Abstract
The World Health Organization classification of lymphoid neoplasms updated in 2008 represents a worldwide consensus on the diagnosis of these tumors and is based on the recognition of distinct diseases, using a multidisciplinary approach. The updated classification refined the definitions of well-recognized diseases, identified new entities and variants, and incorporated emerging concepts in the understanding of lymphoid neoplasms. However, some questions were unresolved, such as the extent to which specific genetic or molecular alterations define certain tumors, and the status of provisional entities, categories for which the World Health Organization working groups felt there was insufficient evidence to recognize as distinct diseases at this time. In addition, since its publication, new findings and ideas have been generated. This review summarizes the scientific rationale for the classification, emphasizing changes that have had an effect on practice guidelines. The authors address the criteria and significance of early or precursor lesions and the identification of certain lymphoid neoplasms largely associated with particular age groups, such as children and the elderly. The issue of borderline categories having overlapping features with large B-cell lymphomas, as well as several provisional entities, is reviewed. These new observations chart a course for future research in the field.
Introduction
Classifications of diseases are the language of medicine, categorizing known entities in a way that facilitates understanding between workers in the field and providing a framework for both clinical practice and the generation of new knowledge. The World Health Organization (WHO) classification of neoplasms of the hematopoietic and lymphoid tissues, published in 2001 and updated in 2008, represents a worldwide consensus on the diagnosis of these tumors, adopted for use by pathologists, clinicians, and basic scientists1 (Table 1). Its worldwide acceptance stems from biologically sound underlying principles and from its clinical relevance, practicality, and reproducibility in diverse international settings. Since 2001, the classification has been used in clinical trials and in pathologic and epidemiologic studies and has provided the basis for new genetic and molecular investigations. The major principle of the classification is the recognition of distinct diseases according to a combination of morphology, immunophenotype, genetic, molecular, and clinical features. The disease entities are stratified according to their cell lineage and, additionally, their derivation from precursor or mature lymphoid cells. These principles in the 2001 WHO classification were based on the Revised European-American Classification of Lymphoid Neoplasms (REAL) published by the International Lymphoma Study Group in 1994.2 The validation of this proposal in a large series of tumors from different parts of the world3 and the publication of the third edition of the WHO classification1 ended a long history of controversies surrounding the classification of lymphomas.4
WHO classification of tumors of hematopoietic and lymphoid tissues
| Mature B-cell neoplasms |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma |
| B-cell prolymphocytic leukemia |
| Splenic marginal zone lymphoma |
| Hairy cell leukemia |
| Splenic lymphoma/leukemia, unclassifiable* |
| Splenic diffuse red pulp small B-cell lymphoma* |
| Hairy cell leukemia variant* |
| Lymphoplasmacytic lymphoma |
| Waldenström macroglobulinemia |
| Heavy chain diseases |
| α Heavy chain disease |
| γ Heavy chain disease |
| μ Heavy chain disease |
| Plasma cell myeloma |
| Solitary plasmacytoma of bone |
| Extraosseous plasmacytoma |
| Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) |
| Nodal marginal zone lymphoma |
| Pediatric nodal marginal zone lymphoma* |
| Follicular lymphoma |
| Pediatric follicular lymphoma* |
| Primary cutaneous follicle centre lymphoma |
| Mantle cell lymphoma |
| Diffuse large B-cell lymphoma (DLBCL), NOS |
| T-cell/histiocyte rich large B-cell lymphoma |
| Primary DLBCL of the CNS |
| Primary cutaneous DLBCL, leg type |
| EBV-positive DLBCL of the elderly* |
| DLBCL associated with chronic inflammation |
| Lymphomatoid granulomatosis |
| Primary mediastinal (thymic) large B-cell lymphoma |
| Intravascular large B-cell lymphoma |
| ALK-positive large B-cell lymphoma |
| Plasmablastic lymphoma |
| Large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease |
| Primary effusion lymphoma |
| Burkitt lymphoma |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma |
| Mature T-cell and NK-cell neoplasms |
| T-cell prolymphocytic leukemia |
| T-cell large granular lymphocytic leukemia |
| Chronic lymphoproliferative disorder of NK cells* |
| Aggressive NK-cell leukemia |
| Systemic EBV-positive T-cell lymphoproliferative disease of childhood |
| Hydroa vacciniforme-like lymphoma |
| Adult T-cell leukemia/lymphoma |
| Extranodal NK/T-cell lymphoma, nasal type |
| Enteropathy-associated T-cell lymphoma |
| Hepatosplenic T-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Mycosis fungoides |
| Sézary syndrome |
| Primary cutaneous CD30+ T-cell lymphoproliferative disorders |
| Lymphomatoid papulosis |
| Primary cutaneous anaplastic large cell lymphoma |
| Primary cutaneous γδ T-cell lymphoma |
| Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma* |
| Primary cutaneous CD4+ small/medium T-cell lymphoma* |
| Peripheral T-cell lymphoma, NOS |
| Angioimmunoblastic T-cell lymphoma |
| Anaplastic large cell lymphoma, ALK-positive |
| Anaplastic large cell lymphoma, ALK-negative* |
| Hodgkin lymphoma |
| Nodular lymphocyte predominant Hodgkin lymphoma |
| Classical Hodgkin lymphoma |
| Nodular sclerosis classical Hodgkin lymphoma |
| Lymphocyte-rich classical Hodgkin lymphoma |
| Mixed cellularity classical Hodgkin lymphoma |
| Lymphocyte-depleted classical Hodgkin lymphoma |
| Histiocytic and dendritic cell neoplasms |
| Histiocytic sarcoma |
| Langerhans cell histiocytosis |
| Langerhans cell sarcoma |
| Interdigitating dendritic cell sarcoma |
| Follicular dendritic cell sarcoma |
| Fibroblastic reticular cell tumor |
| Intermediate dendritic cell tumor |
| Disseminated juvenile xanthogranuloma |
| Posttransplantation lymphoproliferative disorders (PTLDs) |
| Early lesions |
| Plasmacytic hyperplasia |
| Infectious mononucleosis–like PTLD |
| Polymorphic PTLD |
| Monomorphic PTLD (B- and T/NK-cell types)† |
| Classical Hodgkin lymphoma type PTLD† |
| Mature B-cell neoplasms |
| Chronic lymphocytic leukemia/small lymphocytic lymphoma |
| B-cell prolymphocytic leukemia |
| Splenic marginal zone lymphoma |
| Hairy cell leukemia |
| Splenic lymphoma/leukemia, unclassifiable* |
| Splenic diffuse red pulp small B-cell lymphoma* |
| Hairy cell leukemia variant* |
| Lymphoplasmacytic lymphoma |
| Waldenström macroglobulinemia |
| Heavy chain diseases |
| α Heavy chain disease |
| γ Heavy chain disease |
| μ Heavy chain disease |
| Plasma cell myeloma |
| Solitary plasmacytoma of bone |
| Extraosseous plasmacytoma |
| Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) |
| Nodal marginal zone lymphoma |
| Pediatric nodal marginal zone lymphoma* |
| Follicular lymphoma |
| Pediatric follicular lymphoma* |
| Primary cutaneous follicle centre lymphoma |
| Mantle cell lymphoma |
| Diffuse large B-cell lymphoma (DLBCL), NOS |
| T-cell/histiocyte rich large B-cell lymphoma |
| Primary DLBCL of the CNS |
| Primary cutaneous DLBCL, leg type |
| EBV-positive DLBCL of the elderly* |
| DLBCL associated with chronic inflammation |
| Lymphomatoid granulomatosis |
| Primary mediastinal (thymic) large B-cell lymphoma |
| Intravascular large B-cell lymphoma |
| ALK-positive large B-cell lymphoma |
| Plasmablastic lymphoma |
| Large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease |
| Primary effusion lymphoma |
| Burkitt lymphoma |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma |
| B-cell lymphoma, unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classical Hodgkin lymphoma |
| Mature T-cell and NK-cell neoplasms |
| T-cell prolymphocytic leukemia |
| T-cell large granular lymphocytic leukemia |
| Chronic lymphoproliferative disorder of NK cells* |
| Aggressive NK-cell leukemia |
| Systemic EBV-positive T-cell lymphoproliferative disease of childhood |
| Hydroa vacciniforme-like lymphoma |
| Adult T-cell leukemia/lymphoma |
| Extranodal NK/T-cell lymphoma, nasal type |
| Enteropathy-associated T-cell lymphoma |
| Hepatosplenic T-cell lymphoma |
| Subcutaneous panniculitis-like T-cell lymphoma |
| Mycosis fungoides |
| Sézary syndrome |
| Primary cutaneous CD30+ T-cell lymphoproliferative disorders |
| Lymphomatoid papulosis |
| Primary cutaneous anaplastic large cell lymphoma |
| Primary cutaneous γδ T-cell lymphoma |
| Primary cutaneous CD8+ aggressive epidermotropic cytotoxic T-cell lymphoma* |
| Primary cutaneous CD4+ small/medium T-cell lymphoma* |
| Peripheral T-cell lymphoma, NOS |
| Angioimmunoblastic T-cell lymphoma |
| Anaplastic large cell lymphoma, ALK-positive |
| Anaplastic large cell lymphoma, ALK-negative* |
| Hodgkin lymphoma |
| Nodular lymphocyte predominant Hodgkin lymphoma |
| Classical Hodgkin lymphoma |
| Nodular sclerosis classical Hodgkin lymphoma |
| Lymphocyte-rich classical Hodgkin lymphoma |
| Mixed cellularity classical Hodgkin lymphoma |
| Lymphocyte-depleted classical Hodgkin lymphoma |
| Histiocytic and dendritic cell neoplasms |
| Histiocytic sarcoma |
| Langerhans cell histiocytosis |
| Langerhans cell sarcoma |
| Interdigitating dendritic cell sarcoma |
| Follicular dendritic cell sarcoma |
| Fibroblastic reticular cell tumor |
| Intermediate dendritic cell tumor |
| Disseminated juvenile xanthogranuloma |
| Posttransplantation lymphoproliferative disorders (PTLDs) |
| Early lesions |
| Plasmacytic hyperplasia |
| Infectious mononucleosis–like PTLD |
| Polymorphic PTLD |
| Monomorphic PTLD (B- and T/NK-cell types)† |
| Classical Hodgkin lymphoma type PTLD† |
NOS indicates not otherwise specified; ALK, anaplastic lymphoma kinase; HHV8, human herpesvirus 8; and NK, natural killer.
These histologic types are provisional entities for which the WHO Working Group felt there was insufficient evidence to recognize as distinct diseases at this time.
These lesions are classified according to the leukemia or lymphoma to which they correspond.
The conceptual framework of the classification is likened to an evolving organism and is permissive of change as advances in our knowledge occur. Thus, the WHO classification of 2008 refined the definitions of well-recognized diseases, identified new entities and variants, and incorporated new emerging concepts in the understanding of lymphoid neoplasms. However, when published in 2008 some questions were unresolved, such as the extent to which specific genetic or molecular alterations define certain tumors and the status of provisional entities, categories for which the WHO working groups felt there was insufficient evidence to recognize as distinct diseases at this time.
The process of developing the 2008 WHO classification involved working groups of pathologists as well as a clinical advisory committee (CAC) of expert clinicians from around the world. A meeting of the CAC for lymphoid neoplasms together with the editors and lead authors of the WHO book was held at Airlie House, Warrenton, VA, March 8-9, 2007. This report summarizes the process and rationale for the 2008 WHO classification of lymphoid neoplasms, emphasizing those diseases for which changes have had an effect on practice guidelines. In addition, since the 2008 WHO publication, new findings and ideas have been generated. This review emphasizes and expands on these emerging concepts. One such topic is the increased recognition of early or precursor lesions of lymphoid neoplasms. In addition, although clinical features have been a part of disease definition since the REAL classification, it is increasingly recognized that some diseases are largely associated with particular age groups, such as children and the elderly. Finally, the 2008 WHO classification recognizes 2 provisional borderline categories of entities that have overlapping features with diffuse large B-cell lymphoma (DLBCL), as well as several other provisional entities.
New definitions and refinement of diagnostic criteria
The 2008 update of the WHO classification has adopted consensus guidelines for the definition of some well-established diseases, including chronic lymphocytic leukemia (CLL),5 Waldenström macroglobulinemia (WM),6 and plasma cell neoplasms.7 In addition, the WHO/European Organisation for Research and Treatment of Cancer consensus classification of cutaneous lymphomas was largely adopted.8,9 The issue of grading of follicular lymphoma was revisited. The definitions of some categories of T-cell lymphomas, including enteropathy-associated T-cell lymphoma, anaplastic large cell lymphoma (ALCL), and subcutaneous panniculitis-like T-cell lymphoma, have been refined.
Chronic lymphocytic leukemia
The increased sensitivity of immunophenotypic methods has resulted in the incidental detection of clonal lymphoid cell proliferations with an aberrant immunophenotype in healthy persons, even in the absence of clinical lymphocytosis. These proliferations have been termed monoclonal B-cell lymphocytosis (MBL; see “Early lesions in lymphoid neoplasms”).10,11 These observations led the International Workshop on CLL to propose new diagnostic criteria for CLL.5 The requirement for a diagnosis of CLL was modified from a chronic absolute lymphocytosis > 5.0 × 109/L to an absolute count of > 5.0 × 109/L monoclonal B cells with a CLL immunophenotype in the peripheral blood (PB), if there is an absence of disease-related symptoms or cytopenias, or tissue involvement other than BM.5 A diagnosis of small lymphocytic lymphoma (SLL) is made when lymphadenopathy or splenomegaly because of infiltrating CLL cells is found, with < 5 × 109 CLL-type cells in the blood. These criteria were accepted by the WHO CAC meeting in 2007 and were included in the definition of CLL in the 2008 WHO classification.
The selection of the cutoff was arbitrary, without clinical data supporting the number of 5 × 109/L. Recent studies have confirmed that the number of clonal B cells is clinically more relevant than the total lymphocyte count in predicting the outcome of the patients. The B cell count is a continuous variable, and the best thresholds vary around 10-11 × 109/L in different studies.12,13 An additional issue is the definition of SLL, that is, lymph node or other tissue involvement by an infiltrate characteristic of CLL but without the requisite number of PB monoclonal B cells for a diagnosis of CLL. Similarly to PB, small clonal populations of CLL-type B cells may be detected in lymph nodes removed for the diagnosis of other conditions; minimal criteria for the diagnosis of SLL on tissue biopsy specimens may need to be developed if overdiagnosis is to be avoided. At a recent workshop of the European Association for Haematopathology and the Society of Hematopathology (Uppsala, September 2010), it was suggested that, if the lymph node did not show enlargement and at least partial architectural effacement, a diagnosis of “lymph node involvement by monoclonal CLL-type B cells of uncertain clinical significance” may be appropriate. Whether a minimal degree of lymph node involvement is sufficient to warrant a diagnosis of CLL rather than MBL remains to be determined. Because many patients with Rai stage 0 CLL, even as currently defined, are not treated, the change in terminology relates more to how patients are “labeled,” rather than indicating a change in patient management.
Lymphoplasmacytic lymphoma and Waldenström macroglobulinemia
Because many types of small B-lymphoid neoplasms can show plasmacytic differentiation and specific immunophenotypic or genetic markers for LPL remain to be discovered, LPL is still defined in the 2008 WHO as a B-cell neoplasm composed of small lymphocytes, plasmacytoid lymphocytes, and plasma cells, usually involving the BM and sometimes the lymph nodes and spleen, which does not fulfill the criteria for any other B-cell neoplasm with plasmacytic differentiation. In addition, particularly because many marginal zone lymphomas with plasmacytic differentiation also lack defining biomarkers, the 2008 WHO classification openly acknowledges that sometimes only a diagnosis of small B-cell lymphoma with plasmacytic differentiation can be rendered and a differential diagnosis provided. Although documenting an IGH@/PAX5 (t(9;14)) translocation was previously thought to help diagnose a LPL, it is now recognized that it is rarely if ever found in LPL. Del6q occurs in approximately one-half of cases but is not a specific finding.
The definition of WM and its relationship to LPL have also been problematic. The 2008 classification adopted the approach of the second International Workshop on Waldenström's Macroglobulinemia,6 which defined WM as the presence of an IgM monoclonal gammopathy of any concentration associated with BM involvement by LPL. Therefore, LPL and WM are not synonymous, with WM now defined as a subset of LPL. The presence of even a large IgM paraprotein in the absence of a LPL is no longer considered WM, and LPL in the absence an IgM paraprotein is not WM.
Plasma cell neoplasms
Plasma cell myeloma (multiple myeloma).
Plasma cell neoplasms have proven challenging to classify in a biologically correct and clinically useful way. Because the immunoglobulin products of plasma cells are easily detected in the serum and urine, evidence of small clones of plasma cells may be detected by routine laboratory tests in patients who are healthy and may never develop organ damage secondary to the clonal proliferation. In addition, deposition of abnormal secreted immunoglobulin heavy or light chains or both (eg, amyloidosis) in tissues may occur in the presence of a very small plasma cell clone, with organ damage that is because of the deposits, not related to the plasma cell burden. The definition of plasma cell myeloma (PCM) has rested on identifying clinical and laboratory features that predict when a sufficient burden of plasma cells has accumulated that the patient will benefit from treatment. Traditional classifications of PCM, including the third edition of the WHO classification, recognized categories of monoclonal gammopathy with undetermined significance (MGUS), smoldering myeloma, and indolent myeloma. These categories were intended to identify patients who did not require immediate treatment.
In the updated 2008 classification there were no recommendations for changing the definition of MGUS. However, because smoldering and indolent PCM are a continuum, neither can be reliably diagnosed prospectively, and both are asymptomatic, the term asymptomatic PCM is preferred. The presence of radiographically detected bone lesions, even if not symptomatic, would exclude a patient from this category, because these are an indication for treatment. Current guidelines of the International Myeloma Working Group therefore were adopted by the WHO fourth edition7 (Table 2). The diagnosis of PCM, in the absence of myeloma-related end-organ damage (hypercalcemia, renal failure, anemia, bone lesions), requires the presence of a serum M-protein of ≥ 30 g/L or ≥ 10% BM clonal plasma cells, so-called asymptomatic myeloma. Cases not meeting these criteria are considered MGUS. In contrast, if myeloma-related end-organ damage is present, PCM is diagnosed when one detects an M-protein in the serum or urine of any amount and any number of BM clonal plasma cells (usually exceeding 10% of all cells).1
Diagnostic criteria for plasma cell myeloma
| Symptomatic plasma cell myeloma |
| M-protein in serum or urine* |
| BM clonal plasma cells or plasmacytoma† |
| Related organ or tissue impairment heavy chain disease‡ (CRAB) |
| Asymptomatic (smoldering) myeloma |
| M-protein in serum at myeloma levels (> 30 g/L) and/or ≥ 10% clonal plasma cells in BM |
| No related organ or tissue impairment end-organ damage or bone lesions [CRAB] or myeloma-related symptoms |
| Symptomatic plasma cell myeloma |
| M-protein in serum or urine* |
| BM clonal plasma cells or plasmacytoma† |
| Related organ or tissue impairment heavy chain disease‡ (CRAB) |
| Asymptomatic (smoldering) myeloma |
| M-protein in serum at myeloma levels (> 30 g/L) and/or ≥ 10% clonal plasma cells in BM |
| No related organ or tissue impairment end-organ damage or bone lesions [CRAB] or myeloma-related symptoms |
CRAB indicates hypercalcemia, renal insufficiency, anemia, bone lesions.
No level or serum or urine M-protein is included. M-protein in most cases is > 30g/L of IgG or > 25g/L of IgA or > 1g/24 h of urine light chain, but some patients with symptomatic myeloma have levels lower than these.
Monoclonal plasma cell usually exceed 10% of nucleated cells in the marrow, but no minimal levels are designated because ∼ 5% of patients with symptomatic myeloma have < 10% marrow plasma cells.
The most important criteria for symptomatic myeloma are manifestations of end-organ damage including anemia, hypercalcemia, lytic bone lesions, renal insufficiency, hyperviscosity, or recurrent infections.
Since the publication of the third edition of the WHO classification, data on cytogenetic abnormalities have accumulated that identify important prognostic categories of plasma cell myeloma. The cytogenetic categories and risk groups are included in the fourth edition of the WHO. However, diagnosis and treatment of myeloma are currently still based on morphologic and clinical criteria, and cytogenetic analysis is not currently required to establish the diagnosis or to prescribe treatment. However, the WHO working groups agreed that cytogenetic or FISH analyses should be performed in all cases when possible to define prognosis, and these analyses are a mandatory part of the evaluation of cases entered on clinical trials.
Immunoglobulin deposition diseases.
Criteria for the diagnosis of these entities are unchanged; however, the publications of the International Myeloma Working Group and the 2008 WHO fourth edition have given rise to some confusion.1,7 Primary amyloidosis is always the result of a clonal plasma cell neoplasm, but symptoms because of the amyloid deposition usually become clinically evident at a time when the plasma cell tumor burden is relatively low; most cases have < 10% BM plasma cells with low M-protein levels (< 30 g/L), similar to that seen in MGUS.1 If the plasma cell count and M-protein level are consistent with MGUS and the patient's symptoms are entirely attributable to organ damage from amyloid deposition, the resulting organ failure does not constitute a criterion for the diagnosis of symptomatic PCM, and the diagnosis remains primary amyloidosis. Amyloidosis may also occur in the presence of a larger plasma cell burden and other symptoms of myeloma (≤ 10% of patients with myeloma); if the level of M-protein or the plasma cell count is sufficient for a diagnosis of asymptomatic myeloma, the diagnosis is amyloidosis and PCM. Some myeloma cooperative groups prefer a cutoff of 30% BM plasma cells for a diagnosis of PCM in a patient with amyloidosis.14
Follicular lymphoma: grading and variants
Follicular lymphoma (FL) has been traditionally graded into 3 categories since the days of the Rappaport classification.15,16 The REAL and WHO third edition classifications recommended grading (grades 1-3) according to the number of centroblasts (0-5, 6-15, and > 15 per high-power field, respectively). Grade 3 was further subdivided for the purposes of clinical research into 3A (centrocytes still present) and 3B (sheets of centroblasts). Many studies over decades have shown that the proportion of large cells (centroblasts) predicts clinical outcome, with a more aggressive course in cases with increased centroblasts. However, the participants in the 2007 WHO CAC meeting agreed that there were several problems with this scheme. First, it is poorly reproducible among pathologists. Second, it is clear that there are no major biologic or clinical differences between grades 1 and 2, and they are treated similarly. Finally, several publications since 2001 suggested that grade 3B FL is biologically distinct from grades 1-3A, with features suggesting a close relationship to DLBCL (more frequent lack of CD10 and BCL2, expression of IRF4/MUM1, and rearrangement of BCL6 but not BCL2).17-20 These considerations led to the recommendation to group together FL grade 1-3A as “FL” and to create a new category for what has been called FL3B. Although this idea was attractive to both pathologists and clinicians, there were several arguments against it. First, most studies that found a close relationship of FL3B to DLBCL actually included cases with diffuse areas (eg, DLBCL); thus, these cases should not have been classified as FL3B according to the WHO third edition. Second, FL3B (with a purely follicular pattern) comprises only a small fraction of FL grade 3; thus, the clinical behavior of FL grade 3 in many clinical studies is based on the behavior of FL3A. Moreover, Hans et al21 found no difference in outcome between FL3A and FL3B, and another study, also based on cases with a purely follicular growth pattern, found a gene signature in FL3B that was closer to FL than to DLBCL of the germinal center B-cell (GCB) type.22 Thus, the final decision was to combine FL grades 1 and 2 into one category (FL1-2 of 3) and make the distinction between FL3A and FL3B mandatory rather than optional. This issue will need to be revisited when more data are available.
Some studies have suggested that the proliferative index recognized with Ki-67 labeling may represent a complementary aid or even an alternative to the morphologic grading of FL.23,24 However, determination of the proliferation index is also subjective and can be influenced by staining technique, so that this information also needs to be used judiciously.
The genetic hallmark of FL is the t(14;18) translocation and oncogenic BCL2 activation. However, as noted, some grade 3 FLs, particularly grade 3B, do not carry this translocation.25 Similarly, the fourth edition of the WHO classification recognizes some distinctive clinical and genetic subtypes of FL, such as primary duodenal FL and the pediatric type of FL. Primary duodenal FL carries the t(14;18) but usually remains localized to the intestinal mucosa. In contrast, pediatric FL lacks the t(14:18) and usually presents with localized disease. The concept that lymphomas in children often differ from lymphomas in adults is a recurrent theme in the 2008 classification, as will be discussed (see “clinical features in disease definition”).
Several studies since the publication of the updated classification have expanded on these observations, emphasizing that not all B-cell lymphomas with a follicular growth pattern or derived from follicular B cells are part of the same disease process. In particular, several variants of FL lacking the t(14;18) appear to show some distinctive features. Thus, predominantly diffuse FL with frequent deletions of 1p36 have been recognized in patients presenting with localized bulky disease, mainly in the inguinal region.26 Some cases of t(14;18)–negative FL with conventional morphology resemble activated late-stage germinal center B cells by gene expression profiling (GEP),27 whereas some show intrafollicular plasma cell differentiation.28 The current classification retains these t(14;18)–negative cases within the broad group of FL, an issue that will have to be addressed in the future as more clinical and genetic data are obtained.
Newly defined entities and categories
The 2008 classification has delineated some new lymphoma entities. Some had been described previously, but they had been considered morphologic or phenotypic variants of other entities; new evidence supports their distinction as independent diseases.
Diffuse large B-cell lymphomas
The clinical and biologic heterogeneity of DLBCL has been appreciated for some time. The WHO working group delineated some newly defined entities that were based on distinctive clinical, pathologic, or biologic features. This expanded list of diseases has stirred some controversy about the practicality of handling the diversity of these tumors. Some of the new lesions are uncommon or affect limited subsets of patients. However, their recognition serves 2 goals: delineating a more homogeneous group of DLBCL for clinical trials and facilitating the study of rare variants, which may require specialized approaches. Most DLBCLs do not have specific clinical or pathologic features and, therefore, are included in the group of DLBCL, not otherwise specified (NOS). GEP has provided new insights, leading to the identification of 2 principal molecular subtypes, the GCB and activated B-cell (ABC) forms of DLBCL. These subsets are associated with specific genetic alterations, different molecular signaling pathways, and different clinical outcomes.29 A variety of immunohistochemical algorithms have been proposed to delineate these subsets in the routine clinical laboratory.30 The difficulties in reproducibly distinguishing these subsets in the absence of GEP persuaded the WHO committee not to require their distinction in daily clinical practice. However, new therapeutic strategies are being designed to differentially treat GCB and ABC DLBCL.31 These advances will spur the development of novel tools that can be used in the routine clinical laboratory.32
The new classification emphasizes the importance of the location and other clinical aspects in the diagnosis of certain entities (see “Clinical features in disease definition”). Similar to the well-recognized primary mediastinal large B-cell lymphoma (PMBL), other DLBCLs that originate in specific topographic locations are considered specific entities. These tumors include DLBCL of the CNS and primary cutaneous DLBCL, leg type. Primary CNS DLBCL has a particular gene expression and genomic profile that differs from nodal DLBCL, and the patients are managed with different protocols.33-35 The distinction of primary cutaneous DLBCL, leg type, as a specific entity was based on its aggressive clinical behavior and phenotype that differ from the more indolent primary cutaneous follicle center lymphomas, even if the latter is composed mostly of large lymphoid cells. Notably, DLBCL of leg type resembles systemic DLBCL of the ABC subtype by GEP.36,37 We foresee that in the near future the taxonomy of DLBCL will rely more on integrated molecular and genetic parameters rather than on particular clinical features, but it is still possible that the clinical presentation may still determine the management strategy.
The recognition of T-cell/histiocyte-rich large B-cell lymphoma (THRLBCL) as a distinct category of DLBCL highlights the importance of the microenvironment in the biology of some diseases. GEP studies have identified a subgroup of DLBCLs with a high host immune response signature and a bad prognosis that includes most of these cases.38 The morphologic and phenotypic similarities between some THRLBCLs and progressed forms of nodular lymphocyte-predominant Hodgkin lymphomas (NLPHLs) have suggested a possible relationship between these neoplasms. Further studies may clarify whether de novo THRLBCL can be distinguished from such secondary cases.
The new classification recognizes several DLBCL entities characterized by EBV infection of the tumor cells. In addition to lymphomatoid granulomatosis, the WHO now includes EBV-positive DLBCL of the elderly initially described in Asia39-42 and DLBCL associated with chronic inflammation, most often chronic pyothorax.43-46 In these lesions EBV-infected cells frequently express a latency type 3 with positivity for the viral proteins latent membrane protein 1 and EBV nuclear antigen 2, suggesting that the lymphoid proliferation may be related primarily to a decrease in the host immunosurveillance. These cases typically have a rich inflammatory background but are excluded from THRLBCL. EBV-positive DLBCL of the elderly is still considered a provisional entity that requires a more precise definition and distinction from other EBV-related disorders, including reactive lesions, EBV-positive classic Hodgkin lymphoma, and the recently described mucocutaneous ulcer.47-49 The term EBV-positive DLBCL of the elderly emphasizes the tendency of these lesions to present at advanced age, although there is no sharp age limit, and similar lesions may be found in younger persons. To date, reports of these tumors in Western countries are limited.50,51
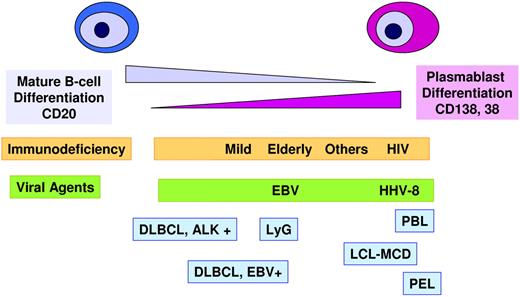
Several types of aggressive B-cell lymphoma included in the 2008 classification have a phenotype resembling terminally differentiated B cells. Cytologically, the cells have immunoblastic or sometimes plasmablastic features, but they exhibit a plasmacytic phenotype with the absence of CD20 and PAX5 and expression of CD38, CD138, and BLIMP1 (Figure 1).52,53 These lymphomas include anaplastic lymphoma kinase (ALK)–positive large B-cell lymphoma, plasmablastic lymphoma (PBL), and the human herpesvirus 8–related primary effusion lymphoma and large B-cell lymphoma associated with multicentric Castleman disease. PBL and human herpesvirus 8–related lymphomas usually occur in patients with immunodeficiency, most often HIV, and the tumors cells are infected by EBV, but usually with a latency type 1. Interestingly, ALK-positive large B-cell lymphoma has the same morphology and phenotype but occurs in younger immunocompetent patients and is EBV negative.54 Some PBLs have been recognized in patients without apparent immunodeficiency, but interestingly these patients are usually elderly.52 The advanced age and the frequent positivity for EBV have stirred some discussion as to whether these tumors should be included in PBL or in EBV-positive DLBCL of the elderly. The WHO considered that these cases should be classified as PBL because the morphology and phenotype, the latency type 1 of the EBV infection, and the scarce T-cell infiltrate are more similar to PBL in other immunodeficiency settings than to EBV-positive DLBCL. In fact, recent studies, including the frequent presence of MYC translocations in PBL, point to a close relationship between PBL and plasmablastic transformation of PCM.55,56 These observations may affect future therapeutic approaches to this disease.
Large B-cell lymphomas with a phenotype of terminal B-cell differentiation. This group of tumors is characterized by a down-regulation of the mature B-cell differentiation program and expression of plasma cell markers. Most of these tumors appear in immunocompromised patients. The tumor cells are often infected by EBV, human herpesvirus 8 (HHV8), or both. ALK+ large B-cell lymphoma (LBCL) occurs in immunocompetent patients and is not virally transformed. LyG indicates lymphomatoid granulomatosis; DLBCL, diffuse large B-cell lymphoma; PBL, plasmablastic lymphoma; PEL, primary effusion lymphoma; LBCL-MCD, large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease.
Large B-cell lymphomas with a phenotype of terminal B-cell differentiation. This group of tumors is characterized by a down-regulation of the mature B-cell differentiation program and expression of plasma cell markers. Most of these tumors appear in immunocompromised patients. The tumor cells are often infected by EBV, human herpesvirus 8 (HHV8), or both. ALK+ large B-cell lymphoma (LBCL) occurs in immunocompetent patients and is not virally transformed. LyG indicates lymphomatoid granulomatosis; DLBCL, diffuse large B-cell lymphoma; PBL, plasmablastic lymphoma; PEL, primary effusion lymphoma; LBCL-MCD, large B-cell lymphoma arising in HHV8-associated multicentric Castleman disease.
Enteropathy-associated T-cell lymphoma
The WHO classification of 2008 has applied more stringent criteria to the diagnosis of enteropathy-associated T-cell lymphoma (EATL), with a concomitant change in terminology from enteropathy-type T-cell lymphoma. It is recognized that a variety of T-cell lymphomas can present with intestinal involvement, but not all are associated with celiac disease. For example, intestinal involvement can be seen at presentation, or with progression, in extranodal natural killer (NK)/T-cell lymphoma and some γδ T-cell lymphomas. To make the diagnosis of EATL, one should have evidence of celiac disease, either clinically, at the genetic level, with the appropriate HLA phenotype, or histologically, in the adjacent uninvolved small bowel mucosa.
A variant of EATL was introduced into the classification, the monomorphic variant of EATL, also called type II. These cases have some distinctive immunophenotypic and genotypic features. The tumor cells are CD8+, CD56+, and MYC amplifications have been shown in a subset of cases.57 The monomorphic variant may occur sporadically without clear risk factors or clinical mani-festations of celiac disease, and it appears to represent a distinct disease entity.
ALK-positive anaplastic large cell lymphoma
The category of ALCL in the 2001 WHO classification included both ALK-positive and ALK-negative tumors and excluded primary cutaneous ALCL. The 2008 classification concluded that current evidence warranted delineation of ALK-positive ALCL as a distinct entity. ALK-positive ALCL occurs mainly in pediatric and young age groups, has a better prognosis than ALK-negative ALCL, and exhibits differences in genetics and GEP.58-60
The categorization of ALK-negative ALCL was more controversial but also felt to be distinguishable from other PTCLs. Recent studies by the International Peripheral T-Cell Lymphoma Project have supported this view, showing that ALK-negative ALCL has an intermediate survival between the better outcome of ALK-positive ALCL and the more aggressive PTCL, NOS.61 In addition, Piva et al59 have shown that the gene signature of ALK-negative ALCL is indeed distinct from that of PTCL, NOS. Given the relatively scarce information about the clinical and biologic features of ALK-negative ALCL, it is designated as a provisional entity.
Early lesions in lymphoid neoplasms
The idea of malignant transformation of cells through a multistep process of accumulating genetic and molecular events is well accepted and recognized in solid tumors. These concepts have been difficult to apply in the lymphoid system in which the cells naturally circulate and colonize different tissues.62 Molecular studies had detected oncogenic chromosomal translocations in the blood or BM cells of otherwise healthy persons. More recently histologic and immunophenotypic correlates of these molecular events have been identified. The new WHO classification addresses the increasing recognition of clonal expansions of lymphoid cells that appear to correspond to early steps in lymphomagenesis. In some cases it is not clear whether these lesions will ever progress to clinically significant disease, or whether they simply correspond to relatively stable “benign lymphoid” clonal proliferations. The identification of these lesions opens new questions as to how to manage these patients.
The recognition of MBL as a potential precursor of CLL and, less frequently, other leukemic lymphoid neoplasms, has stirred several clinical and biologic studies that have altered the traditional diagnosis of CLL. MBL is frequently found in first-degree family members of patients with CLL and in 5% of tested subjects older than 60 years, but the incidence increased to 14% in subjects with lymphocytosis (> 4.0 × 109/L.10,63,64 Population-based studies and the use of highly sensitive detection methods have identified clonal B cells in 12% of the population and > 20% of persons older than 65 years.65 Epidemiologic studies have found evidence of the CLL clone in the blood many years before diagnosis, supporting the idea of a long silent phase.66 The rate of progression of MBL to overt CLL is ∼ 1%-2% per year.64 However, it seems that most persons with MBL will not develop clinically relevant lymphoid neoplasia during their lifespan. MBL detected by random screening shows some biologic differences in the immunoglobulin gene repertoire compared with CLL or other MBLs.65-67 Moreover, patients with MBL may manifest oligoclonal or polyclonal expansions of similar cells. Thus, with aging multiple MBL clones may exist, but only some of them will be selected to progress to clinically relevant disease. The driving forces in the origin, expansion, and selection of these clones are not known.
Clonal B-cell populations with an atypical CLL phenotype (bright CD20/surface immunoglobulin, lack of CD23) or even a non-CLL phenotype (CD5−) have been detected in some healthy persons. Some of these cases carry cytogenetic alterations not characteristic of CLL such as 7q deletions. Thus, stable clonal expansions of B cells of diverse origins may occur,68 a finding of relevance to the observation of in situ lymphoma-like lesions in lymph nodes.
Early possibly neoplastic or preneoplastic proliferations, corresponding to the immunophenotypic and molecular phenotypes of FL or mantle cell lymphoma (MCL), have been observed in tissues. These have been designated as in situ FL or in situ MCL, referring to the fact that the clonal population is restricted in its distribution to its normal anatomic location, the germinal center or mantle zone, respectively (Table 3; Figure 2).69-76 These lesions should be distinguished from partial involvement of the lymph node by overt lymphomas. Cases of in situ FL, or intrafollicular neoplasia, as alternatively termed in the WHO classification, represent expansions of CD10 and BCL2-positive lymphoid cells carrying the t(14;18) translocation found in germinal centers of an otherwise reactive lymph node. The finding is usually incidental, the involved follicles often scattered and generally not completely replaced by BCL2-positive cells. In some patients disseminated FL is discovered on staging, but probably > 50% of the patients do not have evidence of FL beyond the initial node and with existing follow-up.70,72 This situation may represent tissue infiltration of circulating antigen-experienced, clonal expansions of B cells carrying the t(14;18) translocation commonly detected in healthy persons, termed FL-like B cells.77 These circulating t(14;18)–positive clones, which are more prevalent among persons with pesticide exposure, appear to lack additional oncogenic events to develop into an overt lymphoma. It is interesting that some persons may carry several different clones with the t(14;18), although usually one of them largely predominates over the others, suggesting that these clones may arise in a context that facilitates the translocation and subsequent expansion.78 Interestingly, some patients with hepatitis C virus have clones carrying the t(14;18) that may disappear after antiviral therapy.79
Differential features between in situ and partial involvement by follicular and mantle cell lymphoma
| In situ FL/intrafollicular neoplasia* |
| Preserved general architecture |
| Centrocytes strongly positive for BCL2 and CD10 within GC |
| BCL-2 staining in centrocytes usually stronger than in mantle cells or reactive T cells |
| Involved follicles usually scattered, not confluent |
| GCs normal in size and often not completely replaced by BCL-2+ cells |
| Partial involvement by FL |
| Partial effacement of the architecture |
| Affected follicles usually restricted to a limited region of the lymph node |
| Tumor cells may be present in interfollicular areas |
| GCs expanded in size and completely replaced by tumor cells |
| In situ MCL* |
| Preserved general architecture |
| Mantle zones usually not expanded |
| Cyclin D1+ cells restricted to the mantle zones of reactive follicles with only scattered positive cells in interfollicular areas |
| Cyclin D1+ cells tend to accumulate in the inner layers of the mantle zone; not all mantle cells are positive |
| Mantle zone pattern in MCL |
| Architecture preserved or focally effaced |
| Mantle zones usually expanded |
| Cyclin D1+ cells replace virtually all the mantle zone of reactive follicles |
| Focal extension of clusters of tumor cells into interfollicular areas may be seen |
| In situ FL/intrafollicular neoplasia* |
| Preserved general architecture |
| Centrocytes strongly positive for BCL2 and CD10 within GC |
| BCL-2 staining in centrocytes usually stronger than in mantle cells or reactive T cells |
| Involved follicles usually scattered, not confluent |
| GCs normal in size and often not completely replaced by BCL-2+ cells |
| Partial involvement by FL |
| Partial effacement of the architecture |
| Affected follicles usually restricted to a limited region of the lymph node |
| Tumor cells may be present in interfollicular areas |
| GCs expanded in size and completely replaced by tumor cells |
| In situ MCL* |
| Preserved general architecture |
| Mantle zones usually not expanded |
| Cyclin D1+ cells restricted to the mantle zones of reactive follicles with only scattered positive cells in interfollicular areas |
| Cyclin D1+ cells tend to accumulate in the inner layers of the mantle zone; not all mantle cells are positive |
| Mantle zone pattern in MCL |
| Architecture preserved or focally effaced |
| Mantle zones usually expanded |
| Cyclin D1+ cells replace virtually all the mantle zone of reactive follicles |
| Focal extension of clusters of tumor cells into interfollicular areas may be seen |
FL indicates follicular lymphoma; MCL, mantle cell lymphoma; and GC, germinal center.
The terms “FL-like B cells” or “MCL-like B cell of uncertain significance” are recommended as alternatives to “FL in situ” and “MCL in situ” for clinical use in the future.
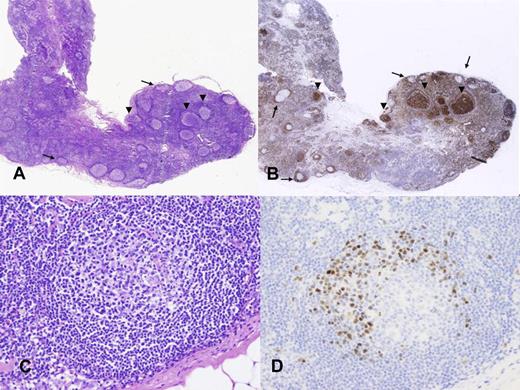
In situ follicular lymphoma and mantle cell lymphoma. (A) Lymph node with a reactive appearance. (B) BCL2 staining shows strong positive cells in some germinal centers (arrows) but not in others (arrowheads). (C) Lymphoid follicle with reactive appearance. (D) Cyclin D1 staining highlights a corona of positive cells in the mantle area. Photographic images were acquired with a Nikon Eclipse 50i microscope equipped with an Olympus (Olympus America Inc) DP71 camera and software. Final image preparation was performed with Adobe Photoshop CS4 extended Version 11.0.2. Original magnifications as follows: panel A(20×/0.1 NA); panel B (20×/0.1 NA); panel C (200×/0.75 NA); panel D (200×/0.75 NA).
In situ follicular lymphoma and mantle cell lymphoma. (A) Lymph node with a reactive appearance. (B) BCL2 staining shows strong positive cells in some germinal centers (arrows) but not in others (arrowheads). (C) Lymphoid follicle with reactive appearance. (D) Cyclin D1 staining highlights a corona of positive cells in the mantle area. Photographic images were acquired with a Nikon Eclipse 50i microscope equipped with an Olympus (Olympus America Inc) DP71 camera and software. Final image preparation was performed with Adobe Photoshop CS4 extended Version 11.0.2. Original magnifications as follows: panel A(20×/0.1 NA); panel B (20×/0.1 NA); panel C (200×/0.75 NA); panel D (200×/0.75 NA).
Early involvement of lymph nodes by cells carrying the t(11;14) translocation and overexpressing cyclin D1 have been reported in several individual cases.69,71,73,74,76 The cyclin D1-expressing cells are predominantly found in the inner area of the mantle zone of the follicles, but usually the rest of the mantle and the follicle have a reactive appearance. The finding is usually incidental in an otherwise reactive lymph node. Some of these patients have circulating t(11;14)–positive cells, but they have not developed a clinically significant neoplasm after several years of follow-up, even without treatment.71,73 However, some cases may progress to overt MCL.76 Similar to the t(14;18) translocation, persisting circulating clones carrying the t(11;14) translocation may be detected in healthy persons, again without evidence of progression.80 However, some patients with clinically detected MCL, usually presenting with leukemic but nonnodal disease, also can have stable disease for many years even without chemotherapy.81 These cases lack chromosomal aberrations other than the t(11;14) and show differential expression of SOX11 and other genes of the high-mobility group of transcription factors, in comparison with conventional MCL.81,82 These observations challenge our current view of the pathogenesis and evolution of MCL and may warrant altered therapeutic strategies on the basis of particular biologic characteristics.
In view of the uncertain clinical behavior of FL in situ and MCL in situ, the terminology of FL- or MCL-like B cells of uncertain significance, in parallel with MGUS, was suggested for these tissue-based lesions at the recent EAHP/SH meeting in Uppsala, Sweden.
Clinical features in disease definition
Clinical features have been always important in lymphoma diagnosis and in certain cases, as in PMBL, an essential element of the definition of the disease. The 2008 WHO classification has expanded this concept and incorporated clinical characteristics, such as age and tumor location, as defining criteria in several newly recognized categories. The issue of location as being a critical factor in disease definition was discussed earlier in the context of aggressive B-cell neoplasms.
Besides location, age has become a significant factor in the recognition of some new lymphoma entities. We previously discussed EBV-positive DLBCL of the elderly. At the other end of the spectrum, some pediatric lymphomas have clinical and pathologic features that differ from the corresponding counterparts in adults. FLs in children tend to present with localized disease in nodal and extranodal sites and are frequently composed of large cells. Despite high-grade (grade 3) cytology, they have a good prognosis with few relapses. The t(14;18) translocation or BCL6 rearrangements are uncommon, although BCL2 protein expression may be found in a subset of the tumors.83 Recurrent breaks in the IGH@ gene are seen in several cases, but the corresponding partners have not been identified.83-85 Some patients have had long survival with only local treatment, and the most appropriate management of these patients is yet to be defined.
Nodal marginal zone lymphoma (NMZL) in children differs from NMZL usually diagnosed in adults. Similarly to pediatric FL, NMZLs in children show a striking male predominance, present as localized disease, and are relatively well controlled with only local therapies.86,87 The biologic characteristics are not well known, but recent genetic studies have shown similar chromosomal aberrations as in the adult counterparts (trisomies 3 and 18 and occasional IGH@ and MALT1 rearrangement) but at lower frequency.88
Florid follicular and marginal zone hyperplasias that occur in children further complicate the diagnosis of pediatric FL and MZL; these cases occasionally have monotypic expression of immunoglobulin light chains, and in some cases evidence of clonality of IG genes at the molecular level. Pediatric patients with FL/MZL should be managed with caution and, as with other in situ type lesions, may represent very early events in neoplasia, with a low risk of clinical effect.89-91
The WHO classification recognized 2 uncommon T-cell lymphoproliferative disorders associated with EBV in children, systemic EBV-positive lymphoproliferative disease of the childhood and hydroa vacciniforme-like lymphoma.48,92,93 These disorders have a particular geographic distribution, more frequently affecting Asians and indigenous populations of Latin America. Hydroa vacciniforme-like lymphoma is a proliferation of clonal T cells or less frequently NK cells infected by EBV with a latency type 1. The disease has an indolent clinical course with long periods of recurrent skin lesions in sun-exposed areas that tend to regress spontaneously. After several years the process may resolve or progress to systemic disease. Mosquito bite allergy is a closely related disorder, usually of NK-cell derivation. Systemic EBV-positive T-cell lymphoproliferative disease is an aggressive condition with rapid evolution to multiple-organ failure and death. The disease has overlapping features with aggressive NK-cell leukemia, but the cells have a T-cell phenotype and clonal TCR rearrangement. It may emerge in a background of chronic active EBV infection and progress from a polyclonal, to oligoclonal, to monoclonal EBV-driven proliferation.94 These lesions may occur less often in young adults.
Gray zones between Hodgkin lymphoma and primary mediastinal large B-cell lymphoma
In the past 20 years there has been a greater appreciation of morphologic and immunophenotypic overlap between some large B-cell lymphomas and classic Hodgkin lymphoma (CHL), particularly nodular sclerosis CHL of the mediastinum and PMBL.95 GEP studies have shown further that PMBL and CHL share a common gene expression signature, supporting a close biologic relationship.96,97 Several reports had recognized the simultaneous or sequential diagnosis of otherwise conventional PMBL and CHL in the same patient.98 Tumors with transitional or intermediate morphologic and phenotypic features between these 2 entities were described (Figure 3).95,99 These observations suggested that a true biologic gray zone between these 2 entities could exist, a finding supported by profiling at the epigenetic level.100 The 2008 WHO classification incorporates these new ideas and recognizes a provisional category of B-cell neoplasms with features intermediate between DLBCL and CHL. The category does not include the composite or sequential cases of both neoplasms. Other intermediate forms between CHL and DLBCL, as may be seen with EBV transformation, represent a different biologic phenomenon. With the use of this concept it appears that these tumors occur predominantly in young men and have more aggressive behavior than either PMBL or NSCHL. The optimal therapeutic management of these lymphomas has not been determined. Recent biologic studies have begun to yield insights into the mechanisms modulating the plasticity of the neoplastic B cells and tumor microenvironment in CHL and DLBCL,100,101 and they may provide data relevant to improved therapies.102
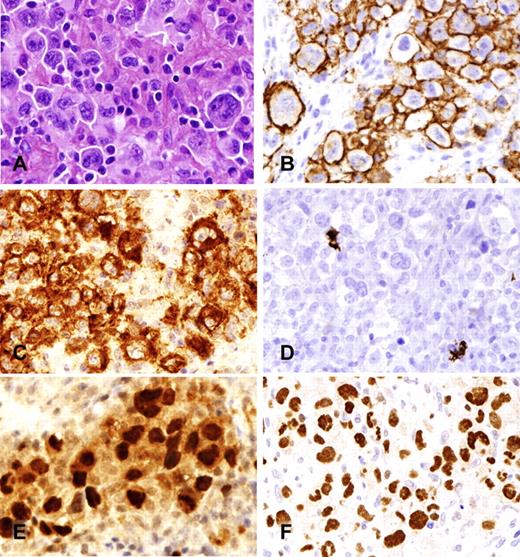
B-cell lymphoma unclassifiable, intermediate between diffuse large B-cell lymphoma and Hodgkin lymphoma. (A) Neoplastic cells resemble Hodgkin/Reed-Sternberg cells (H&E) but show retention of a full B-cell program. (B) CD20 is uniformly positive. (C) CD30 is positive, but CD15 (D) is negative. Both PAX5 (E) and OCT-2 (F) are strongly expressed. Photomicrographic images were acquired with a Nikon Eclipse 50i microscope equipped with an Olympus (Olympus America Inc) DP71 camera and software. Final image preparation was performed with Adobe Photoshop CS4 extended Version 11.0.2. Original magnifications as follows: panels A-F (400×/0.95 NA).
B-cell lymphoma unclassifiable, intermediate between diffuse large B-cell lymphoma and Hodgkin lymphoma. (A) Neoplastic cells resemble Hodgkin/Reed-Sternberg cells (H&E) but show retention of a full B-cell program. (B) CD20 is uniformly positive. (C) CD30 is positive, but CD15 (D) is negative. Both PAX5 (E) and OCT-2 (F) are strongly expressed. Photomicrographic images were acquired with a Nikon Eclipse 50i microscope equipped with an Olympus (Olympus America Inc) DP71 camera and software. Final image preparation was performed with Adobe Photoshop CS4 extended Version 11.0.2. Original magnifications as follows: panels A-F (400×/0.95 NA).
Gray zones between Burkitt and diffuse large B-cell lymphoma
The diagnostic criteria for Burkitt lymphoma (BL) and DLBCL have been relatively well defined for many years. However, pathologists have always encountered cases with intermediate features between these categories that have been difficult to classify. The different names that these tumors have received over the years such as Burkitt-like lymphoma, small noncleaved cell lymphoma, non-Burkitt type, and high-grade B-cell lymphoma, among others, reflect their imprecise definition and limits in our conceptual understanding. Not surprisingly, these borderline cases have been among the least reproducible diagnoses, even among expert pathologists.3 Two recent GEP studies of BLs have provided evidence that the difficulties among pathologists to recognize the border between BL and DLBCL reflect a true biologic gray zone. One study found the molecular signature of BL in a group of cases diagnosed as DLBCL or high grade B-cell lymphomas.103 Despite the molecular signature of BL, these cases differed clinically and genetically from classic BL. They were identified in older patients, had an equal male/female ratio, and complex karyotypes, including simultaneous t(8;14) and t(14;18) translocations (“double-hit” tumors; Figure 4). The clinical behavior was aggressive.104 Similarly, a second GEP study of BL found a subset of tumors with an intermediate expression profile between BL and DLBCL. These cases also had complex karyotypes and MYC translocations with a non-IG gene partner, both uncommon features in typical BL.105
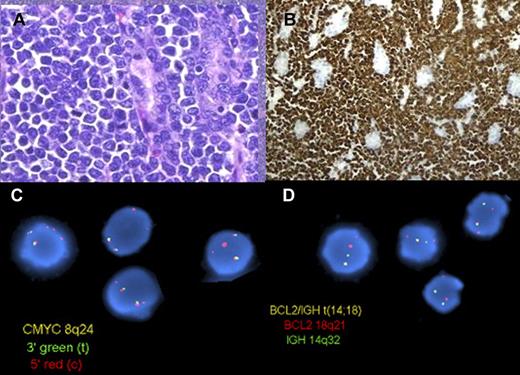
B-cell lymphoma unclassifiable, intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma, with translocations involving MYC and BCL2 (double hit). (A) A high-grade lymphoma involves the gastric mucosa of a patient with a history of low-grade follicular lymphoma (H&E). (B) At higher magnification, the cells are medium-sized and monomorphic with a high mitotic rate (H&E). (C) The cells are strongly positive for Bcl2 (Bcl2 immunoperoxidase stain). (D) FISH on interphase nuclei using a dual-color dual fusion probe for the BCL2 and IGH loci (top) shows one abnormal (yellow) signal in most cells, indicating a translocation involving the BCL2 and IGH genes. FISH on interphase nuclei with the use of a dual-color break-apart probe for the MYC locus (bottom) shows 1-2 yellow signals (normal) and 2-3 red signals, indicating a break at the MYC locus, with loss of the 3′ end of MYC, consistent with an unbalanced or complex MYC rearrangement. Panels A, B, and C were provided by Dr Aliyah Sohani, Department of Pathology, Massachusetts General Hospital. Panel D is reprinted from Snuderl et al.106
B-cell lymphoma unclassifiable, intermediate between diffuse large B-cell lymphoma and Burkitt lymphoma, with translocations involving MYC and BCL2 (double hit). (A) A high-grade lymphoma involves the gastric mucosa of a patient with a history of low-grade follicular lymphoma (H&E). (B) At higher magnification, the cells are medium-sized and monomorphic with a high mitotic rate (H&E). (C) The cells are strongly positive for Bcl2 (Bcl2 immunoperoxidase stain). (D) FISH on interphase nuclei using a dual-color dual fusion probe for the BCL2 and IGH loci (top) shows one abnormal (yellow) signal in most cells, indicating a translocation involving the BCL2 and IGH genes. FISH on interphase nuclei with the use of a dual-color break-apart probe for the MYC locus (bottom) shows 1-2 yellow signals (normal) and 2-3 red signals, indicating a break at the MYC locus, with loss of the 3′ end of MYC, consistent with an unbalanced or complex MYC rearrangement. Panels A, B, and C were provided by Dr Aliyah Sohani, Department of Pathology, Massachusetts General Hospital. Panel D is reprinted from Snuderl et al.106
The WHO classification of 2008 assigned these high-grade B-cell lymphomas that are not readily classified as either BL or DLBCL to an intermediate group. They are relatively rare, occur predominantly in adults, frequently have a germinal center phenotype (CD20+, BCL6+, CD10+), MYC translocations (sometimes with a non-IG partner), and a high proliferation rate that resembles BL. However, they exhibit atypical features for BL such as BCL2 protein expression. Most are aggressive B-cell lymphomas with double-hit translocations.106,107 The borderline cases are not considered a specific entity but a working category, because better guidelines are needed for their recognition and clinical management. Biologically and clinically, they are distinct from both conventional DLBCL and BL. To avoid converting this category into a wastebasket, tumors with typical DLBCL morphology and MYC rearrangement or a high proliferative index or a combination should not be included in this “intermediate” group. These observations suggest that routine evaluation of DLBCL for MYC and other molecular alterations may be advised in the future.
Open questions and future challenges
The 2008 WHO classification of lymphoid neoplasms has been a major consensus effort in updating new knowledge, concepts, and criteria in the taxonomy of these neoplasms. However, many questions still remain.
Similar to DLBCL, peripheral T-cell lymphomas (PTCLs) NOS probably include multiple entities, but we lack criteria and biomarkers to recognize them. The recent identification of follicular Th cells has allowed us to relate angioimmunoblastic lymphoma, one of the major types of PTCL, to this type of germinal center T cell.108,109 A follicular variant of T-cell lymphoma has been recognized that also seems to originate from these cells, with some cases carrying the t(5;9) translocation, fusing ITK to SYK.110,111 However, it is not clear if all PTCL-expressing follicular Th markers correspond to the same entity.112 Despite the recognition of some specific subtypes, most PTCLs still remain under a broad category of NOS. In contrast to what occurred among DLBCL, GEP has not dramatically changed the PTCL, NOS scenario by allowing specific subtype identification. Although GEP identified a cytotoxic subgroup with more aggressive behavior,58 a similar conclusion had been suggested previously in a series analyzed by immunohistochemistry.113 Our lack of success is probably secondary to the relative rarity of PTCL, the heterogeneity of the cellular infiltrates that comprise neoplastic and reactive cells, and our limited knowledge of the normal cellular counterparts. Nevertheless, GEP studies have provided some information about novel therapeutic targets111,114 that are potentially important in light of the lack of sensitivity to conventional chemotherapies.115
The major premise of the REAL and WHO classifications has been that lymphomas were distinct and nonoverlapping disease entities, which could be defined with the use of using a multiple parameter approach that was based on clinical, morphologic, and biologic features. However, several recent observations challenge the idea of a precise separation between entities in certain situations. The identification of the gray zones between CHL and PMBL highlights how tumor cells may cross boundaries between current categories, suggesting that some entities may just be ends of a spectrum in the pathogenetic pathway of the tumor cells and their relationship with the microenvironment. The increasing recognition of a clonal relationship between the different components of composite lymphomas such as FL and MALT lymphoma116 or Hodgkin lymphoma and FL or MCL117 underscore the complex ontogeny of these tumors, which may share a common cell of origin and initial transformation events. Recent studies have shown identical clonal rearrangements in T-cell lymphoblastic leukemias and subsequent Langerhans cell histiocytosis or in FLs lymphomas and associated histiocytic tumors, indicating a common clonal origin in tumors of different lineages.118-120 This phenomenon recapitulates in a clinical setting the plasticity of hematopoietic cells observed in experimental models, in which modulation of specific transcription factors can reprogram cells to enter disparate differentiation pathways.121
The biology of lymphoid cells and the tumors derived from these cells is complex. The application of new technologies has always helped to improve our understanding and to translate the new knowledge into clinical applications. Microarray platforms are one of the first tools to bring a broad genomic perspective to the study of lymphomas. GEP and chromosomal array studies have confirmed the molecular and genetic identity of many different diseases. Intriguingly, they have helped to discover only a limited number of new possible entities such as the GCB and ABC molecular subtypes of DLBCL or some categories of T-cell lymphoblastic leukemia, indicating that the classic pathologic and immunophenotypic approach to the study of lymphomas has been a robust and successful tool of disease discovery.4 However, genetic and genomic approaches have proved instrumental in defining the molecular identities of diseases, providing new prognostic models, and identifying molecular pathogenetic pathways that may be targets of new therapeutic drugs. Wide genome screening of genetic alterations is starting to provide insights into the genetic susceptibility of some entities and the discovery of a broader spectrum of oncogenic mutations relevant in the pathogenesis of these tumors than previously known.122-125 The next generation of sequencing technologies with the possibility of elucidating the whole genome sequence of large number of tumors at an affordable cost is the next step starting to produce early results.101,126,127 The new technologies promise new insights into basic biology, allowing us to refine our clinical and pathologic perspectives. Classifications, as a reference framework between research and clinical practice, will continue to evolve, keeping pace with new discoveries and knowledge.
Appendix
Chairs and members of the Clinical Advisory Committee for the WHO Classification of Lymphoid Neoplasms were the following: N. L. Harris, Boston, MA; E. Campo, Barcelona, Spain; E. Montserrat, Barcelona, Spain; S. Horning, Palo Alto, CA (Chairs); K. C. Anderson, Boston, MA; J. O. Armitage, Omaha, NE; P. L. Bergsagel, Scottsdale, AZ; C. D. Bloomfield, Columbus, OH; D. Catovsky, Sutton, United Kingdom; F. Cavalli, Bellinzona, Switzerland; J. K. C. Chan, Hong Kong, China; W. C. Chan, Omaha, NE; B. Cheson, Washington, DC; B. Coiffier, Pierre-Bénite, France; J. Connors, Vancouver, BC; G. Delsol, Toulouse, France; V. Diehl, Köln, Germany; M. A. Dimopoulos, Athens, Greece; A. Engert, Köln, Germany; B. Falini, Perugia, Italy; R. I. Fisher, Rochester, NY; P. Gaulard, Créteil, France; A. Hagenbeek, Amsterdam, The Netherlands; W. Hiddemann, Munich, Germany; R. Hoppe, Palo Alto, CA; M. M. Hudson, Memphis, TN; P. Isaacson, London, United Kingdom; R. Jaffe, Pittsburgh, PA; E. Jaffe, Bethesda, MD; P. Johnson, Southampton, United Kingdom; M. J. Keating, Houston, TX; E. Kimby, Huddinge, Sweden; P. Kluin, Croningen, The Netherlands; R. Liang, Hong Kong, China; D. Linch, London, United Kingdom; M. Link, Palo Alto, CA; T. A. Lister, London, United Kingdom; R. McKenna, Minneapolis, MN; S. Nakamura, Nagoya, Japan; M. Pfreundschuh, Homburg, Germany; S. Pileri, Bologna, Italy; M. A. Piris, Madrid, Spain; S. Poppema, Groningen, The Netherlands; K. R. Rai, New Hyde Park, NY; S. T. Rosen, Chicago, IL; G. A. Salles, Pierre-Benité, France; M. A. Shipp, Boston, MA; H. Stein, Berlin, Germany; S. Swerdlow, Pittsburgh, PA; J. Thiele, Cologne, Germany; S. P. Treon, Boston, MA; K. Tsukasaki, Nagasaki, Japan; J. Vardiman, Chicago, IL; R. Warnke, Stanford, CA; H. J. Weinstein, Boston, MA; R. Willemze, Leiden, The Netherlands, W. Wilson, Bethesda, MD; M. Yamaguchi, Tsu Mie, Japan; A. Zelenetz, New York, NY; P. L. Zinzani, Bologna, Italy; and E. Zucca, Locarno, Switzerland.
Acknowledgments
The preparatory works and the meeting of the Clinical Advisory Committee for the World Health Organization classification were supported in part by the Associazione S.P.E.S. Onlus, Bologna; Friends of José Carreras International Leukemia Foundation; National Cancer Institute; and the Office of Rare Diseases, National Institutes of Health; and unrestricted educational grants from Hoffman-La Roche and Genentech.
National Institutes of Health
Authorship
Contribution: E.C., S.H.S., N.L.H., S.P., H.S., and E.S.J. contributed conceptually to the development of the classification, and wrote and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Elaine S. Jaffe, Hematopathology Section, Laboratory of Pathology, National Cancer Institute, 10 Center Dr MSC 1500, Bethesda, MD 20892; e-mail: elainejaffe@nih.gov.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal