
Cxcr2 mediates G-CSF–induced mobilization and increased motility of neutrophils in vivo. (A) Time course of mean blood levels ± SEM of Cxcr2-binding chemokines determined by ELISA after systemic application of G-CSF in mice (2 independent experiments with 3 mice per sample point in each experiment). (B) Levels of circulating neutrophils in the blood of Cxcr2+/+ or Cxr2−/− mice treated with either PBS or G-CSF. Data represent one experiment with 5 mice per group. (C) Levels of circulating neutrophils in the blood of G-CSF–mobilized mice after pre-injection of Cxcr2 antiserum or control NRS 48 hours previously. Representative fluorescence-activated cell sorting histogram and the spectrum of results from all individual animals analyzed are shown. (D) Velocity and migratory activity of neutrophils in the BM of mice treated with either NRS or anti-Cxcr2 48 hours before injection of G-CSF. Motility values were measured by intravital microscopy 1 hour after injection of G-CSF in 5 independently analyzed animals per group; ns = nonsignificant. In panels B through D, the horizontal bar indicates the mean value. The accompanying video is provided as supplemental Video 5.
Cxcr2 mediates G-CSF–induced mobilization and increased motility of neutrophils in vivo. (A) Time course of mean blood levels ± SEM of Cxcr2-binding chemokines determined by ELISA after systemic application of G-CSF in mice (2 independent experiments with 3 mice per sample point in each experiment). (B) Levels of circulating neutrophils in the blood of Cxcr2+/+ or Cxr2−/− mice treated with either PBS or G-CSF. Data represent one experiment with 5 mice per group. (C) Levels of circulating neutrophils in the blood of G-CSF–mobilized mice after pre-injection of Cxcr2 antiserum or control NRS 48 hours previously. Representative fluorescence-activated cell sorting histogram and the spectrum of results from all individual animals analyzed are shown. (D) Velocity and migratory activity of neutrophils in the BM of mice treated with either NRS or anti-Cxcr2 48 hours before injection of G-CSF. Motility values were measured by intravital microscopy 1 hour after injection of G-CSF in 5 independently analyzed animals per group; ns = nonsignificant. In panels B through D, the horizontal bar indicates the mean value. The accompanying video is provided as supplemental Video 5.
We next tested whether triggering of Cxcr2 was mandatory for neutrophil mobilization by investigating the responsiveness of Cxcr2−/− animals to G-CSF. These mice have a higher baseline level of circulating neutrophils13 (Figure 3B), but, interestingly, the administration of G-CSF did not further increase their numbers, whereas control littermate mice responded normally (Figure 3B). To assess the effect of blocking Cxcr2 on neutrophil activity in the BM itself in a genetic background that did not directly influence BM physiology, we made use of a polyclonal Cxcr2 antiserum that has previously been shown to potently inhibit neutrophil recruitment into an inflamed central nervous system or during viral encephalitis.14 Indeed, the Cxcr2 antiserum did almost completely inhibit the stress mobilization of neutrophils to the peripheral blood of wild-type mice by G-CSF (Figure 3C). Furthermore, in the presence of the Cxcr2-blocking antiserum in Lys-EGFP mice, the G-CSF–induced neutrophil velocity in the BM was significantly reduced and the percentage of migrating cells was lower by trend compared with NRS-treated control animals, albeit not reaching the level of significance (Figure 3D). Furthermore, neutrophil entry into sinuses was blocked (supplemental Video 5). In contrast to the depleting anti–Gr-1 antibody RB6-8C5,23 the Cxcr2 antiserum did not deplete neutrophils from the BM (supplemental Figure 3). Thus, the induced release of Cxcr2-binding chemokines by G-CSF provides a selective trigger for enhanced motility of neutrophils in the BM, which is a prerequisite for their mobilization into local sinuses and the circulation. These data suggested that G-CSF, acting as a BM stimulant, was centrally dependent on the expression of the KC/MIP-2 receptor Cxcr2 on neutrophils for their danger mobilization.
Cxcr2 ligands are expressed by megakaryocytes and the BM vasculature
We next sought to identify the cellular source of the chemokine that is active in mobilizing Cxcr2-expressing neutrophils. Immunohistochemistry of BM sections showed that KC (Figure 4A) was strongly expressed by megakaryocytes positioned next to sinus vessels that were also chemokine positive. Because megakaryocytes can nonspecifically bind antibodies, it was important to demonstrate that an isotype control showed no detectable background levels of staining in these experiments (Figure 4A). Anti–MIP-2 directed against the Cxcr2-binding KC homolog was also expressed by megakaryocytes (Figure 4B), with an IgG2b isotype mAb staining negatively (data not shown). Two hours after G-CSF injection, the detectable expression of KC and MIP-2 in both megakaryocytes and endothelial cells was highly significantly reduced (Figure 4B-C), implying that the chemokines were released into the immediate surroundings and potentially into the blood. The loss of MIP-2 from megakaryocytes and vasculature but the lack of MIP-2 in plasma were notable (Figure 3B). An explanation might lie in the differential engagement of KC versus MIP-2 with interceptor chemokine receptors such as DARC expressed by erythrocytes and the vasculature.24 These immunohistochemical observations were backed up by the demonstration using qRT-PCR that the Cxcr2 ligands KC (Cxcl1), MIP-2 (Cxcl2), and CXCL3, but not CXCL5 and CXCL7, were substantially elevated in BM samples from mice taken 90 minutes after a systemic dose of G-CSF (Figure 4D). This suggested that sustained production of the Cxcr2 ligands in BM followed their initial release.
Immunohistology of murine BM identifies megakaryocytes as producers of the neutrophil chemoattractants KC and MIP-2. (A) Three KC-positive megakaryocytes (arrowheads) surround a KC-expressing BM vessel in naive BM (representative image of 4 independent experiments with 3 mice per treatment group). The lower image was stained with an IgG2a isotype control mAb. Megakaryocytes are indicated by arrowheads. (B) Expression of KC and MIP-2 on megakaryocytes and associated vessels 2 hours after injection of mice with either control PBS or G-CSF (representative image of 4 independent experiments with 3 mice per treatment group). Arrowheads indicate megakaryocytes. (C) Quantification of loss of KC and MIP-2 expression by image analysis from megakaryocytes after G-CSF or PBS treatment of mice as in panel B (AU = arbitrary units, n = 16-20 megakaryocytes from 4 independent experiments for each condition, the horizontal bar indicates the mean value). (D) Fold increase in Cxcr2 chemokine mRNAs in murine BM 2 hours after systemic G-CSF treatment as detected by qRT-PCR. Data are means ± standard deviation (SD) of a total of 6 animals measured separately in 3 independent experiments each time against 2 PBS-treated control mice. (E) Production of KC and MIP-2 by primary purified BM mouse megakaryocytes over 4 hours ± G-CSF (mean ± SD, 3 separate experiments per condition). (F) Production of IL-8 by the human megakaryocyte cell line MEG-01 over a 24-hour period (mean ± SD, 3 separate experiments per condition). Also shown is the lack of effect of coincubation of megakaryocytes with G-CSF at 20 and 50 μg/mL.
Immunohistology of murine BM identifies megakaryocytes as producers of the neutrophil chemoattractants KC and MIP-2. (A) Three KC-positive megakaryocytes (arrowheads) surround a KC-expressing BM vessel in naive BM (representative image of 4 independent experiments with 3 mice per treatment group). The lower image was stained with an IgG2a isotype control mAb. Megakaryocytes are indicated by arrowheads. (B) Expression of KC and MIP-2 on megakaryocytes and associated vessels 2 hours after injection of mice with either control PBS or G-CSF (representative image of 4 independent experiments with 3 mice per treatment group). Arrowheads indicate megakaryocytes. (C) Quantification of loss of KC and MIP-2 expression by image analysis from megakaryocytes after G-CSF or PBS treatment of mice as in panel B (AU = arbitrary units, n = 16-20 megakaryocytes from 4 independent experiments for each condition, the horizontal bar indicates the mean value). (D) Fold increase in Cxcr2 chemokine mRNAs in murine BM 2 hours after systemic G-CSF treatment as detected by qRT-PCR. Data are means ± standard deviation (SD) of a total of 6 animals measured separately in 3 independent experiments each time against 2 PBS-treated control mice. (E) Production of KC and MIP-2 by primary purified BM mouse megakaryocytes over 4 hours ± G-CSF (mean ± SD, 3 separate experiments per condition). (F) Production of IL-8 by the human megakaryocyte cell line MEG-01 over a 24-hour period (mean ± SD, 3 separate experiments per condition). Also shown is the lack of effect of coincubation of megakaryocytes with G-CSF at 20 and 50 μg/mL.
Given the fact that megakaryocytes had previously been associated mainly with platelet production,25 our finding that they might also be involved in the regulation of neutrophil traffic led us to investigate this possibility in more detail. The conclusion that megakaryocytes can indeed be a physiologic source of neutrophil-attracting chemokines was further supported by our demonstration that isolated primary mouse megakaryocytes produced KC and MIP-2 in vitro (Figure 4E). In contrast, primary neutrophils and macrophages were unable to produce KC/MIP-2 even after treatment with G-CSF (supplemental Figure 4A). However, the macrophages were able to produce these chemokines after lipopolysaccharide stimulation, as shown previously.20 In agreement with these observations, the human megakaryocyte cell line MEG-01 released abundant amounts of IL-8, a human KC/MIP-2 homolog, in vitro (Figure 4F). Furthermore, Eash et al described endothelial cells and osteoblasts as sources of Cxcr2 ligands.22 Because the Cxcr2 ligands were identified in their study using expression profiling of sorted BM cells after 7 days of systemic G-CSF treatment, megakaryocytes may not have survived tissue disruption or, as in our study, they may have had a role only in the immediate stress response within 1-2 hours after G-CSF exposure. Our immunohistologic data (Figure 4A-B) also suggest that endothelial cells acutely release Cxcr2 chemokines under G-CSF stimulation.
The nature of the G-CSF target cell(s) has remained elusive. The constant presence of G-CSF had no impact on KC/MIP-2 release by megakaryocytes (Figure 4E-F), suggesting that they are not direct targets of the cytokine. Osteoblasts that line the endosteal wall within BM are G-CSF receptor positive,26 and may therefore directly respond to G-CSF in terms of Cxcr2 ligand release. Neutrophils also express the receptor for G-CSF, but its expression on these cells is not required for their efficient mobilization.3,27 This is in agreement with our finding that the velocity of neutrophils freshly explanted from the BM of mice and embedded within a 3-D collagen matrix model displayed motility resembling the migratory behavior in vivo, with no impact of G-CSF addition (supplemental Figure 4B).
TPO triggers megakaryocytes to release the chemokines KC and MIP-2
Because megakaryocytes did not directly respond to G-CSF, we needed to identify a signaling molecule that was able to trigger the release of KC/MIP-2 from these cells. Megakaryocytes are best known as platelet producers, and the hematopoietic cytokine TPO is the major stimulant of this process25 ; its roles extend from acting as the main colony-stimulating factor in megakaryopoiesis to controlling the number of mature megakaryocytes able to form pro-platelets.28 In vitro, both primary mouse megakaryocytes (Figure 5A) and the human MEG-01 cell line (Figure 5B) were induced to produce significantly more Cxcr2 ligands in response to TPO than without stimulation. The time frame of this response corresponded well with the kinetics of the G-CSF–induced danger mobilization in vivo, which suggested a potential role for TPO in the release of neutrophil-attracting chemokines by megakaryocytes. Endothelial cells can also express the TPO receptor and may therefore be similarly targeted29 ; however, it is presently unclear whether such a mechanism is active in endothelial cells of the BM.
TPO links G-CSF to megakaryocytes. (A) Time course of KC production by purified mouse BM megakaryocytes cultured in vitro ± TPO (TPO, mean ± SEM of 3 separate experiments per treatment). (B) Time course of IL-8 production by the human megakaryocyte cell line MEG-01 after culture ± TPO (mean ± SEM of 3 separate experiments per treatment). (C) Expression of TPO on BM cells 2 hours after injection of mice with either control PBS or G-CSF (images are representative for data obtained in 2 independent experiments with 3 mice per treatment group). Scale bar = 20 μm. (D) The effect on PMN mobilization at 2 hours after a single intravenous injection of 1 μg of TPO compared with a control injection of PBS. Data represent 1 experiment with 4-5 mice per group. The horizontal bar indicates the mean value. (E) Neutrophil mobilization 2 hours after a single injection of G-CSF in littermate versus Mpl−/− animals. Data represent one experiment with 5-7 mice per group. The horizontal bar indicates the mean value.
TPO links G-CSF to megakaryocytes. (A) Time course of KC production by purified mouse BM megakaryocytes cultured in vitro ± TPO (TPO, mean ± SEM of 3 separate experiments per treatment). (B) Time course of IL-8 production by the human megakaryocyte cell line MEG-01 after culture ± TPO (mean ± SEM of 3 separate experiments per treatment). (C) Expression of TPO on BM cells 2 hours after injection of mice with either control PBS or G-CSF (images are representative for data obtained in 2 independent experiments with 3 mice per treatment group). Scale bar = 20 μm. (D) The effect on PMN mobilization at 2 hours after a single intravenous injection of 1 μg of TPO compared with a control injection of PBS. Data represent 1 experiment with 4-5 mice per group. The horizontal bar indicates the mean value. (E) Neutrophil mobilization 2 hours after a single injection of G-CSF in littermate versus Mpl−/− animals. Data represent one experiment with 5-7 mice per group. The horizontal bar indicates the mean value.
To function as a mechanistic link during neutrophil mobilization, a measurable change in the expression level of TPO within the BM in response to G-CSF would be expected. To test this assumption, we investigated the expression of TPO in the BM of mice before and after a systemic injection of G-CSF by immunohistology. Indeed, whereas the detectable levels of TPO were very low in unstimulated BM, we found a strong up-regulation of the signal in distinct cells within 2 hours after systemic application of G-CSF (Figure 5C). Furthermore, using ELISA, we observed a significant increase of TPO protein in total BM cells taken from mice 30 minutes after a systemic dose of G-CSF over controls treated with PBS (35-40 pg/mL, n = 2 independent experiments). These data further underscored a role for TPO in the release of the neutrophil-attracting chemokines and provided evidence that TPO is a possible link between G-CSF–responsive target cell(s) and megakaryocyte function.
To directly test the potential activity of TPO in neutrophil mobilization in vivo, we systemically injected TPO intravenously into mice, measuring the mobilization of neutrophils 2 hours later. Confirming our histologic and cell-culture observations, a single injection of TPO led to a significant mobilization of neutrophils in vivo, bypassing the need for G-CSF and causing neutrophil mobilization directly when injected in vivo (Figure 5D). Finally, the injection of G-CSF into mice lacking Mpl,12 the receptor for TPO, led to an insignificant mobilization of neutrophils compared with littermate mice (Figure 5E). Therefore, the use of this genetic model further underscored a role for TPO in acute G-CSF–mediated neutrophil mobilization.
Discussion
This study is the first to address the important phenomenon of neutrophil stress mobilization using a direct imaging approach, thus allowing investigation of the effects of the central hematopoietic cytokine G-CSF on individual cells. We demonstrate that G-CSF rapidly and specifically induces increased motility of neutrophils in vivo within the BM and their emigration into local blood vessels. This was not known previously, and it immediately suggests a physiologic framework as to how these cells, which are critically important for the initiation of many immune responses, can be rapidly made available from a central source, where they wait in a resting state. It will be very important in the future to determine how other BM-resident cell types such as macrophages, dendritic cells, and memory B cells respond to suitable recruitment triggers at the single-cell level. Furthermore, our novel technical approach allows in vivo imaging of cells in the tibia, a long-bone compartment that is the usual source of neutrophils isolated for experimental studies in mice. This is a helpful addition to the imaging of BM-resident cells, which has so far concentrated on calvarial BM.30,31 The calvaria is generated by intramembranous ossification with specific features that are not found in long bones, which are generated by endochondral ossification.32 Future studies should thus address whether there are specific differences in the response of cells within either BM compartment toward systemic triggers or whether cells in both compartments behave similarly.
An unexpected finding in this study was the intense level of vascularization close to the endosteum, which also allowed direct blood recruitment of neutrophils. Concepts of stem cell emigration during hematopoiesis suggest the migration of these cells to the center of the bone, where they enter central blood vessels.33 According to our imaging data, entry into blood vessels is possible throughout the entire BM matrix and also very close to the endosteal surface, at least for neutrophils. This might be one reason that neutrophil mobilization is so rapid; again, it remains to be determined whether this is a specific feature of long bones.
Our study also provides new details of how neutrophils are mobilized from BM after a stimulus such as G-CSF. We show that the neutrophil-attracting, Cxcr2-binding chemokines KC and MIP-2 are released in the BM shortly after G-CSF administration, and are required for the induction of neutrophil motility and their entry into local blood sinuses. In the work of Link et al,22 the increased production of Cxcr2 ligands was demonstrated only after 7 days of treatment with G-CSF, and thus did not show the immediate stress-mobilization response that was the focus of our study. We extend these data by providing a cell-biologic explanation of how Cxcr2 triggering mediates increased neutrophil mobilization. Our study also gives a new perspective to the role of megakaryocytes, which until now have only been known as platelet producers.25 Our findings suggest an important new role for this cell type, together with the BM vasculature and potentially osteoblasts, as gatekeepers of neutrophil mobilization in the BM matrix. Focusing on megakaryocytes in this study, we show that these cells release KC/MIP-2 in vitro after TPO stimulation. Histologically, megakaryocyte expression of Cxcr2-binding chemokines is decreased in vivo after a systemic increase of G-CSF, which implies the release of preformed chemokines into the surrounding microenvironment. At the same time, production of the chemokines is enhanced at the mRNA level in the BM. This is coincident with an increase in neutrophil motility in the BM that is absent when Cxcr2 is blocked. Our histologic data also suggest that the BM vasculature is able to rapidly secrete Cxcr2-ligands after G-CSF treatment, indicating a role for these BM endothelial cells in stress mobilization.
These data suggest that the acute release of TPO can provide a link by which G-CSF induces the release of the chemokines KC and MIP-2 from megakaryocytes and other BM-resident cells such as endothelial cells and, ultimately, the mobilization of neutrophils. Long-term experimental exposure to TPO via a retroviral vector infection in vivo gives rise to a fatal myeloproliferative syndrome,34 and TPO and G-CSF have been shown in several animal models to have synergistic effects,35,36 showing that the link between TPO and neutrophils is on another level.
Previous work has highlighted the essential nature of the Cxcr4-Cxcl12 bond that serves to retain neutrophils in the BM and the effect of G-CSF on this interaction.8,22 After cleavage of Cxcr4-Cxcl12 bonds restraining the neutrophils within the BM, the next step for neutrophil release is their detection of the Cxcr2-chemokines that initiate neutrophil motility and migration directed toward the BM sinuses. Based on our findings, G-CSF can act as a relay signal using TPO and TPO-responsive cells as amplifiers to mobilize the large numbers of neutrophils needed to fight peripheral infections.15 This concept would also provide an explanation for the known synergism between the Cxcr4 antagonist AMD3100 and G-CSF for the release of neutrophils, because AMD3100 effectively uncouples neutrophils from their Cxcr4 bonds to the BM stroma, whereas G-CSF would increase the amount of available, migration-inducing Cxcr2 ligands. For this concept to work, it would be necessary for megakaryocytes to participate in generating a chemokine gradient from the BM matrix across the endothelial layer into the bloodstream. It has been shown that megakaryocytes produce circulating platelets by hanging long, cellular protrusions into the streaming blood.25 Liberation of chemokines from such protrusions could directly secrete KC/MIP-2 into the bloodstream and thus generate a higher concentration there. If we further assume that endothelial cells produce Cxcr2 chemokines directly within the bloodstream, it is not difficult to imagine how a chemokine gradient would be produced. Further, endothelial cells, at least those of brain blood vessels, can respond to TPO.29 In addition to producing a gradient of Cxcr2 ligands in the blood, it might also be very relevant for megakaryocytes to increase the amount of Cxcr2 chemokines within the BM matrix, so that the general motility of neutrophils is initially increased, thereby enhancing the “hit rate” with blood vessels nearby. Our videos indeed show a strongly increased motility of neutrophils in vivo with an enhanced endothelial encounter resulting as a side effect. Finally, because platelets do express the TPO receptor37 and contain KC and MIP-2 in α granules,38 they might also add to an increased concentration of these chemokines in the blood after TPO release caused by G-CSF.
In summary, our data show that megakaryocytes can operate to regulate emergency levels of neutrophils. We have identified a network of key signaling molecules and chemokines controlling neutrophil release that is strategically positioned in the BM cavity adjacent to vessels. It will be important to consider the impact of this cellular axis in response to external perturbations such as peripheral infections requiring immune defense, as well as its involvement in the release from the BM of other mature leukocytes or cells from the hematopoietic stem-cell compartment. Our novel approach will allow the exploration of these biologically and clinically central questions with a new level of sophistication.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dominique Bonnet, Facundo Batista, Caetano Reis e Sousa, and Ulrich Gunzer for critical reading of the manuscript and Luca Manetta for help with Figure 4C.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG; SFB 854, GU 769/1-3, GU769/2-1, GU769/4-1 priority program “Immunobone”); from the European Union (NEST Mamocell to M.G.); from Cancer Research UK (to N.H.); and from the DFG (GE2063/1) and the National Institutes of Health (HL076604 and DK077762 to H.G.). H.G. is a New Investigator in Aging of The Ellison Medical Foundation.
National Institutes of Health
Authorship
Contribution: A.K., K.D.F., M.H., C.v.d.B., E.N., M.P.H., T.E.L., L.M., H.G., and A.E.H. performed experiments; O.W., R.M.R., and B.S. provided vital models and tools; and M.G. and N.H. conceived of the study, oversaw experimentation, interpreted the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation of C.v.d.B. is Department of Transfusion Medicine, University Clinic Göttingen, Göttingen, Germany.
Correspondence: Matthias Gunzer, Otto-von-Guericke University, Institute of Molecular and Clinical Immunology, Leipziger Str 44, 39120 Magdeburg, Germany; e-mail: matthias.gunzer@med.ovgu.de; or Nancy Hogg, Cancer Research UK, Leukocyte Adhesion Laboratory, 44 Lincoln's Inn Fields, London WC2A 3PX, United Kingdom; e-mail: nancy.hogg@cancer.org.uk.
References
Author notes
A.K. and K.D.F. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal