To the editor:
We previously reported that peripheral blasts, performance status (PS), red blood cell (RBC) transfusion requirement, and International Prognostic Scoring System (IPSS) cytogenetic risk independently predicted inferior overall survival (OS) in 282 consecutive IPSS high- and intermediate-2–risk myelodysplastic syndromes (MDS) patients treated with azacitidine (AZA).1 These 4 criteria could be integrated in a 3-group prognostic score that significantly discriminated between high-, intermediate-, and low-risk groups, with median OS of 6.1, 15.0 months, and not reached, respectively. This prognostic score was validated in the AZA arm of the AZA-001 trial2 and in 2 smaller cohorts.3,4 Median follow-up of the 3 validation cohorts was 21, 8, and 15 months, respectively, and 26 months in our initial report. We report here an update of our cohort as of November 1, 2011, made to assess the long-term outcome of patients with favorable risk in our proposed prognostic score and to determine the characteristics of long-term survivors.
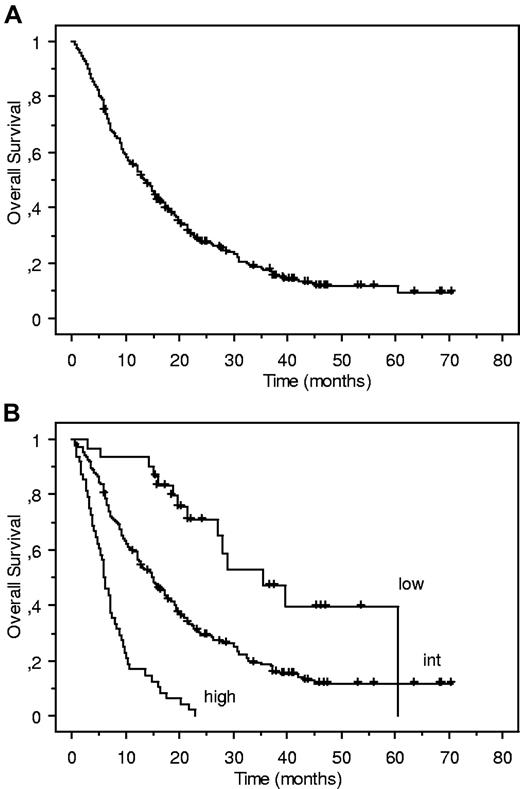
In the updated analysis, with a median follow-up of 41.3 months, 54 of the 282 patients were still alive and the median number of AZA cycles received was 6 (range 1-53). Median OS was 13.5 months, and the 3-year estimate of OS was 17.5% (95% confidence interval [CI]: 12.6%-22.4%). Sixteen of the 30 patients in the favorable risk group were still alive and median OS of this group was reached at 32.1 months. Our proposed prognostic score remained highly valid (Figure 1; log-rank test: P < 10−4). Censoring at the time of allogeneic stem cell transplantation did not affect those results.
Updated Kaplan-Meier estimates of overall survival (OS) of our previously reported cohort of 282 higher-risk myelodysplastic syndromes (MDS) patients treated with azacitidine, with a median follow-up of 41.3 months. (A) Global cohort (n = 282).1 (B) Cohort according to our risk stratification: low (n = 30, median OS: 32.1 month); intermediate (int; n = 191, median OS: 15.0 months); high (n = 48; median OS: 6.1 month; log-rank test: P < 10−4).
Updated Kaplan-Meier estimates of overall survival (OS) of our previously reported cohort of 282 higher-risk myelodysplastic syndromes (MDS) patients treated with azacitidine, with a median follow-up of 41.3 months. (A) Global cohort (n = 282).1 (B) Cohort according to our risk stratification: low (n = 30, median OS: 32.1 month); intermediate (int; n = 191, median OS: 15.0 months); high (n = 48; median OS: 6.1 month; log-rank test: P < 10−4).
Thirty-four patients (M/F: 20/14), with a median age of 69 years (range 42-86) had an OS of at least 3 years after AZA onset. At AZA onset, their World Health Organization diagnosis was refractory cytopenia with multilinage dysplasia, refractory anemia with excess blasts (RAEB)–1, RAEB-2, and AML with 20%-30% blasts in 1, 6, 21, and 6 cases, respectively. Four of them had therapy-related MDS. PS was < 2 in 30 cases (88%). Cytogenetic risk was low, intermediate, high, and not available in 18 cases (53%; all with normal karyotype), 8 cases (24%), 7 cases (21%: 3 with isolated monosomy 7 and 4 with complex abnormalities), and 1 case (2%). Only 22 of the 34 patients (64%) had responded to AZA according to International Working Group 2006 criteria,5 including 7 (21%) complete responses (CR), 1 (3%) partial response (PR), 4 (12%) marrow CR, and 10 (29%) stable disease with hematologic improvement. Three years after AZA onset, 6 (18%) of the 34 patients were still receiving AZA, 8 (24%) had been allografted, and 15 (44%) had received other treatments including intensive chemotherapy (n = 2), low-dose chemotherapy (n = 2), investigational drugs (n = 6), or decitabine (n = 5).
Altogether this updated report indicates that long-term survival with AZA in higher-risk MDS can be predicted using our daily-practice scoring system. Long-term survival can be achieved even in some of the patients with poor risk features including therapy-related cases, cases with 20%-30% blasts or with high-risk karyotype, and some patients who do not achieve CR or PR. However, for the majority of patients, long-term outcome remains poor, prompting the investigation of second-line therapies.
Authorship
Contribution: R.I. and S.T. collected the data; R.I. performed statistical analyses and drafted the manuscript; and R.I., L.A. and P.F. designed the study and wrote the manuscript. The other authors enrolled the patients and collected the data. All authors approved the final manuscript.
Conflict-of-interest disclosure: P.F. receives research funding from Celgene and R.I. receives honoraria from Celgene. The remaining authors declare no competing financial interests.
Correspondence: Pierre Fenaux, MD, PhD, Service d'Hématogie Clinique Hôpital Avicenne, AP-HP, Université Paris 13, 125 route de Stalingrad 93009 Bobigny, France; e-mail: pierre.fenaux@avc.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal