Abstract
Weibel-Palade bodies (WPBs) are elongated secretory organelles specific to endothelial cells that contain von Willebrand factor (VWF) and a variety of other proteins that contribute to inflammation, angiogenesis, and tissue repair. The remarkable architecture of WPBs is because of the unique properties of their major constituent VWF. VWF is stored inside WPBs as tubules, but on its release, forms strikingly long strings that arrest bleeding by recruiting blood platelets to sites of vascular injury. In recent years considerable progress has been made regarding the molecular events that underlie the packaging of VWF multimers into tubules and the processes leading to the formation of elongated WPBs. Mechanisms directing the conversion of tightly packaged VWF tubules into VWF strings on the surface of endothelial cells are starting to be unraveled. Several modes of exocytosis have now been described for WPBs, emphasizing the plasticity of these organelles. WPB exocytosis plays a role in the pathophysiology and treatment of von Willebrand disease and may have impact on common hematologic and cardiovascular disorders. This review summarizes the major advances made on the biogenesis and exocytosis of WPBs and places these recent discoveries in the context of von Willebrand disease.
Introduction
In 1964 Ewald Weibel and George Palade used transmission electron microscopy (EM) to discover that “a hitherto unknown rod-shaped cytoplasmic component which consists of a bundle of fine tubules, enveloped by a tightly fitted membrane, was regularly found in endothelial cells of small arteries in various organs in rat and man.”1 Subsequent studies confirmed the presence of those organelles in a variety of vertebrates, including hagfish,2 which suggests they were present at least 500 million years ago. These organelles have a diameter of 0.1-0.3 μm, length of 1-5 μm, and characteristic longitudinal striations (Figure 1).3 In cross section they consist of electron dense tubules with an inside diameter of 12 nm, surrounded by a less dense matrix, and packed in parallel bundles that are surrounded by a lipid bilayer. We now refer to these organelles as Weibel-Palade bodies (WPBs).
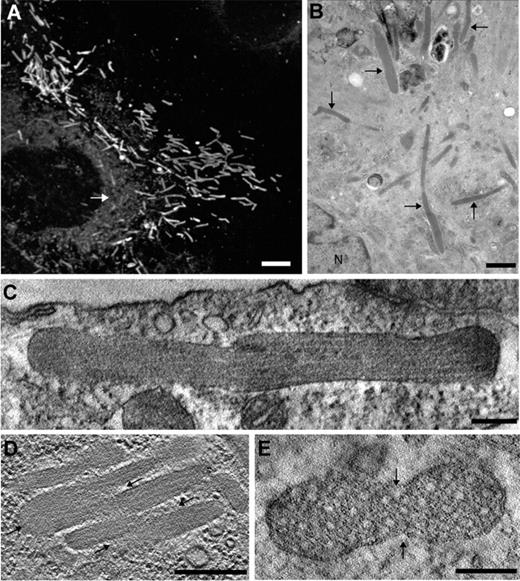
Morphology of WPBs in nonstimulated cells. (A) Immunofluorescence image of a human umbilical vein endothelial cell (HUVEC) labeled with an antibody against VWF, revealing the numerous elongated WPBs throughout the cell. Note the reticular staining representing the ER (white arrow). Scale bar represents 5 μm. (B) EM image of a HUVEC showing several WPBs (arrows). N, nucleus. Scale bar, 1 μm. (C-E) Digital slices through electron tomograms of WPBs displaying irregular shapes suggesting homotypic fusion between WPBs.3 (C) The delimiting membrane of the WPB is not straight along the long axis of the WPB. Scale bar, 200 nm. (D) Several WPBs are aligned against each other and are connected at some extremities (arrows). Scale bar, 200 nm. (E) Transverse section of an atypical WPB with an oval shape displaying 2 indentations (arrows). Scale bar, 100 nm.
Morphology of WPBs in nonstimulated cells. (A) Immunofluorescence image of a human umbilical vein endothelial cell (HUVEC) labeled with an antibody against VWF, revealing the numerous elongated WPBs throughout the cell. Note the reticular staining representing the ER (white arrow). Scale bar represents 5 μm. (B) EM image of a HUVEC showing several WPBs (arrows). N, nucleus. Scale bar, 1 μm. (C-E) Digital slices through electron tomograms of WPBs displaying irregular shapes suggesting homotypic fusion between WPBs.3 (C) The delimiting membrane of the WPB is not straight along the long axis of the WPB. Scale bar, 200 nm. (D) Several WPBs are aligned against each other and are connected at some extremities (arrows). Scale bar, 200 nm. (E) Transverse section of an atypical WPB with an oval shape displaying 2 indentations (arrows). Scale bar, 100 nm.
At the time of their discovery, the biologic function of WPBs was unknown, although their conserved ultrastructure and wide distribution suggested that they must play a significant role in vertebrate endothelium. Almost 20 years later WPBs were shown to contain von Willebrand factor (VWF),4 a multimeric hemostatic protein that is secreted into the blood in response to a variety of agonists and mediates platelet adhesion at sites of vascular injury. Inherited defects in VWF cause von Willebrand disease (VWD), the most common inherited bleeding disorder.5 Interestingly, VWF is also produced by megakaryocytes and platelets,6 the latter containing tubular structures similar to those found in WPBs but eccentrically localized in the α-granule, and shown to be positive for VWF by immunogold EM techniques.7 In fact, VWF is the only protein readily detected in WPBs after biosynthetic labeling of endothelial cells.8 Several other proteins have been identified as constituents of WPBs including tissue-type plasminogen activator (tPA), P-selectin, interleukin-8 (IL-8), eotaxin-3, angiopoietin-2, osteoprotegerin, endothelin-1, endothelin-converting enzyme, and calcitonin gene-related peptide.9,10 In addition, clotting factor VIII is stored with VWF in the endothelial cells of some tissues such as lung.11,12 Therefore, WPBs contain a supply of mediators that could be deployed in response to signaling molecules or mechanical stress, allowing vascular endothelial cells to influence hemostasis, inflammation, angiogenesis, and vascular tone. This review will focus on recent advances in understanding the biogenesis, composition, ultrastructure, and secretion of WPBs that illuminate their biologic function.
Biosynthesis of VWF and the biogenesis of WPBs
VWF is a prerequisite for the existence of WPBs. Endothelial cells of VWF-deficient animals do not contain WPBs.13-15 The expression of recombinant VWF in other cell types induces them to produce WPB-like granules that are similarly cigar-shaped and striated.16,17 These data demonstrate that VWF has the intriguing property of inducing the elongated organelle in which it is stored.
The VWF precursor is composed of a signal peptide followed by conserved structural domains in the order D1-D2-D′-D3-A1-A2-A3-D4-B1-B2-B3-C1-C2-CK. Domains D1D2 (741 aa, 100 kDa) represent the propeptide, and the other domains ranging from domain D′ to the cysteine knot (CK) domain (2050 aa, 260 kDa) represent mature VWF. In the endoplasmic reticulum (ER), pro-VWF assembles into dimers (also called protomers) in a “tail-to-tail” fashion by forming interchain disulfide bonds between CK domains.18
After arrival in the trans-Golgi network (TGN), dimers of pro-VWF assemble into multimers by forming “head-to-head” interchain disulfide bonds between D3 domains and subsequently the propeptide is cleaved, probably by furin.18,19 Multimerization depends on the decrease in pH between the ER (pH 7.2) and TGN (pH 6.2)20 and is blocked by treatment of cells with a weak base.21
The VWF multimers and propeptide condense into tubules and become incorporated into nascent vesicles with clathrin/AP-1 coats22,23 that protrude from the TGN. Targeting to the WPB requires the VWF propeptide.17 VWF tubules have only been identified in the TGN and not in other cisternae of the Golgi, suggesting that they form at this stage.23 WPB formation can be prevented by overexpression of a dominant-negative AP180 construct that blocks clathrin coat formation or by siRNA knockdown of AP-1. Thus, a clathrin/AP-1 coat is essential for the packaging of VWF tubules and possibly for their formation.
The AP-1 effectors aftiphilin and γ-synergin are recruited to forming WPBs and exhibit partial colocalization with immature perinuclear WPBs. Depletion of either protein with siRNA does not alter the number or morphology of WPBs but increases basal secretion and reduces the agonist-stimulated secretion of VWF.24 Therefore, the transient acquisition of a clathrin/AP-1 coat and associated effector proteins is required to specify the regulated secretory phenotype of mature WPBs. In summary, the biogenesis of WPBs requires the presence of VWF and the formation of clathrin/AP-1 coats.
Recruitment of other WPB cargo in the TGN
Several other proteins are incorporated into WPBs in the TGN: tPA, angiopoietin-2, osteoprotegerin, and various cytokines, as well as P-selectin, a transmembrane protein with a large lumenal domain and short cytoplasmic tail. The P-selectin lumenal domain alone is sufficient to direct incorporation into WPBs, possibly because it interacts with the D′D3 domains of VWF.25 In addition, a YGVF motif in the P-selectin cytoplasmic tail can target heterologous proteins to WPBs. Interestingly, P-selectin is also produced by platelets and is recruited to the α-granule.26 Although VWF and P-selectin are specific for the WPBs and α-granules, deficiency of VWF disrupts the targeting of P-selectin to endothelial WPBs but has no effect on targeting P-selectin to platelet α-granules. Conversely, deficiency of P-selectin has no effect on the targeting of VWF.13 Like P-selectin, osteoprotegerin appears to be incorporated into WPBs through direct interaction with VWF, in this case with the VWF A1-domain.27,28 Thus, both P-selectin and osteoprotegerin are actively sorted into WPBs by virtue of their ability to interact with VWF.
Several investigators have addressed sorting of the tetraspanin CD63 to WPBs. CD63 is delivered to maturing WPBs by a mechanism that requires AP3, an adaptor that participates in targeting of proteins from early endosomes to lysosomes and related organelles.25,29 Sorting of endosomal proteins to post–Golgi secretory granules is a characteristic of lysosome-related organelles such as melanosomes and α-granules30,31 and suggests that WPBs share some properties with lysosome-related organelles.32
Angiopoietin-2 is an autocrine regulator of Tie-2 signaling that is markedly induced in endothelial cells by vascular endothelial growth factor or hypoxia.33 Angiopoietin-2 functions mainly as a Tie-2 antagonist, inhibiting angiogenesis while promoting inflammation.34 Angiopoietin-2 is incorporated into WPBs, probably in the TGN, but only into WPBs that lack P-selectin and thus the storage of angiopoietin-2 and P-selectin appears to be mutually exclusive.35
The relevance of cytokine storage in WPBs has recently been questioned. The efficiency of sorting to WPBs was determined for tPA, IL-8, monocyte chemoattractant protein-1, and growth regulated oncogene-α in comparison with enhanced green fluorescent protein, a nonmammalian protein with no intrinsic targeting information. For each cytokine, the fraction recovered in WPBs was no greater than that of enhanced green fluorescent protein, < 5%. Such inefficient storage suggests that most cytokines are not specifically targeted to WPBs but are incidentally included and inefficiently removed possibly because of their ability to bind to VWF at low pH as observed for IL-8.36
Maturation and morphology of WPBs
After budding from the TGN, immature WPBs remain in a perinuclear location, where they acquire additional membrane proteins and then disperse throughout the cytoplasm. Maturation is accompanied by loss of the clathrin/AP-1 coat,22,23 aftiphilin, and γ-synergin,24 as well as a further decrease in pH to approximately pH 5.4.37 The separation between VWF tubules is relatively large within immature WPBs, which are much less electron dense than mature WPBs.3,23,38,39 The budding of clathrin-coated vesicles from immature WPBs probably removes selected proteins and facilitates condensation of mature WPB contents by removing excess membrane.
A growing list of membrane proteins distinguishes mature (cytoplasmic) from immature (perinuclear) WPBs. As mentioned previously, CD63 first appears in endosomes and is subsequently recycled to preformed WPBs.25 WPBs also acquire several small GTPases, including Rab27A,40 RalA,41,42 and Rab3D.43 Rab27A interacts with MyRIP, a Rab effector protein that binds myosin VIIa or myosin Va and may link WPBs to the actin cytoskeleton. Rab27A and MyRIP are recruited sequentially: Rab27A first decorates WPBs uniformly and then MyRIP appears preferentially at the tips of WPBs that associate with actin filaments at the cell periphery. Depletion of either Rab27A or MyRIP causes a loss of peripheral WPB localization and an increase in both basal and stimulated VWF secretion, suggesting that Rab27A and MyRIP prevent premature secretion of WPBs.44
Similarly, overexpression of Rab3D or constitutively active Rab3D (Q81L) inhibits agonist-induced secretion and causes the formation of large spherical WPBs. Transfection with dominant-negative Rab3D (T36N or N135I) causes WPBs to disappear.43 These results suggest that Rab3D inhibits exocytosis and controls the formation and shape of WPBs, consistent with studies of Rab3D knockout mice showing that Rab3D is involved in the biogenesis of pancreatic secretory granules.45 In contrast, transfection with constitutively active RalA (G23V) promotes exocytosis and leads to the disappearance of most WPBs.41,42 These findings indicate that the biogenesis and function of WPBs require the coordinated activity of multiple small GTPases.
In addition to heterogeneity of cargo and membrane proteins, WPBs exhibit striking variations in morphology and flexibility that correlate with their state of maturation. Analysis of endothelial cells by electron tomography3 or transmission EM23 shows that some WPBs have kinked or branched shapes (Figure 1). The VWF tubules appear discontinuous or kinked at these junctions, suggesting they may be flexible, and video microscopy of living endothelial cells confirms that WPBs do frequently flex at such bends.23,46 Some images show electron dense material between juxtaposed WPBs or focal narrowing at the site of bending, which suggests that complex irregular shapes result from homotypic fusion between WPBs.3 This form of homotypic fusion that occurs in the absence of regulated exocytotic activity (see “Exocytosis”) most likely represents a maturation step during WPB biogenesis. The functional significance of WPB flexing is unclear, but it seems plausible that it enhances the maneuverability of WPBs within the confined space of the endothelial cell cytoplasm.
The morphology of VWF tubules within WPBs reflects their mode of assembly and maturation. The tips of tubules frequently touch the WPB membrane, but the sides of tubules are separated from the membrane by a distance that often appears constant along the length of a tubule.3,23,47
VWF tubules can extend the entire length of a WPB but are sometimes truncated (Figure 2).3,47,48 Tubules within immature WPBs tend to be curved and disorganized, but in mature WPBs the tubules usually are regularly arranged, with a majority running parallel from end to end of the WPB.3,23,47 Tomographic reconstructions of entire WPBs indicate that VWF tubules gently twist inside the mature WPB, which could mean that tubules are spring-loaded. These studies suggest that tightly packed VWF tubules are stiffer than individual tubules, which are flexible and can rearrange during the maturation of WPBs.
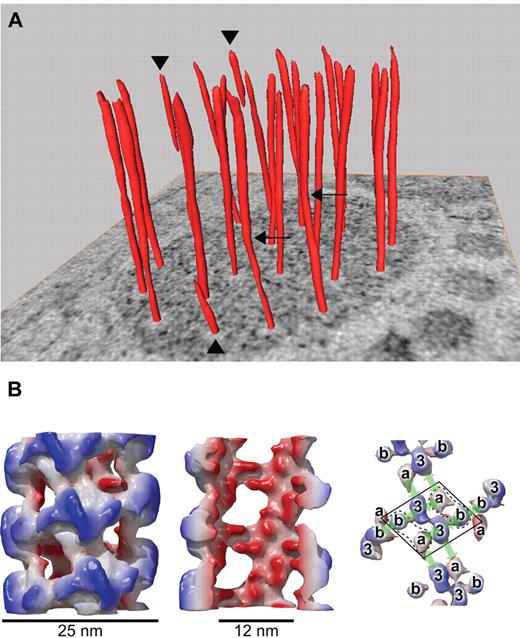
Helical arrangement of VWF in tubular striations of WPBs. (A) The orderly twisting of the tubules within WPBs is illustrated by the surface rendered tubules on a tomographic slice. Three tubules stop halfway into the WPB (arrowheads) and 2 tubules display kinks (arrows). (B) Reconstruction of VWF tubules assembled in vitro at pH 6.2 from purified dimeric D′D3 and D1D2 domains. Left, whole tubule demonstrating an outside diameter of 25 nm. Middle, cutaway view shows the inner diameter of the tubule of 12 nm. Right, arrangement of the different domains within the helix. The D′D3 domain in the center is flanked by 2 D1D2 domains (because the orientation of the propeptide is not yet known, a and b represent either D1 or D2). The arrows indicate 4 distinct noncovalent domain-domain interactions. Panel B was adapted from Huang et al.48
Helical arrangement of VWF in tubular striations of WPBs. (A) The orderly twisting of the tubules within WPBs is illustrated by the surface rendered tubules on a tomographic slice. Three tubules stop halfway into the WPB (arrowheads) and 2 tubules display kinks (arrows). (B) Reconstruction of VWF tubules assembled in vitro at pH 6.2 from purified dimeric D′D3 and D1D2 domains. Left, whole tubule demonstrating an outside diameter of 25 nm. Middle, cutaway view shows the inner diameter of the tubule of 12 nm. Right, arrangement of the different domains within the helix. The D′D3 domain in the center is flanked by 2 D1D2 domains (because the orientation of the propeptide is not yet known, a and b represent either D1 or D2). The arrows indicate 4 distinct noncovalent domain-domain interactions. Panel B was adapted from Huang et al.48
Changes with WPB maturation include a striking increase in electron density and a corresponding decrease in the separation between the electron dense tubules. Immature and mature WPBs have average diameters of approximately 220 nm and 150 nm, respectively,23 which implies an approximately 45% progressive compaction of WPB contents.
Assembly and structure of VWF tubules
The packing of VWF into tubules does not require dimerization of pro-VWF subunits in the ER. RIN 5F cells expressing recombinant VWF truncated before the CK domain do not make pro-VWF dimers but still form elongated WPB-like organelles with longitudinal striations and tubular cross sections.17 In human embryonic kidney 293 (HEK293) cells, expression of VWF domains D1D2D′D3 is sufficient to store VWF tubules in WPBs, and removal of the A1 domain still allows the formation of elongated storage granules.49 Therefore, the N-terminal D1D2D′D3 domains of VWF are sufficient for targeting and tubular packing in WPBs.
VWF tubules can be assembled reversibly in vitro with the same N-terminal domains of VWF.48 At pH 7.4, similar to the ER, 2 D1D2 propeptides and one disulfide-linked dimeric D′D3 fragment form a calcium ion-dependent complex. At pH 6.2, characteristic of the TGN, these complexes assemble into hollow, fenestrated, right-handed helical tubules with 4.2 repeating units per turn and a variable pitch of 9-12 nm (Figure 2). The tubules have an inside diameter of 12 nm, outside diameter of 25 nm, and wall thickness of 6 nm, comparable with VWF tubules in WPBs.48 No other cellular proteins or components of WPBs are required, indicating that VWF subunits contain all the information needed for self-assembly into tubules.
VWF within endothelial WPBs is organized similarly. High-resolution EM images show that VWF tubules in endothelial cells have cross striations with a spacing of 11-14 nm,3,47 consistent with the 11-nm average pitch of tubules assembled in vitro. In addition, cryo-EM of endothelial cells demonstrates that VWF tubules within WPBs are helices with the same inside (12 nm) and outside (24 nm) diameter, and fenestrated structure as tubules made from pure VWF fragments. The pitch (12 nm) of tubules within mature WPBs is less variable, probably because of the greater straightness and rigidity of the tubules.47 The constant dimensions of VWF tubules, made with VWF D′D3 dimers in vitro or full-length VWF in vivo, suggests that all VWF domains C-terminal to the D3 domain are located outside of the helical tubule and comprise the matrix that separates tubules from each other and the WPB membrane.
Mutations in VWD and defects in WPB biogenesis
The biosynthesis of VWF involves multimerization in the Golgi, targeting to WPBs, recruitment of selected cargo, and tubular packing. Mutations in the D1D2D′D3 region of VWF that cause various subtypes of VWD (ISTH-SSC VWF online database50 ) affect some of these steps (Table 1)12,15,16,49,51-60 and illuminate the mechanism of WPB formation.
Multimerization, targeting, and tubulation for selected VWD mutations
| Domain/mutation . | VWD type . | Multimers . | Stored* . | Granule shape† . | Tubules . | Cell line‡ . | References . |
|---|---|---|---|---|---|---|---|
| D1 | |||||||
| Tyr87Ser | 2A (IIC) | No | Yes | – | – | AtT-20 | Rosenberg et al51 |
| Yes | Round | – | HEK293 | Michaux et al49 | |||
| Yes | – | – | VWD-AECs | Haberichter et al15 | |||
| Arg273Trp | 2A | No | Yes/no§ | Round | Disordered | HEK293 | Michaux et al52 , Allen et al59 |
| D2 | |||||||
| del437-442 | 2A | No | No | na | na | AtT-20 | Haberichter et al53 |
| Asn528Ser | 2A (IIC) | No | No | na | na | AtT-20, HEK293, VWD-AECs | Haberichter et al54 |
| Furin site | |||||||
| Arg763Gly | 2N | Yes | Yes§ | Rod | Normal | CV-1 | Voorberg et al16 |
| Yes | Rod | – | HEK293 | van den Biggelaar et al57 | |||
| No§ | na | na | RIN 5F | Journet et al56 | |||
| No | na | na | AtT-20 | Journet et al56 | |||
| 2269_2270delCT | Yes | – | – | AtT-20 | Rosenberg et al12 | ||
| D′D3 | |||||||
| Cys788Arg | 2A/2N | No | Yes§ | Rod (short) | Normal | HEK293 | Allen et al60 , Michaux et al52 |
| Thr791Met | 2N | Yes | Yes | Rod | – | HEK293 | van den Biggelaar et al57 |
| Arg816Trp | 2N | Yes | Yes | Rod | – | HEK293 | van den Biggelaar et al57 |
| Arg854Gln | 2N | Yes | Yes | Rod | – | HEK293 | van den Biggelaar et al57 |
| Arg854Trp | 2N/2A | No | No | na | na | HEK293 | Castaman et al58 |
| Cys1060Arg | 2N | Yes | Yes | Rod | – | HEK293 | van den Biggelaar et al57 |
| Cys1157Phe | 2A | No | Yes/no | – | – | AtT-20 | Hommais et al55 |
| Cys1225Gly | 2A/2N | No | Yes§ | Rod (short) | Normal | HEK293 | Allen et al60 , Michaux et al52 |
| Cys1234Trp | 2A | No | Yes/no | – | – | AtT-20 | Hommais et al55 |
| Domain/mutation . | VWD type . | Multimers . | Stored* . | Granule shape† . | Tubules . | Cell line‡ . | References . |
|---|---|---|---|---|---|---|---|
| D1 | |||||||
| Tyr87Ser | 2A (IIC) | No | Yes | – | – | AtT-20 | Rosenberg et al51 |
| Yes | Round | – | HEK293 | Michaux et al49 | |||
| Yes | – | – | VWD-AECs | Haberichter et al15 | |||
| Arg273Trp | 2A | No | Yes/no§ | Round | Disordered | HEK293 | Michaux et al52 , Allen et al59 |
| D2 | |||||||
| del437-442 | 2A | No | No | na | na | AtT-20 | Haberichter et al53 |
| Asn528Ser | 2A (IIC) | No | No | na | na | AtT-20, HEK293, VWD-AECs | Haberichter et al54 |
| Furin site | |||||||
| Arg763Gly | 2N | Yes | Yes§ | Rod | Normal | CV-1 | Voorberg et al16 |
| Yes | Rod | – | HEK293 | van den Biggelaar et al57 | |||
| No§ | na | na | RIN 5F | Journet et al56 | |||
| No | na | na | AtT-20 | Journet et al56 | |||
| 2269_2270delCT | Yes | – | – | AtT-20 | Rosenberg et al12 | ||
| D′D3 | |||||||
| Cys788Arg | 2A/2N | No | Yes§ | Rod (short) | Normal | HEK293 | Allen et al60 , Michaux et al52 |
| Thr791Met | 2N | Yes | Yes | Rod | – | HEK293 | van den Biggelaar et al57 |
| Arg816Trp | 2N | Yes | Yes | Rod | – | HEK293 | van den Biggelaar et al57 |
| Arg854Gln | 2N | Yes | Yes | Rod | – | HEK293 | van den Biggelaar et al57 |
| Arg854Trp | 2N/2A | No | No | na | na | HEK293 | Castaman et al58 |
| Cys1060Arg | 2N | Yes | Yes | Rod | – | HEK293 | van den Biggelaar et al57 |
| Cys1157Phe | 2A | No | Yes/no | – | – | AtT-20 | Hommais et al55 |
| Cys1225Gly | 2A/2N | No | Yes§ | Rod (short) | Normal | HEK293 | Allen et al60 , Michaux et al52 |
| Cys1234Trp | 2A | No | Yes/no | – | – | AtT-20 | Hommais et al55 |
ER indicates endoplasmic reticulum; na, not applicable; and VWF, von Willebrand factor.
Storage in secretory granules (Yes), retention in ER (No), or mainly retention in ER with some granule storage (Yes/no) was determined by immunofluorescence, except where localization by electron microscopy is indicated (§).
Shape determined by immunofluorescence, in some cases confirmed by electron microscopy. For VWF mutants that were not stored, granule shape is not applicable (na).
Cell line listed was used to investigate VWF targeting and storage. Multimerization and storage were not always studied in the same cells, but no discordance among cell lines has been observed for VWF multimerization.
Localization by electron microscopy.
VWD type 2A is characterized by the absence of large VWF multimers, and several VWD type 2A mutations in the VWF propeptide have interesting effects on WPB biogenesis (Table 1). The mutation Y87S in the D1 domain causes a profound defect in multimer assembly.51 VWF Y87S is targeted to storage granules in a variety of cell types, and expression in HEK293 cells leads to storage of VWF in round granules, suggesting a defect in tubular packing of the mutant VWF.15,49,51 When expressed in human endothelial cells, VWF Y87S causes endogenous WPBs to become spherical, indicating that the mutant VWF disrupts the tubular packing of wild-type VWF.49 The mutation R273W causes most of the protein to be retained in the ER. The small amount that reaches the Golgi is targeted to spherical granules with disordered tubular packing of VWF.52 These domain D1 mutations indicate that targeting to storage vesicles does not require VWF multimerization, which involves the formation of intersubunit disulfide bonds between D3 domains.
VWD type 2A mutations in the D2 and D3 domains are dominated by intracellular retention in the ER. The in-frame deletion of 6 amino acid residues in VWF del437-442 causes colocalization in the ER with GRP78, with no VWF multimerization or WPB formation in AtT-20 cells.53 The mutation VWF N528S also causes ER retention, defective multimerization, and the absence of storage organelles when expressed in AtT-20 cells, HEK293 cells, or aortic endothelial cells from dogs with severe VWD.54 The effects of VWF N528S may be because of the creation of a new N-linked glycosylation site. The adjacent VWF sequence is 521CGLCGNYN528, and the N528S substitution may allow the glycosylation of Asn526. A patient with the homozygous VWF N528S mutation did not respond to desmopressin (DDAVP; Rhone Poulenc Rorer, now Aventis Pharma) with an increase in plasma VWF, which suggests that failure to store mutant VWF N528S in transfected cells correlates with failure of storage and regulated secretion in vivo.54
Heterozygous D3 domain mutations C1157F and C1234W were identified in 2 families with VWD type 2A.55 When expressed in COS-7 cells, both mutant proteins were mostly retained in the ER and had severe defects in multimer assembly, which is often observed for VWF mutations at conserved Cys residues. However, in AtT-20 cells some recombinant VWF C1157F or C1234W was targeted to storage granules (Table 1).55
Mutations at the furin cleavage site between the propeptide and mature VWF subunit have uncovered some differences in WPB biogenesis among cell lines. Cleavage of pro-VWF after Arg763 occurs in the TGN and is required for the high-affinity binding of factor VIII to VWF. The mutation R763G blocks cleavage of pro-VWF. This mutation was discovered in a heterozygous patient with VWD type 2N characterized by a very low plasma factor VIII, normal VWF:Ag and VWF:RCo, and a full range of VWF multimers.61 Recombinant VWF R763G was stored in rod-shaped WPBs when expressed in CV-1 or HEK293 cells but was not stored detectably in RIN 5F or AtT-20 cells, despite normal multimer assembly16,56,57 (Table 1). Therefore, propeptide cleavage appears to be necessary for WPB formation in some but not all cell lines. However, a more complex mutation at the furin cleavage site, deletion 2269_2270delCT, results in alternative splicing across intron 17 and causes persistence of pro-VWF.12,62 When expressed in AtT-20 cells, this variant is targeted to storage granules, in contrast to the R763G mutation, indicating that the persistence of pro-VWF per se does not prevent granular storage. Whether VWF propeptide cleavage is necessary for storage in endothelial cells is unknown.
VWD type 2N mutations in the D′D3 region of VWF impair factor VIII binding to VWF, and most are compatible with normal multimerization and recruitment of P-selectin to rod-shaped WPBs.57 The exceptions include mutations C788R, R854W, and C1225G (Table 1).52,58 When expressed in HEK293 cells, VWF R854W did not assemble into large multimers, was secreted poorly, and was not stored in secretory granules.58 In COS-7 cells, VWF C788R had a similar severe defect in multimer assembly and secretion, whereas VWF C1225G formed multimers with almost normal efficiency. In HEK293 cells, both VWF C788R and VWF C1225G were stored in WPBs that were somewhat shorter, but tubular packing appeared normal.52
The effect of factor VIII on WPB biogenesis suggests a mechanism by which proteins that interact with VWF may disrupt tubular packing. Recombinant factor VIII expressed in human endothelial cells was stored with VWF and caused the endogenous WPBs to become spherical.57 Unexpectedly, several VWD type 2N mutations that markedly impair factor VIII-VWF binding did not prevent the storage of factor VIII with VWF in HEK293 cells, and both proteins were stored in spherical organelles.57 This apparently paradoxical result suggests that factor VIII might bind more tightly to VWF type 2N variants in the TGN (pH 6.2) than in standard factor VIII-VWF binding assays (pH 7.4). A similar study in AtT-20 cells provided different results. Factor VIII was stored in granules with wild-type VWF, but not with type 2N variants caused by either mutation R854Q or a splicing defect that prevents VWF propeptide cleavage.12 Some factor VIII appears to be stored with VWF in patients with VWD type 2N because both plasma VWF and factor VIII increase after DDAVP. The magnitude of the factor VIII response appears to be substantially greater for the mutation R854Q, which causes a modest decrease in factor VIII binding, compared with the mutation R816W, which profoundly impairs factor VIII binding.63
Mutagenesis of VWF has provided more precise information about the role of D domains in VWF targeting and tubular packing. Both the D1 and D2 domains have a conserved CGLC motif that resembles the active sites of protein disulfide isomerases, and these motifs are required for VWF multimerization. The insertion of an extra Gly residue into the CGLC motif of domain D1 prevents multimerization but allows VWF storage in rod-shaped granules within AtT-20 cells. Thus, the formation of intersubunit disulfide bonds between D3 domains depends on specific sequences in the D1 domain, but multimerization is not a prerequisite for tubular VWF packing.64
The D1D2D′D3 portions of canine and human VWF are 87% identical in amino acid sequence, and the construction of canine-human chimeric proteins has been useful to identify interactions that contribute to WPB biogenesis.65 For example, canine propeptide supports the multimerization of human mature VWF but not targeting to WPBs. Residue 416 is Arg in the canine D2 domain and Gln in the human, and the mutation R416Q enables the canine propeptide to support both multimerization and storage in WPBs of human mature VWF. Storage in WPBs also is restored by changing Thr869 in the D3 domain of human mature VWF to Ala, matching the sequence of canine VWF at this position. These complementary results imply that some interactions between D2 and D3 domains are dispensable for multimerization but necessary for targeting and tubular packing of VWF in WPBs.65
The results available to date for the expression of VWD mutations and other variants in heterologous cells indicate that multimerization is not a prerequisite for the formation of WPB-like organelles and that tubulation of VWF is not restricted to elongated WPBs. The structural requirements for tubular storage in platelet α-granules have not been investigated but are likely to be similar.
Exocytosis
Many secretagogues that mediate release of WPBs act on G protein-coupled receptors that trigger 1 of 2 major signaling pathways.10 Several agonists, including thrombin and histamine, increase intracellular free Ca2+ through a phospholipase C-dependent mechanism, whereas other agonists such as epinephrine and vasopressin increase intracellular cyclic adenosine monophosphate (cAMP). Interactions between cell surface receptors and crosstalk between signaling pathways can fine-tune endothelial responses. For example, thrombin cleaves and activates the G protein-coupled receptor PAR1, which induces WPB release through a pathway that increases intracellular Ca2+. However, occupancy of the endothelial cell protein C receptor prevents thrombin-induced release of VWF, P-selectin, and angiopoietin-2, suggesting that protein C switches the phenotype of thrombin-stimulated endothelium from proinflammatory to anti-inflammatory by blocking PAR-1–dependent exocytosis of WPBs.66
Exocytosis depends on the interaction of WPBs with microtubules and actin (Figure 3).67 Microtubules are oriented in a polarized fashion with “minus” ends clustered at a perinuclear microtubule-organizing center (MTOC) and “plus” ends toward the cell periphery. Microtubules and plus-end kinesin motors are necessary for long-range transport of WPBs from the TGN to peripheral locations in the cell, where a complex of MyRIP and Rab27A appears to mediate anchoring of WPBs to actin filaments.44 VWF secretion induced by Ca2+-elevating agonists is reported to be inhibited by disruption of microtubules with colchicine or nocodazole but potentiated by disruption of actin with cytochalasin E. In contrast, cAMP-mediated secretion is not affected by disruption of either microtubules or actin filaments, suggesting that only Ca2+-elevating agonists induce transport from the TGN to the cell membrane.44,67,68 In addition, Ca2+-elevating agonists can deplete the cell of almost all WPBs, whereas cAMP-elevating agonists selectively release a pool of mature WPBs that contain VWF but little or no P-selectin.69
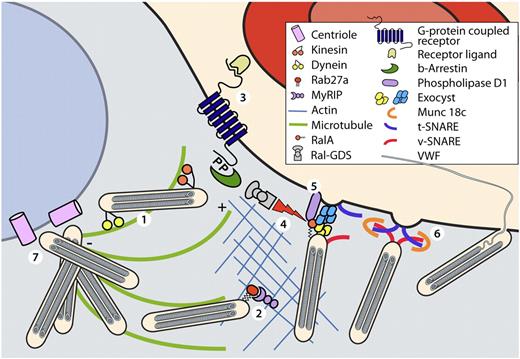
Molecular components regulating WPB excytosis. In resting endothelial cells WPBs are linked to microtubules and the actin cytoskeleton (1). Plus end directed movement of WPBs along microtubules is mediated by the motor protein kinesin. WPB are anchored to the actin cytoskeleton by the Rab27A/MyRIP complex (2). Ligand binding to G-protein coupled receptors initiates signal transduction pathways (3). G-protein–coupled receptors are subsequently internalized in a β-arrestin-mediated manner. Dissociation of the RalGDS/β-arrestin liberates RalGDS, which activates the small GTPase Ral. Activation of Ral mediates assembly of the exocyst complex (4). Simultaneously, Ral-dependent activation of phospholipase D1 induces accumulation of fusogenic lipids (5) that facilitate fusion of WPBs after the Munc18c-assisted assembly of the SNARE-complex (6). After stimulation of endothelial cells with cAMP-elevating agonists dynein-mediated minus-end directed movement of WPBs along microtubule promotes clustering of WPBs at the MTOC (7).
Molecular components regulating WPB excytosis. In resting endothelial cells WPBs are linked to microtubules and the actin cytoskeleton (1). Plus end directed movement of WPBs along microtubules is mediated by the motor protein kinesin. WPB are anchored to the actin cytoskeleton by the Rab27A/MyRIP complex (2). Ligand binding to G-protein coupled receptors initiates signal transduction pathways (3). G-protein–coupled receptors are subsequently internalized in a β-arrestin-mediated manner. Dissociation of the RalGDS/β-arrestin liberates RalGDS, which activates the small GTPase Ral. Activation of Ral mediates assembly of the exocyst complex (4). Simultaneously, Ral-dependent activation of phospholipase D1 induces accumulation of fusogenic lipids (5) that facilitate fusion of WPBs after the Munc18c-assisted assembly of the SNARE-complex (6). After stimulation of endothelial cells with cAMP-elevating agonists dynein-mediated minus-end directed movement of WPBs along microtubule promotes clustering of WPBs at the MTOC (7).
After stimulation of endothelial cells with cAMP-elevating agonists, remaining WPBs cluster near the MTOC in a process that depends on dynein/dynactin, a minus-end motor complex that moves vesicles in the opposite direction from kinesin.10,46,70 It has been suggested that immature WPBs are not anchored to the actin cytoskeleton by Rab27A/MyRIP,44 which would be consistent with the agonist-induced movement of immature WPBs away from the plasma membrane and toward the MTOC.
Intracellular membrane fusion involves the proteins NSF (N-ethylmaleimide-sensitive factor) and SNAP (soluble NSF attachment protein), as well as a selection from among many SNARE (SNAP receptor) and Sec1/Munc18-like proteins that determine the specificity of membrane fusion (Figure 3).71 Interactions between SNAREs on the target membrane (t-SNARE) and vesicle (v-SNARE) promote fusion by pulling the membranes together. The Sec1/munc18-like proteins bind to SNARE complexes to direct their fusogenic action,71 after which NSF and SNAP disassemble the SNARE complex for recycling.
WPB exocytosis appears to be mediated by syntaxin-4 (t-SNARE), SNAP-23 (a t-SNARE, not to be confused with SNAP), vesicle-associated membrane protein-3 (VAMP3, a v-SNARE), and Munc18c.72,73 Phosphorylation of Munc18c and syntaxin-4 during agonist-induced release of WPBs may regulate assembly of the SNARE complex.72,74 Tethering of vesicles to the plasma membrane is required before fusion, and the evolutionarily conserved “exocyst-complex” has been proposed to mediate WPB tethering,75 although direct involvement of exocyst proteins in WPB exocytosis has not been reported.
Not many steps that couple the activation of G protein-coupled receptors to the fusion of WPBs with the plasma membrane are known, but RalA may be involved. RalA is a positive effector of endothelial cell exocytosis induced by thrombin or epinephrine.41,42 RalA is regulated by the guanine exchange factor RalGDS, which is required for WPB exocytosis.76 In resting endothelial cells, RalGDS is inactive and bound to β-arrestin. Thrombin activates PAR1 and causes intracellular Ca2+ to increase, which promotes the calmodulin-dependent dissociation of RalGDS/β-arrestin, which activates RalGDS.76 In other cell types, activated RalGDS translocates from the cytoplasm to the plasma membrane, where it binds the membrane-tethered GTPase Ras and activates RalA,77 which then activates phospholipase D1.78 Similar events in endothelial cells would link G protein-coupled receptor signaling to the RalA-dependent activation of phospholipase D1 and assembly of exocyst complex.76 Phospholipase D1 catalyzes the conversion of phosphatidylcholine into choline and phosphatidic acid, a fusogenic lipid that promotes SNARE-complex mediated fusion of WPBs with the plasma membrane, and phospholipase D1 is required for the exocytosis of WPBs.79 Therefore, RalA is positioned to connect G protein-coupled receptor signaling to WPB exocytosis through the activation of phospholipase D1.
Heterogenous WPBs enable specialized secretory responses
Differential recruitment of cargo proteins to WPBs, slow recruitment of membrane proteins during WPB maturation, and tissue-specific differences in endothelial cell phenotype lead to a heterogeneous population of WPBs.2,40 These processes contribute to the emerging concept of WPB plasticity, which can enable specialized secretory responses depending on the applied stimulus, recent history and location of the cell.10
Endothelial cells can exhibit different modes of exocytosis. Live-cell imaging has shown release of green fluorescent protein–tagged VWF from single WPBs,37,46,80-82 and single WPB exocytosis has been confirmed by transmission EM (Figure 4).83,84 Another mode of exocytosis has been described in which a WPB fuses transiently with the plasma membrane in a “lingering kiss” event, creating a small 10- to 12-nm pore through which small molecules such as IL-8 and CD63 are released whereas larger molecules, such as VWF and P-selectin, are retained.80 Recently, multigranular exocytosis has been proposed to be a significant mode of exocytosis in which WPBs coalesce into a “secretory pod” before fusing with the plasma membrane (Figure 4).85 Why these 3 modes of regulated exocytosis coexist is currently a matter of speculation.
Schematic representation of 3 different modes of regulated exocytosis described for WPBs. (A) “Conventional” exocytosis in which single WPBs fuse with the plasma membrane, thereby releasing their cargo. For simplicity, the release of only 2 different cargo molecules is depicted: membrane-bound P-selectin released into the plasma membrane, and VWF released into the blood, where it can form long strings. The content of multiple exocytosed WPBs may contribute to the formation of VWF strings. Binding of the VWF strings to platelets, collagen, and integrin αvβ3 is not shown. (B) Lingering-kiss exocytosis is shown, where single WPBs fuse with the plasma membrane via a small fusion pore of approximately 12 nm in diameter.80 Because of the small size of the fusion pore, larger cargo proteins such as P-selectin and VWF are retained, whereas the smaller membrane-bound CD63 and soluble IL-8 are released. Lingering-kiss exocytosis of WPBs is characterized by the rounding up of the WPBs. The partially emptied and rounded WPBs are presumed to retract from the plasma membrane, but their fate is unknown. (C, D) Multigranular exocytosis where before exocytosis, WPBs coalesce into large intracellular membrane vesicles, termed secretory pods. As depicted in panel C, the coalescence of WPBs into secretory pods is mediated by interposing tiny vesicles termed nanovesicles.85 Within the secretory pods, VWF loses its characteristic tubular organization. Clathrin-coated profiles on the secretory pods suggest remodeling via a clathrin-mediated pathway. As illustrated in panel D, secretory pods fuse with the plasma membrane and release VWF strings via a large pore with a diameter of 1-2 μm. Whether P-selectin and other cargo molecules are released or selectively retained during multigranular exocytosis, remains to be established.
Schematic representation of 3 different modes of regulated exocytosis described for WPBs. (A) “Conventional” exocytosis in which single WPBs fuse with the plasma membrane, thereby releasing their cargo. For simplicity, the release of only 2 different cargo molecules is depicted: membrane-bound P-selectin released into the plasma membrane, and VWF released into the blood, where it can form long strings. The content of multiple exocytosed WPBs may contribute to the formation of VWF strings. Binding of the VWF strings to platelets, collagen, and integrin αvβ3 is not shown. (B) Lingering-kiss exocytosis is shown, where single WPBs fuse with the plasma membrane via a small fusion pore of approximately 12 nm in diameter.80 Because of the small size of the fusion pore, larger cargo proteins such as P-selectin and VWF are retained, whereas the smaller membrane-bound CD63 and soluble IL-8 are released. Lingering-kiss exocytosis of WPBs is characterized by the rounding up of the WPBs. The partially emptied and rounded WPBs are presumed to retract from the plasma membrane, but their fate is unknown. (C, D) Multigranular exocytosis where before exocytosis, WPBs coalesce into large intracellular membrane vesicles, termed secretory pods. As depicted in panel C, the coalescence of WPBs into secretory pods is mediated by interposing tiny vesicles termed nanovesicles.85 Within the secretory pods, VWF loses its characteristic tubular organization. Clathrin-coated profiles on the secretory pods suggest remodeling via a clathrin-mediated pathway. As illustrated in panel D, secretory pods fuse with the plasma membrane and release VWF strings via a large pore with a diameter of 1-2 μm. Whether P-selectin and other cargo molecules are released or selectively retained during multigranular exocytosis, remains to be established.
In other cell types, multigranular exocytosis may intensify and localize a secretory response. For example, eosinophils are thought to kill parasites more efficiently and with less damage to the host tissue by releasing cytotoxic proteins focally by multigranular exocytosis.86 In endothelial cells, the formation of secretory pods may facilitate VWF string formation by accumulating the content of multiple WPBs before release. However, exocytosis of single WPBs may prevail when the release of other WPB constituents is required, such as the exposure of P-selectin to initiate leukocyte binding. Finally, in lingering-kiss exocytosis, bioactive cargo molecules are selectively retained or released on the basis of their size. Therefore, different modes of exocytosis may enable the release of subsets of molecules from WPBs in response to distinct physiologic conditions.
On exocytosis, the propeptide, which is not covalently bound to VWF in WBPs, is rapidly released,82 whereas secreted VWF multimers form strings that can remain associated with cells for a considerable time.48,49 VWF string formation is triggered by the neutral extracellular pH and is impaired if the pH within the WPBs is increased by pretreatment with monensin, which also causes the disappearance of VWF tubules and the rounding up of WPBs. These observations suggest that VWF becomes entangled if it unfurls prematurely in WPBs and then cannot form strings. However, VWF does unfurl within multigranular secretory pods that nevertheless discharge VWF strings on exocytosis, which suggests that the preservation of tubular packaging is not a prerequisite for the assembly of VWF strings. The different consequences of disrupting VWF tubules in monensin-treated WPBs and in multigranular secretory pods may be reconciled by considering the time scales of these phenomena. Changes in WPB structure appear to require prolonged exposure to monensin (1 hour), which may allow extensive changes in the tubular packing of VWF that do not occur over the relatively rapid formation and exocytosis of secretory pods (minutes). Some VWD mutations are associated with round WPBs, and it will be interesting to determine whether these organelles contain VWF tubules and can secrete VWF strings.
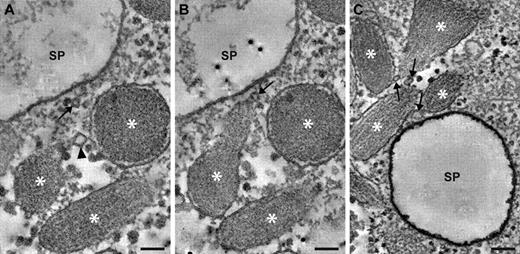
The unfurling of VWF in secretory pods is presumably triggered by an increase in lumenal pH, although the cause of these changes is unknown. Fusion between WPBs and other organelles could modify the pH, but fusion between WPBs and lysosomes or endosomes is unlikely because secretory pods lack the endosome/lysosome marker, lysosomal-associated membrane protein 1. In addition, fusion with acidic lysosomes or endosomes would be unlikely to increase the pH of WPBs. However, secretory pod formation was accompanied by fusion with tiny 30- to 40-nm vesicles, termed “nanovesicles” (Figures 4–5), that might affect the pH. More data on the origin and composition of nanovesicles will be needed to assess their role in multigranular exocytosis.
Nanovesicles at sites of multigranular exocytosis. Examples of nanovesicles are shown in digital slices through electron tomograms recorded from secretagogue-challenged HUVECs. (A) 2 WPBs are connected via a nanovesicle (arrowhead). WPBs are indicated by asterisks. The WPBs are in proximity of a secretory pod (SP) that is only partially shown. A seemingly solitary nanovesicle is indicated with an arrow. (B) Adjacent slice through the same tomogram as in panel A, showing a third nanovesicle (arrow) in contact with a WPB. This nanovesicle is also connected to the nanovesicle that appears solitary in panel A. (C) 4 WPBs in proximity of a secretory pod (SP), and interconnecting via 3 nanovesicles (arrows). Scale bars represent 100 nm.
Nanovesicles at sites of multigranular exocytosis. Examples of nanovesicles are shown in digital slices through electron tomograms recorded from secretagogue-challenged HUVECs. (A) 2 WPBs are connected via a nanovesicle (arrowhead). WPBs are indicated by asterisks. The WPBs are in proximity of a secretory pod (SP) that is only partially shown. A seemingly solitary nanovesicle is indicated with an arrow. (B) Adjacent slice through the same tomogram as in panel A, showing a third nanovesicle (arrow) in contact with a WPB. This nanovesicle is also connected to the nanovesicle that appears solitary in panel A. (C) 4 WPBs in proximity of a secretory pod (SP), and interconnecting via 3 nanovesicles (arrows). Scale bars represent 100 nm.
The vascular endothelium consists of a very flat monolayer of cells—the thickness of capillary endothelial cells ranges between 0.17 and 0.23 μm.87 Elongated WPBs usually align parallel to the cell membrane, which preserves the flatness of the endothelium. Tubular storage of VWF, which is solely responsible for the elongated shape of WPBs, could therefore be viewed as a mechanism to accommodate a bulky secretory organelle in a very thin cell. It follows that the rounding up of WPBs and particularly of large secretory pods could lead to the bulging of endothelial cells that might constrict blood flow or cause turbulence, especially in the microvasculature.
The strings that are secreted by endothelial cells are composed of ultralarge VWF multimers and can be several millimeters long,88 which raises the question as to the mechanism of assembly of these structures. A helical model for tubular packing suggests that a 5-μm tubule within a WPB could be composed of a single VWF multimer of approximately 3900 VWF subunits that would be 240 μm long when fully extended.48 Much longer strings would be expected to consist of several VWF multimers. In fact, VWF strings on the surface of secretagogue-challenged endothelial cells in vitro85,89 and in vivo49,90 can branch and form bundles that are much thicker than the 7-nm width of a single VWF multimer, indicating a complex association of several VWF multimers (Figure 6).
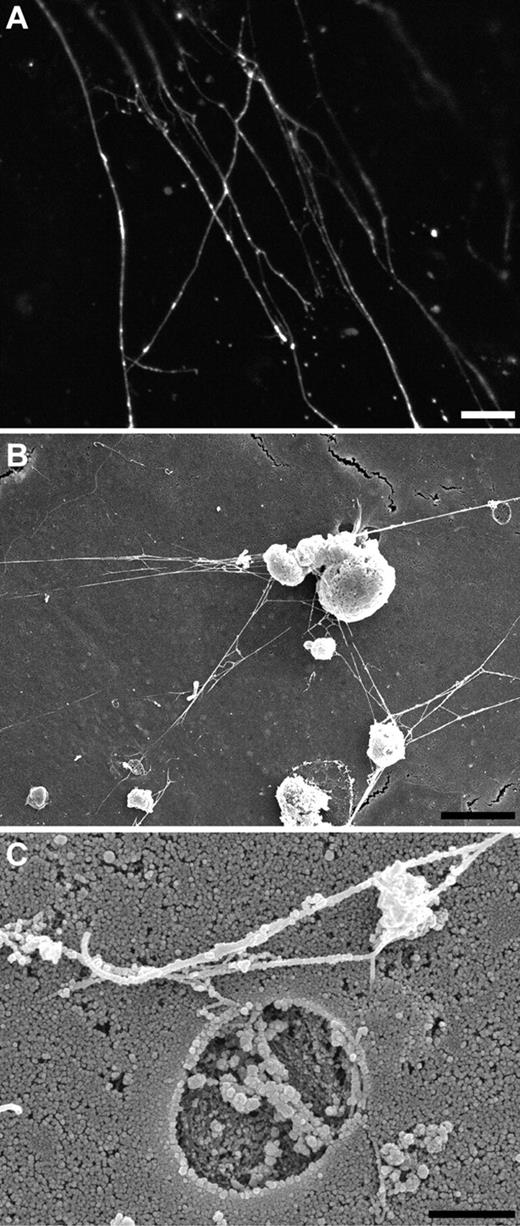
Morphology of VWF strings. (A) Immunofluorescence image of a stimulated HUVEC labeled with an antibody against VWF showing numerous VWF strings branching at the surface of the cell. Scale bar represents 5 μm. (B) Scanning EM of VWF strings at the surface of stimulated HUVECs displaying numerous branching VWF strings. Note the presence of pore in the membrane (right top corner). Scale bar, 5 μm. (C) Scanning EM of a pore in the membrane representing the site of exocytosis of WPB. VWF strings are branching and bundling at the surface of the cell. Scale bar, 1 μm.
Morphology of VWF strings. (A) Immunofluorescence image of a stimulated HUVEC labeled with an antibody against VWF showing numerous VWF strings branching at the surface of the cell. Scale bar represents 5 μm. (B) Scanning EM of VWF strings at the surface of stimulated HUVECs displaying numerous branching VWF strings. Note the presence of pore in the membrane (right top corner). Scale bar, 5 μm. (C) Scanning EM of a pore in the membrane representing the site of exocytosis of WPB. VWF strings are branching and bundling at the surface of the cell. Scale bar, 1 μm.
VWF strings attach to the surface of endothelial cells and provide a platform for adhesion of platelets (Figure 6).88 In addition, VWF strings attach to collagen at sites of tissue injury.91 The mechanism by which VWF strings are anchored to the plasma membrane is still under debate, but integrin αvβ3 and P-selectin are potential candidates for anchoring, and this attachment is shear dependent.89,90
WPBs in the pathophysiology and treatment of disease
The relationship between altered WPB-biogenesis and disease is quite direct in the case of VWD. Changes in the magnitude and quality of WPB exocytosis influence the plasma level of VWF and other prothrombotic and proinflammatory mediators, which in turn contribute to the pathophysiology of cardiovascular disorders. For example, mild exercise as well as infusion of epinephrine leads to a rise in VWF.92,93 Defective nitric oxide synthesis associated with endothelial dysfunction increases exocytosis of VWF73,94 and may increase plasma levels of VWF and factor VIII, which are associated with an increased risk of arterial thrombosis in coronary heart disease, stroke, and venous thromboembolism.95-97
Interestingly, genetic association studies implicate several proteins that may be components of the WPB secretory machinery in the control of plasma VWF levels. The G12E polymorphism in the AVPR2 increases its affinity for vasopressin approximately 3-fold and is associated with increased plasma levels of VWF and factor VIII.98 Meta-analysis of 5 genome-wide association studies identified polymorphisms in syntaxin-binding protein 5 and syntaxin-2 that are associated with VWF and factor VIII levels.99 These proteins have not been implicated previously in the release of WPBs, but they interact with SNARE complex proteins that do participate such as SNAP23 and syntaxin 4,100 suggesting that syntaxin-binding protein 5 and syntaxin-2 could be considered for such a role.99
We already exploit the regulated secretion of WPBs for the treatment of hemorrhagic disorders. The synthetic vasopressin analog desmopressin (DDAVP)101 promotes exocytosis of WPBs by triggering the vasopressin 2 receptor on endothelial cells102 and is widely used to increase VWF and factor VIII levels in patients with VWD or hemophilia A.5 Conversely, inhibition of WPB exocytosis may prove useful to prevent tissue injury. For example, administration of a cell-permeable inhibitor of NSF was shown to block WPB exocytosis and also to reduce VWF levels and limit myocardial infarct size in a mouse model of myocardial ischemia/reperfusion injury.103 Continuing advances in understanding the biogenesis and secretion of WPBs are likely to identify additional targets for therapeutic intervention in a variety of hematologic and cardiovascular disorders.
Acknowledgments
This work was supported by the Dutch Organization for Scientific Research (NWO TOP-grant 91209006) and by National Institutes of Health grant HL72917.
National Institutes of Health
Authorship
Contribution: K.M.V., J.V., and J.E. conceived the article; K.M.V., J. A.V., and J.V. designed figures; and all authors contributed to the writing of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr J. Eikenboom, Department of Thrombosis and Hemostasis, Einthoven Laboratory for Experimental Vascular Medicine, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: h.c.j.eikenboom@lumc.nl.