Abstract
Phosphatidylinositol-3-kinase p110δ serves as a central integration point for signaling from cell surface receptors known to promote malignant B-cell proliferation and survival. This provides a rationale for the development of small molecule inhibitors that selectively target p110δ as a treatment approach for patients with B-cell malignancies. We thus identified 5-fluoro-3-phenyl-2-[(S)-1-(9H-purin-6-ylamino)-propyl]-3H-quinazolin-4-one (CAL-101), a highly selective and potent p110δ small molecule inhibitor (half-maximal effective concentration [EC50] = 8nM). Using tumor cell lines and primary patient samples representing multiple B-cell malignancies, we have demonstrated that constitutive phosphatidylinositol-3-kinase pathway activation is p110δ-dependent. CAL-101 blocked constitutive phosphatidylinositol-3-kinase signaling, resulting in decreased phosphorylation of Akt and other downstream effectors, an increase in poly(ADP-ribose) polymerase and caspase cleavage and an induction of apoptosis. These effects have been observed across a broad range of immature and mature B-cell malignancies, thereby providing a rationale for the ongoing clinical evaluation of CAL-101.
Introduction
Dysregulation of the phosphatidylinositol-3-kinase (PI3K) pathway plays an important role in the etiology of human malignancies, including those of hematologic origin.1,2 In B-cell malignancies, aberrant PI3K signaling may be the result of constitutive B-cell receptor (BCR) activation and/or the response to proliferation and survival factors present in bone marrow and lymph node microenvironments.3-5 Activation of the PI3K pathway by cell surface receptors is directly mediated by class I isoforms p110α, p110β, p110δ, and p110γ. The p110δ isoform is highly expressed in cells of hematopoietic origin, being predominantly detected in leukocytes.6 Genetic and pharmacologic approaches that specifically inactivate the p110δ isoform have demonstrated its important role in B-cell signaling.7-9 We identified 5-fluoro-3-phenyl-2-[(S)-1-(9H-purin-6-ylamino)-propyl]-3H-quinazolin-4-one (CAL-101), a potent and selective inhibitor of p110δ, in a kinome-wide screen using purified enzymes and in cell-based PI3K isoform-specific assays. To investigate the therapeutic potential of p110δ inhibition by CAL-101 in B-cell tumors, we used malignant B-cell tumor lines and primary tumor cells.
Methods
Cell culture conditions
Cell lines were cultured in RPMI 1640 medium (ATCC) supplemented with 10% fetal bovine serum, 100 U/L penicillin-streptomycin, and incubated at 37°C/5% CO2. Blood samples were obtained after written informed consent by the Declaration of Helsinki according to local institutionally approved protocols by the Calistoga Pharmaceuticals Institutional Review Board. Mononuclear cells were isolated from peripheral blood or bone marrow by density centrifugation over Ficoll. Cells were analyzed on a FACSAria flow cytometer (BD Biosciences) using lineage- and blast-specific cell-surface markers, confirming that neoplastic cells accounted for more than 90% of the total cell population in all patient samples analyzed.
In vitro kinase profiling
All biochemical in vitro protein kinase assays were analyzed using the SelectScreen kinase inhibitor assay service (Invitrogen).
Western blot and ELISA analysis
Whole-cell lysates were analyzed on 10% polyacrylamide gels. Transfer to nitrocellulose, blocking, probing with antibodies, and chemiluminescence were performed as previously described.10 Total Akt1, total S6, phospho-Akt1 (Ser473), phospho-S6 (Ser 235/236), cleaved caspase-3, and cleaved poly(ADP-ribose) polymerase were measured using the PathScan Sandwich ELISA kit (Cell Signaling Technology) following the manufacturer's protocol.
Cell viability assays
Cell viability was assessed using Cell Titer Aqueous One Solution Cell Proliferation Assay reagent (Promega) following the manufacturer's protocol. Fluorescence was measured with the Spectramax M5 plate reader (Molecular Devices).
Flow cytometry
Apoptosis was measured by annexin V–fluorescein isothiocyanate (FITC)/7-amino-actinomycin D (7-AAD) labeling followed by fluorescence flow cytometry as previously described.10 Intracellular flow cytometric cells were labeled with either anti–CD19-FITC or anti–CD5-FITC as well as anti–phospho-Akt T308 (Alexa Fluor 488), anti–phospho-Akt Ser473 (Alexa Fluor 488), or an isotype-matched control antibody (mouse IgG1 Alexa Fluor 488 conjugate). Cells were assayed by flow cytometry using the Cytomics FC 500MPL cytometer, and data were collected and analyzed using CXP Version 2.2 software (Beckman Coulter).
Results and discussion
CAL-101 is a potent selective inhibitor of PI3Kδ
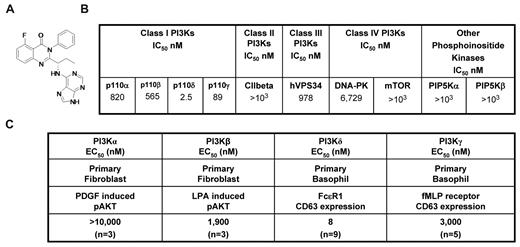
CAL-101 (Figure 1A) is an oral p110δ inhibitor currently under clinical evaluation in patients with B-cell malignancies. CAL-101 was 40- to 300-fold more selective for p110δ relative to other PI3K class I enzymes (IC50 p110δ = 2.5nM; p110α, p110β, and p110γ IC50 were 820, 565, and 89nM, respectively; Figure 1B). Greater selectivity (400- to 4000-fold) was seen against related kinases C2β, hVPS34, DNA-PK, and mTOR (Figure 1B), whereas no activity was observed against a panel of 402 diverse kinases at 10μM (Ambit KinomeScan, data not shown).
CAL-101 inhibits PI3Kδ with high selectivity. (A) Chemical structure of CAL-101. (B) CAL-101 in vitro activity profiles (half-maximal inhibitory concentration values) against recombinant enzymes of class I, II, III, and IV PI3Ks. CAL-101 was diluted in dimethyl sulfoxide at a concentration of 10mM, and 10-point kinase inhibitory activities were measured over a concentration range (5.0-104nM) with adenosine triphosphate at a concentration consistent with each enzymes Kms. (C) Potency of CAL-101 in PI3K class I isoform-specific cell-based assays. For the analysis of p110α and p110β signaling, murine embryo fibroblasts were stimulated with PDGF or LPA and soluble protein was analyzed by Western blotting for Akt and pAkt473 levels. For the analysis of p110δ and p110γ signaling, basophil activation was measured in isolated peripheral blood mononuclear cell or whole blood using the Flow2 CAST kit according to the manufacturer's standardized methods (Buhlman Laboratories AG). p110δ was activated with anti-FCϵRI, and p110γ was activated with formyl-methionyl-leucyl-phenylalanine. To monitor the basophil cell population and cellular activation, anti–CD63-FITC and anti–CCR3-phycoerythrin antibodies were added to each sample. Cells were fixed and analyzed on a FC500MPL flow cytometer (Beckman Coulter).
CAL-101 inhibits PI3Kδ with high selectivity. (A) Chemical structure of CAL-101. (B) CAL-101 in vitro activity profiles (half-maximal inhibitory concentration values) against recombinant enzymes of class I, II, III, and IV PI3Ks. CAL-101 was diluted in dimethyl sulfoxide at a concentration of 10mM, and 10-point kinase inhibitory activities were measured over a concentration range (5.0-104nM) with adenosine triphosphate at a concentration consistent with each enzymes Kms. (C) Potency of CAL-101 in PI3K class I isoform-specific cell-based assays. For the analysis of p110α and p110β signaling, murine embryo fibroblasts were stimulated with PDGF or LPA and soluble protein was analyzed by Western blotting for Akt and pAkt473 levels. For the analysis of p110δ and p110γ signaling, basophil activation was measured in isolated peripheral blood mononuclear cell or whole blood using the Flow2 CAST kit according to the manufacturer's standardized methods (Buhlman Laboratories AG). p110δ was activated with anti-FCϵRI, and p110γ was activated with formyl-methionyl-leucyl-phenylalanine. To monitor the basophil cell population and cellular activation, anti–CD63-FITC and anti–CCR3-phycoerythrin antibodies were added to each sample. Cells were fixed and analyzed on a FC500MPL flow cytometer (Beckman Coulter).
In fibroblasts, the receptor tyrosine kinase platelet-derived growth factor receptor signals through p110α and the G-protein–coupled receptor for lysophosphatidic acid (LPA) signals through p110β.11 We stimulated murine embryonic fibroblasts with PDGF or LPA and monitored phosphorylation of Akt to measure pathway activation. CAL-101 reduced PDGF-induced pAkt by only 25% at 10μM, whereas the positive control, PI-103,12 had a half-maximal effective concentration (EC50) of 90nM (Figure 1C; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CAL-101 inhibited LPA-induced pAkt with an EC50 of 1.9μM (Figure 1C; supplemental Figure 1B). Expression of p110δ and p110γ is normally restricted to leukocytes. In basophils, FcϵRI signals through p110δ, whereas formyl-methionyl-leucyl-phenylalanine (fMLP) signals through G-protein–coupled receptor-p110γ,13,14 and activation through either stimulus results in surface expression of CD63 that can be monitored by flow cytometry. CAL-101 blocked FcϵRI p110δ-mediated CD63 expression with an EC50 of 8nM, whereas formyl-methionyl-leucyl-phenylalanine activation of p110γ was inhibited with an EC50 of 3.0μM (Figure 1C; supplemental Figure 1C). Thus, in cell-based assays, CAL-101 had 240- to 2500-fold selectivity for p110δ over the other class I PI3K isoforms.
Sensitivity of primary cells from patients with lymphoid and myeloid leukemias to CAL-101
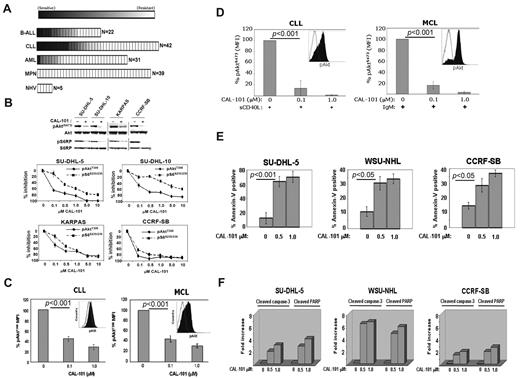
Primary leukemia cells from patients with a variety of malignancies were tested for sensitivity to CAL-101 in vitro. In total, 134 leukemia patient and 5 control peripheral blood mononuclear cells samples were tested. We observed significant sensitivity (defined as an EC50 < 1μM) to CAL-101 in 23% of B-cell acute lymphoblastic leukemia (B-ALL) samples (5 of 22), 26% of chronic lymphocytic leukemia (CLL) samples (11 of 42), 3% of acute myeloid leukemia (AML) samples (1 of 31), and 0% of myeloproliferative neoplasm (MPN) samples (0 of 39). Normal peripheral blood mononuclear cells showed no sensitivity to CAL-101 (Figure 2A). Thus, CAL-101 exhibited far greater activity in B-ALL and CLL cells compared with acute myeloid leukemia and MPN cells, suggesting a greater therapeutic potential for patients with B-cell malignancies.
CAL-101 inhibits PI3K signaling and cellular viability. (A) CAL-101 screening of primary cells from patients with ALL, CLL, acute myeloid leukemia (AML), or myeloproliferative neoplasm (MPN) or from normal healthy volunteers (NHV). Cell viability was determined using an 3-carboxymethoxyphenyl)-2-(4-sulphenyl)-2H-tetrazolium assay, and the sensitivity of each sample relative to untreated cells was calculated for estimation of the EC50. The heat map readout shown was generated using GenePattern Version 3.2 software (Broad Institute) and indicates the percentage EC50 for each sample relative to the maximum drug concentration tested (10μM). (B) CAL-101 inhibition of p110δ blocks PI3K signaling in malignant B-cell lines and primary patient tumor cells. Serum-starved cells were incubated with 1μM CAL-101, and total cell lysates were subjected to Western blot analysis using anti–phospho-AktS473, anti-Akt, anti-phospho S6S235/236, and anti-S6 antibodies (supplemental data). Starved cells were incubated with vehicle or serial dilution of CAL-101 for 1 hour, and pAktT308, pS6S235/236, total Akt, and total S6 were detected by PathScan sandwich ELISA (supplemental data). (C-D) CLL (n = 5) and MCL (n = 5) patient whole blood samples were subjected to Ficoll-Hypaque separation. Isolated cells were incubated in RPMI with vehicle or serial dilutions of CAL-101 before fixation and staining with anti–phospho-AktT308 Alexa Fluor 488 or isotype-matched Alexa Fluor 488 antibody. Cells pretreated with CAL-101 or vehicle for 1 hour and then stimulated with 10 μg/mL anti-IgM (BCR activation), or 50 ng/mL sCD40L (CD40 stimulation) for 10 minutes before fixation and staining with anti–phospho-AktS473 Alexa Fluor 488 or isotype-matched Alexa Fluor 488 antibody. FITC-CD5+ cells were gated and analyzed by 2-color flow cytometry to quantify intracellular p-AktT308 levels using the Beckman Coulter Cytomics FC 500MPL using CXP Version 2.2 software. Bar graphs represent the percentage difference in mean fluorescence intensity values between isotype-matched control Ig and phospho-AktT308. (E) CAL-101 induces apoptosis in diffuse large B-cell lymphoma, follicular lymphoma, and B-ALL cell lines. Cells were treated with vehicle or 0.5μM or 1.0μM CAL-101 for 24 hours. The percentage of apoptotic cells was determined by annexin V–FITC/7-AAD staining followed by 2-color flow cytometric analysis. Percentages represent both annexin V–FITC/7-AAD negative and annexin V–FITC/7-AAD double-positive. (F) Cells were cultured in RPMI/10% fetal bovine serum with CAL-101 or vehicle alone for 24 hours, and cells were lysed and analyzed by PathScan Sandwich 96-well ELISA for the detection of cleaved caspase-3 and cleaved poly(ADP-ribose) polymerase as indicated (supplemental data). Results are expressed as mean ± SD. Statistically significant differences between means were determined using a one-way analysis of variance. Data are expressed as the fold change and are representative of 3 separate experiments.
CAL-101 inhibits PI3K signaling and cellular viability. (A) CAL-101 screening of primary cells from patients with ALL, CLL, acute myeloid leukemia (AML), or myeloproliferative neoplasm (MPN) or from normal healthy volunteers (NHV). Cell viability was determined using an 3-carboxymethoxyphenyl)-2-(4-sulphenyl)-2H-tetrazolium assay, and the sensitivity of each sample relative to untreated cells was calculated for estimation of the EC50. The heat map readout shown was generated using GenePattern Version 3.2 software (Broad Institute) and indicates the percentage EC50 for each sample relative to the maximum drug concentration tested (10μM). (B) CAL-101 inhibition of p110δ blocks PI3K signaling in malignant B-cell lines and primary patient tumor cells. Serum-starved cells were incubated with 1μM CAL-101, and total cell lysates were subjected to Western blot analysis using anti–phospho-AktS473, anti-Akt, anti-phospho S6S235/236, and anti-S6 antibodies (supplemental data). Starved cells were incubated with vehicle or serial dilution of CAL-101 for 1 hour, and pAktT308, pS6S235/236, total Akt, and total S6 were detected by PathScan sandwich ELISA (supplemental data). (C-D) CLL (n = 5) and MCL (n = 5) patient whole blood samples were subjected to Ficoll-Hypaque separation. Isolated cells were incubated in RPMI with vehicle or serial dilutions of CAL-101 before fixation and staining with anti–phospho-AktT308 Alexa Fluor 488 or isotype-matched Alexa Fluor 488 antibody. Cells pretreated with CAL-101 or vehicle for 1 hour and then stimulated with 10 μg/mL anti-IgM (BCR activation), or 50 ng/mL sCD40L (CD40 stimulation) for 10 minutes before fixation and staining with anti–phospho-AktS473 Alexa Fluor 488 or isotype-matched Alexa Fluor 488 antibody. FITC-CD5+ cells were gated and analyzed by 2-color flow cytometry to quantify intracellular p-AktT308 levels using the Beckman Coulter Cytomics FC 500MPL using CXP Version 2.2 software. Bar graphs represent the percentage difference in mean fluorescence intensity values between isotype-matched control Ig and phospho-AktT308. (E) CAL-101 induces apoptosis in diffuse large B-cell lymphoma, follicular lymphoma, and B-ALL cell lines. Cells were treated with vehicle or 0.5μM or 1.0μM CAL-101 for 24 hours. The percentage of apoptotic cells was determined by annexin V–FITC/7-AAD staining followed by 2-color flow cytometric analysis. Percentages represent both annexin V–FITC/7-AAD negative and annexin V–FITC/7-AAD double-positive. (F) Cells were cultured in RPMI/10% fetal bovine serum with CAL-101 or vehicle alone for 24 hours, and cells were lysed and analyzed by PathScan Sandwich 96-well ELISA for the detection of cleaved caspase-3 and cleaved poly(ADP-ribose) polymerase as indicated (supplemental data). Results are expressed as mean ± SD. Statistically significant differences between means were determined using a one-way analysis of variance. Data are expressed as the fold change and are representative of 3 separate experiments.
CAL-101 inhibits constitutive PI3K signaling and induces apoptosis
Diffuse large B-cell lymphoma (SU-DHL-5), follicular lymphoma (KARPAS-422), and B-ALL (CCRF-SB) cell lines showed pAktS473 expression in an initial assay. In these cell lines, CAL-101 produced a concentration-dependent reduction in pAktS473, pAktT308, and the downstream target S6 with an EC50 of 0.1 to 1.0μM, demonstrating a central role for p110δ in constitutive PI3K signaling (Figure 2B). Patient-derived malignant B cells (5 of 5 CLL and 5 of 5 mantle cell lymphoma [MCL] samples) displayed constitutive levels of pAktT308, which was significantly reduced by CAL-101 (EC50 < 100nM; Figure 2C). In these cells, phosphorylation of Akt was low or undetectable at S473. Because Akt phosphorylation at both sites is required for full kinase activity, we investigated whether tumor microenvironment signals could cause further p110δ-dependent PI3K pathway activation through S473 phosphorylation. Treatment of patient CLL or MCL cells with sCD40L or BCR crosslinking caused rapid induction of pAktS473 that was completely inhibited by CAL-101 at 0.1 to 1.0μM (Figure 2D). Comparable observations were made using MCL cell lines (supplemental Figure 2). Similarly, knockdown of p110δ in CLL cells by siRNA resulted in inhibition of CD40-induced Akt phosphorylation and a decrease in the prosurvival factor Mcl-1 (supplemental Figure 3), supporting the role of the p110δ isoform.
The functional role of p110δ was evaluated by measuring apoptosis in SU-DHL-5, WSU-NHL (follicular lymphoma), and CCRF-SB tumor cell lines. CAL-101 treatment resulted in a 3- to 5-fold increase in annexin V staining, indicating a significant level of apoptosis induction (Figure 2E). We also observed a dose-dependent 2- to 8-fold increase in both caspase 3 and poly(ADP-ribose) polymerase cleavage at 24 hours with CAL-101 treatment compared with vehicle controls (Figure 2F).
We have thus described the biochemical and cellular activity of CAL-101, a selective and potent inhibitor of p110δ; such targeted inhibition has the potential to avoid adverse effects that may result from nonselective PI3K inhibition. Further, we have demonstrated an essential role for PI3K p110δ in constitutive PI3K signaling that is required for the survival of malignant B cells. Oncogenic mutations in components of the PI3K pathway are infrequent in B-cell malignancies, and much less is known about the importance of constitutive PI3K signaling in these tumors. A potential mechanism for PI3K pathway activation in this setting is tonic antigen-independent BCR signaling that requires p110δ for the transduction of proliferation and survival signals.8 In this regard, CAL-101 blocks constitutive oncogenic signaling, resulting in apoptosis, and inhibits survival signals provided by the microenvironment. Our studies have identified a novel targeted approach for the treatment of patients with B-cell malignancies and provided the rational for ongoing clinical studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jerry Evarts for supplying CAL-101, Heather Webb for helpful discussion, Mike Gallatin for advice, Albert Yu for critical reading of the manuscript, and the clinical investigators as part of our CAL-101 phase I study who provided CLL cells for analysis in this study.
Authorship
Contribution: B.J.L. planned the research, performed experiments, analyzed data, drafted the first and subsequent drafts of the paper, and approved the final version of the paper; S.A.M., A.K., and B.S. were involved in planning components of the research, performing experiments, and approved the final version of the paper; S.E.H.H. performed experiments; A.J.J. provided primary cells and reviewed drafts; J.C.B. was involved in planning components of the research, reviewed drafts, and approved the final version of the paper; J.W.T. and M.M.L. obtained patient samples, performed experiments, reviewed drafts of the paper, and approved the final version of the paper; M.D. and B.J.D. were involved in planning components of the research and approved the final version of the paper; K.D.P. was involved in planning components of the research; R.G.U. and N.A.G. planned research, supervised the research, analyzed data, reviewed drafts, and approved the final version of the paper.
Conflict-of-interest disclosure: B.J.L., S.A.M., A.K., B.S., K.D.P., R.G.U., and N.A.G. are employees of Calistoga Pharmaceuticals. B.J.D. is a consultant for Calistoga Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Brian J. Lannutti, Calistoga Pharmaceuticals, Inc, 2101 4th Ave, Ste 1960, Seattle, WA 98121; e-mail: blannutti@calistogapharma.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal