Abstract
FOXN1 deficiency is a primary immunodeficiency characterized by athymia, alopecia totalis, and nail dystrophy. Two infants with FOXN1 deficiency were transplanted with cultured postnatal thymus tissue. Subject 1 presented with disseminated Bacillus Calmette-Guérin infection and oligoclonal T cells with no naive markers. Subject 2 had respiratory failure, human herpes virus 6 infection, cytopenias, and no circulating T cells. The subjects were given thymus transplants at 14 and 9 months of life, respectively. Subject 1 received immunosuppression before and for 10 months after transplantation. With follow up of 4.9 and 2.9 years, subjects 1 and 2 are well without infectious complications. The pretransplantation mycobacterial disease in subject 1 and cytopenias in subject 2 resolved. Subject 2 developed autoimmune thyroid disease 1.6 years after transplantation. Both subjects developed functional immunity. Subjects 1 and 2 have 1053/mm3 and 1232/mm3 CD3+ cells, 647/mm3 and 868/mm3 CD4+ T cells, 213/mm3 and 425/mm3 naive CD4+ T cells, and 10 200 and 5700 T-cell receptor rearrangement excision circles per 100 000 CD3+ cells, respectively. They have normal CD4 T-cell receptor β variable repertoires. Both subjects developed antigen-specific proliferative responses and have discon-tinued immunoglobulin replacement. In summary, thymus transplantation led to T-cell reconstitution and function in these FOXN1 deficient infants.
Introduction
The nude/severe combined immunodeficiency (SCID) phenotype due to deficiency of the transcription factor FOXN1 was first described in mice by Flanagan in 1966, who noted an absence of hair, poor growth, early mortality, and susceptibility to infection.1 Subsequent investigations revealed that the immunodeficiency resulted from athymia caused by mutations in the WHN gene, since renamed FOXN1.2-4 The first human cases of FOXN1 deficiency were reported by Pignata in 2 children with athymia, reduced T-cell numbers, absence of hair, and nail dysplasia.5 One child died and the other received bone marrow transplantation without reconstitution of the naive T-cell pool.6 In the same community, 4 other children with alopecia had died early in life from severe infections, which suggested that they, too, had the same FOXN1 mutation.7
Here we report 2 unrelated infants who presented with congenital athymia due to the human nude/SCID phenotype resulting from mutations in FOXN1. We treated the 2 infants with FOXN1 deficiency with thymus transplantation, taking advantage of previous experience using this therapy to achieve immunoreconstitution in infants with athymia secondary to complete DiGeorge anomaly.8 In this report, we describe the presentation of the research subjects and their clinical and immune outcomes.
Methods
Research subjects
Both subjects were enrolled in protocols that were approved by the Duke University Health System Institutional Review Board and were reviewed by the Food and Drug Administration (FDA) under an Investigational New Drug application.8 The parents of each subject provided written informed consent in accordance with the Declaration of Helsinki. The clinical trial registration numbers are NCT00579709 “Thymus Transplantation with Immunosuppression” for subject 1 and NCT00576407 “Thymus Transplantation in DiGeorge Syndrome” for subject 2.
Thymus transplantation
Unrelated allogeneic thymus tissue, routinely discarded during cardiac surgery, was collected from infants less than 9 months of age. The tissue was used for transplantation after informed consent was obtained under protocols approved by the Duke Institutional Review Board and reviewed by the FDA. Detailed descriptions of the procedure are published.8-10
Immune testing
Standard flow cytometry and proliferation assays were performed as previously described. In brief, antibodies for flow cytometry included CD3, CD4, CD8, CD14, CD16, CD19, CD45, CD45RA, CD56, and CD62L (all from BD Biosciences). The T-cell receptor β variable (TCRBV) analysis by flow cytometry used the β Mark TCR Vβ repertoire kit (#IM3497; Immunotech, Beckman Coulter). The proliferative response to phytohemagglutinin (PHA) was performed in triplicate using 100 000 cells per well with 3 concentrations of mitogen. Tritiated thymidine incorporation was measured on days 3 and 4. Cultures with purified protein derivative (PPD, 5 μg/mL; Statens Serum Institut), Candida albicans (40 μg/mL; Greer Laboratories), varicella zoster virus (VZV; 1 μg/mL; Virusys Corp), and tetanus toxoid (20 μg/mL; Virusys Corp) were performed in quadruplicate and then pulse-labeled and harvested on day 6.
Spectratyping was performed using RNA isolated from cells separated magnetically with CD3, CD4, or CD8 microbeads (Miltenyi Biotec). After capillary gel electrophoresis, the data (Gene Scan Software; Applied Biosystems) were uploaded onto a web accessible analysis program, SpA.11-13 The result was reported as the Kullback-Leibler divergence (DKL) score. High scores reflect oligoclonal repertoires (highly divergent from normal), whereas low scores reflect polyclonal repertoires.13
Signal joint (sj) T-cell receptor rearrangement excision circle (TREC) analyses were performed as described.11
To evaluate for maternal engraftment, DNA was obtained from isolated circulating T cells in the subject, from maternal peripheral blood, and from the subject's own buccal swab. The hospital laboratory compared the samples using multiplex polymerase chain reaction amplification for 8 microsatellite markers followed by electrophoretic separation of each sample. The limit of detection was 2%.
Results
Subject 1
Subject 1 was born at term to Portuguese parents who were distant cousins. The female infant had nail dystrophy and no hair. Genetic analysis revealed a homozygous nonsense mutation at residue 255 (R255X) in exon 4 (formerly exon 5) in FOXN1.
Slowly progressive Bacillus Calmette-Guérin (BCG) adenitis and mild erythroderma had been apparent at 3 months of life. At day 157 of life, the subject was admitted with respiratory failure and noted to have marked posterior cervical adenitis and inguinal adenitis. Gastric and bronchoalveolar lavage secretions grew Mycobacterium bovis resistant to isoniazid; treatment included streptomycin, rifampicin, ethambutol, itraconazole, trimethoprim/sulfamethoxazole, and intravenous immunoglobulin (Ig).
The initial immune evaluations were performed when the subject presented with respiratory failure and the subsequent pretransplantation evaluations are included in Table 1. Although circulating T cells were present, naive T cells were profoundly low. The striking feature of the immune profiles in the first year of life was the expansion of double negative (CD4−CD8−) T cells. The T-cell receptor (TCR) γδ population composed approximately one-third of the double-negative population (unpublished data, M.L.M. and A.E.S., June 2010); the remainder of the double-negative T cells were thus TCRαβ-positive.
Presenting immunophenotypes before transplantation
| Subject/day of life (day before transplant) . | CD3+/mm3 . | CD4+/mm3 . | CD8+/mm3 . | CD3+ CD4−CD8−/mm3 . | Naive CD4+, %* . | CD19/mm3 . | CD16+ and/or CD56+, CD3−/mm3 . | PHA (bkg), cpm† . | Eosinophils/mm3 . |
|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | |||||||||
| 166 (−258) | 2219 | 660 | 612 | 678 | < 1 | 3940 | 51 | 53 | |
| 215 (−209) | 6250 | 1950 | 1737 | 1507 | < 1 | 1507 | 886 | 4189 (654) | 4155 |
| 254 (−170) | 2296 | 745 | 576 | 749 | < 1 | 1307 | 487 | 2582 (584) | 2436 |
| 297 (−127) | 1463 | 446 | 246 | 752 | < 1 | 1946 | 1667 | 18 912 (103) | 1613 |
| 408 (−16) | 964 | 404 | 146 | 411 | < 2 | 3632 | 582 | 63 733 (413) | 468 |
| 418 (−6) | 1010 | 473 | 155 | 381 | < 1 | 2734 | 409 | 104 246 (154) | 510 |
| Subject 2 | |||||||||
| 140 (−126) | 2‡ | 315 | 273 | ||||||
| 252 (−14) | 1‡ | 638 | 167 | 36 | |||||
| 255 (−11) | 635 (205) | 76 | |||||||
| Subject/day of life (day before transplant) . | CD3+/mm3 . | CD4+/mm3 . | CD8+/mm3 . | CD3+ CD4−CD8−/mm3 . | Naive CD4+, %* . | CD19/mm3 . | CD16+ and/or CD56+, CD3−/mm3 . | PHA (bkg), cpm† . | Eosinophils/mm3 . |
|---|---|---|---|---|---|---|---|---|---|
| Subject 1 | |||||||||
| 166 (−258) | 2219 | 660 | 612 | 678 | < 1 | 3940 | 51 | 53 | |
| 215 (−209) | 6250 | 1950 | 1737 | 1507 | < 1 | 1507 | 886 | 4189 (654) | 4155 |
| 254 (−170) | 2296 | 745 | 576 | 749 | < 1 | 1307 | 487 | 2582 (584) | 2436 |
| 297 (−127) | 1463 | 446 | 246 | 752 | < 1 | 1946 | 1667 | 18 912 (103) | 1613 |
| 408 (−16) | 964 | 404 | 146 | 411 | < 2 | 3632 | 582 | 63 733 (413) | 468 |
| 418 (−6) | 1010 | 473 | 155 | 381 | < 1 | 2734 | 409 | 104 246 (154) | 510 |
| Subject 2 | |||||||||
| 140 (−126) | 2‡ | 315 | 273 | ||||||
| 252 (−14) | 1‡ | 638 | 167 | 36 | |||||
| 255 (−11) | 635 (205) | 76 | |||||||
bkg indicates medium-plus-cells background.
For subject 1, naive T cells were defined by the phenotype CD4+CD45RA+CD27+ for the first 3 values done in the referring center and by the phenotype CD4+CD45RA+CD62L+ in the transplant center. Naive CD8 cells (unpublished) were similarly very low.
The first 2 PHA assays for subject 1 were performed in the referring center; the lower limit of normal is 20 000 counts per minute (cpm). The remaining PHA assays in this table were performed at the transplantation center laboratory in which the lower limit of normal is 75 000 cpm.
The T-cell numbers were too low for accurate determination of the naive percentages.
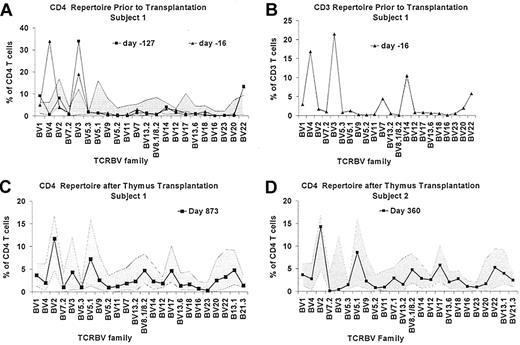
The TCRBV repertoire was evaluated before thymus transplantation by flow cytometry and spectratyping. The CD4 TCRBV repertoire assessment of subject 1 by flow cytometry on day 297 of life (127 days before thymus transplantation) showed expansions of T cells expressing BV3 and BV22 (Figure 1A); the spectratyping analysis of CD4 RNA was markedly oligoclonal (Figure 2A).13 Additional flow cytometry evaluations of TCRBV repertoire are shown in Figure 1A and B. Lastly, sjTREC analysis on day 409 of life (15 days before transplantation) revealed < 100 TRECs per 100 000 CD3+ T cells. Overall, these data were consistent with a lack of thymic function in subject 1.
T cells are oligoclonal in subject 1 before transplantation but are polyclonal after transplantation in both subjects by flow cytometry. TCR diversity for CD4 (A) and CD3 (B) T cells was assessed for subject 1 before transplantation. (C) The latest diversity assessment in subject 1 is shown; (D) the latest assessment in subject 2 is shown. Note that the y-axis in the CD4 in panel A differs from the y-axis in CD4 panels C-D. (A,C-D) The shaded area represents the normal range ± 3 SD based on data from 19 healthy adults.
T cells are oligoclonal in subject 1 before transplantation but are polyclonal after transplantation in both subjects by flow cytometry. TCR diversity for CD4 (A) and CD3 (B) T cells was assessed for subject 1 before transplantation. (C) The latest diversity assessment in subject 1 is shown; (D) the latest assessment in subject 2 is shown. Note that the y-axis in the CD4 in panel A differs from the y-axis in CD4 panels C-D. (A,C-D) The shaded area represents the normal range ± 3 SD based on data from 19 healthy adults.
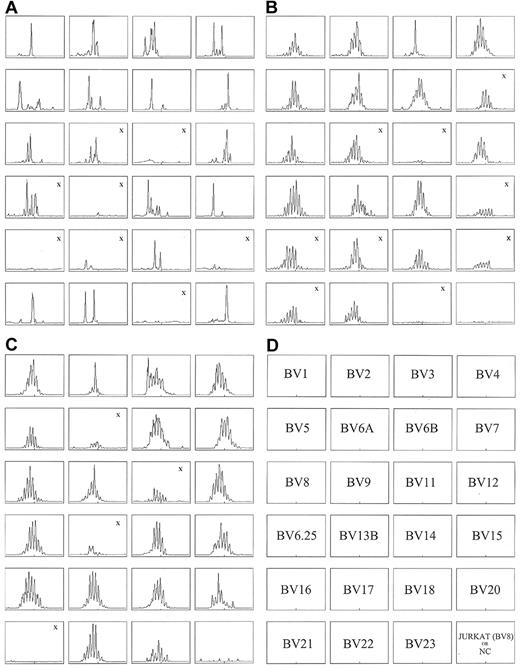
CD4 RNA spectratyping shows oligoclonality in subject 1 before transplantation and polyclonality in both subjects after transplantation. Subject 1 at (A) day −127 and (B) day 873 after transplantation; subject 2 at (C) day 368 after transplantation. The DKL score for subject 1 pretransplantation (A) is 1.38 compared with the DKL score of 0.19 after transplantation (B). For subject 2, the posttransplantation DKL was 0.08 (C). Lower DKL scores reflect greater diversity in the TCR repertoire. The “X” indicates panels with insufficient RNA concentrations. These panels were not included in the calculation of the DKL score. NC indicates negative control.
CD4 RNA spectratyping shows oligoclonality in subject 1 before transplantation and polyclonality in both subjects after transplantation. Subject 1 at (A) day −127 and (B) day 873 after transplantation; subject 2 at (C) day 368 after transplantation. The DKL score for subject 1 pretransplantation (A) is 1.38 compared with the DKL score of 0.19 after transplantation (B). For subject 2, the posttransplantation DKL was 0.08 (C). Lower DKL scores reflect greater diversity in the TCR repertoire. The “X” indicates panels with insufficient RNA concentrations. These panels were not included in the calculation of the DKL score. NC indicates negative control.
The T-cell proliferative responses to PHA were initially low but increased unexpectedly to more than 100 000 counts per minute before transplantation (Table 1). Evaluation for possible maternal engraftment was performed at the time of diagnosis and 9 days before transplantation. No evidence of circulating maternal T cells was found.
Beginning before transplantation, the subject was treated with cyclosporine, steroids, and rabbit anti-thymocyte globulin as described previously.8 The immunosuppression was initiated because of the increased T-cell proliferation in response to PHA, the large numbers of oligoclonal double-negative T cells, and the increased levels of T-cell activation markers (unpublished data, M.L.M., June 2010). One dose of daclizumab, 1 mg/kg, was also given shortly before transplantation. The female O+ blood type subject was transplanted with cultured thymus tissue from an unrelated female A− blood type infant who was < 1 month of age. The human leukocyte antigen (HLA) types for the subject and thymus donor are shown in Table 2.
HLA typing of subjects and thymus donors
| . | HLA-A . | HLA-B . | HLA-C . | HLA-DRB1 . | HLA-DQB1 . |
|---|---|---|---|---|---|
| Subject 1 | 2601, 3101 | 3503, 3801 | 1203 | 0701, 1201 | 0202, 0301* |
| Thymus 1 | 0101, 2301 | 0801, 4901 | 0701 | 1101, 1301 | 0301*, 0603 |
| Subject 2 | 2402, 2501 | 1501, 4402 | 0303, 0501 | 0404, 1301 | 0302, 0603 |
| Thymus 2 | 0101, 0201 | 0801, 4002 | 0202, 0701 | 0301, 0408 | 0201, 0301 |
| . | HLA-A . | HLA-B . | HLA-C . | HLA-DRB1 . | HLA-DQB1 . |
|---|---|---|---|---|---|
| Subject 1 | 2601, 3101 | 3503, 3801 | 1203 | 0701, 1201 | 0202, 0301* |
| Thymus 1 | 0101, 2301 | 0801, 4901 | 0701 | 1101, 1301 | 0301*, 0603 |
| Subject 2 | 2402, 2501 | 1501, 4402 | 0303, 0501 | 0404, 1301 | 0302, 0603 |
| Thymus 2 | 0101, 0201 | 0801, 4002 | 0202, 0701 | 0301, 0408 | 0201, 0301 |
Specificities shared between the recipient and the thymus donor.
Clinical course after transplantation
Subject 1 was weaned off steroids and cyclosporine by 10 months after transplantation. Pneumocystis pneumonia prophylaxis and Ig replacement were stopped at 33 months after transplantation.
The subject had several serious infections in addition to M bovis. A severe rotavirus infection was present from the time of admission for transplantation until discharge. After transplantation, she developed a Klebsiella pneumoniae urine infection on day 19; pneumocystis pneumonia requiring oxygen therapy on day 53 (despite pentamidine prophylaxis); a central venous catheter infection with blood cultures positive for K oxytoca and Entero-coccus faecalis at 6 months; and varicella at 8 months that was treated with intravenous acyclovir with an uneventful course. By 18 months after transplantation, adenopathy from BCG had resolved. The antimycobacterial medications were stopped 33 months after transplantation.
Immune results after thymus transplantation
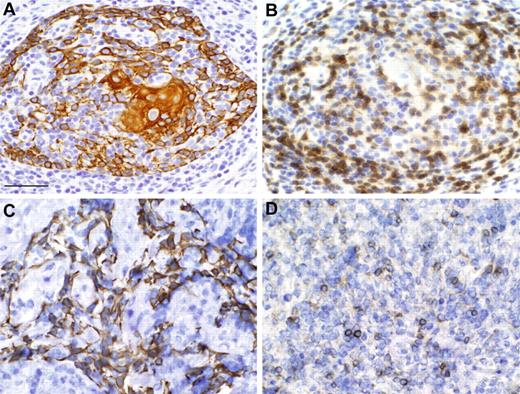
A biopsy of the allograft was performed on day 99 after thymus transplantation. Figure 3A-B shows evidence of thymopoiesis with a Hassall body and medullary thymocytes.
Biopsy evaluation of thymus allografts shows thymopoiesis. Subject 1 (A-B) and subject 2 (C-D). Cytokeratin reactivity (A,C) and CD3 reactivity (B,D). Scale bar, 50 μm. The microscope was an Olympus VANOX AHBS3. The magnification was 40× using a 40× numerical aperture objective lens (Olympus SPLAN 40×). The photomicrograph was taken at room temperature. Neither imaging media nor fluorochromes were used. The camera used was an Olympus DP70 digital imaging camera. Acquisition software was Olympus DP Controller. Subsequently, Adobe Photoshop 6.0 was used to compose this figure.
Biopsy evaluation of thymus allografts shows thymopoiesis. Subject 1 (A-B) and subject 2 (C-D). Cytokeratin reactivity (A,C) and CD3 reactivity (B,D). Scale bar, 50 μm. The microscope was an Olympus VANOX AHBS3. The magnification was 40× using a 40× numerical aperture objective lens (Olympus SPLAN 40×). The photomicrograph was taken at room temperature. Neither imaging media nor fluorochromes were used. The camera used was an Olympus DP70 digital imaging camera. Acquisition software was Olympus DP Controller. Subsequently, Adobe Photoshop 6.0 was used to compose this figure.
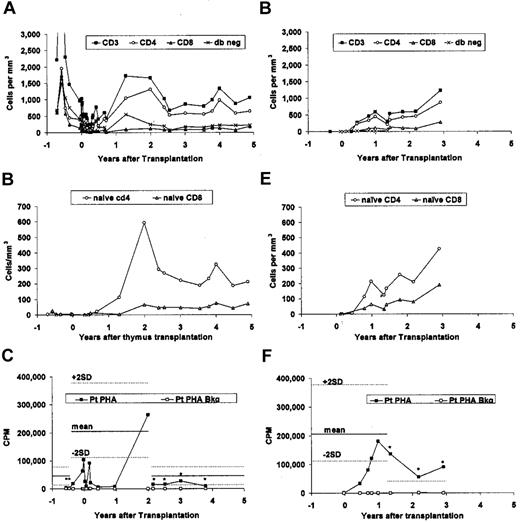
Subject 1 had presented with circulating T cells that were predominantly CD4−CD8− (Table 1). By 6 months after transplantation, the CD4 T cells outnumbered the double-negative T cells (Figure 4A). Naive T cells began increasing 1 year after transplantation (Figure 4B). The most recent naive CD4 count at 4.9 years after transplantation is 213 cells/mm3 (normal 420-1500, 10th-90th percentile).16 No thymus donor T cells nor maternal T cells were detected when tested at day 101 after transplantation.
T-cell subtype populations and PHA responses before and after thymus transplantation. Subject 1 is shown in (A-C); and subject 2 is shown in (D-F). (A,D) T-cell phenotypes are shown; (B,E) naive T cells are shown; and (C,F) proliferative responses to PHA is shown. (A-B) The data points starting at 2.1 years were obtained by the referring hospital laboratory. (D-E) The first 3 data points and the data points starting at 1.4 years after transplantation were obtained by the referring hospital laboratory. The phenotypes of the naive CD4+ and CD8+ T cells are as described in Table 1. (C,F) The asterisks indicate the values obtained in the referring hospital laboratories. The mean (solid line) and ± 2 SD (dotted lines) for healthy adult data are shown (C) for the referring and transplant center laboratories. (F) The lower limits of the normal PHA response observed in the referring hospital laboratory is indicated by the single dotted line.
T-cell subtype populations and PHA responses before and after thymus transplantation. Subject 1 is shown in (A-C); and subject 2 is shown in (D-F). (A,D) T-cell phenotypes are shown; (B,E) naive T cells are shown; and (C,F) proliferative responses to PHA is shown. (A-B) The data points starting at 2.1 years were obtained by the referring hospital laboratory. (D-E) The first 3 data points and the data points starting at 1.4 years after transplantation were obtained by the referring hospital laboratory. The phenotypes of the naive CD4+ and CD8+ T cells are as described in Table 1. (C,F) The asterisks indicate the values obtained in the referring hospital laboratories. The mean (solid line) and ± 2 SD (dotted lines) for healthy adult data are shown (C) for the referring and transplant center laboratories. (F) The lower limits of the normal PHA response observed in the referring hospital laboratory is indicated by the single dotted line.
Antigen-specific T-cell responses developed after transplantation. At 17 months after transplantation, subject 1 developed a proliferative response to PPD (Table 3). An antigen-specific proliferative response to VZV was detected when assayed at 45 months after transplantation (Table 3). The proliferative response to PHA is normal (Figure 4C).
T-cell proliferative responses to antigens
| Subject/antigen . | Month after transplantation . | Antigen-stimulated cells, cpm . | Unstimulated cells, cpm . | Stimulation index . |
|---|---|---|---|---|
| Subject 1 | ||||
| PPD | 7 | 686 | 700 | 1 |
| 17 | 16 167 | 630 | 26 | |
| 36 | 31 063 | 243 | 128 | |
| 45 | 22 019 | 430 | 34 | |
| C albicans | 17 | 645 | 630 | 1 |
| 45 | 6732 | 430 | 10 | |
| Tetanus toxoid | 45 | 15 240 | 430 | 24 |
| VZV | 45 | 17 938 | 430 | 28 |
| Subject 2 | ||||
| Tetanus toxoid | 15 | 18 500 | 3000 | 6 |
| 35.4 | 27 000 | 700 | 39 | |
| C albicans | 15 | 20 500 | 3000 | 7 |
| 35.4 | 74 500 | 700 | 106 |
| Subject/antigen . | Month after transplantation . | Antigen-stimulated cells, cpm . | Unstimulated cells, cpm . | Stimulation index . |
|---|---|---|---|---|
| Subject 1 | ||||
| PPD | 7 | 686 | 700 | 1 |
| 17 | 16 167 | 630 | 26 | |
| 36 | 31 063 | 243 | 128 | |
| 45 | 22 019 | 430 | 34 | |
| C albicans | 17 | 645 | 630 | 1 |
| 45 | 6732 | 430 | 10 | |
| Tetanus toxoid | 45 | 15 240 | 430 | 24 |
| VZV | 45 | 17 938 | 430 | 28 |
| Subject 2 | ||||
| Tetanus toxoid | 15 | 18 500 | 3000 | 6 |
| 35.4 | 27 000 | 700 | 39 | |
| C albicans | 15 | 20 500 | 3000 | 7 |
| 35.4 | 74 500 | 700 | 106 |
The CD4 TCRBV repertoire as assessed by spectratyping improved significantly after transplantation (Figure 2B) compared with the previous analysis (Figure 2A). The spectratyping results correlated with an improvement in the flow cytometric assessment of the repertoire, which no longer contained expansions of VB3, VB4, VB14, or VB22 (Figure 1C). The sjTREC analysis at 2 years after transplantation showed 10 220 TRECs per 100 000 CD3+ T cells, a value within the normal range for age.17
Subject 1 stopped Ig replacement approximately 2.7 years after transplantation. By this time, subject 1 had developed normal serum IgA and IgM levels, both of which had been undetectable early in life (Table 4). Serum IgG levels were within the normal range 4 of 5 times when tested after discontinuation of Ig therapy (Table 4). At 3.2 years after transplantation, the subject de-monstrated an excellent response to tetanus toxoid immunization (Table 4).
B-cell function in subjects after stopping immunoglobulin replacement
| Subject/age, y . | Time after tx, y . | Serum IgG, mg/dL . | Serum IgA, mg/dL . | Serum IgM, mg/dL . | Serum IgE, IU/mL . | Other titers . |
|---|---|---|---|---|---|---|
| Subject 1* | ||||||
| 0.4 | −0.7 | 126 | < 22 | < 17 | 409 | |
| 4.0 | 2.8 | 761 | 68 | 104 | ||
| 4.2 | 3.0 | 775 | 93 | 80 | ||
| 4.3 | 3.1 | 686 | 93 | 103 | 13 | Tetanus titer (prevaccine): 1 μg/mL |
| 4.4 | 3.2 | Tetanus titer (postvaccine): 135 μg/mL | ||||
| 4.7 | 3.5 | 568 | 82 | 116 | ||
| 5.5 | 4.4 | 831 | 110 | 162 | ||
| Normal, age 4-6 y | 640-1420 | 52-220 | 40-180 | < 52 | ||
| Subject 2* | ||||||
| 0.4 | −0.3 | 292 | 15 | 55 | ||
| 2.1 | 1.4 | 477 | 26 | 99 | ||
| 2.1 | 1.4 | Tetanus titer: 1.24 IU/m†; hemophilus titer: 17.4 μg/mL (> 1†); polio #1: 15 (< 5‡); polio #2: > 160 (< 5‡); polio #3: 80 (< 5‡) | ||||
| 2.3 | 1.6 | 432 | 32 | 103 | ||
| 2.5 | 1.8 | 408 | 39 | 87 | ||
| 2.9 | 2.2 | 504 | 39 | 110 | ||
| 3.6 | 2.9 | 506 | 96 | 112 | HBs: 70 mIU/mL (< 10‡); rubella: 154 IU/mL (< 10‡) | |
| 3.6 | 2.9 | Anti-A: 1:32 (IgM)†; anti-B: 1:16 (IgM)† | ||||
| 3.7 | 2.9 | Mumps: 351 (< 50‡); measles: 265 (< 70‡) | ||||
| 3.7 | 2.9 | 7.7 IU/mL | ||||
| Normal, age 2 y | 582-1200 | 46-157 | 54-155 | 0-126 | ||
| Normal, age 3 y | 582-1200 | 46-157 | 54-155 | 3-135 |
| Subject/age, y . | Time after tx, y . | Serum IgG, mg/dL . | Serum IgA, mg/dL . | Serum IgM, mg/dL . | Serum IgE, IU/mL . | Other titers . |
|---|---|---|---|---|---|---|
| Subject 1* | ||||||
| 0.4 | −0.7 | 126 | < 22 | < 17 | 409 | |
| 4.0 | 2.8 | 761 | 68 | 104 | ||
| 4.2 | 3.0 | 775 | 93 | 80 | ||
| 4.3 | 3.1 | 686 | 93 | 103 | 13 | Tetanus titer (prevaccine): 1 μg/mL |
| 4.4 | 3.2 | Tetanus titer (postvaccine): 135 μg/mL | ||||
| 4.7 | 3.5 | 568 | 82 | 116 | ||
| 5.5 | 4.4 | 831 | 110 | 162 | ||
| Normal, age 4-6 y | 640-1420 | 52-220 | 40-180 | < 52 | ||
| Subject 2* | ||||||
| 0.4 | −0.3 | 292 | 15 | 55 | ||
| 2.1 | 1.4 | 477 | 26 | 99 | ||
| 2.1 | 1.4 | Tetanus titer: 1.24 IU/m†; hemophilus titer: 17.4 μg/mL (> 1†); polio #1: 15 (< 5‡); polio #2: > 160 (< 5‡); polio #3: 80 (< 5‡) | ||||
| 2.3 | 1.6 | 432 | 32 | 103 | ||
| 2.5 | 1.8 | 408 | 39 | 87 | ||
| 2.9 | 2.2 | 504 | 39 | 110 | ||
| 3.6 | 2.9 | 506 | 96 | 112 | HBs: 70 mIU/mL (< 10‡); rubella: 154 IU/mL (< 10‡) | |
| 3.6 | 2.9 | Anti-A: 1:32 (IgM)†; anti-B: 1:16 (IgM)† | ||||
| 3.7 | 2.9 | Mumps: 351 (< 50‡); measles: 265 (< 70‡) | ||||
| 3.7 | 2.9 | 7.7 IU/mL | ||||
| Normal, age 2 y | 582-1200 | 46-157 | 54-155 | 0-126 | ||
| Normal, age 3 y | 582-1200 | 46-157 | 54-155 | 3-135 |
tx indicates transplantation; and HBs, antibody to hepatitis B surface antigen.
Subject 1 stopped immunoglobulin replacement at 3.9 years of age, 2.7 years after transplantation; subject 2 stopped immunoglobulin replacement at 1.8 years of age, 1.1 years after transplantation.
Normal response.
Control value indicates absence of immunity.
Subject 2
Subject 2 was born at term to unrelated parents of mixed French/African origin who came from nearby communities. The male infant had no hair and dystrophic nails. Genetic analysis revealed a homozygous missense mutation in exon 6 of FOXN1, C987T (R320W). The subject was not given BCG vaccination at birth. At 3 months of life, the infant presented in respiratory distress and was mechanically ventilated for 15 days. No microorganism was recovered by bronchoalveolar lavage, but he was treated with antibiotics including trimethoprim/sulfamethoxazole and liposomal amphotericin. One month later, after transfer to a tertiary hospital, human herpes virus 6 (HHV6) infection, was detected (140 000 copies/mL) associated with mild anemia and neutropenia. Thrombocytopenia, felt likely to be related to HHV6 infection, developed at 8 months of life. The initial immune evaluation at 4 months of life revealed no T cells and no proliferative response to PHA (Table 1). Flow cytometric evaluations of TCRBV repertoire and sjTREC analysis were not performed in subject 2 due to lack of T cells before transplantation.
Subject 2 did not receive any immunosuppression. Thymus transplantation was performed at 9 months of life. The male O+ blood type subject received thymus tissue from a type O+ female who was less than 9 months old. The HLA types for the subject and thymus donor are shown in Table 2.
Clinical course after transplantation
The HHV6 viral load was 110 000 copies/mL at 1 month after transplantation and dropped to 600 copies/mL 18 months later. The thrombocytopenia resolved 3 months after transplantation. At 4 months after transplantation, the subject developed a polymicrobial central line infection. At month 11 after transplantation, he developed a mild but chronic urticaria. Ig replacement and trimethoprim/sulfamethoxazole prophylaxis were stopped at 13 and 16 months after transplantation, respectively. At 1.6 years after transplantation, subject 2 developed autoimmune hypothyroidism with positive anti-thyroglobulin, anti–thyroid peroxidase, and anti–thyroid stimulating hormone receptor antibodies. Three years after thymus transplantation, the subject is well without recurrent or chronic infection. He has a normal life and normal growth with thyroid hormone replacement.
Immune results after thymus transplantation
Subject 2 underwent a biopsy of the allograft on day 53 after thymus transplantation. The biopsy showed lacy cytokeratin and the presence of CD3+ thymocytes (Figure 3C-D). Scattered thymocytes were Ki-67 (nuclear proliferation marker) and CD1a positive (not shown). These markers are characteristic of cortical thymocytes.
T-cell numbers began to increase at 5.5 months after thymus transplantation (Figure 4D). Naive T cells appeared by 9 months (Figure 4E). At 16.5 months after transplantation, all T cells were shown to be genetically host. The most recent total CD4+ T-cell and naive CD4+ T-cell numbers (obtained at 2.9 years after transplantation) are in the normal range for the age of the subject (3.6 years of life).16 The total CD8+ T-cell and naive CD8+ T-cell numbers remain below the 10th percentile for age.16
The CD4 TCRBV repertoire spectratype analysis was polyclonal when tested at 1 year after thymus transplantation, comparable with those of healthy controls (Figure 2C). The flow cytometric evaluation of the CD4 TCRBV repertoire was similarly polyclonal (Figure 1D). An sjTREC assessment at 1 year revealed 5700 TRECs per 100 000 CD3+ cells.
The subject demonstrated a normal T-cell response to PHA response by 9 months after transplantation (Figure 4F) and antigen proliferative responses by 15 months after transplantation (tetanus toxoid and C albicans; Table 3). Both responses remained normal over time. The subject was given a live measles, mumps, and rubella vaccine 33 months after transplantation without any adverse sequelae.
Antibody function was tested after Ig replacement was stopped 1.1 years after transplantation. Table 4 shows the serum Ig levels and several antibody responses to immunizations. Although the serum IgG levels are slightly low compared with the age-matched range, all specific antibody titers tested were within the pro-tective range.
Discussion
We report here for the first time the use of allogeneic thymus transplantation for the treatment of athymia and its associated lack of naive T cells in 2 human subjects with the nude/SCID phenotype due to FOXN1 mutations. The 2 subjects are well 5 and 3 years posttransplantation. They both developed functional T- and B-cell immune reconstitution.
The subjects were diagnosed after severe infections (disseminated BCG in subject 1, and a severe respiratory infection of unknown etiology in subject 2). They presented with absence of naive T cells, total alopecia, and nail dystrophy. FOXN1 deficiency was suspected and genetically confirmed in both subjects. Subject 1 was homozygous for the same mutation previously described in southern Italy7 and present in the first FOXN1-deficient human described.7,18 Subject 2 was homozygous for a novel missense mutation, C987T (R320W) in exon 6. This mutation is in the middle of the forkhead domain that is involved in DNA binding and is highly conserved among species.3,19 This homozygous mutation would likely abolish FOXN1 activity, although protein function was not tested.
The presentation of subject 1 to the transplantation center bore a striking resemblance to the presentation of infant patients with atypical complete DiGeorge anomaly.20 Infants with complete DiGeorge anomaly characteristically present with a heart defect, hypoparathyroidism, and athymia. These athymic infants with complete DiGeorge anomaly represent less than 5% of all infants with DiGeorge anomaly.21-23 The diagnosis of athymia is based on the absence of naive T cells. Some patients with complete DiGeorge anomaly develop a rash and circulating oligoclonal T cells after birth.20 They are said to have “atypical” complete DiGeorge anomaly.20 In occasional patients, the oligoclonal T cells infiltrate the liver or the gut and lead to graft-versus-host–like disease in these organs. This presentation resembles that of Omenn syndrome.20,24
Similar to patients with atypical complete DiGeorge anomaly, subject 1 presented to the transplant center at 13.7 months of life with oligoclonal T cells (which were predominantly double-negative T cells), absence of naive T cells, and a T-cell proliferative response to PHA within the normal range (although the PHA response had initially been low per the laboratory standards at the referring institution). This subject had lymphadenopathy, although this finding was likely related to the underlying M bovis infection, and eosinophilia. The skin manifestations in subject 1 were different from those in atypical complete DiGeorge anomaly, because subject 1 presented only with mild erythroderma. The proliferative response to PHA observed in subject 1 was unusually high compared with other infants with athymia. Only 3 patients with complete DiGeorge anomaly (of 60 transplanted with thymus tissue) have developed PHA responses > 100 000 counts per minute before transplantation (unpublished data, M.L.M., June 2010).
We find it interesting that the first subject reported with FOXN1 deficiency had an Omenn syndrome-like appearance with erythroderma and lymphadenopathy associated with circulating T cells that did not proliferate to mitogens, including anti-CD3.5 That subject's FOXN1-deficient sibling also had erythroderma and circulating T cells that did not proliferate in culture. These features suggest that these 2 subjects, who were reported previously, had circulating oligoclonal T cells. Subject 1, who had the same mutation as the previously reported patients, also presented with erythroderma. This phenotype contrasts with subject 2, who carries a different mutation and had no circulating T cells.
The oligoclonal T cells of atypical complete DiGeorge anomaly and Omenn/atypical FOXN1 deficiency may have an extrathymic origin or may arise secondary to a nest of thymus epithelium able to support atypical development of T cells. Studies of nude mice have also demonstrated the presence of oligoclonally expanded T cells.25,26 The mechanisms for the proliferation and lack of homeostasis by these oligoclonal T cells are poorly understood.20
Because of athymia in FOXN1 deficiency, thymus transplant was chosen as the appropriate treatment, although bone marrow transplantation had been performed in one child with the nude/SCID phenotype due to FOXN1 deficiency.6 That child did not develop naive T cells, as might be expected given the absence of a thymus.6
In determining the strategy to use for thymus transplantation in the 2 subjects presented in this report, we drew on our experience with infant patients who have complete DiGeorge anomaly. Immunosuppression has not been necessary in patients with typical complete DiGeorge anomaly who have few if any T cells.8,11 Thus, immunosuppression was not used for subject 2. Atypical complete DiGeorge anomaly patients, who have oligoclonal T-cell expansions, have required immunosuppression to prevent graft rejection.8,14 The same immunosuppression regimen was used for subject 1.
Just as seen in thymus transplantation for complete DiGeorge anomaly patients, both FOXN1-deficient subjects developed naive T cells, T-cell function, and diverse TCR repertoires after thymus transplantation. The development of an in vitro proliferative T-cell response to PPD in subject 1 was temporally associated with the clearance of BCG infection (Table 3). Subject 2 also developed in vitro proliferative T-cell responses against antigens, namely tetanus toxoid and C albicans (Table 3).
The kinetics of appearance of T cells and the ultimate T-cell numbers of the 2 FOXN1-deficient subjects fall within the ranges seen for infants with complete DiGeorge anomaly who receive postnatal allogeneic thymus transplants8,10 Naive T cells in subject 1 developed later than in most patients with complete DiGeorge anomaly who are given immunosuppression.10 A slower development of naive T cells in subject 1 was expected given the presence of M bovis infection.27,28 Subject 2 also showed slightly delayed development of naive T cells compared with most subjects with typical complete DiGeorge anomaly10 who usually develop naive T cells before 6 months after transplantation.8 Of note, in these subjects and the infants with complete DiGeorge anomaly who are given thymus transplantation, the CD8+ T-cell numbers are sub-stantially below the 10th percentile for age in the first years after thymus transplantation.8 As in the infants with complete DiGeorge anomaly, the low CD8 numbers have not resulted in clinical infection.
In subject 1, concern arose that the preexisting infection with M bovis would suppress thymopoiesis.27,28 The most recent T-cell count for this subject (1053 cells/mm3 at 58 months after transplantation) indicates that the thymus transplantation has been successful in restoring relatively normal T-cell numbers. The success in this subject gives hope for future athymic subjects who have mycobacterial infection.
Our data indicate that B-cell function was restored after thymus transplantation. Both subjects were able to discontinue Ig replacement, maintain normal serum Ig levels, and generate protective antigen specific titers. Of particular note, both subjects received the measles, mumps, and rubella vaccine without any adverse events. Normal postvaccine antibody responses to these 3 viruses were confirmed in subject 2 (Table 4).
It is remarkable that functional immunity developed in subject 1 with only one HLA match (HLA-DQB1) and in subject 2 without any HLA matches. This is similar to the findings in infants with complete DiGeorge anomaly for whom matching for HLA class I and class II has not been found to affect CD4 or CD8 T-cell counts after thymus transplantation.9
The mechanisms involved in positive and negative thymic selection after unmatched thymus transplantation are not clear. Classically, it has been hypothesized that cortical thymic epithelium is necessary for positive selection to occur.29 Murine studies suggest that recipient bone marrow-derived cells, such as antigen presenting cells30,31 or thymocytes,32,33 may also play a role in positive selection in the thymus. Alternatively, circulating host-derived epithelial progenitors34-37 may migrate to the thymus. These recipient epithelial cells could then provide signals for positive selection of the developing thymocytes. In our subjects, even though the thymus graft is unmatched to the recipient, the recipients develop T cells that proliferate in response to antigens presented by recipient antigen presenting cells (Table 3) and provide help for B-cell antibody production leading to protective antibody titers after vaccination (Table 4).
Regarding negative selection, dendritic cells have been shown to be involved.38,39 Thus, it is likely that recipient bone marrow-derived dendritic cells that colonize the thymic graft may play a role. This putative mechanism for negative selection appears to be able to prevent the development of a graft-versus-host disease–like syndrome mediated by the genetically host T cells that develop in the thymus.
Negative selection in the thymus does not prevent all autoimmune disease. Subject 2 developed autoimmune thyroid disease at 1.6 years after transplantation. The urticaria seen in subject 2 may have been related to the presence of antithyroid antibodies, as observed in approximately 30% of patients with chronic urticaria.40,41 Thyroid disease (Hashimoto or Graves) has occurred in 16 of 60 patients with complete DiGeorge anomaly after thymus transplantation8,10 (and unpublished data, M.L.M., June 2010). The mechanism for the increased prevalence of thyroid disease remains unclear. These data further emphasize the importance of continuing surveillance of these subjects for autoimmune disease.
In summary, after thymus transplantation in 2 FOXN1-deficient subjects, naive T cells and diverse TCR repertoires developed in parallel with normalization of T-cell proliferative responses and Ig levels. More importantly, the associated clearance of the ongoing disseminated infections raises the expectation that this therapeutic approach may have long-term clinical benefit for subjects with athymia secondary to FOXN1 deficiency. Overall, thymus transplantation offers a promising treatment for FOXN1 deficiency (nude/SCID).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The assistance of surgeons James Jaggers, Andrew Lodge, Henry Rice, Michael Skinner, and Jeffrey Hoehner is appreciated, as is the technical assistance of Marilyn Alexieff, Jie Li, Chia-San Hsieh, Jennifer Lonon, and Julie Smith and the clinical research assistance of Stephanie Gupton and Alice Jackson. We appreciate the assistance of Drs Michael Cook and Scott Langdon in the Duke Comprehensive Cancer Center flow cytometry and sequencing facilities. The clinical care by the faculty and fellows of the Duke Pediatric Allergy and Immunology Division are appreciated. We are indebted to the medical and nursing staff of Unité d'Immunologie et d'Hématologie Pédiatriques, Hôpital Necker, Paris who cared for subject 2. We acknowledge the collaboration of Ana Carvalho and Catarina Nascimento, from the Intensive Care Unit and J. C. Gomes Pedro, Director of the Pediatric Department of Hospital Universitário de Santa Maria, Alcinda Melo for assistance with flow cytometry, and Russell Foxall for the critical discussion of the manuscript.
Funding sources included National Institutes of Health (NIH; grant nos. R01AI47040 and R01AI54843 to M.L.M. and M01RR30 NCRR, Clinical Research, to Duke University) and grants from “Fundação para a Ciência e a Tecnologia” (FCT) and “Programa Operacional Ciência e Inovação 2010” (POCI2010) PIC/83068 to R.M.V. and PTDC/66248 to A.E.S. A.S.A. received a scholarship from FCT. Thymus transplantation for subjects 1 and 2 was financially supported by the Portuguese and French national health services, respectively. M.L.M. is a member of the Duke Comprehensive Cancer Center.
National Institutes of Health
Authorship
Contribution: M.L.M. designed research, performed research, contributed new reagents, collected data, analyzed and interpreted data, and wrote the manuscript; J.G.M. analyzed and interpreted clinical data for subject 1 and established the indication for thymic transplant; B.N. collected and analyzed data, performed research, and edited the manuscript; B.H.D. analyzed and interpreted data; E.A.M. designed research protocols and performed research; I.K.C. performed research and reviewed the manuscript; A.S.A. collected, analyzed and interpreted data from subject 1; S.L.S. analyzed and interpreted clinical data for subject 1 and established the indication for thymic transplantation; C. Pignata identified the FOXN1 mutation in subject 1 by genomic sequencing; G.d.S.B. identified the FOXN1 mutation in subject 2 by genomic sequencing; R.M.V. analyzed and interpreted data from subject 1; C. Picard performed research and evaluated immunologic data from subject 2; M.D. performed clinical follow-up and collection of data from subject 2; N.M. performed clinical follow-up and collection of data from subject 2; A.F. analyzed and interpreted data; and A.E.S. designed research and analyzed and interpreted the data.
Conflict-of-interest disclosure: M.L.M. receives funding from the NIH and the FDA and has a patent pending for culture conditions for thymus tissue for transplantation. B.H.D. and I.K.C. receive funding from the NIH, and E.A.M. receives funding from the NIH and the FDA. The remaining authors declare no competing financial interests.
Correspondence: M. Louise Markert, Duke University Medical Center, Box 3068, 6 Circuit Dr, Research Park IV, Rm 109B, Durham, NC 27710; e-mail: marke001@mc.duke.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal