Abstract
Prognostic factors for response and survival in higher-risk myelodysplastic syndrome patients treated with azacitidine (AZA) remain largely unknown. Two hundred eighty-two consecutive high or intermediate-2 risk myelodysplastic syndrome patients received AZA in a compassionate, patient-named program. Diagnosis was RA/RARS/RCMD in 4%, RAEB-1 in 20%, RAEB-2 in 54%, and RAEB-t (AML with 21%-30% marrow blasts) in 22%. Cytogenetic risk was good in 31%, intermediate in 17%, and poor in 47%. Patients received AZA for a median of 6 cycles (1-52). Previous low-dose cytosine arabinoside treatment (P = .009), bone marrow blasts > 15% (P = .004), and abnormal karyotype (P = .03) independently predicted lower response rates. Complex karyotype predicted shorter responses (P = .0003). Performance status ≥ 2, intermediate- and poor-risk cytogenetics, presence of circulating blasts, and red blood cell transfusion dependency ≥ 4 units/8 weeks (all P < 10−4) independently predicted poorer overall survival (OS). A prognostic score based on those factors discriminated 3 risk groups with median OS not reached, 15.0 and 6.1 months, respectively (P < 10−4). This prognostic score was validated in an independent set of patients receiving AZA in the AZA-001 trial (P = .003). Achievement of hematological improvement in patients who did not obtain complete or partial remission was associated with improved OS (P < 10−4). In conclusion, routine tests can identify subgroups of patients with distinct prognosis with AZA treatment.
Introduction
Myelodysplastic syndromes (MDSs) are marrow stem cell disorders characterized by ineffective hematopoiesis, leading to blood cytopenias and a high risk of progression to acute myeloid leukemia (AML).1
The hypomethylating agent, azacitidine (AZA), has been approved in the United States and Europe for the treatment of higher risk MDS (ie, intermediate-2 and high risk, according to the International Prognostic Scoring System [IPSS],2 ) and in AML with 20%-30% marrow blasts (refractory anemia with excess blasts in transformation [RAEB-t], according to French-American-British classification). AZA induces 50%-60% responses in those patients, including 10%-20% complete remissions (CRs), 10%-20% partial remissions (PRs), and the remaining being marrow CR and stable disease (SD) with hematological improvement (HI; with correction of 1 or several cytopenias in the absence of CR or PR criteria) according to International Working Group (IWG) 2006 criteria.3 In a multicenter, randomized trial, AZA significantly improved overall survival (OS) in higher risk MDS (including RAEB-t/AML), compared with conventional treatments, including supportive care, low-dose cytosine arabinoside (LD AraC), and intensive anthracycline AraC chemotherapy (IC),4,5 and currently appears as the standard of care in those patients, at least in those who are not candidates to allogeneic stem cell transplantation (alloSCT). Prognostic factors of response and OS after hypomethylating agents (ie, AZA and decitabine) remain, however, largely unknown.6-13
We took advantage of a large multicenter cohort of higher risk MDS patients treated with AZA in a patient-named compassionate program to address this issue.
Methods
Patients
Following approval of AZA by the US Food and Drug Administration (FDA) for the treatment of MDS in 2004 and before European Medicines Agency (EMEA) approval at the end of 2008, the French health agency (French Health Products Safety Agency; AFSSAPS) opened a compassionate, patient-named program (authorization for temporary utilization [ATU] program) of AZA in higher risk MDS and poor-risk AML, in cooperation with the Groupe Francophone des Myelodysplasies (GFM). All IPSS intermediate-2 or high-risk MDS patients (de novo or secondary) could be included. Applications were reviewed by an expert before acceptance, and patients were included after informed consent in accordance with the Declaration of Helsinki. All protocols were approved by the institutional review board of the GFM.
Between September 2004 and January 2009, 931 patients were included in this program. One hundred ninety-six were excluded due to duplicate identities (n = 51), AZA not started (n = 96), previous hypomethylating agents (n = 20), AZA used only as maintenance (n = 20), combination with chemotherapy (n = 1), and unconfirmed diagnosis of MDS (n = 8). The remaining 735 patients had received at least 1 cycle of AZA and were treated in the 42 centers that sent to GFM data for all their patients included in the program (other centers were not retained to avoid bias). The present analysis was restricted to IPSS intermediate-2 or high-risk MDS and to AML with ≤ 30% marrow blasts (RAEB-t), excluding AML with > 30% blasts (n = 318), MDS/myeloproliferative neoplasm (n = 33), patients previously treated with IC (n = 38), alloSCT (n = 4), patients with IPSS low/intermediate-1 (n = 48), or missing IPSS (n = 12). All data were collected at the reference date of December 1, 2009.
Cytogenetics
Treatment
AZA was planned to be administered subcutaneously at the approved FDA/EMEA schedule (75 mg/m2/d during 7 days every 28 days) for at least 4 cycles. However, a 5-day administration was allowed in centers where AZA could not be administered during weekends, and daily-dose reductions were allowed in case of older age, Eastern Cooperative Oncology Group performance status (ECOG PS) > 2, and renal failure. Delays in cycles or dose reduction were recommended in case of grade 4 cytopenias. The schedule reported is the one planned at AZA onset, not taking into consideration schedule modifications occurring during the course of treatment. All responders after 4-6 cycles of AZA were to continue treatment until progression. RBC and platelet transfusion thresholds were in agreement with AFSSAPS recommendations: hemoglobin (Hb) < 8 g/dL or higher (9-10 g/dL) in case of comorbidity and platelets < 20 G/L or higher in case of bleeding or concomitant use of anticoagulant or antiplatelet therapy, respectively.
Response criteria and study endpoints
Response was evaluated after 4-6 cycles by blood count and marrow aspirate. CR, PR, marrow CR, SD, HI, and progression were defined according to IWG 2006 criteria.16 Response was analyzed on an intent-to-treat basis, considering patients receiving fewer than 4 cycles of AZA without documented response or progression as treatment failure, regardless of the cause of interruption.
Only patients with Hb < 11 g/dL or requiring RBC transfusions, with absolute neutrophil count (ANC) < 1.0 G/L, and with platelets < 100 G/L before onset of AZA were considered eligible for an assessment of HI of the erythroid, neutrophil, and platelet lineages (HI-E, HI-N, and HI-P), respectively. Response duration was measured from the date of marrow evaluation in patients achieving mCR, PR, or CR, and from the date of the first blood count meeting HI criteria in patients who achieved SD with HI, until the date of progression. OS was measured from the onset of AZA. Patients eligible for an assessment of HI and who achieved mCR or SD, with or without HI, were included in the time-dependent model of OS.
Statistical analysis
Predictive factors for response were analyzed using the Mann-Whitney rank-sum test or Fisher exact test for univariate comparisons. Variables with P-values below .20 in univariate analysis were included in a multivariate logistic regression analysis. The backward-elimination method was used to obtain the final model. Interaction terms were found not significant.
For censored variables (ie, response duration, OS), survival life tables were established with the Kaplan-Meier method. Univariate and multivariate analyses were performed with log-rank tests and proportional hazard Cox models, respectively, the latter including all variables with P < .05 in univariate analysis. Analysis of response duration was stratified by response category. A predictive score for survival was designed using the variables from the multivariate model. Risk categories derived from this score were tested with the log-rank test.
A validation set for this predictive score, consisting of patients included in the AZA arm of AZA 001 international trial, was analyzed. Patient-level data for this validation set was provided by the AZA-001 trial writing committee.4 All patients randomized to AZA in that trial were included in the validation cohort, excluding 4 patients who did not receive the drug, 11 patients with chronic myelomonocytic leukemia, and 3 with IPSS intermediate-1, leaving a total of 161 patients. Risk categories were derived and tested as in the development of the ATU cohort. Cox models with time-dependent covariates were performed according to Therneau and Grambsch.17 The proportional hazard assumptions were tested by graphic representation of Schoenfeld residuals.18
All analyses were performed with Statview Version 5.0 (SAS) and R 2.10.1 software programs.
Results
Baseline characteristics of the study population
The study population included 282 patients from 42 centers (Table 1). Median age was 71 years. World Health Organization diagnosis included 12 (4%) cases of refractory anemia (RA), RA with ringed sideroblasts (RARS), or refractory cytopenia with multilineage dysplasia (RCMD) and 56 (20%) cases of RAEB-1, 151 (54%) RAEB-2, and 63 (22%) RAEB-t/AML; 74 patients (26%) had secondary MDS, including 13 occurring after myeloproliferative neoplasm treated with hydroxyurea and 61 therapy-related MDS cases (ie, with a primary tumor treated with chemo- and/or radiotherapy). Cytogenetic risk was good in 88 (31%), intermediate in 46 (17%), and poor in 133 (47%) patients, respectively, and karyotype was a failure in 15 (5%). Poor-risk karyotypes included 103 (77%) complex karyotypes (≥ 3 aberrations; median, 6 anomalies), 25 (19%) with noncomplex -7/del7q, and 5 (4%) -7/del7q by fluorescence in situ hybridization in patients with karyotype failure. IPSS was intermediate-2 in 153 (54%), high in 122 (43%), and undetermined (but at least intermediate-2) in 7 (2%) of the patients with missing cytogenetics. Baseline Hb level < 11 g/dL or RBC transfusion dependency (TD), ANC < 1.0 G/L, and platelets < 100 G/L were present in 91%, 55%, and 74% of the patients, respectively. Median duration of MDS before the onset of AZA was 5 months; 29 and 91 patients had previously received LD AraC and an erythropoiesis-stimulating agent (ESA), respectively.
Baseline patient characteristics (n = 282)
| . | N or median . | % or range . |
|---|---|---|
| Age (y) | 71 | 20-89 |
| Sex | ||
| Male | 173 | 61 |
| Female | 109 | 39 |
| Secondary MDS | 74 | 26 |
| WHO diagnosis | ||
| RA/RARS/RCMD | 12 | 4 |
| RAEB1 | 56 | 20 |
| RAEB2 | 151 | 54 |
| AML (RAEB-t) | 63 | 22 |
| Cytogenetic risk (IPSS) | ||
| Favorable | 88 | 31 |
| Intermediate | 46 | 17 |
| Unfavorable | 133 | 47 |
| Failure | 15 | 5 |
| IPSS risk | ||
| Intermediate-2 | 153 | 54 |
| High | 122 | 43 |
| Na (int-2 or high) | 7 | 2 |
| ECOG PS | ||
| 0-1 | 217 | 77 |
| ≥ 2 | 56 | 20 |
| Na | 9 | 3 |
| Hb < 11 g/dL and/or RBC transfusion dependence | 256 | 91 |
| RBC transfusion dependence | ||
| ≥ 4 RBC units/8 w | 128 | 45 |
| 1-3 RBC units/8 w | 60 | 21 |
| No RBC transfusion dependence | 91 | 32 |
| Na | 3 | 1 |
| ANC < 1.0 G/L | 154 | 55 |
| PLT < 100 G/L | 209 | 74 |
| PB blasts | ||
| Present | 127 | 45 |
| Absent | 147 | 52 |
| Na | 8 | 3 |
| Interval from diagnosis (mo) | 5 | 0-143 |
| Prior treatment with LD AraC | ||
| Yes | 29 | 10 |
| No | 243 | 86 |
| Na | 10 | 4 |
| Prior treatment with ESA | ||
| Yes | 91 | 32 |
| No | 190 | 67 |
| Na | 1 | 1 |
| AZA schedule | ||
| Standard (75 mg/m2/d during 7 d every 28 d) | 202 | 72 |
| < 75 mg/m2/d for 7 d/cycle | 16 | 6 |
| 75 mg/m2/d for 5 d/cycle | 55 | 19 |
| < 75 mg/m2/d for 5 d/cycle | 9 | 3 |
| Number of AZA cycles (median, range) | 6 | 1-52 |
| Median follow-up (mo, range) | 26 | 6-52 |
| . | N or median . | % or range . |
|---|---|---|
| Age (y) | 71 | 20-89 |
| Sex | ||
| Male | 173 | 61 |
| Female | 109 | 39 |
| Secondary MDS | 74 | 26 |
| WHO diagnosis | ||
| RA/RARS/RCMD | 12 | 4 |
| RAEB1 | 56 | 20 |
| RAEB2 | 151 | 54 |
| AML (RAEB-t) | 63 | 22 |
| Cytogenetic risk (IPSS) | ||
| Favorable | 88 | 31 |
| Intermediate | 46 | 17 |
| Unfavorable | 133 | 47 |
| Failure | 15 | 5 |
| IPSS risk | ||
| Intermediate-2 | 153 | 54 |
| High | 122 | 43 |
| Na (int-2 or high) | 7 | 2 |
| ECOG PS | ||
| 0-1 | 217 | 77 |
| ≥ 2 | 56 | 20 |
| Na | 9 | 3 |
| Hb < 11 g/dL and/or RBC transfusion dependence | 256 | 91 |
| RBC transfusion dependence | ||
| ≥ 4 RBC units/8 w | 128 | 45 |
| 1-3 RBC units/8 w | 60 | 21 |
| No RBC transfusion dependence | 91 | 32 |
| Na | 3 | 1 |
| ANC < 1.0 G/L | 154 | 55 |
| PLT < 100 G/L | 209 | 74 |
| PB blasts | ||
| Present | 127 | 45 |
| Absent | 147 | 52 |
| Na | 8 | 3 |
| Interval from diagnosis (mo) | 5 | 0-143 |
| Prior treatment with LD AraC | ||
| Yes | 29 | 10 |
| No | 243 | 86 |
| Na | 10 | 4 |
| Prior treatment with ESA | ||
| Yes | 91 | 32 |
| No | 190 | 67 |
| Na | 1 | 1 |
| AZA schedule | ||
| Standard (75 mg/m2/d during 7 d every 28 d) | 202 | 72 |
| < 75 mg/m2/d for 7 d/cycle | 16 | 6 |
| 75 mg/m2/d for 5 d/cycle | 55 | 19 |
| < 75 mg/m2/d for 5 d/cycle | 9 | 3 |
| Number of AZA cycles (median, range) | 6 | 1-52 |
| Median follow-up (mo, range) | 26 | 6-52 |
Treatment modalities and response (IWG 2006 criteria)
Two hundred two (72%) patients received AZA at the FDA/EMEA-approved schedule (75 mg/m2/d for 7 days), and 80 patients (28%) received reduced schedules because of difficulties in weekend injections (n = 53), renal failure (n = 8), age > 75 years (n = 7), PS > 2 (n = 4), ANC < 1.0 G/L (n = 4), and physician's preference (n = 4).
The median number of cycles of AZA received was 6 (1-52; Table 1). The best response was CR in 38 (14%), PR in 9 (3%), marrow CR in 32 (11%, 16 of them with concomitant HI, but without CR criteria), SD with HI in 43 (15%), SD without HI in 61 (22%), and progressive disease (PD) in 52 (18%; Table 2). Forty-seven patients (17%) received less than 4 cycles of AZA without documented response or progression. The cause of interruption was early death in 13, severe sepsis in 15, severe bleeding in 5, prolonged cytopenias in 9, stroke in 2, hepatic failure in 1, occurrence of polychondritis in 1, and consent withdrawal in 2 patients. Median duration of response was 10.4, 9.8, 8.0, and 7.9 months for CR, PR, mCR, and SD with HI, respectively, without significant difference (Table 2; P = .93).
Response to azacitidine according to IWG 2006 criteria in the intent-to-treat cohort (n = 282)
| IWG 2006 response . | Response achievement . | Duration (mo) . | ||
|---|---|---|---|---|
| n . | % . | Median . | Range . | |
| Complete response (CR) | 38 | 14 | 10.4 | 1-24+ |
| Partial response (PR) | 9 | 3 | 9.8 | 1-13 |
| Marrow CR (mCR) | 32 | 11 | 8.0 | 2-38+ |
| Stable disease with hematological improvement | 43 | 15 | 7.9 | 2-28+ |
| Stable disease without hematological improvement | 61 | 22 | ||
| Progressive disease | 52 | 18 | ||
| Failure to achieve 4 cycles of AZA | 47 | 17 | ||
| Overall response rate (CR + PR + mCR + SD with HI) | 122 | 43 | 9.5 | 1-38+ |
| IWG 2006 response . | Response achievement . | Duration (mo) . | ||
|---|---|---|---|---|
| n . | % . | Median . | Range . | |
| Complete response (CR) | 38 | 14 | 10.4 | 1-24+ |
| Partial response (PR) | 9 | 3 | 9.8 | 1-13 |
| Marrow CR (mCR) | 32 | 11 | 8.0 | 2-38+ |
| Stable disease with hematological improvement | 43 | 15 | 7.9 | 2-28+ |
| Stable disease without hematological improvement | 61 | 22 | ||
| Progressive disease | 52 | 18 | ||
| Failure to achieve 4 cycles of AZA | 47 | 17 | ||
| Overall response rate (CR + PR + mCR + SD with HI) | 122 | 43 | 9.5 | 1-38+ |
Predictive factors of response and response duration
To determine baseline characteristics that could predict overall response, the 282 patients were dichotomized according to IWG best response as overall responders (CR, PR, marrow CR, and SD with HI) or nonresponders (SD without HI, PD, and failure to complete 4 cycles, whatever the reason; Table 3). In a multivariate model with all factors found significant in univariate analysis (Table 3), previous treatment with LD AraC (odds ratio [OR] = 0.26 [0.10-0.71]; P = .009), bone marrow blasts > 15% (best cut-off for marrow blast %; OR = 0.44 [0.26-0.77]; P = .004), and abnormal karyotype (OR = 0.4 [0.2-0.7]; P = .03) were associated with a significantly lower overall response rate (OR = 2.5 [1.4-4.5]; P = .03). There was no significant interaction between previous treatment with LD AraC and disease evolution (P = .55). Ten of 25 (40%) patients with noncomplex -7/del7q and 39 of 103 (38%) patients with complex karyotype responded. Four of 12 (33%) patients with noncomplex del5q/-5 responded. Reduced schedules of AZA were associated with 41% responses vs 44% for the standard schedule (P = .69).
Prognostic factors of response achievement and response duration
| . | Response achievement (n = 282) . | Response duration (months, n = 122) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate . | Multivariate analysis . | |||||||
| Response rate . | % . | P . | OR (95% CI) . | P . | Median . | Range . | P . | HR (95% CI) . | P . | |
| Age | .99 | .94 | ||||||||
| > 70 y | 67/156 | 43 | 9.2 | 2-28+ | ||||||
| ≤ 70 y | 55/126 | 44 | 9.5 | 2-38+ | ||||||
| ECOG PS | .02 | .73 | ||||||||
| 0-1 | 104/217 | 48 | 9.5 | 1-38+ | ||||||
| ≥ 2 | 17/56 | 30 | 9.5 | 2-24 | ||||||
| MDS type | .99 | .58 | ||||||||
| Secondary | 32/74 | 43 | 7.1 | 1-38+ | ||||||
| De novo | 90/208 | 43 | 9.5 | 1-28+ | ||||||
| WHO diagnosis | .14 | .04 | ||||||||
| RA/RARS/RCMD | 8/12 | 67 | 4.6 | 2-21+ | ||||||
| RAEB1 | 25/56 | 45 | 5.4 | 2-22 | ||||||
| RAEB2 | 68/151 | 45 | 9.8 | 1-38+ | ||||||
| AML (RAEB-t) | 21/63 | 33 | 10.9 | 1-24 | ||||||
| Interval from diagnosis | .99 | .31 | ||||||||
| > 6 mo | 55/126 | 44 | 9.6 | 2-38+ | ||||||
| ≤ 6 mo | 67/156 | 43 | 9.7 | 2-22+ | ||||||
| Prior LD AraC | .03 | .009 | .06 | |||||||
| Yes | 7/29 | 24 | 0.26 [0.10-0.71] | 17.7 | 4-28+ | |||||
| No | 111/243 | 46 | 8.9 | 1-38+ | ||||||
| Prior ESA | .25 | .41 | ||||||||
| Yes | 44/91 | 48 | 8.5 | 2-24+ | ||||||
| No | 78/190 | 41 | 9.8 | 1-38+ | ||||||
| Cytogenetic risk | .36 | .02 | ||||||||
| Favorable | 43/88 | 49 | 13.7 | 2-28 | ||||||
| Intermediate | 20/46 | 43 | 6.5 | 2-22+ | ||||||
| Unfavorable | 52/133 | 39 | 6.9 | 1-38+ | ||||||
| IPSS | .05 | .84 | ||||||||
| Intermediate-2 | 75/153 | 49 | 8.2 | 2-28+ | ||||||
| High | 45/122 | 37 | 9.3 | 1-38+ | ||||||
| -7/del7q | .19 | .03 | ||||||||
| Yes | 32/87 | 37 | 6.7 | 1-22 | ||||||
| No | 82/180 | 46 | 9.7 | 1-38+ | ||||||
| del5q/-5 | .36 | .11 | ||||||||
| Yes | 36/93 | 39 | 6.7 | 1-38+ | ||||||
| No | 77/172 | 45 | 9.7 | 1-28+ | ||||||
| +8 | .99 | .96 | ||||||||
| Yes | 17/39 | 44 | 7.7 | 1-22 | ||||||
| No | 97/225 | 43 | 9.5 | 1-38+ | ||||||
| del17p/-17 | .07 | .05 | ||||||||
| Yes | 7/28 | 25 | 4.7 | 1-18 | ||||||
| No | 105/234 | 45 | 9.5 | 1-38+ | ||||||
| Complex karyotype | .25 | .0003 | .0003 | |||||||
| Yes | 39/103 | 38 | 4.6 | 1-38+ | 2.64 [1.6-4.9] | |||||
| No | 74/162 | 46 | 10.3 | 2-28+ | ||||||
| Normal karyotype | .08 | .003 | .02 | |||||||
| Yes | 41/80 | 51 | 13.7 | 2-28+ | ||||||
| No | 73/186 | 39 | 0.4 [0.2-0.7] | 6.9 | 1-38+ | |||||
| Transfusion dependence | .4 | .47 | ||||||||
| ≥ 4 RBC units/8 w | 69/151 | 46 | 7.5 | 1-38+ | ||||||
| 0-3 RBC units/8 w | 52/128 | 41 | 10.3 | 1-28+ | ||||||
| ANC | .33 | .14 | ||||||||
| ≥ 1.0 G/L | 52/130 | 40 | 7.9 | 1-24+ | ||||||
| < 1.0 G/L | 68/152 | 46 | 9.5 | 1-38+ | ||||||
| Platelets | .21 | .80 | ||||||||
| ≥ 100 G/L | 35/70 | 50 | 10.3 | 2-22+ | ||||||
| < 100 G/L | 86/212 | 41 | 8.2 | 1-38+ | ||||||
| PB blasts | .04 | .03 | ||||||||
| Present | 47/127 | 37 | 6.6 | 1-24 | ||||||
| Absent | 73/147 | 50 | 10.2 | 2-38+ | ||||||
| Marrow blasts | .01 | .004 | .18 | |||||||
| > 15% | 42/121 | 35 | 0.44 [0.26-0.77] | 15.2 | 1-24+ | |||||
| ≥ 15% | 80/161 | 50 | 8.0 | 1-38+ | ||||||
| . | Response achievement (n = 282) . | Response duration (months, n = 122) . | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis . | Multivariate analysis . | Univariate . | Multivariate analysis . | |||||||
| Response rate . | % . | P . | OR (95% CI) . | P . | Median . | Range . | P . | HR (95% CI) . | P . | |
| Age | .99 | .94 | ||||||||
| > 70 y | 67/156 | 43 | 9.2 | 2-28+ | ||||||
| ≤ 70 y | 55/126 | 44 | 9.5 | 2-38+ | ||||||
| ECOG PS | .02 | .73 | ||||||||
| 0-1 | 104/217 | 48 | 9.5 | 1-38+ | ||||||
| ≥ 2 | 17/56 | 30 | 9.5 | 2-24 | ||||||
| MDS type | .99 | .58 | ||||||||
| Secondary | 32/74 | 43 | 7.1 | 1-38+ | ||||||
| De novo | 90/208 | 43 | 9.5 | 1-28+ | ||||||
| WHO diagnosis | .14 | .04 | ||||||||
| RA/RARS/RCMD | 8/12 | 67 | 4.6 | 2-21+ | ||||||
| RAEB1 | 25/56 | 45 | 5.4 | 2-22 | ||||||
| RAEB2 | 68/151 | 45 | 9.8 | 1-38+ | ||||||
| AML (RAEB-t) | 21/63 | 33 | 10.9 | 1-24 | ||||||
| Interval from diagnosis | .99 | .31 | ||||||||
| > 6 mo | 55/126 | 44 | 9.6 | 2-38+ | ||||||
| ≤ 6 mo | 67/156 | 43 | 9.7 | 2-22+ | ||||||
| Prior LD AraC | .03 | .009 | .06 | |||||||
| Yes | 7/29 | 24 | 0.26 [0.10-0.71] | 17.7 | 4-28+ | |||||
| No | 111/243 | 46 | 8.9 | 1-38+ | ||||||
| Prior ESA | .25 | .41 | ||||||||
| Yes | 44/91 | 48 | 8.5 | 2-24+ | ||||||
| No | 78/190 | 41 | 9.8 | 1-38+ | ||||||
| Cytogenetic risk | .36 | .02 | ||||||||
| Favorable | 43/88 | 49 | 13.7 | 2-28 | ||||||
| Intermediate | 20/46 | 43 | 6.5 | 2-22+ | ||||||
| Unfavorable | 52/133 | 39 | 6.9 | 1-38+ | ||||||
| IPSS | .05 | .84 | ||||||||
| Intermediate-2 | 75/153 | 49 | 8.2 | 2-28+ | ||||||
| High | 45/122 | 37 | 9.3 | 1-38+ | ||||||
| -7/del7q | .19 | .03 | ||||||||
| Yes | 32/87 | 37 | 6.7 | 1-22 | ||||||
| No | 82/180 | 46 | 9.7 | 1-38+ | ||||||
| del5q/-5 | .36 | .11 | ||||||||
| Yes | 36/93 | 39 | 6.7 | 1-38+ | ||||||
| No | 77/172 | 45 | 9.7 | 1-28+ | ||||||
| +8 | .99 | .96 | ||||||||
| Yes | 17/39 | 44 | 7.7 | 1-22 | ||||||
| No | 97/225 | 43 | 9.5 | 1-38+ | ||||||
| del17p/-17 | .07 | .05 | ||||||||
| Yes | 7/28 | 25 | 4.7 | 1-18 | ||||||
| No | 105/234 | 45 | 9.5 | 1-38+ | ||||||
| Complex karyotype | .25 | .0003 | .0003 | |||||||
| Yes | 39/103 | 38 | 4.6 | 1-38+ | 2.64 [1.6-4.9] | |||||
| No | 74/162 | 46 | 10.3 | 2-28+ | ||||||
| Normal karyotype | .08 | .003 | .02 | |||||||
| Yes | 41/80 | 51 | 13.7 | 2-28+ | ||||||
| No | 73/186 | 39 | 0.4 [0.2-0.7] | 6.9 | 1-38+ | |||||
| Transfusion dependence | .4 | .47 | ||||||||
| ≥ 4 RBC units/8 w | 69/151 | 46 | 7.5 | 1-38+ | ||||||
| 0-3 RBC units/8 w | 52/128 | 41 | 10.3 | 1-28+ | ||||||
| ANC | .33 | .14 | ||||||||
| ≥ 1.0 G/L | 52/130 | 40 | 7.9 | 1-24+ | ||||||
| < 1.0 G/L | 68/152 | 46 | 9.5 | 1-38+ | ||||||
| Platelets | .21 | .80 | ||||||||
| ≥ 100 G/L | 35/70 | 50 | 10.3 | 2-22+ | ||||||
| < 100 G/L | 86/212 | 41 | 8.2 | 1-38+ | ||||||
| PB blasts | .04 | .03 | ||||||||
| Present | 47/127 | 37 | 6.6 | 1-24 | ||||||
| Absent | 73/147 | 50 | 10.2 | 2-38+ | ||||||
| Marrow blasts | .01 | .004 | .18 | |||||||
| > 15% | 42/121 | 35 | 0.44 [0.26-0.77] | 15.2 | 1-24+ | |||||
| ≥ 15% | 80/161 | 50 | 8.0 | 1-38+ | ||||||
In univariate analysis, longer response duration was observed in RAEB-2 and RAEB-t/AML, compared with RAEB-1 and RA/RARS/RCMD (P = .04), in patients without peripheral blood (PB) blasts (P = .03) and in patients with normal karyotype (P = .02), while shorter response was seen with complex karyotype (P = .0003), del17p/-17 (P = .05), and -7/del7q (P = .03; Table 3). By multivariate analysis, only complex karyotype was predictive of shorter response duration (median 4.6 vs 10.3 months in other patients; P = .0003).
Prognostic factors of OS
After a median follow-up of 26 months, 199 deaths had occurred (including 150 due to AML progression), and median OS was 13.5 months. Eighteen patients died before the completion of 4 cycles (6%). In univariate analysis (Table 4; Figure 1), ECOG PS ≥ 2 (P < 10−4), high IPSS (P = .004), secondary MDS (P = .001), presence of peripheral blasts (P < 10−4), RBC TD ≥ 4 RBC units/8 weeks at inclusion (P < 10−4), baseline platelets < 100 G/L (P = .02), and intermediate- or poor-risk karyotype (P < 10−4) were associated with shorter survival. Among patients with poor-risk cytogenetics, there was a trend for improved survival in the 25 patients with noncomplex -7/del7q, compared with the 103 patients with complex karyotypes (median, 11.0 vs 8.3 months; P = .08). Age as a continuous variable (P = .24), marrow blast percent, whatever the cut-off, ANC < 1.0 G/L, prior treatment with LD AraC or ESA, and disease duration or reduced AZA schedule (median, 10.3 vs 14.3 months; P = .10) did not significantly influence OS.
Prognostic factors of overall survival
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Median OS . | P . | HR (95% CI) . | P . | |
| Age | .38 | |||
| > 70 y | 12.7 | |||
| ≤ 70 y | 15.0 | |||
| ECOG PS | < .0001 | < .0001 | ||
| 0-1 | 15.7 | 1 | ||
| ≥ 2 | 7.1 | 2.0 [1.4-2.9] | ||
| MDS type | .002 | |||
| Secondary | 9.2 | |||
| De novo | 15.3 | |||
| WHO diagnosis | .32 | |||
| RA/RARS/RCMD | 11.0 | |||
| RAEB1 | 13.1 | |||
| RAEB2 | 15.2 | |||
| AML (RAEB-t) | 9.7 | |||
| Interval from diagnosis | .05 | |||
| > 6 mo | 15.8 | |||
| ≤ 6 mo | 10.3 | |||
| Prior LD AraC | .75 | |||
| Yes | 14.9 | |||
| No | 13.1 | |||
| Prior ESA | .73 | |||
| Yes | 13.4 | |||
| No | 13.3 | |||
| Cytogenetic risk | < .0001 | < .0001 | ||
| Favorable | 22.4 | 1 | ||
| Intermediate | 15.0 | 1.4 [0.8-2.3] | .23 | |
| Unfavorable | 8.8 | 3.0 [2.0-4.3] | < .0001 | |
| IPSS risk | .004 | |||
| Intermediate-2 | 16.1 | |||
| High | 9.4 | |||
| Transfusion dependence | < .0001 | < .0001 | ||
| ≥ 4 RBC units/8 weeks | 10.3 | 1.9 [1.4-2.6] | ||
| 0-3 RBC units/8 weeks | 19.2 | 1 | ||
| ANC | .18 | |||
| ≥ 1.0 G/L | 12.0 | |||
| < 1.0 G/L | 15.1 | |||
| Platelets | .02 | |||
| ≥ 100 G/L | 19.6 | |||
| < 100 G/L | 12.3 | |||
| PB blasts | < .0001 | < .0001 | ||
| Present | 9.4 | 2.0 [1.5-2.7] | ||
| Absent | 19.8 | 1 | ||
| Bone marrow blasts | .11 | |||
| > 15% | 10.9 | |||
| ≤ 15% | 15.4 | |||
| . | Univariate analysis . | Multivariate analysis . | ||
|---|---|---|---|---|
| Median OS . | P . | HR (95% CI) . | P . | |
| Age | .38 | |||
| > 70 y | 12.7 | |||
| ≤ 70 y | 15.0 | |||
| ECOG PS | < .0001 | < .0001 | ||
| 0-1 | 15.7 | 1 | ||
| ≥ 2 | 7.1 | 2.0 [1.4-2.9] | ||
| MDS type | .002 | |||
| Secondary | 9.2 | |||
| De novo | 15.3 | |||
| WHO diagnosis | .32 | |||
| RA/RARS/RCMD | 11.0 | |||
| RAEB1 | 13.1 | |||
| RAEB2 | 15.2 | |||
| AML (RAEB-t) | 9.7 | |||
| Interval from diagnosis | .05 | |||
| > 6 mo | 15.8 | |||
| ≤ 6 mo | 10.3 | |||
| Prior LD AraC | .75 | |||
| Yes | 14.9 | |||
| No | 13.1 | |||
| Prior ESA | .73 | |||
| Yes | 13.4 | |||
| No | 13.3 | |||
| Cytogenetic risk | < .0001 | < .0001 | ||
| Favorable | 22.4 | 1 | ||
| Intermediate | 15.0 | 1.4 [0.8-2.3] | .23 | |
| Unfavorable | 8.8 | 3.0 [2.0-4.3] | < .0001 | |
| IPSS risk | .004 | |||
| Intermediate-2 | 16.1 | |||
| High | 9.4 | |||
| Transfusion dependence | < .0001 | < .0001 | ||
| ≥ 4 RBC units/8 weeks | 10.3 | 1.9 [1.4-2.6] | ||
| 0-3 RBC units/8 weeks | 19.2 | 1 | ||
| ANC | .18 | |||
| ≥ 1.0 G/L | 12.0 | |||
| < 1.0 G/L | 15.1 | |||
| Platelets | .02 | |||
| ≥ 100 G/L | 19.6 | |||
| < 100 G/L | 12.3 | |||
| PB blasts | < .0001 | < .0001 | ||
| Present | 9.4 | 2.0 [1.5-2.7] | ||
| Absent | 19.8 | 1 | ||
| Bone marrow blasts | .11 | |||
| > 15% | 10.9 | |||
| ≤ 15% | 15.4 | |||
Prognostic variables of overall survival (OS; Kaplan-Meier curves). (A) ECOG performance status. (B) IPSS cytogenetic risk. (C) RBC transfusion dependency. (D) PB blasts.
Prognostic variables of overall survival (OS; Kaplan-Meier curves). (A) ECOG performance status. (B) IPSS cytogenetic risk. (C) RBC transfusion dependency. (D) PB blasts.
In multivariate analysis, PS ≥ 2 (hazard ratio [HR] = 2.0 [95% confidence interval: 1.4-2.9]; P < 10−4), intermediate and unfavorable IPSS cytogenetic risk (intermediate: HR = 1.4 [0.8-2.3]; poor: HR = 3.0 [2.0-4.3]; P < 10−4), presence of circulating blasts (HR = 2.0 [1.5-2.7]; P < 10−4), and RBC TD ≥ 4 RBC units/8 weeks (HR = 1.9 [1.4-2.6]; P < 10−4) retained independent adverse prognostic values. Censoring the 43 patients allografted after AZA treatment at the time of alloSCT did not modify this prognostic model.
A scoring system was designed based on those 4 prognostic factors: 1 point was attributed to each of ECOG PS ≥ 2, presence of circulating blasts, and RBC TD ≥ 4 RBC units/8 weeks; 1 and 2 points were for intermediate- and poor-risk cytogenetics, respectively. This allowed us to segregate patients into 3 risk categories: low (score = 0), intermediate (score = 1-3), and high (score = 4-5). Due to some missing values, the score could be determined in 269 patients. Median OS was not reached in the low-risk (n = 30), was 15.0 months in the intermediate-risk (n = 191), and was 6.1 months in the high-risk (n = 48) groups, respectively (Figure 2; P < 10−4).
Prognostic score for overall survival. The score was computed (for each patient) based on the presence of PS ≥ 2 (1 point), presence of circulating blasts (1 point), RBC TD ≥ 4 RBC units/8 weeks (1 point), and intermediate- and high-risk cytogenetics (1 and 2 points, respectively). Kaplan-Meier curves of OS for low (score = 0), intermediate (score = 1-3), and high (score = 4-5) risk patients in the development (ATU) and validation (AZA-001) cohorts
Prognostic score for overall survival. The score was computed (for each patient) based on the presence of PS ≥ 2 (1 point), presence of circulating blasts (1 point), RBC TD ≥ 4 RBC units/8 weeks (1 point), and intermediate- and high-risk cytogenetics (1 and 2 points, respectively). Kaplan-Meier curves of OS for low (score = 0), intermediate (score = 1-3), and high (score = 4-5) risk patients in the development (ATU) and validation (AZA-001) cohorts
This prognostic score was then applied to an independent validation cohort consisting of patients randomized to AZA and having received AZA, excluding chronic myelomonocytic leukemia and IPSS intermediate-1 patients (n = 161) in the AZA-001 trial.4 Proportions of patients with RBC transfusion dependence ≥ 4 units/8 weeks (46%) or with circulating blasts (50%) were similar to the ATU development cohort (46% [P > .9] and 46% [P = .5], respectively). However ECOG PS was ≥ 2 in only 7% of the patients in the AZA-001 cohort vs 20% in the ATU cohort P < 10−3), while cytogenetics was good, intermediate, poor, and failed in 46%, 20%, 28%, and 6% vs 31%, 17%, 47%, and 5%, respectively; P < 10−3), so that both parameters were more “favorable” in the AZA-001 trial. A prognostic group could be attributed to 152 (94%) of those 161 patients, due to failed cytogenetics in 9 patients. Twenty-three (15%), 114 (75%), and 15 (10%) patients were classified in the low-, intermediate-, and high-risk groups, respectively. This distribution tended to be skewed toward better risk categories, compared with the ATU cohort (P = .06). Median follow-up of the validation cohort was 21 months. Median OS was not reached at 21.4 and 9.3 months, respectively, in the 3 groups (Figure 2; P = .003). Finally, after exclusion from the ATU cohort of patients with therapy-related MDS, PS ≥ 3, prior LD araC exposure, and reduced AZA schedule (all features absent in the AZA-001 cohort), there were 18, 77, and 20 patients in the low-, intermediate-, and high-risk groups, respectively. Median OS was comparable in the 2 cohorts for low- (both not reached; P = .78) and intermediate-risk patients (ATU: 17.9 months; AZA-001: 21.4 months; P = .22). Median OS in high-risk patients remained inferior in the ATU cohort patients (ATU: 6.2 months; AZA-001: 9.3 months; P = .03).
Influence of hematological improvement on OS
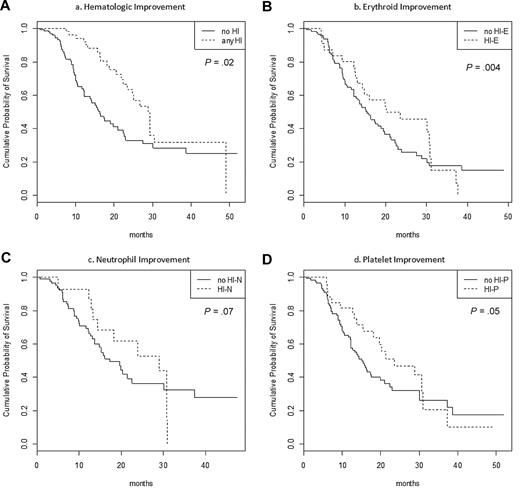
To determine whether correction of cytopenias improved OS in patients failing to achieve CR or PR, a time-dependent Cox model assessing the prognostic impact of achievement of HI was designed. This analysis was restricted to the 151 patients who achieved mCR or stable disease and were assessable for HI, including 131, 114, and 88 with baseline significant anemia, thrombocytopenia, and neutropenia (as defined above), respectively.
In patients with mCR or SD, achievement of any type of HI was associated with improved OS (Table 5; Figure 3; HR = 0.54 [0.34-0.87]; P = .02). This effect remained significant in multivariate analysis, including PS, cytogenetic risk, presence of circulating blasts, and transfusion dependence ≥ 4 RBC units/8 weeks (HR = 0.42 [0.29-0.62]; P < 10−4).
Time-dependent Cox model of OS in stable disease or marrow CR patients according to the achievement or not of hematological improvement
| . | Evaluable . | n (%) . | HR (95% CI) . | P . |
|---|---|---|---|---|
| Any HI | 151 | 62 (41%) | 0.54 [0.34-0.87] | .02 |
| HI-E | 131 | 33 (25%) | 0.56 [0.38-0.83] | .004 |
| HI-N | 88 | 31 (27%) | 0.65 [0.40-1.00] | .07 |
| HI-P | 114 | 18 (20%) | 0.67 [0.45-1.00] | .05 |
| . | Evaluable . | n (%) . | HR (95% CI) . | P . |
|---|---|---|---|---|
| Any HI | 151 | 62 (41%) | 0.54 [0.34-0.87] | .02 |
| HI-E | 131 | 33 (25%) | 0.56 [0.38-0.83] | .004 |
| HI-N | 88 | 31 (27%) | 0.65 [0.40-1.00] | .07 |
| HI-P | 114 | 18 (20%) | 0.67 [0.45-1.00] | .05 |
Impact of hematological improvement on OS in a time-dependent model in patients with baseline cytopenia(s) who achieved SD or mCR (n = 151). (A) Achievement of any HI. (B) Achievement of HI-E in patients with baseline Hb < 11 g/dL or RBC transfusion dependency (n = 131). (C) Achievement of HI-P in patients with baseline platelets < 100 G/L (n = 114). (E) Achievement of HI-P in patients with baseline ANC < 1.0 G/L (n = 88).
Impact of hematological improvement on OS in a time-dependent model in patients with baseline cytopenia(s) who achieved SD or mCR (n = 151). (A) Achievement of any HI. (B) Achievement of HI-E in patients with baseline Hb < 11 g/dL or RBC transfusion dependency (n = 131). (C) Achievement of HI-P in patients with baseline platelets < 100 G/L (n = 114). (E) Achievement of HI-P in patients with baseline ANC < 1.0 G/L (n = 88).
When analyzing the type of HI observed, achieving HI-E was significantly associated with improved survival (P = .004), while achieving HI-P or HI-N had only borderline favorable significance (P = .05 and P = .07, respectively).
Discussion
In this large cohort of higher risk MDS treated with AZA, a bone marrow blast count < 15%, a normal karyotype, and no previous treatment with LD araC independently predicted better response to AZA, while patients with complex karyotypes had shorter response durations. RBC transfusion dependence, poor ECOG performance status, intermediate and unfavorable IPSS cytogenetic risk, and presence of circulating blasts all independently predicted shortened OS. Those factors could be combined in a simple prognostic score defining 3 patient subsets with significantly different survival. Finally, in patients failing to achieve CR or PR with AZA, achievement of HI, notably of HI-E, was associated to improved OS.
Previous prognostic analyses of outcome with hypomethylating agents have been carried out, mostly with decitabine. Similar survival was found in IPSS intermediate-2 and high-risk patients, and in RAEB and RAEB-t patients in a phase II trial.11 In a pooled analysis of phase II studies, no predictive factor of response was identified, but response duration was inversely related to IPSS and OS was better with high-risk, compared with intermediate-risk, cytogenetics.12 In the M.D. Anderson series, prior therapy and longer MDS duration predicted inferior CR rates, while patients with chromosome 5 or 7 abnormalities, prior therapy, and older age had shorter survival.13 In a series of mostly lower risk MDS patients treated with AZA, the IPSS and World Health Organization–Based Prognostic Scoring System classifications retained their known prognostic impact on OS, and high lactate dehydrogenase values predicted shorter response duration and survival.8
Our findings are complementary to those of the AZA-001 trial, which demonstrated a significant survival improvement of AZA over conventional care regimens (especially best supportive care and LD AraC), but did not analyze prognostic factors of response and OS in the AZA arm of the trial.4 OS, in our cohort, was shorter than in the AZA arm of the AZA-001 trial (median, 13 vs 24.4 months), but our patients had, on average, higher risk features, including 26% secondary cases (vs none in the AZA-001 trial), poorer PS (PS ≥ 2 in 20% vs 7%), more frequent poor-risk cytogenetics (47% vs 28% in the AZA-001 trial), and prior LD AraC treatment in 10% of the cases (compared with none in the AZA-001 trial).
Those differences probably reflect differences frequently observed between prospective, randomized, clinical trials with relatively stringent inclusion criteria, and less selected, somewhat more “real life” cohorts included in compassionate programs. Our median number of AZA cycles was 6, compared with 9 in the AZA-001 trial. Early discontinuation of AZA in the present study was mostly due to hematologic complications (eg, sepsis, bleeding), and the early death rate was higher than that observed in the AZA-001 trial, again possibly reflecting the average higher risk of our cohort.
Twenty-eight percent of our patients received attenuated schedules of AZA, mainly due to difficulties in performing injections during weekends in some centers, and, less often, due to older age or renal failure, whereas all patients in the AZA-001 trial had received a full-dose schedule (75 mg/m2/d, 7 days every 4 weeks). Even though the AZA schedule had no obvious significant impact on response or OS in our study, allowing the inclusion of those patients in our prognostic models, the nonsignificant trend for reduced OS in patients receiving reduced schedules warrants caution in clinical practice.
A significant unfavorable prognostic impact of increased marrow blasts on response was found for a cut-off value of 15%, but not with the usual cut-offs of 10% and 20%. The impact of cytogenetic analysis on response was apparent only when considering normal vs abnormal karyotypes, and we could not identify specific chromosomal abnormalities associated with AZA failure. The 40% response rate of noncomplex -7/del7q is in line with previous encouraging results obtained with hypomethylating agents in this patient subset.6,9,10,19,20 Patients with complex karyotypes were found to have shorter median response duration, suggesting that in those patients, AZA can only be a transient therapeutic option prior to, for example, alloSCT.
For OS, PS, and IPSS cytogenetic risk, RBC TD and the presence of peripheral blasts were independent prognostic factors. The impact of PS probably reflects the poor outcome of very frail patients who are usually excluded from clinical trials. The survival impact of RBC TD had previously been demonstrated in MDS overall, including higher risk patients, but not in patients treated with hypomethylating agents.21 The presence of peripheral blasts has been proposed as a poor prognostic factor in untreated lower risk MDS.22 We extend this finding to higher risk patients, where the prognostic impact of circulating blasts was independent of marrow blast percentage, possibly suggesting that the presence of PB blasts is associated with a particular MDS profile. Routine pathological examination of bone marrow biopsies was not available to assess the impact of myelofibrosis in response to AZA in the present cohort, an impact not analyzed in previous studies.
We found that IPSS cytogenetic classification retained prognostic significance for OS in patients treated with AZA. The size of the cohort did not allow us to assess the recently characterized prognostic value of rare cytogenetic lesions.23 The adverse OS of patients with poor-risk karyotype, notably of complex karyotypes involving chromosome 5 or 17 lesions, suggests that, although those patients may respond better to hypomethylating agents than to chemotherapy,4,24 they still have a poor prognosis with hypomethylating agents. On the other hand, both our results and previously published data suggest that the relatively favorable outcome of patients with -7/del7q is restricted to patients without complex karyotypes. For example, detailed cytogenetic results of the AZA-001 trial, so far only published in abstract form, found a median survival of 24.5 months in patients with noncomplex -7/del 7q, compared with 5.3 months in patients with -7/del 7q and at least 2 other cytogenetic abnormalities.25
Combining PS, RBC TD, presence of PB blasts, and cytogenetic risk in a simple prognostic score allowed us to identify 3 groups of patients with significantly distinct OS.
We were also able to validate this model in an independent cohort of higher risk MDS, constituted of patients receiving AZA in the international phase III randomized trial comparing AZA and conventional care regimens (ie, the AZA-001 trial). Interestingly, this patient population was more selected than our ATU program population, in that only patients with PS ≤ 2, de novo MDS cases, and patients having never received any cytotoxic chemotherapy (including LD araC) could be included. Furthermore, all patients were to receive AZA at full dose in this trial. Those differences certainly contributed to the different survival of patients between the 2 cohorts, because after exclusion of patients with those features from the ATU cohort, median OS was comparable in the 2 cohorts in patients of the low- or intermediate-risk groups. Validation of our score in a cohort with somewhat different characteristics suggests its relatively wide applicability.
Finally, using a time-dependent survival model that allows assessment of the prognostic contribution of events occurring after the onset of AZA, we found that patients who achieved HI without criteria of CR or PR, especially those achieving HI-E, had better survival. Similar results have been reported in the AZA-001 trial, although, so far, only in abstract form,7 and with decitabine.11,26 Such findings suggest that improvement of survival by AZA is, in part, attributable to the correction of anemia, or, more probably, that AZA allows disease regression to an earlier stage, with less cytopenias and a lower risk of progression to AML.
In conclusion, our results suggest that a few routine prognostic factors can predict, to a large extent, response and survival with AZA. They identify a subgroup of patients with relatively favorable survival with AZA and, conversely, suggest that alternative therapeutic strategies should be sought in patients with high-risk features, notably complex karyotypes. Finally, we stress the importance of including HI as a meaningful endpoint in future clinical trials of hypomethylating agents in higher risk MDS. On the other hand, our study did not include other biological makers than karyotype, which may be crucial to further predict response to hypomethylating agents. Currently, however, the prognostic value of gene methylation at baseline or during treatment remains controversial,9,27-29 while the prognostic value of other biological markers, such as microRNA (eg, miR-29b19 ) or gene (eg, phosphoinositide/phospholipase C beta30 ) expression levels, will have to be confirmed in multicenter studies.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
Results presented, in part, at the 51st American Society of Hematology Annual Meeting, December, 2009, New Orleans, LA.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank investigators of the GFM group who enrolled patients in this study and the French health agency (AFSSAPS).
This work was supported, in part, by an unrestricted research grant from Celgene to the GFM group.
Authorship
Contribution: R.I. and S.T. collected the data and wrote the manuscript; R.I. performed statistical analysis; L.A., P.F., C.G., and S.B. designed the study and wrote the manuscript; C.L.B. provided the AZA 001 database; and the remaining authors enrolled the patients and collected the data.
Conflict-of-interest disclosure: P.F. received research funding from Celgene. C.L.B. is a Celgene employee. The remaining authors declare no competing financial interests.
For a list of additional GFM participants, please see the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Correspondence: Pierre Fenaux, Service d'Hématogie Clinique Hôpital Avicenne, AP-HP, Université Paris 13, Inserm U848, 125 route de Stalingrad, 93009 Bobigny, France; e-mail: Pierre.fenaux@avc.aphp.fr.
References
Author notes
R.I. and S.T. contributed equally to this work.