Abstract
Fas ligand (FasL) not only induces apoptosis in Fas receptor-bearing target cells, it is also able to transmit signals into the FasL-expressing cell via its intracellular domain (ICD). Recently, we described a Notch-like proteolytic processing of FasL that leads to the release of the FasL ICD into the cytoplasm and subsequent translocation into the nucleus where it may influence gene transcription. To study the molecular mechanism underlying such reverse FasL signaling in detail and to analyze its physiological importance in vivo, we established a knockout/knockin mouse model, in which wild-type FasL was replaced with a deletion mutant lacking the ICD. Our results demonstrate that FasL ICD signaling impairs activation-induced proliferation in B and T cells by diminishing phosphorylation of phospholipase C γ, protein kinase C, and extracellular signal-regulated kinase 1/2. We also demonstrate that the FasL ICD interacts with the transcription factor lymphoid-enhancer binding factor-1 and inhibits lymphoid-enhancer binding factor-1–dependent transcription. In vivo, plasma cell numbers, generation of germinal center B cells, and, consequently, production of antigen-specific immunoglobulin M antibodies in response to immunization with T cell–dependent or T cell–independent antigen are negatively affected in presence of the FasL ICD, suggesting that FasL reverse signaling participates in negative fine-tuning of certain immune responses.
Introduction
Fas ligand (FasL, CD95L, CD178, TNSF6) is a 40-kDa glycosylated type II transmembrane protein that belongs to the tumor necrosis factor (TNF) family. Its extracellular portion harbors a C-terminal TNF homology domain that assembles as homotrimers and mediates interactions with receptors. Interestingly, the 77-amino acid (aa)–long N-terminal mouse FasL intracellular domain (ICD) contains several conserved signaling motifs, such as a tandem casein kinase I phosphorylation site (aa 17-21), a proline-rich domain (PRD; aa 45-69) that serves as a docking site for SH3 domain-containing proteins, and a tyrosine phosphorylation site located at aa 7.1
The FasL/Fas receptor system is renowned as a potent inducer of apoptosis in the receptor-bearing cell and is especially important for numerous immune system functions, including removal of target cells by natural killer and cytotoxic T cells, (self) elimination of effector cells after the proliferative phase of an immune response (activation-induced cell death), and maintenance of immune-privileged sites.2,3 Naturally occurring homozygous mutations of the Fas (lpr) and the FasL (gld) genes, which abolish Fas-mediated apoptosis, lead to development of a severe lymphoproliferative disease.4
Because of its potent proapoptotic potential and important function, expression and activity of FasL is tightly regulated at transcriptional and posttranslational levels and is restricted to a few cell types, such as immune effector cells and cells of immune-privileged sites (eg, eyes, testis, brain). In contrast, the Fas receptor is expressed in a wide variety of tissues, including the lymphoid tissues, liver, heart, and ovary.1,5
In addition to its pro-death function, the Fas/FasL system also transduces nonapoptotic signals into the Fas-expressing cells. Among others, Fas is required for cell survival, proliferation and activation of T cells, liver regeneration, and neurogenesis.6,7
A number of studies have presented evidence for alternative signal transduction via “reverse signaling” into the FasL-bearing cells, although conflicting data have been published regarding the functional implications (for review see Voss et al1 ). First evidence for the existence of retrograde FasL signaling stems from 2 studies that demonstrated either costimulation of murine CD8+ cytotoxic T lymphocyte (CTL) cell lines upon FasL cross-linking8 or inhibition of activation-induced proliferation in murine CD4+ T cells.9 In both cases, FasL reverse signaling could only be observed under conditions of suboptimal T-cell receptor (TCR)–stimulation and in the presence of signaling-competent FasL. According to in vitro experiments, the FasL PRD domain is sufficient for costimulation of T cells, which includes FasL recruitment into rafts, enhancement of AKT, extracellular signal-regulated kinase 1/2 (ERK1/2), JNK and FasL phosphorylation, activation of nuclear factor of activated T cells (NFAT) and activator protein 1, and increased interferon-γ production.10 Remarkably, inhibition of T-cell activation by FasL reverse signaling has been associated with reduced phosphorylation of ERK1/2, linker of activated T cells, phospholipase C γ (PLCγ), and ζ-chain–associated protein kinase 70, and with diminished TCR internalization.11 Considering these conflicting results, it is clear that a physiological in vivo model system is required to elucidate the mechanism and role of FasL reverse signaling.
Recently, we discovered a Notch-like processing of FasL by the proteases a disintegrin and metalloprotease 10 (ADAM10) and signal peptide peptidase-like 2a (SPPL2a), which leads to the release of the FasL ICD into the cytoplasm and subsequent translocation to the nucleus where it may influence gene transcription.12 These results strongly suggest that the intracellular FasL domain is responsible for reverse signal transmission into the FasL-bearing cell.
Several FasL binding partners have been identified, the majority of which interact with the FasL PRD domain via their SH3 domains (for review see Voss et al1 ). We reported a ternary complex consisting of FasL, the SH3 domain-containing adaptor protein PSTPIP (proline, serine, threonine phosphatase interacting protein) and the phosphatase PTP-PEST (protein tyrosine phosphatase, nonreceptor type, 12; PTPN12). According to our data, formation of this complex reduces FasL cell surface expression by promoting its intracellular storage, which correlates with a decreased FasL-dependent killing capacity.13
As the precise molecular mechanism underlying FasL reverse signaling and its physiological relevance have not been addressed at the endogenous protein level in vivo, we have established a knockout/knockin mouse model in which wild-type FasL was replaced with a deletion mutant lacking the intracellular portion (FasL ΔIntra). In this study, we show that signaling via the FasL ICD dampens the activation-induced proliferative response of lymphocytes by impairing PLCγ, protein kinase C (PKC) and ERK1/2 phosphorylation and by affecting target gene regulation. We found that interaction of the FasL ICD with the transcription factor lymphoid-enhancer binding factor-1 (Lef-1) regulates Lef-1–dependent transcription. In vivo T cell–dependent (TD) immunization with 3-hydroxy 4-nitrophenylacetyl chicken gamma globulin (NP-CGG) revealed elevated numbers of plasma cells (PCs) and a significantly increased generation of germinal center B cells (GCs) in response to the immunization in homozygous FasL ΔIntra mice, leading to increased titers of NP-specific immunoglobulin M (IgM) antibodies in the serum. Similarly, T cell–independent (TI)–immunization with NP-Ficoll led to significantly higher PC numbers and NP-specific IgM antibody titers in mutant mice. Our in vivo findings based on endogenous FasL protein levels demonstrate that FasL reverse signaling functions as a negative modulator of certain immune responses.
Methods
Generation of FasL ΔIntra animals, genotyping of mice
Mice were housed and handled according to the federal animal welfare guidelines, and all mice studies were approved by the state ethics committee. The mouse FasL gene was subcloned from a 129Sv genomic BAC clone into the pBluescript vector (Stratagene). The AfeI/PstI fragment, which comprises most of exon 1, was replaced with a polymerase chain reaction (PCR)–generated fragment in which aa 2-74 from the N terminus of FasL were deleted. A tk-neo cassette flanked by 2 loxP sites was placed into the blunted EcoRI site in intron 1, and a diphtheria toxin A gene cassette was placed downstream of the 3′ homology arm for negative selection (see Figure 1A). The linearized targeting construct was electroporated into the 129 × 1/SvJ-derived mouse embryonic stem (ES) cell line TBV2. G418-resistant clones were screened for homologous recombination at the FasL locus by Southern blot analysis of a BamHI fragment covering exon 1 and 2 (see supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Successfully targeted ES cells were injected into C57Bl/6 blastocysts and transferred into pseudopregnant CD-1 foster mothers to obtain chimeric animals. Germline transmission in mice generated by mating chimeric males with C57Bl/6 females was detected by coat color and reconfirmed by Southern analysis of tail DNA.
To remove the floxed tk-neo selection cassette in the germ line, mice were crossed with the early deleter transgenic mouse strain CMV-Cre. FasL ΔIntra mice were backcrossed into the C57Bl/6N genetic background, and offspring were routinely genotyped by PCR with 2 μL of the genomic DNA as template, Red Taq genomic DNA Polymerase (Sigma-Aldrich), and primers amplifying the region around the remaining loxP site in intron 1 of the mutated allele (forward 5′-CCATGAAATCATTTTATGGTTGGGG-3′; reverse 5′-GAACAAGACACAAAATTGATTTTCAT-3′). PCR cycling conditions included an annealing temperature of 57°C.
For expression of the mutant FasL protein in the Fas-deficient lpr,lpr background, FasL ΔIntra mice were mated with lpr animals (FasB6.MRL-Faslpr/J) to obtain homozygous lpr,lpr offspring that express either wild-type or truncated FasL (lpr,lpr/FasL wild-type,FasL wild-type and lpr,lpr/FasL ΔIntra,FasL ΔIntra). To genotype for the lpr mutation, a 3-primer strategy was used using the following primer sequences: lpr-common 5′-GTAAATAATTGTGCTTCGTCAG-3′, lpr-wild-type 5′-CAAATCTAGGCATTAACAGTG-3′, lpr-mutant 5′-TAGAAAGGTGCACGGGTGTG-3′. Primers were used at 20μM each and annealed at 59°C according to the protocol provided by The Jackson Laboratory.
Additional detailed experimental procedures are described in the supplemental Data section.
Results
Generation of knockout/knockin mice that express mutant FasL lacking the intracellular domain
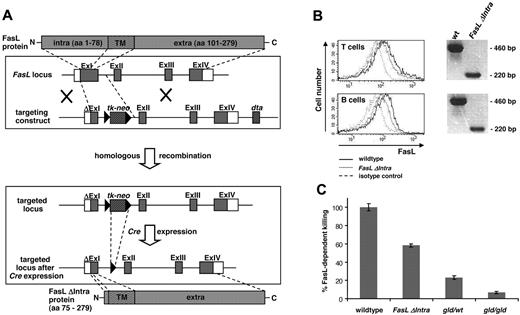
To study reverse signaling via the FasL ICD in vivo, the mouse line FasL ΔIntra was established that expresses a truncated FasL molecule without the N-terminal ICD instead of the wild-type protein. Exon 1 was shortened by the DNA sequences coding for FasL aa 2-74 in the targeting construct, and a tk-neo cassette flanked by loxP sites was placed into intron 1 to allow selection of ES cells that had undergone monoallelic homologous recombination (Figure 1A). Correctly targeted ES clones (supplemental Figures 1-2), were injected into C57Bl/6 blastocytes and transferred into CD1 foster mice. Breeding of the resulting heterozygous FasL knockout/knockin mice with CMV-Cre transgenic animals resulted in constitutive elimination of the tk-neo cassette in the germ line (supplemental Figure 2). Correct insertion of the desired mutation at the FasL locus was confirmed by sequence analysis of genomic DNA (supplemental Figure 3). For the following studies, FasL ΔIntra mice were bred into a pure C57Bl/6N genetic background.
Establishment and validation of knockout/knockin mice that lack the intracellular FasL domain. (A) Targeting strategy for the establishment of FasL ΔIntra mice. DNA sequences coding for the first 73 amino acids were deleted in exon 1, and a tk-neo selection cassette flanked by loxP sites was inserted into intron 1. Subsequent excision of this cassette by Cre recombinase left a single loxP site in the genome. (B) T and B lymphocytes from homozygous FasL ΔIntra mice express the truncated FasL molecule lacking the intracellular domain. Left panel, surface expression of full-length and mutant FasL molecules at the surface of activated lymphocytes isolated from wild-type and homozygous FasL ΔIntra mice. Freshly isolated lymphocyte populations from spleen were stimulated with plate-coated 5 μg/mL anti-CD3 antibody (T cells), or with 10 μg/mL LPS (B cells) for 24 hours. One representative experiment of 4 is shown. Wild-type cells are represented by the solid lines, FasL ΔIntra cells by the dotted lines, and the isotype control by the wide dashed lines. Right panel, RT-PCR analysis reveals FasL ΔIntra mRNA expression in T and B cells. Total RNA was isolated from purified splenic wild-type and FasL ΔIntra T and B cells, which had been stimulated for 24 hours with 1 μg/mL anti-CD3 (T cells) or for 4 hours with 1 μg/mL anti-IgM (B cells). The primer pair used for RT-PCR amplification of the FasL ICD allowed to distinguish cDNA from genomic DNA template (intron-spanning). (C) T cells isolated from homozygous FasL ΔIntra mice retain the ability to kill in a FasL-dependent manner. The killing capacity of restimulated T cell blasts (10 μg/mL anti-CD3 antibody, 24 hours) from wild-type, FasL ΔIntra and both homozygous and heterozygous gld mice was determined by coculture experiments. Fas-sensitive, CFSE-labeled A20 target cells were used at an effector:target ratio of 2.5:1 for 6 hours in the presence of 10 μg/mL anti-CD3 antibody. Killing capacity is expressed as the percentage of apoptotic subG1 A20 target cells minus the percentage of dead cells observed in the presence of 100 μg/mL Fas:Fc. Columns represent the mean and bars the SE of 4 individual experiments.
Establishment and validation of knockout/knockin mice that lack the intracellular FasL domain. (A) Targeting strategy for the establishment of FasL ΔIntra mice. DNA sequences coding for the first 73 amino acids were deleted in exon 1, and a tk-neo selection cassette flanked by loxP sites was inserted into intron 1. Subsequent excision of this cassette by Cre recombinase left a single loxP site in the genome. (B) T and B lymphocytes from homozygous FasL ΔIntra mice express the truncated FasL molecule lacking the intracellular domain. Left panel, surface expression of full-length and mutant FasL molecules at the surface of activated lymphocytes isolated from wild-type and homozygous FasL ΔIntra mice. Freshly isolated lymphocyte populations from spleen were stimulated with plate-coated 5 μg/mL anti-CD3 antibody (T cells), or with 10 μg/mL LPS (B cells) for 24 hours. One representative experiment of 4 is shown. Wild-type cells are represented by the solid lines, FasL ΔIntra cells by the dotted lines, and the isotype control by the wide dashed lines. Right panel, RT-PCR analysis reveals FasL ΔIntra mRNA expression in T and B cells. Total RNA was isolated from purified splenic wild-type and FasL ΔIntra T and B cells, which had been stimulated for 24 hours with 1 μg/mL anti-CD3 (T cells) or for 4 hours with 1 μg/mL anti-IgM (B cells). The primer pair used for RT-PCR amplification of the FasL ICD allowed to distinguish cDNA from genomic DNA template (intron-spanning). (C) T cells isolated from homozygous FasL ΔIntra mice retain the ability to kill in a FasL-dependent manner. The killing capacity of restimulated T cell blasts (10 μg/mL anti-CD3 antibody, 24 hours) from wild-type, FasL ΔIntra and both homozygous and heterozygous gld mice was determined by coculture experiments. Fas-sensitive, CFSE-labeled A20 target cells were used at an effector:target ratio of 2.5:1 for 6 hours in the presence of 10 μg/mL anti-CD3 antibody. Killing capacity is expressed as the percentage of apoptotic subG1 A20 target cells minus the percentage of dead cells observed in the presence of 100 μg/mL Fas:Fc. Columns represent the mean and bars the SE of 4 individual experiments.
The truncated FasL molecule is expressed on the surface of activated FasL ΔIntra lymphocytes and is capable of inducing apoptosis
First, we analyzed whether the truncated FasL protein lacking the intracellular domain was expressed by activated lymphocytes of the FasL ΔIntra mouse model, and whether it retained its killing capability. Reverse transcription PCR (RT-PCR) analysis using intron-spanning primer pairs and RNA isolated from both wild-type and mutant T and B cells revealed that FasL ΔIntra mRNA is expressed in both cell types, and flow cytometric analysis of activated T and B cells confirmed comparable FasL cell surface expression in wild-type and FasL ΔIntra lymphocytes (Figure 1B and supplemental Figure 4).
Coculture with Fas-expressing A20 target cells confirmed that cells derived from homozygous FasL ΔIntra mice retained the ability to kill in a FasL-dependent manner (Figure 1C), although less efficient than wild-type cells. This decrease in cytotoxicity is consistent with our earlier finding that FasL only displays maximum killing potential when it is localized in rafts, which requires the presence of the ICD.14 However, FasL ΔIntra T cells are clearly more potent at inducing Fas-mediated apoptosis than T cells isolated from heterozygous or homozygous gld mice. Only the latter develop the lymphoproliferative disease and accumulate an aberrant CD3+B220+CD4−CD8− T-cell population and autoantibodies, while the remaining FasL killing capacity in gld,wt heterozygous mice is already sufficient to completely prevent gld-associated pathology.4 Systemic first-line phenotyping at the German Mouse Clinic (GMC;15,16 ) revealed that unchallenged homozygous FasL ΔIntra mice do not display any phenotypic anomalies, exhibit normal T- and B-cell populations, no abnormality in Ig levels, and do not develop a gld-like lymphoproliferative disease (data not shown). The expression levels of Fas on the cell surface of lymphocytes are also normal (eg, see supplemental Figure 9A for T cells). Therefore, FasL ΔIntra mice represent a suitable model to study the consequences of FasL reverse signaling deficiency in vivo.
Absence of the intracellular FasL domain leads to increased activation-induced lymphocyte proliferation
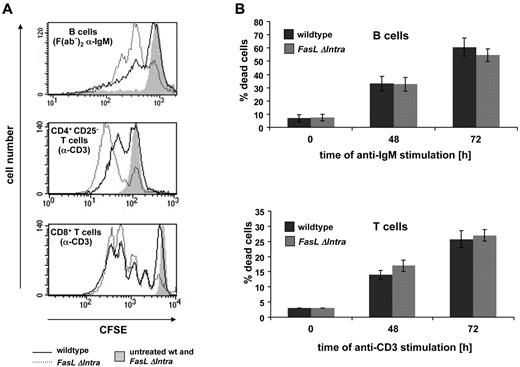
Because the intracellular FasL domain has been implicated in the regulation of T-cell proliferation in several in vitro studies,8-11 we investigated activation-induced lymphocyte proliferation in reverse signaling-deficient FasL ΔIntra mice. For this purpose, naive CD4+ or CD8+ T cells and B cells were isolated from the spleen, stained with carboxyfluorescein succinimidyl ester (CFSE) and activated for 72 hours with anti-CD3 or F(ab′)2 anti-IgM antibody. Proliferation of living cells was then quantified by flow cytometry. Surprisingly, T cells lacking the FasL ICD proliferated more vigorously than wild-type cells. In B cells, this increase in activation-induced proliferation was even stronger, as seen for both, anti-IgM (Figure 2A) and anti-CD40 (supplemental Figure 5) antibody stimulation. Interestingly, differences in the proliferative capacity of CD4+ T cells could only be detected ex vivo after depletion of CD4+CD25+ regulatory T cells (Tregs). In the presence of Tregs, the proliferative response of FasL ΔIntra T cells was indistinguishable from wild-type cells (data not shown). Increased proliferation of T and B cells expressing mutant FasL was not due to diminished activation-induced cell death as measured by annexin V/propidium iodide staining (Figure 2B). These data imply that reverse signaling via the FasL ICD dampens lymphocyte proliferation.
Increased ex vivo lymphocyte proliferation in the absence of the intracellular FasL domain. (A) CFSE-labeled lymphocytes were either treated for 72 hours with 5 μg/mL coated anti-CD3 antibody (T cells), for 72 hours with 2.5 μg/mL soluble F(ab′)2 anti-IgM antibody (B cells) or were left untreated (filled gray peaks, negative control). B cells (top panel), CD4+ CD25− helper T cells without regulatory T cells (middle panel) and CD8+ cytotoxic T cells (bottom panel) were isolated from splenocytes. Activation-induced proliferation of living cells (propidium iodide negative) was measured by FACS analysis, as represented by the decrease in CFSE fluorescence intensity. The solid line represents wild-type cells while the dotted line represents cells from homozygous FasL ΔIntra mice. One representative experiment of 5 (T cells) or 1 of 4 (B cells) is shown. (B) The observed differences in proliferation are not due to differences in activation-induced cell death. Lymphocytes used for the CFSE proliferation assays were simultaneously seeded under identical conditions for annexinV/PI staining. The number of dead cells was quantified 48 and 72 hours after stimulation with anti-IgM (B cells) or plate-bound anti-CD3 (T cells) antibodies by FACS analysis. Dark gray columns represent wild-type and light gray columns FasL ΔIntra cells. The columns display the mean and bars the SE of 5 individual experiments.
Increased ex vivo lymphocyte proliferation in the absence of the intracellular FasL domain. (A) CFSE-labeled lymphocytes were either treated for 72 hours with 5 μg/mL coated anti-CD3 antibody (T cells), for 72 hours with 2.5 μg/mL soluble F(ab′)2 anti-IgM antibody (B cells) or were left untreated (filled gray peaks, negative control). B cells (top panel), CD4+ CD25− helper T cells without regulatory T cells (middle panel) and CD8+ cytotoxic T cells (bottom panel) were isolated from splenocytes. Activation-induced proliferation of living cells (propidium iodide negative) was measured by FACS analysis, as represented by the decrease in CFSE fluorescence intensity. The solid line represents wild-type cells while the dotted line represents cells from homozygous FasL ΔIntra mice. One representative experiment of 5 (T cells) or 1 of 4 (B cells) is shown. (B) The observed differences in proliferation are not due to differences in activation-induced cell death. Lymphocytes used for the CFSE proliferation assays were simultaneously seeded under identical conditions for annexinV/PI staining. The number of dead cells was quantified 48 and 72 hours after stimulation with anti-IgM (B cells) or plate-bound anti-CD3 (T cells) antibodies by FACS analysis. Dark gray columns represent wild-type and light gray columns FasL ΔIntra cells. The columns display the mean and bars the SE of 5 individual experiments.
FasL reverse signaling dampens the immune response following TD and TI immunization
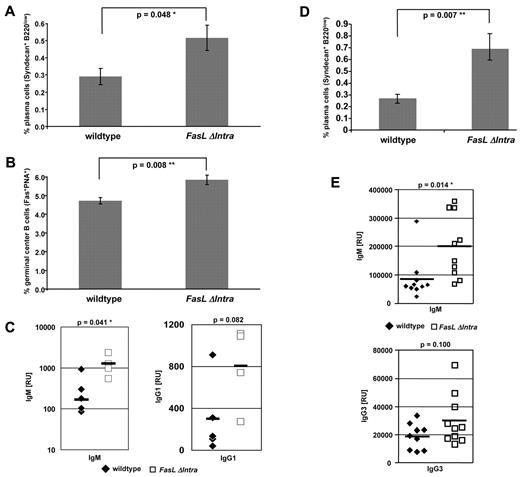
Immune responses against antigens are mediated by the expansion of antigen-specific lymphocyte populations. Because FasL reverse signaling influenced activation-induced proliferation in lymphocytes ex vivo, we assessed whether FasL ICD-dependent signaling would also affect B-cell proliferation following antigen exposure in vivo. For this purpose, wild-type and homozygous FasL ΔIntra mice were immunized with NP-CGG,17 and the following formation of germinal centers was analyzed 14 days later by fluorescence-activated cell sorting (FACS). We observed elevated numbers of Syndecan+B220low PCs and a significant higher increase in Fas+PNA+ GCs in FasL ΔIntra mice (Figure 3A-B), a finding that correlated with significantly increased titers of NP-specific IgM and a trend toward elevated NP-specific IgG1 serum antibodies (Figure 3C). In the same animals, no differences were detected in the amounts of B220+IgMhighIgDlow marginal zone B cells and B220+IgM+IgD+ follicular B cells (data not shown). Likewise, no alterations were found in the T:B cell ratio and in the number of activated B cells before and 14 days after immunization (data not shown).
Signaling via the FasL ICD modulates the immune response following immunization with NP-CGG. Wild-type and FasL ΔIntra mice were immunized with 100 μg of the TD antigen NP-CGG (left panel) or with 50 μg of the TI antigen NP-Ficoll (right panel; both intraperitoneal injections) and killed 14 days (NP-CGG) or 7 days (NP-Ficoll) later. The amount of Syndecan+B220low plasma B cells (A,D) and CD95+PNA+ GCs (B) was determined by FACS analysis of splenocytes. The data represent results from 2 immunization experiments, each time with 4 (NP-CGG) or 5 (NP-Ficoll) mice per group. The columns represent mean values of the individual animals, and the bars indicate the standard error. (C,E) Titers of antigen-specific, NP17-binding IgM, IgG1 (C), or IgG3 (E) antibodies in the serum of immunized animals were determined by ELISA. Each symbol represents 1 mouse, and the vertical bar indicates the mean value per group. Immunglobulin titers in the serum of wild-type mice are represented by filled hash keys and titers in FasL ΔIntra mice by open rectangles.
Signaling via the FasL ICD modulates the immune response following immunization with NP-CGG. Wild-type and FasL ΔIntra mice were immunized with 100 μg of the TD antigen NP-CGG (left panel) or with 50 μg of the TI antigen NP-Ficoll (right panel; both intraperitoneal injections) and killed 14 days (NP-CGG) or 7 days (NP-Ficoll) later. The amount of Syndecan+B220low plasma B cells (A,D) and CD95+PNA+ GCs (B) was determined by FACS analysis of splenocytes. The data represent results from 2 immunization experiments, each time with 4 (NP-CGG) or 5 (NP-Ficoll) mice per group. The columns represent mean values of the individual animals, and the bars indicate the standard error. (C,E) Titers of antigen-specific, NP17-binding IgM, IgG1 (C), or IgG3 (E) antibodies in the serum of immunized animals were determined by ELISA. Each symbol represents 1 mouse, and the vertical bar indicates the mean value per group. Immunglobulin titers in the serum of wild-type mice are represented by filled hash keys and titers in FasL ΔIntra mice by open rectangles.
To exclude that the higher PC numbers and IgM antibody titers are due to an impaired T cell–dependent negative selection of GC B cells, we immunized wild-type and FasL ΔIntra mutant mice with the TI antigen NP-Ficoll (Figure 3D-E). Similar to the TD immunization, we observed significantly elevated numbers of PCs (Figure 3D) and titers of NP-specific IgM antibodies (Figure 3E top panel) and a trend toward higher levels of NP-specific IgG3 antibodies (Figure 3E bottom panel) 7 days after immunization. Besides NP-specific IgM levels, only titers of antigen-specific IgG1 or IgG3 antibodies were determined, as the majority of B cells switch to these Ig isotypes following TD (IgG1) or TI (IgG3) immunization.18,19
These observations suggest that signaling via the FasL ICD participates in the regulation of T cell–dependent and independent immune responses by dampening lymphocyte activation and expansion.
The intracellular FasL domain regulates ERK1/2 activation and proliferation via PLCγ2 and PKC in B cells
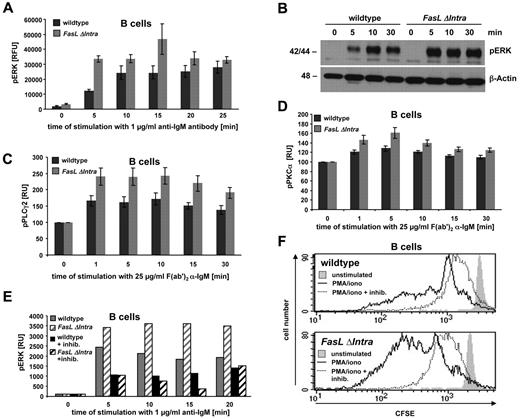
B-cell receptor (BCR) and TCR signaling involves activation of the ERK/mitogen-activated protein kinase (MAPK) cascade, which can be assessed by monitoring ERK1/2 phosphorylation.20 To identify a molecular correlate for the differential activation-induced proliferation of FasL ΔIntra and wild-type lymphocytes, naive splenic B cells were stimulated with anti-IgM for various time points, and ERK1/2 activation was quantified by enzyme-linked immunosorbent assay (ELISA). Five minutes after BCR cross-linking, the relative amount of phosphorylated ERK1/2 was significantly higher in FasL ΔIntra cells compared with wild-type B cells, and phospho-ERK1/2 levels remained elevated throughout the entire time course of the experiment (Figure 4A). This finding was confirmed by an antiphospho-ERK1/2 Western blot analysis of cell lysates prepared from activated FasL ΔIntra and wild-type B cells (Figure 4B).
The FasL ICD dampens ERK1/2 activity during B cell activation by inhibiting PKCα phosphorylation. (A) Increased ERK1/2 phosphorylation in activated homozygous FasL ΔIntra B cells. Naive splenic B cells were stimulated with 1 μg/mL soluble anti-IgM antibody for the indicated time points and analyzed for ERK1/2 phosphorylation by ELISA. The diagram displays the relative induction of ERK1/2 activation over time during anti-IgM treatment, measured as the increase in relative fluorescence intensity of phosphorylated ERK1/2 compared with total ERK1/2 protein. Wild-type cells are represented by the dark gray columns and homozygous FasL ΔIntra cells by light gray columns. Mean values of 4 independent experiments are shown, and the error bars indicate the SE. (B) Western Blot analysis of total cell lysates prepared from wild-type and homozygous FasL ΔIntra splenic B cells that were stimulated for 0, 5, 10, and 30 minutes with 1 μg/mL anti-IgM antibody. Phosphorylated ERK1/2 was detected with a rabbit anti–mouse pERK1/2 antibody; β-actin served as a loading control. (C,D) The activating phosphorylation of PLCγ2 and PKCα is repressed by the FasL ICD. Activation of PLCγ2 (C) and PKCα (D) after stimulation of naive wild-type and homozygous FasL ΔIntra splenic B cells with 25 μg/mL F(ab′)2 anti-IgM antibody for the indicated times was evaluated by intracellular staining of the phosphorylated proteins, followed by FACS analysis. The relative geometric mean fluorescent intensity (MFI) of activated cells was calculated against the MFI of untreated cells. Mean data of 11 (PLCγ2) and 12 (PKCα) independent experiments are shown together with standard errors. (E) ERK1/2 activation in activated mouse B cells is PKCα-dependent. ERK phosphorylation, in response to B cell stimulation with 1 μg/mL anti-IgM antibody, was determined by ELISA, either in the presence or absence of the PKC inhibitor GF109203x hydrochloride (1μM). Wild-type cells are illustrated by solid and homozygous FasL ΔIntra cells by striped columns. The gray color represents cells without and the black color the corresponding inhibitor-treated cells. (F) PKCα regulates FasL reverse signaling-influenced B cell proliferation. Proliferation of wild-type and homozygous FasL ΔIntra naive splenic B cells in response to stimulation with 100 ng/mL PMA and 0.2 μg/mL ionomycine in the presence (1μM) or absence of the PKC inhibitor GF109203x hydrochloride (Sigma-Aldrich) was measured after 72 hours by a FACS-based CFSE dilution assay. Nonstimulated cells are represented by the light gray-filled histogram. The solid line shows activated B cells without PKC inhibitor, the dotted line indicates the activated inhibitor-treated cells. One representative experiment of 4 is shown.
The FasL ICD dampens ERK1/2 activity during B cell activation by inhibiting PKCα phosphorylation. (A) Increased ERK1/2 phosphorylation in activated homozygous FasL ΔIntra B cells. Naive splenic B cells were stimulated with 1 μg/mL soluble anti-IgM antibody for the indicated time points and analyzed for ERK1/2 phosphorylation by ELISA. The diagram displays the relative induction of ERK1/2 activation over time during anti-IgM treatment, measured as the increase in relative fluorescence intensity of phosphorylated ERK1/2 compared with total ERK1/2 protein. Wild-type cells are represented by the dark gray columns and homozygous FasL ΔIntra cells by light gray columns. Mean values of 4 independent experiments are shown, and the error bars indicate the SE. (B) Western Blot analysis of total cell lysates prepared from wild-type and homozygous FasL ΔIntra splenic B cells that were stimulated for 0, 5, 10, and 30 minutes with 1 μg/mL anti-IgM antibody. Phosphorylated ERK1/2 was detected with a rabbit anti–mouse pERK1/2 antibody; β-actin served as a loading control. (C,D) The activating phosphorylation of PLCγ2 and PKCα is repressed by the FasL ICD. Activation of PLCγ2 (C) and PKCα (D) after stimulation of naive wild-type and homozygous FasL ΔIntra splenic B cells with 25 μg/mL F(ab′)2 anti-IgM antibody for the indicated times was evaluated by intracellular staining of the phosphorylated proteins, followed by FACS analysis. The relative geometric mean fluorescent intensity (MFI) of activated cells was calculated against the MFI of untreated cells. Mean data of 11 (PLCγ2) and 12 (PKCα) independent experiments are shown together with standard errors. (E) ERK1/2 activation in activated mouse B cells is PKCα-dependent. ERK phosphorylation, in response to B cell stimulation with 1 μg/mL anti-IgM antibody, was determined by ELISA, either in the presence or absence of the PKC inhibitor GF109203x hydrochloride (1μM). Wild-type cells are illustrated by solid and homozygous FasL ΔIntra cells by striped columns. The gray color represents cells without and the black color the corresponding inhibitor-treated cells. (F) PKCα regulates FasL reverse signaling-influenced B cell proliferation. Proliferation of wild-type and homozygous FasL ΔIntra naive splenic B cells in response to stimulation with 100 ng/mL PMA and 0.2 μg/mL ionomycine in the presence (1μM) or absence of the PKC inhibitor GF109203x hydrochloride (Sigma-Aldrich) was measured after 72 hours by a FACS-based CFSE dilution assay. Nonstimulated cells are represented by the light gray-filled histogram. The solid line shows activated B cells without PKC inhibitor, the dotted line indicates the activated inhibitor-treated cells. One representative experiment of 4 is shown.
We then tried to further characterize the signaling pathway leading to differential ERK1/2 activation in FasL ΔIntra and wild-type B cells. Cross-linking of the BCR leads to activation of the protein tyrosine kinases Syk and Btk, which are both required for PLCγ2 activation.21 This ultimately results in PKC phosphorylation and triggering of the MAPK pathway upon phosphorylation of Raf and/or MEK. We analyzed the amount of phosphorylated PLCγ2 and PKCα by intracellular FACS staining over a time period of 30 minutes following activation of naive B cells with F(ab′)2 anti-IgM antibody. Interestingly, we observed a significant higher induction of PLCγ2 and PKCα activity in B cells derived from homozygous FasL ΔIntra mice compared with wild-type cells (Figure 4C-D). We confirmed the differences in the amount of activated PKCα by Western Blot analysis (supplemental Figure 6). The importance of PKC activation for B-cell stimulation is underlined by the strong inhibition of ERK1/2 phosphorylation (Figure 4E) and proliferation (Figure 4F) of naive wild-type and homozygous FasL ΔIntra B cells when the cells were simultaneously treated with a pan-PKC inhibitor (GF109203x hydrochloride).
Collectively, our data confirm that in activated FasL ΔIntra B cells, phosphorylation of PLCγ2, PKC, and ERK1/2 is increased compared with wild-type cells, leading to augmented proliferation. Our data suggest that the FasL ICD diminishes activation-induced lymphocyte proliferation by interfering with early molecular events following cross-linking of the BCR.
The intracellular FasL domain interacts with Lef-1 and inhibits Lef-1–dependent transcription
In a yeast-2-hybrid screen using the FasL ICD as bait, we identified the transcription factor Lef-1 as a putative interaction partner of the cytoplasmic FasL domain (data not shown). To confirm the interaction, we performed in vitro glutathione S-transferase (GST) pull-down assays. A full-length Lef-1 GST fusion protein was able to co-precipitate in vitro translated full-length FasL and the sole FasL ICD (Figure 5A left and middle panels), and GST-FasL ICD could coprecipitate in vitro translated Lef-1 (Figure 5A right panel). We verified the FasL-Lef-1 interaction in coimmuno-precipitation experiments with lysates prepared from transfected HEK293T cells (Figure 5B).
The intracellular FasL domain binds to Lef-1 and influences its transcriptional activity. (A) In vitro GST pull-down experiments reveal FasL-Lef1 interaction. Top panel, pull-down of in vitro translated 35 [S] FasL (left), FasL ICD (middle), and Lef-1 (right) proteins with the indicated bacterially expressed GST fusion proteins. The black arrows mark in vitro translated proteins that specifically interact with the GST fusion molecules. GST-PSTPIP serves as a positive control for FasL binding13 and GST protein only as a general negative control. Bottom panel, Coomassie-stained protein gels showing purified GST fusion proteins. Arrows indicate the GST fusion proteins used for the above GST pull-down experiment. (B) Coimmunoprecipitation of overexpressed Flag-FasL and Lef-1 proteins. HEK 293T cells were transfected with Lef-1 and FasL or empty vector (pcDNA3.1). Forty-eight hours later, cell lysates were prepared and immunoprecipitations performed with anti-Flag M2 (binding to Flag-tagged FasL) or anti-FasL (NOK1) antibodies. Western Blot analysis with the anti–Lef-1 antibody revealed for both coimmunoprecipitation reactions binding of Flag-FasL to Lef-1 (top panel). Control incubation of the membrane with anti-Flag antibody confirmed the presence and successful immunoprecipitation of Flag-FasL (middle panel right side); the input Western Blot analysis with anti–Lef-1 antibody (bottom panel) demonstrated presence of transfected Lef-1. (C) The FasL ICD represses Lef-1/β-catenin activity in a Luciferase reporter assay. HEK293T cells were cotransfected with the indicated increasing amounts of the FasL ICD together with optimal (TOP) or mutated (FOP, negative control) Firefly luciferase reporter constructs for Lef-1 activity. Sixteen hours after cultivation of the cells in the presence of 30mM LiCl to inhibit GSK3β, Lef-1–dependent Luciferase activity was quantified in the cell lysates. Firefly luciferase activity was normalized to Renilla luciferase activity, and the value obtained for TOP plasmid without FasL ICD was set to 1. All samples were measured in triplicates. Control transfections with TOP plasmid alone without LiCl did not induce luciferase activity (data not shown). The columns represent the mean of 3 individual experiments, and error bars indicate the standard deviation. Statistical significance was assessed using Student t test (*P < .5; **P < .01; ***P < .001). (D) RNA and protein expression analysis of Lef-1 in mature B cells. Top panel, RT-PCR experiment with intron-spanning Lef-1–specific PCR primers and total RNA isolated from wild-type and FasL ΔIntra splenic B cells that were stimulated with 1 μg/mL anti-IgM antibody for 4 hours. RNA prepared from Lef-1–transfected HEK 293T cells served as a positive control, while water without RNA template was used as a negative control. Bottom panel, histogram of intracellular FACS staining confirms expression of Lef-1 in B cells. Naive splenic B cells were fixed, permeabilized, and incubated subsequently with anti–Lef-1 and secondary antibody. The solid curve represents wild-type cells, the dotted curve represents the FasL ΔIntra B cells, and the filled gray curve depicts the isotype control. One representative experiment of 6 is displayed.
The intracellular FasL domain binds to Lef-1 and influences its transcriptional activity. (A) In vitro GST pull-down experiments reveal FasL-Lef1 interaction. Top panel, pull-down of in vitro translated 35 [S] FasL (left), FasL ICD (middle), and Lef-1 (right) proteins with the indicated bacterially expressed GST fusion proteins. The black arrows mark in vitro translated proteins that specifically interact with the GST fusion molecules. GST-PSTPIP serves as a positive control for FasL binding13 and GST protein only as a general negative control. Bottom panel, Coomassie-stained protein gels showing purified GST fusion proteins. Arrows indicate the GST fusion proteins used for the above GST pull-down experiment. (B) Coimmunoprecipitation of overexpressed Flag-FasL and Lef-1 proteins. HEK 293T cells were transfected with Lef-1 and FasL or empty vector (pcDNA3.1). Forty-eight hours later, cell lysates were prepared and immunoprecipitations performed with anti-Flag M2 (binding to Flag-tagged FasL) or anti-FasL (NOK1) antibodies. Western Blot analysis with the anti–Lef-1 antibody revealed for both coimmunoprecipitation reactions binding of Flag-FasL to Lef-1 (top panel). Control incubation of the membrane with anti-Flag antibody confirmed the presence and successful immunoprecipitation of Flag-FasL (middle panel right side); the input Western Blot analysis with anti–Lef-1 antibody (bottom panel) demonstrated presence of transfected Lef-1. (C) The FasL ICD represses Lef-1/β-catenin activity in a Luciferase reporter assay. HEK293T cells were cotransfected with the indicated increasing amounts of the FasL ICD together with optimal (TOP) or mutated (FOP, negative control) Firefly luciferase reporter constructs for Lef-1 activity. Sixteen hours after cultivation of the cells in the presence of 30mM LiCl to inhibit GSK3β, Lef-1–dependent Luciferase activity was quantified in the cell lysates. Firefly luciferase activity was normalized to Renilla luciferase activity, and the value obtained for TOP plasmid without FasL ICD was set to 1. All samples were measured in triplicates. Control transfections with TOP plasmid alone without LiCl did not induce luciferase activity (data not shown). The columns represent the mean of 3 individual experiments, and error bars indicate the standard deviation. Statistical significance was assessed using Student t test (*P < .5; **P < .01; ***P < .001). (D) RNA and protein expression analysis of Lef-1 in mature B cells. Top panel, RT-PCR experiment with intron-spanning Lef-1–specific PCR primers and total RNA isolated from wild-type and FasL ΔIntra splenic B cells that were stimulated with 1 μg/mL anti-IgM antibody for 4 hours. RNA prepared from Lef-1–transfected HEK 293T cells served as a positive control, while water without RNA template was used as a negative control. Bottom panel, histogram of intracellular FACS staining confirms expression of Lef-1 in B cells. Naive splenic B cells were fixed, permeabilized, and incubated subsequently with anti–Lef-1 and secondary antibody. The solid curve represents wild-type cells, the dotted curve represents the FasL ΔIntra B cells, and the filled gray curve depicts the isotype control. One representative experiment of 6 is displayed.
Lef-1 is a member of the LEF/TCF transcription factor family that mediates the nuclear response to Wnt signaling, which is increasingly being recognized as important for hematopoiesis, lymphopoiesis, and immune function.22 To study the functional consequences of the FasL-Lef-1 interaction, we quantified Lef-1–dependent transcription in the presence of various FasL ICD concentrations using the TOP/FOP Lef-1 reporter system (Figure 5C). After cotransfecting FasL ICD with either TOP or FOP luciferase reporter plasmids into HEK293T cells, Lef-1 signaling was stimulated with LiCl, which mimics canonical Wnt signaling, and Luciferase reporter activity was quantified. Interestingly, the FasL ICD repressed Lef-1–dependent transcription of the luciferase gene in a concentration-dependent manner. Similar results were obtained with the specific GSK3β inhibitor SB-216763 instead of LiCl to induce Lef-1/β-catenin–mediated transcription (supplemental Figure 7). Collectively, our data demonstrate that the FasL ICD interacts with Lef-1 to regulate Lef-1–dependent transcription.
While elevated expression of Lef-1 and an important function for Lef-1–dependent Wnt signaling has been shown in B-cell progenitors,23,24 less information is known about expression and function of Lef-1 in mature B lymphocytes. Therefore, we analyzed Lef-1 expression in mature splenic B cells. RT-PCR analysis with RNA prepared from activated B cells revealed the presence of Lef-1 mRNA (Figure 5D top panel). In addition, we could detect significant Lef-1 protein expression in both wild-type and FasL ΔIntra B cells by intracellular FACS staining (Figure 5D bottom panel). The expression of both proteins, FasL and Lef-1, in B and T cells enables regulation of Lef-1–dependent Wnt signaling by the FasL ICD in lymphocytes.
FasL reverse signaling regulates proliferation-associated and Wnt pathway–related target genes
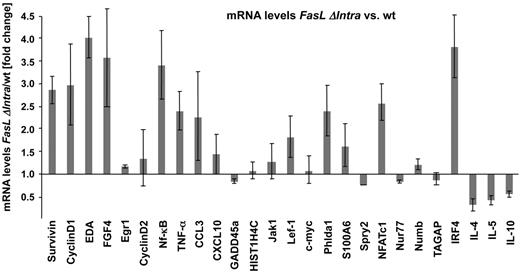
Based on the observed interaction of the FasL ICD with Lef-1 and its influence on the transcriptional activity of a transfected Lef-1 reporter construct, we analyzed global gene expression patterns in activated B cells from wild-type and FasL ΔIntra mice. We observed profound differences in the mRNA levels of several candidate genes, including Wnt pathway-associated genes (data not shown). The differential expression of a number of genes in wild-type and FasL ΔIntra B cells was then verified by quantitative RT-PCR. Several genes implicated in lymphocyte proliferation and activation, such as NFATc1,25 NF-κB1/p50,26 and interferon regulatory factor 4 (IRF4)27 were significantly up-regulated in the absence of the FasL ICD (Figure 6), a finding that correlates with the increased proliferative capacity of mutant FasL ΔIntra lymphocytes. Furthermore, increased expression of Wnt-related genes, such as Cyclin D1,28 Survivin,29 ectodysplasin A (EDA),30 and fibroblast growth factor 4 (FGF 4)31 was confirmed in FasL ΔIntra B cells. Interleukin-4 (IL-4) is negatively controlled by direct binding of Lef-1 to the IL-4 promoter.32 Consequently, we found IL-4 to be down-regulated 2-fold in FasL ΔIntra B cells lacking the Lef-1–inhibiting FasL ICD. These data support the model of Notch-like reverse signaling via FasL influencing gene transcription and the regulation of Wnt signaling by interaction of the FasL ICD with Lef-1 in lymphocytes.
The FasL ICD regulates expression of proliferation-associated and Wnt signaling target genes. Differential expression of selected genes, which represent direct targets of the Wnt signaling pathway and/or that are involved in lymphocyte activation and proliferation, is shown in wild-type and FasL ΔIntra B cells. Gene expression in both cell populations was quantified by real-time PCR with cDNA derived from stimulated splenic B cells (1 μg/mL anti-IgM antibody for 4 hours). Each sample was assessed in triplicate and normalized to the housekeeping gene Hprt. The relative change of gene expression in FasL ΔIntra cells was calculated with the comparative threshold cycle method (2−ΔΔCt). The columns for each gene represent the mean of 4 independent experiments, and the black bars indicate SEs.
The FasL ICD regulates expression of proliferation-associated and Wnt signaling target genes. Differential expression of selected genes, which represent direct targets of the Wnt signaling pathway and/or that are involved in lymphocyte activation and proliferation, is shown in wild-type and FasL ΔIntra B cells. Gene expression in both cell populations was quantified by real-time PCR with cDNA derived from stimulated splenic B cells (1 μg/mL anti-IgM antibody for 4 hours). Each sample was assessed in triplicate and normalized to the housekeeping gene Hprt. The relative change of gene expression in FasL ΔIntra cells was calculated with the comparative threshold cycle method (2−ΔΔCt). The columns for each gene represent the mean of 4 independent experiments, and the black bars indicate SEs.
Discussion
TNF receptor family members are expressed by a wide variety of cells, whereas the majority of the 19 identified TNF ligands are found exclusively on cells belonging to the immune system. Most of the molecules function as membrane-bound proteins, and at least 10 TNF family members receive and deliver (co-)stimulatory signals through their respective receptors.33
FasL has been shown to mediate reverse signal transduction following receptor binding, however, there is conflicting in vitro data concerning the functional outcome.8-11 Here we aimed to elucidate the physiological consequences of FasL reverse signaling at the endogenous protein level in vivo. For this purpose, a knockout/knockin mouse model deficient for FasL reverse signaling was established, in which wild-type FasL was replaced by a FasL deletion mutant lacking the complete intracellular domain (FasL ΔIntra). We demonstrated that activated lymphocytes from homozygous FasL ΔIntra mice express a functional truncated FasL membrane-bound protein with the capacity to induce apoptosis in Fas receptor-positive target cells. Furthermore, ex vivo experiments revealed that activation-induced proliferation is significantly increased in B cells, CD8+ CTLs, and CD4+ T cells derived from homozygous FasL ΔIntra mice. To our knowledge, this is the first time that FasL reverse signaling has been demonstrated in B cells. The activated FasL-mutant lymphocytes display increased phosphorylation of ERK1/2, PLCγ2, and PKCα, demonstrating that reverse signaling via the FasL ICD impedes early events following antigen stimulation of BCRs and TCRs. Interestingly, the increased proliferation of FasL ΔIntra T cells is reduced to wild-type levels in the presence of inhibitory CD4+ CD25+ regulatory T cells,34 which indicates that additional control mechanisms act in vivo. As we did not find obvious differences in the in vivo proliferation of Vβ8+ T cells following injection of wild-type and FasL ΔIntra mice with the superantigen SEB, our data substantiates the notion that regulatory T cells display an inhibitory influence on the increased activation-induced proliferation of FasL ΔIntra T cells (Oehme et al35 and data not shown). An alternative explanation for the SEB result builds on the fact that according to several in vitro studies, the influence of FasL reverse signaling on T-cell proliferation is only observable under conditions of suboptimal stimulation.8-11 If TCR signaling is induced, for example, by high amounts of anti-CD3 antibody, the proliferative behavior does not change upon co-triggering of FasL. SEB exerts a very strong T-cell stimulus and may override inhibitory FasL reverse signaling.
The hyperactivation of homozygous FasL ΔIntra lymphocytes in the absence of the FasL ICD that we observed ex vivo is expected to affect immune regulation in vivo. Indeed, the immune response following immunization with the TD antigen NP-CGG differs between FasL ΔIntra and wild-type mice. The number of PCs and the increase in GCs is significant higher in the absence of the FasL ICD and leads to elevated NP-specific IgM antibody titers, thereby representing an in vivo finding that confirms the modulation of immune responses by FasL reverse signaling. Similarly, immunization with the TI antigen NP-Ficoll resulted in significant higher numbers of PCs and titers of NP-specific IgM antibodies, thereby ruling out that the phenotype observed after TD immunization reflects an impaired negative selection of mutant GC B cells. This notion is also supported by a comparable sensitivity of wild-type and mutant-activated B cells toward FasL/Fas-dependent cell death induced in coculture experiments with T cell blasts, and by a similar costimulatory potential (ie, CD40L expression) of activated wild-type and FasL ΔIntra T cells (supplemental Figure 8). According to these results, the observed differences between the immune responses of immunized wild-type and FasL ΔIntra mice most likely can be explained by a lack of inhibitory FasL reverse signaling in the mutant mice. However, we cannot completely exclude that at least part of the in vivo phenotype following TD and TI immunization is due to a reduced killing efficiency of the truncated FasL.
In addition to its critical pro-death function, the Fas receptor transmits important nonapoptotic signals, which can lead to survival and proliferation of the receptor-bearing cells,6,7 and therefore, the consequences of FasL activation experiments could also be assigned to disturbance of nonapoptotic Fas receptor signaling. However, the increased activation-induced proliferation we observed in homozygous FasL ΔIntra lymphocytes is due to diminished FasL reverse signaling and not to increased pro-proliferative Fas receptor signaling. This conclusion is supported by ex vivo proliferation assays with Fas-deficient B cells isolated from wild-type and homozygous FasL ΔIntra mice, both in the lpr,lpr genetic background. Although nonapoptotic signaling via Fas is excluded in these cells, we again noticed increased proliferation in the FasL mutant cells lacking the FasL ICD (supplemental Figure 9B). In addition, nonapoptotic Fas signaling delivers a proliferative (and not an inhibitory) stimulus,6 therefore, the increased activation-induced proliferation of FasL ΔIntra lymphocytes that we observed would have to be explained by increased proliferative Fas signaling. However, we did not obtain evidence of enlarged Fas receptor triggering in the mutant cells: the number of Fas receptor expressing activated T cells and Fas expression levels were not augmented (supplemental Figure 9A), and the killing of target cells by FasL ΔIntra T cells was slightly decreased. These observations thereby argue against the notion of enhanced Fas triggering.
In our studies, we aimed to elucidate the molecular signaling events downstream of FasL triggering. The presence of the cytoplasmic FasL domain in wild-type cells causes diminished activation-induced proliferation of splenic B cells by decreasing the activating phosphorylation of PLCγ2, PKC, and ERK1/2, which subsequently influences target gene expression. Using various protein binding assays, we showed that an interaction occurs between the FasL ICD and the transcription factor Lef-1, which causes inhibition of Lef-1 transcriptional activity. FasL expression in mature B cells has been confirmed in different studies36-38 (for review see Lundy39 ). Although Lef-1 expression is down-regulated during B cell development,24 we could detect Lef-1 mRNA by RT-PCR and Lef-1 protein by intracellular FACS staining in mature murine B cells. Interestingly, we measured an up-regulation of several proliferation-relevant genes in FasL ΔIntra B cells (eg, IRF4, which plays an important role in the germinal center reaction).27 Because IRF4 is an important regulator of PC differentiation, increased IRF4 levels in B cells of FasL ΔIntra mice might contribute to the higher percentages of PCs in the spleen following immunization with NP-CGG.
Some of the genes up-regulated in activated FasL ΔIntra B cells represent well-known Lef-1 target genes (eg, NFAT,25 NF-κB,26 CycD1,28 Survivin,29 EDA,30 and FGF431 ). This transcriptional up-regulation correlates with and accounts for the increased proliferation of these cells. The exact molecular mechanism by which the FasL ICD inhibits Lef-1 transcriptional activity remains unclear. The binding site for FasL within the Lef-1 molecule does not overlap with the N-terminal β-catenin binding site,40 implying that direct competition between the FasL ICD and β-catenin for Lef-1 binding seems implausible. Unlike most of the known FasL binding partners, Lef-1 does not contain an SH3 domain and therefore does not bind to the PRD of FasL, but rather to its extreme N terminus.
In addition, FasL may influence ERK1/2 activity and target gene expression via another pathway, independently of Lef-1. We have previously reported the existence of a ternary intracellular protein complex consisting of FasL, the adaptor protein PST-PIP and the protein tyrosine phosphatase PTP-PEST.13 Our studies demonstrated enhanced binding of PSTPIP to the FasL PRD after FasL stimulation with an agonistic antibody. PTP-PEST has been described as a negative regulator of T-cell activation.41-43 PTP-PEST functions by dephosphorylating TCR-proximal signaling molecules such as Lck, Shc, Pyk2, Fak, Cas, and PLC, resulting in the inactivation of some signal transduction pathways, including Ras signaling.
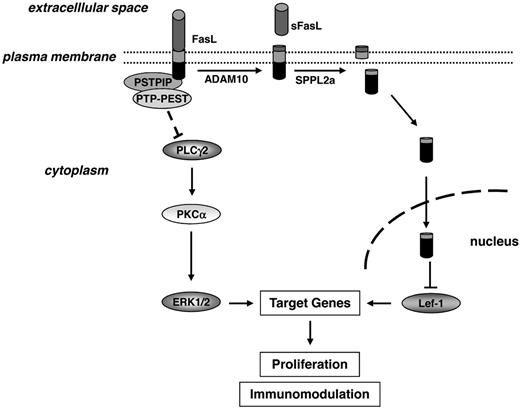
Collectively, our data suggest a model in which reverse signaling via FasL attenuates transcription of genes important for proliferation by influencing at least 2 pathways (Figure 7): in one pathway, interaction of full-length FasL with PSTPIP recruits the negative ERK1/2/MAPK regulator PTP-PEST. Meanwhile, FasL also impedes proliferation following proteolysis by ADAM10 and SPPL2a, which results in liberation of the intracellular FasL domain and its binding to Lef-1 for negative regulation of Lef-1–dependent pro-proliferative Wnt signaling. Both pathways lead to diminished lymphocyte proliferation following antigen receptor stimulation.
Model of FasL reverse signaling and the underlying molecular mechanism. At least 2 signaling pathways might be involved in transmitting the antiproliferative stimulus into the FasL-bearing cell. Recruitment of the tyrosine phosphatase PTP-PEST by the FasL-binding adaptor protein PSTPIP may lead to inhibition of ERK1/2 activity. In parallel, triggering of FasL followed by proteolytic cleavage of the molecule by ADAM10 and SPPL2a results in liberation of the FasL ICD into the cytoplasm, from where it translocates to the nucleus and influences gene transcription by binding to and inhibiting the transcription factor Lef-1.
Model of FasL reverse signaling and the underlying molecular mechanism. At least 2 signaling pathways might be involved in transmitting the antiproliferative stimulus into the FasL-bearing cell. Recruitment of the tyrosine phosphatase PTP-PEST by the FasL-binding adaptor protein PSTPIP may lead to inhibition of ERK1/2 activity. In parallel, triggering of FasL followed by proteolytic cleavage of the molecule by ADAM10 and SPPL2a results in liberation of the FasL ICD into the cytoplasm, from where it translocates to the nucleus and influences gene transcription by binding to and inhibiting the transcription factor Lef-1.
Our in vivo results are consistent with other studies suggesting an inhibitory role for FasL reverse signaling in T-cell proliferation,9,11 and they are in contrast to reports claiming a costimulatory function.8,10 Interestingly, we also found the ERK1/2-MAPK pathway to be affected by removal of the FasL ICD, but with the opposite tendency; while Sun et al10 observe increased ERK1/2 activation upon FasL costimulation, we noticed diminished ERK1/2 phosphorylation as a result of FasL reverse signaling. In the experiments showing costimulation by FasL triggering, T-cell lines were used, some of which were engineered to overexpress a rather artificial mouse FasL ICD fusion construct.10 Cross-linking was induced by anti-FasL or Fas:Fc,8,10 which may generate an unphysiologically strong (or weak) signal, leading to costimulation rather than inhibition; however, Paulsen et al observed decreased T-cell proliferation upon FasL engagement with the same reagents.11 In our knockout/knockin system with endogenous protein expression, FasL reverse signaling is initiated by its natural inducers, which results in decreased activation-induced proliferation of primary T and B cells ex vivo and decreased numbers of GC and PC numbers upon immunization. Nevertheless, we performed ex vivo activation studies with wild-type and FasL ΔIntra T and B cells (stimulated with anti-CD3 or F(ab′)2 anti-IgM antibodies, respectively) in the presence of coated Fas:Fc for 48 hours. We observed an inhibition of activation-induced proliferation of both T and B wild-type cells by Fas:Fc, whereas FasL triggering by Fas:Fc had no influence on the proliferation of reverse signaling-incompetent FasL ΔIntra cells (supplemental Figure 10). This is in agreement with our previous result and with data presented by Paulsen et al,11 according to which FasL reverse signaling inhibits activation-induced proliferation of lymphocytes.
Several publications present evidence that the nascent FasL molecule is targeted, via its PRD, to specialized secretory lysosomes present in lymphocytes.44,45 The lysosomes then undergo fusion with the plasma membrane at the site of contact with the target cell to deliver their cytotoxic cargo. However, analysis of our knockout/knockin mouse model, which completely lacks the FasL ICD, did not provide in vivo evidence for an important role of FasL PRD-dependent sorting of the molecule. Constitutive expression of truncated FasL at the cell surface of T, B, and natural killer cells due to the lack of the PRD domain would probably result in massive induction of apoptosis. Such increased cell killing (leading to pathological consequences) appears likely because, in contrast to human FasL, membrane-bound FasL is the primary functional form of murine FasL.46 It is because of this that we would not expect increased shedding of surface FasL to compensate for the constitutive delivery and expression of FasL at the cell surface. In addition, our experiments with ex vivo isolated cells did not reveal significant differences in the FasL-dependent killing of target cells by CTLs, and the in vivo FasL expression and killing level in different cell types is large enough to prevent development of the gld phenotype. In agreement with our findings, 2 independent lines of evidence suggest the possibility that FasL is sorted to the cell surface of hematopoietic effector cells by alternative pathways independent of the FasL ICD. First, recent studies did not confirm colocalization of FasL with classical markers or components of secretory lysosomes, suggesting storage in other compartments.47-49 An alternative pathway for FasL sorting to the cell surface has been suggested, possibly involving the FasL binding proteins Grb250 and Adaptin 2.51 Furthermore, removal of the PRD has been correlated with increased FasL cell surface expression on rat basophil leukemia cells. However 70% of these mutant FasL molecules remain intracellular,44 arguing against constitutive delivery of FasL in the absence of the cytoplasmic domain.
In summary, our FasL ΔIntra mouse model data confirms an inhibitory function for FasL reverse signaling in lymphocyte activation, which may prevent hyperactivation of lymphocytes in response to antigen stimulation. It is worth speculating whether this immune modulation by FasL signal transduction is important for the termination of immune responses and for the prevention of overwhelming inflammation in autoimmunity or allergy, a theory that will be tested in adequate mouse models in the future.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Susanne Bösser, Gabriele Marschall-Schröter, Sandra Geissler, Kerstin Kutzner, and Christine Fürmann for excellent technical assistance.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG; ZO110/4-1; ZO110/2-1/2, to M.Z.), the German National Academic Foundation (to K.L.), the European Union (EUMODIC LSHG-2006-037188, German Mouse Clinic), and the German National Genome Research Network (NGFN project N1KR-S12T23, to M.Z.; NGFNplus 01GS0850 to M.H.dA., 01GS0852 to D.B., 01GS0868 to M.O.).
Authorship
Contribution: V.K. and G.M. established the mouse model; K.L., I.M.M., K.R., F.T., D.S., L.J.S., and U.Z.-S. performed experiments and analyzed the data; W.M. performed the FasL ICD Y2H screen; A.A.P., T.A., D.B., M.O., M.H., and J.B. conducted a systematic phenotypic analysis of the FasL ΔIntra mouse model; M.H.deA., V.G.-D., H.F., F.J.T.S., A.-O.H., and U.Z.-S. contributed to the design of the experiments and the analysis of data; and K.L. and M.Z. conceived the study and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for Vladimir Kirkin is Merck-Serono/Research and Development/Oncology, In Vivo Pharmacology, Merck KGaA, Darmstadt, Germany. The current affiliation for Wiebke Milani is Institut für Physiologie und Pathophysiologie, University of Marburg, Marburg, Germany. The current affiliation for Geert Michel is Charité- Universitätsmedizin Berlin, Campus Benjamin Franklin, Transgene Technologien, Berlin, Germany.
Correspondence: Martin Zörnig, Chemotherapeutisches For-schungsinstitut Georg-Speyer-Haus, Paul-Ehrlich-Strasse 42-44, D-60596 Frankfurt, Germany; e-mail: zoernig@em.uni-frankfurt.de.

![Figure 5. The intracellular FasL domain binds to Lef-1 and influences its transcriptional activity. (A) In vitro GST pull-down experiments reveal FasL-Lef1 interaction. Top panel, pull-down of in vitro translated 35[S] FasL (left), FasL ICD (middle), and Lef-1 (right) proteins with the indicated bacterially expressed GST fusion proteins. The black arrows mark in vitro translated proteins that specifically interact with the GST fusion molecules. GST-PSTPIP serves as a positive control for FasL binding13 and GST protein only as a general negative control. Bottom panel, Coomassie-stained protein gels showing purified GST fusion proteins. Arrows indicate the GST fusion proteins used for the above GST pull-down experiment. (B) Coimmunoprecipitation of overexpressed Flag-FasL and Lef-1 proteins. HEK 293T cells were transfected with Lef-1 and FasL or empty vector (pcDNA3.1). Forty-eight hours later, cell lysates were prepared and immunoprecipitations performed with anti-Flag M2 (binding to Flag-tagged FasL) or anti-FasL (NOK1) antibodies. Western Blot analysis with the anti–Lef-1 antibody revealed for both coimmunoprecipitation reactions binding of Flag-FasL to Lef-1 (top panel). Control incubation of the membrane with anti-Flag antibody confirmed the presence and successful immunoprecipitation of Flag-FasL (middle panel right side); the input Western Blot analysis with anti–Lef-1 antibody (bottom panel) demonstrated presence of transfected Lef-1. (C) The FasL ICD represses Lef-1/β-catenin activity in a Luciferase reporter assay. HEK293T cells were cotransfected with the indicated increasing amounts of the FasL ICD together with optimal (TOP) or mutated (FOP, negative control) Firefly luciferase reporter constructs for Lef-1 activity. Sixteen hours after cultivation of the cells in the presence of 30mM LiCl to inhibit GSK3β, Lef-1–dependent Luciferase activity was quantified in the cell lysates. Firefly luciferase activity was normalized to Renilla luciferase activity, and the value obtained for TOP plasmid without FasL ICD was set to 1. All samples were measured in triplicates. Control transfections with TOP plasmid alone without LiCl did not induce luciferase activity (data not shown). The columns represent the mean of 3 individual experiments, and error bars indicate the standard deviation. Statistical significance was assessed using Student t test (*P < .5; **P < .01; ***P < .001). (D) RNA and protein expression analysis of Lef-1 in mature B cells. Top panel, RT-PCR experiment with intron-spanning Lef-1–specific PCR primers and total RNA isolated from wild-type and FasL ΔIntra splenic B cells that were stimulated with 1 μg/mL anti-IgM antibody for 4 hours. RNA prepared from Lef-1–transfected HEK 293T cells served as a positive control, while water without RNA template was used as a negative control. Bottom panel, histogram of intracellular FACS staining confirms expression of Lef-1 in B cells. Naive splenic B cells were fixed, permeabilized, and incubated subsequently with anti–Lef-1 and secondary antibody. The solid curve represents wild-type cells, the dotted curve represents the FasL ΔIntra B cells, and the filled gray curve depicts the isotype control. One representative experiment of 6 is displayed.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/2/10.1182_blood-2010-07-292722/4/m_zh89991064230005.jpeg?Expires=1767806155&Signature=Ll5DoH1tU-oauMsa~Dd4U3Udq-K1Uzzgt7GfIaC0uCO8uQ5oYKq3nRKvXdGqo3n~7~izljb-h~QqvZ-7lyy~UU2gB3E1O1pXokgEy4mvlnJhLniXXqEVgewSJMHwAdoA2SDtgkwYSD2ybil3WawfBSHid7IsRw20iQZHQJZ~1Ne4kFd9qkL0dI2-Dw5ied3dJE3OpM9tHRni~jir9HsqSyaCvLXii-BrCxZrepzZipiXODTCnB5vGaovM46BUg1GTTidxtMr21NvASnLuqrYbgobD8uXNbjrqdYKGgWJmVKwbM3ruaHN14W5jn7xSA-IrrTEO30s6OJd-UlR6CT6Bg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)