Abstract
Graft-versus-host disease (GVHD) is initiated and maintained by antigen-presenting cells (APCs) that prime alloreactive donor T cells. APCs are therefore attractive targets for GVHD prevention and treatment. APCs are diverse in phenotype and function, making understanding how APC subsets contribute to GVHD necessary for the development of APC-targeted therapies. Langerhans cells (LCs) have been shown to be sufficient to initiate skin GVHD in a major histocompatibility complex–mismatched model; however, their role when other host APC subsets are intact is unknown. To address this question, we used mice genetically engineered to be deficient in LCs by virtue of expression of diphtheria toxin A under the control of a BAC (bacterial artificial chromosome) transgenic hu-man Langerin locus. Neither CD8- nor CD4-mediated GVHD was diminished in recipients lacking LCs. Similarly, CD8- and CD4-mediated GVHD, including that in the skin, was unaffected if bone marrow came from donors that could not generate LCs, even though donor LCs engrafted in control mice. Engraftment of donor LCs after irradiation in wild-type hosts required donor T cells, with immunofluorescence revealing patches of donor and residual host LCs. Surprisingly, donor LC engraftment in Langerin-diphtheria toxin A (DTA) transgenic hosts was independent of donor T cells, suggesting that a Langerin+ cell regulates repopulation of the LC compartment.
Introduction
Allogeneic hematopoietic stem cell transplantation (alloSCT) can be a life-saving therapy for hematologic malignancies and acquired or inherited nonmalignant disorders of blood cells such as sickle-cell anemia and aplastic anemia. Mature donor T cells in allografts contribute to the efficacy of alloSCT and are pivotal for reconstituting T-cell immunity, particularly in adult patients who have incomplete and delayed generation of progenitor-derived T cells. They also mediate a potent antineoplastic effect called graft-versus-leukemia. Unfortunately, donor T cells can broadly target nonmalignant host tissues in a process called graft-versus-host disease (GVHD).1 Because of GVHD, all alloSCT patients receive some type of prophylactic immunosuppression, either by depleting T cells from the allograft or, more commonly, by using pharmacologic agents that inhibit T-cell function. However, pharmacologic immunosuppression is incompletely effective and renders recipients more susceptible to serious pathogen infections. Novel approaches are clearly needed to reduce GVHD while preserving the beneficial effects of donor T cells.
GVHD is initiated by antigen-presenting cells (APCs) that prime alloreactive donor T cells.1-6 Recipient APCs that survive conditioning are essential for GVHD in major histocompatibility complex (MHC)–mismatched transplantations and in CD8-mediated GVHD across only minor histocompatibility antigens (miHAs).1,2,7,8 While not sufficient for GVHD initiation, donor APCs contribute to CD8-mediated GVHD.5 Donor and host APCs also have nonredundant roles in CD4-mediated GVHD across miHAs.6 Thus, both host and donor APCs would be logical targets for suppressing GVHD.
The first step in developing APC-targeted therapies is to determine how different APC populations contribute to GVHD. APCs, which include dendritic cells (DCs), B cells, macrophages, and basophils, are diverse cells that have in common the ability to prime T cells. DCs are perhaps the most efficacious in priming naive T cells, which upon activation are potent inducers of GVHD.9-12 Consistent with this, in an MHC-mismatched model, infused wild-type recipient splenic DCs were shown to partially restore GVHD to mice unable to initiate GVHD due to genetic defects in their APCs.3 However, ideally, the roles of an APC subset would be studied when all other APCs are intact, which would be the scenario in any clinical attempt to specifically impair host APCs before transplantation, rather than in an approach that adds back a specific cell type to a deficient environment.
Multiple subsets of DCs have been described,9,10,13 and presumably these subsets evolved to perform unique functions. Consistent with this, APC subsets have been shown to have distinct roles in various model systems.14-23 In some of these models, the potency of a given subset has been correlated with its access to or ability to present antigen.24 However, this principle may not be as applicable in alloSCT, in which all recipient and most donor DCs are likely to present host alloantigens.
DCs can also be discriminated by whether they are resident in secondary lymphoid tissues or in parenchymal tissues and by their precise locations within these tissues.10,25,26 Tissue-resident DCs have been hypothesized to be sentinels that acquire antigen, become activated, and then migrate to secondary lymphoid tissues, where they can present antigen directly to T cells or transfer antigen to lymphoid-resident DCs that in turn activate T cells. DCs in secondary lymphoid tissues, which include those that migrated from the periphery,25,27 are presumed to be essential for priming naive T cells, which do not readily enter nonlymphoid tissues. Consistent with this, GVHD is reduced in mice that almost completely lack secondary lymphoid tissues.28,29 Tissue-resident DCs from different organs could indirectly present relatively tissue-restricted minor histocompatibility antigens or may program T cells to be more likely to traffic to a specific tissue,30-32 including the skin.32 If this is the case, then DCs in different locations could have nonredundant roles in shaping the alloimmune response and the resulting GVHD phenotype. Aside from their roles after migration to lymph nodes (LNs), tissue DCs could modify immune responses in situ by further activating or even suppressing infiltrating alloreactive T cells initially primed in secondary lymphoid tissues.
LCs are a distinct subset of tissue DCs restricted to the epidermis.13 They are characterized by the expression of the C-type lectin receptor Langerin.33 Mice also express Langerin in a subset of nonepidermal DCs, including dermal DCs, which can be distinguished from epidermal LCs by the absence of CD11b and EpCAM.34-36 It had long been hypothesized that LCs are essential for adaptive immune responses against antigens in the skin, including contact hypersensitivity and skin graft rejection.37 However, contrary to this view, we and others used mice deficient in LCs to show that LCs are not required for contact hypersensitivity reactions and skin graft rejection.20,23,38-41 Unexpectedly, some contact-hypersensitivity and skin graft-rejection responses were augmented in the absence of LCs.23,39
LCs have been of interest in alloSCT because the skin is the most commonly affected and most easily accessible site of GVHD. In human alloSCT, LCs can remain recipient in origin even after the rest of the hematopoietic system has largely converted from being host derived to being donor derived.42,43 This property was elegantly demonstrated in mouse bone marrow transplantation (BMT) models wherein LCs remained host derived in irradiated recipients of allogeneic T cell–depleted donor BM, whereas the addition of donor T cells induced the replacement of host LCs with donor-derived LCs.44 The same group used MHC-mismatched donor→host BM chimeras, in which LCs were the only residual recipient APCs, as hosts in a second GVHD-inducing transplantation from the same donor in a model in which host APCs were required for GVHD.8 Such chimeras developed cutaneous GVHD, demonstrating that recipient LCs are sufficient to cause skin GVHD when all other APCs are donor in origin.45 These results imply a direct interaction between donor T cells and host LCs in draining LNs, skin, or both.
However, these studies did not address the role of recipient LCs when other types of host APCs are present, which is the scenario at the time of transplantation. That recipient LCs could directly prime donor T cells made it reasonable, if not likely, that LCs would have a nonredundant role in GVHD. The role of donor-derived LCs has also been unexplored, which is relevant given that alloreactive T cells augment donor LC engraftment44 and that, clinically, GVHD often continues long after donor-type LCs would be expected to have engrafted.
To study the roles of recipient LCs in GVHD under conditions in which all other APCs are intact, we used LC-deficient mice that specifically lack only epidermal LCs by virtue of expressing the DTA chain under the control of the human Langerin locus in BAC (bacterial artificial chromosome) transgenic mice (Langerin-DTA mice).23 Cells that express the DTA gene do not develop, but because the human Langerin gene is only expressed in mouse epidermal LCs,23 other mouse Langerin+ cells, including Langerin+ dermal DCs, are intact.34 These mice were used as recipients in 2 alloBMT models, both of which absolutely require host APCs for GVHD induction. To investigate donor-derived LCs in GVHD, we used LC-deficient mice as BM donors in 2 other GVHD models in which donor-derived APCs have established roles in patho-genesis.5,6 We also used Langerin-DTA mice to determine whether Langerin+ cells regulate LC turnover in BM transplantation.
Methods
Mice
Langerin-DTA mice were generated as described in Kaplan et al23 and backcrossed at least 6 generations to B6 or B6 Ly5.1 mice, at least 2 additional generations to B6.C (H-2d) and 5 generations to C3H.SW, prior to use in BMT experiments. C3H.SW (H-2b) and B6bm12 mice were bred at Yale University from mice originally purchased from The Jackson Laboratory. Transgenic animals were genotyped by polymerase chain reaction of tail DNA (forward primer: 5′-GAGGCAAATGATTGGCATTCTAC-3′; reverse primer: 5′-CTGGAAAATTCAAGAAGAGCCT-3′). RAG1−/− mice were obtained from The Jackson Laboratory.
Cell purifications
CD8 cells were purified from LNs via negative selection using biotin-conjugated antibodies against CD4 (clone GK1.5; laboratory prepared), B220 (clone 6B2; laboratory prepared), and CD11b (clone M1/70; from BD Pharmingen), followed by streptavidin-conjugated magnetic beads (Miltenyi Biotec) and separation on a magnetic cell sorter (autoMACS;Miltenyi Biotec). CD8 cells were more than 90% pure, with CD4 T-cell contamination of less than 2% (not shown). T-cell depletion of BM was performed with anti-Thy1.2 microbeads and the autoMACS. CD4 cells were purified the same way, except anti-CD4 was omitted and replaced with anti-CD8 (clone TIB105; laboratory prepared).
BMT
All BMTs were performed according to protocols approved by the Yale University Institutional Animal Care and Use Committee. In the C3H.SW (H-2b)→B6 (H-2b) model, transgene-positive (Tg+) or transgene-negative (Tg−) littermates (8-12 weeks in age) received 1000 cGy of radiation, followed by reconstitution with 7 × 106 T cell–depleted C3H.SW BM from wild-type, Tg+, or Tg− littermate controls with or without 2-3 × 106 purified C3H.SW CD8+ T cells. In the B6bm12→B6 model, Tg+ or Tg− littermates received 800 cGy of radiation and 107 B6bm12 BM cells, with or without donor splenocytes containing 106 CD4 cells. In the B6.C (H-2d)→BALB/c (H-2d) model, BALB/c mice received 800 cGy of radiation and were reconstituted with 107 T cell–depleted BM from Tg+ B6.C mice or Tg− littermate controls, with or without 106 purified CD4 cells from Tg+ B6.C mice. C3H.SW→B6 Tg+ or B6 RAG1−/−→Tg+ chimeras for analysis of LC turnover were prepared as in GVHD experiments. Mice were weighed 2-3 times per week; weights from mice that died or that were killed due to predefined measures of morbidity were included in averages for subsequent time points at the last value recorded. Skin disease was scored when mice were weighed. For the C3H.SW→B6 model, minimal criteria for clinical skin disease was fur loss in an area > 1 cm2. Skin scoring in the B6.C→BALB/c model was as described in Anderson et al.6
Histopathology
All surviving mice were killed between days 33 and 50, and samples from back skin, ear, small intestine, colon, and liver were harvested. Tissues were fixed in 10% phosphate-buffered formalin and stained with hematoxylin and eosin. Skin pathology was scored as previously described.6,7,12 In the liver, portal inflammation, inflammatory bile duct damage, central perivenulitis, and lobular necro-inflammatory activity were semiquantitatively evaluated on a scale of 0-3. Weighted scores were calculated by multiplying the portal inflammation and central perivenulitis scores by a factor of 1. Bile duct injury and lobular necro-inflammatory activity were multiplied by a factor of 0.5. Total scores for each animal were obtained by adding the weighted scores together. Features evaluated for colon GVHD included overall inflammation/cryptitis, average number of apoptotic bodies per 10 crypts in the most severely affected areas, crypt abscesses, crypt loss, and ulceration. Each parameter was scored on a scale of 0-3. Weighted scores for each parameter were calculated as follows: inflammation/cryptitis × 0.9, apoptotic bodies per 10 crypts × 0.1, neutrophilic cryptitis/crypt abscess × 0.4, crypt loss × 1, and ulceration × 2. The total score for each animal was derived by adding together each of the weighted scores.
Immunofluorescence
Epidermal sheets were prepared by treating mouse ears with hair remover (Nair; Church and Dwight) for 5 minutes, splitting into dorsal and ventral halves, and then affixing them to slides (epidermis side down) with double-sided adhesive tape (3M). Slides were incubated in 10mM EDTA (ethylenediaminetetraacetic acid) in phosphate-buffered saline for 2 hours at 37°C, followed by physical removal of the dermis. Tissues were fixed in acetone for 5 minutes at 4°C and stained with anti MHCII fluorescein isothiocyanate (clone M5; BioLegend), followed by anti-fluorescein/Oregon Green Alexa Fluor 488 (Invitrogen) and biotin-conjugated anti-Langerin (eBioscience), followed by streptavidin–Alexa Fluor 647 (Invitrogen). In some experiments, sheets were also stained with antibodies against CD45.2 PE (clone 104; BioLegend), followed by anti-PE Alexa Fluor 568 (purified anti-PE; Rockland Immunochemicals; laboratory conjugated). Images were acquired with an automated wide-field microscope (Eclipse Ti; Nikon) and a charge-coupled device camera (Retiga 2000R; QImaging) using NIS Elements Version 3.10 software (Nikon). Emitted light was collected through 460/50-, 525/50-, 605/70-, and 700/75-nm bandpass filters. Composites of single-channel images were created with Adobe Photoshop Version 10.0.
LC-migration assay
Mouse ears were mechanically split with forceps and then incubated in 0.5% trypsin (Invitrogen) and 5mM EDTA in phosphate-buffered saline for 90 minutes at 37°C. Peeled epidermal sheets were cultured in the presence of 10 ng/mL of granulocyte-macrophage colony-stimulating factor (BD Pharmingen) and 10 ng/mL of tumor necrosis factor-α (PeproTech). After a 48-hour incubation at 37°C, cells that had migrated were collected and analyzed by flow cytometry.
Flow cytometry
Cells that migrated from ear preparations were incubated with ethidium monoazide (Invitrogen) to allow exclusion of dead cells, and then stained with antibodies against CD45.2 (APC-Cy7, clone 104; BioLegend) and MHCII (Alexa Fluor 488, clone M5; BioLegend), fixed and permeabilized, and then stained with biotin-conjugated anti-Langerin (clone eBioL31; eBioscience) followed by streptavidin-PE.
Measurement of serum cytokines
Serum cytokines were measured using the Bioplex kit Th1/Th2 panel (Bio-Rad) and a Luminex 100 analyzer (Luminex).
Statistics
Differences in weights were calculated by Student t test. Significance for comparisons of pathology scores, clinical GVHD scores, and cytokine levels were made using the Mann-Whitney test. Differences in incidences of skin GVHD were calculated by log-rank test. Error bars in weight measurements are standard error measurements (Prism Version 4.0c software; GraphPad).
Results
Recipient LCs are not required for GVHD in the C3H.SW→B6 strain pairing
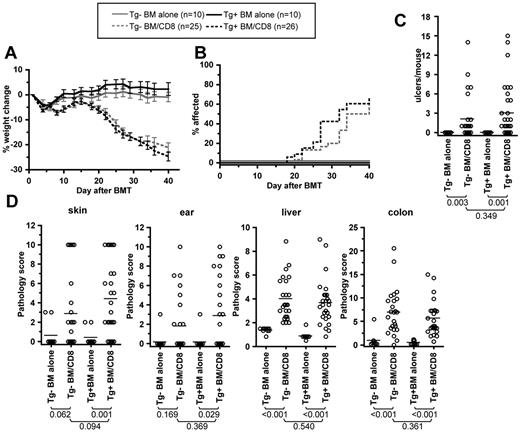
We first assessed the role of recipient LCs in the C3H.SW→B6 strain paring, in which we have previously shown that recipient APCs are necessary and sufficient for GVHD induced by CD8+ T cells.5,7 B6 Tg+ mice or Tg− littermate controls (ie, LC-deficient or not) were irradiated and reconstituted with C3H.SW BM with or without 2-3 × 106 C3H.SW CD8+ T cells. Both Tg+ and Tg− CD8 recipients developed similar clinical GVHD as measured by weight loss (Figure 1A), incidence of clinical skin disease (Figure 1B), and number of skin ulcerations (Figure 1C).
Recipient Langerhans cells are not required for clinical or histologic GVHD in the C3H.SW→B6 model. Tg+ and Tg− hosts were irradiated and reconstituted with C3H.SW BM with or without 2-3 × 106 purified C3H.SW CD8 cells. Shown are data from 1 of 2 experiments with comparable results. Tg+ and Tg− CD8-recipients had similar weight change (A; P > .170 comparing Tg+ and Tg− CD8 recipients at all time points; P < .01 comparing each CD8 group to its respective BM alone control from day +20 onward), incidence of clinical skin disease (B; P = .299 comparing Tg+ and Tg− CD8 recipients; P < .001 comparing each CD8 recipient to its BM alone control), and number of skin ulcers (C). (D) GVHD pathology scores in skin, ear, liver, and colon. The P values for number of skin ulcers and pathology scores are shown in the figure.
Recipient Langerhans cells are not required for clinical or histologic GVHD in the C3H.SW→B6 model. Tg+ and Tg− hosts were irradiated and reconstituted with C3H.SW BM with or without 2-3 × 106 purified C3H.SW CD8 cells. Shown are data from 1 of 2 experiments with comparable results. Tg+ and Tg− CD8-recipients had similar weight change (A; P > .170 comparing Tg+ and Tg− CD8 recipients at all time points; P < .01 comparing each CD8 group to its respective BM alone control from day +20 onward), incidence of clinical skin disease (B; P = .299 comparing Tg+ and Tg− CD8 recipients; P < .001 comparing each CD8 recipient to its BM alone control), and number of skin ulcers (C). (D) GVHD pathology scores in skin, ear, liver, and colon. The P values for number of skin ulcers and pathology scores are shown in the figure.
Tg+ and Tg− CD8-recipients had similar GVHD pathology in all tissues (Figure 1D and supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). However, compared with recipients of BM only, histopathologic skin and ear GVHD only occurred in Tg+ CD8 recipients (P < .03), with borderline P values compared with Tg− mice that did or did not receive CD8 cells. Nonetheless, if scores are compared with the combined Tg+ and the Tg− BM-alone groups, which did not differ in their histology scores, Tg− CD8 recipients had significant pathology of skin (P = .02) and borderline pathology of ear (P = .07). Skin samples analyzed for histopathologic changes were taken from the interscapular area to compare histology in a standard region in all mice. The clinical score, which was similar in the Tg+ and Tg− recipients, captured disease that occurred elsewhere. Indeed, supplementary skin specimens from clinically affected areas (in addition to the standard location) in several mice that had pathology scores of 0 in the interscapular sample had histopathology consistent with GVHD (not shown). In summary, recipient LCs were dispensable for pathologic GVHD of all target organs examined, and in fact their absence may have minimally facilitated GVHD in the skin.
Recipient LCs are not required for GVHD in the B6bm12→B6 strain pairing
We next determined whether recipient LCs were required for GVHD in the MHCII-mismatched B6→B6bm12 strain pairing in which host APCs are also essential.2 Tg+ and Tg− recipients of donor CD4 cells lost weight relative to control recipients of BM only, and the weight changes in Tg+ and Tg− CD4 recipients were not significantly different at any time point (Figure 2A; results from 2 independent experiments). Both Tg+ and Tg− CD4 recipients developed similar small intestine and colon GVHD (Figure 2B and Supplemental Figure 2). Neither clinical nor pathologic cutaneous GVHD developed (not shown), which is typical for this strain pairing. As an additional quantitative indicator of donor T-cell activation, we measured serum interferon-γ and tumor necrosis factor-α on day +7. Levels were significantly elevated in both Tg+ and Tg− CD4 recipients compared with recipients of no T cells (Figure 2C), but were similar in both CD4-recipient groups. Thus, as was the case in the C3H.SW→B6 model, recipient LCs were not required for GVHD when all other recipient APCs were intact.
Recipient Langerhans cells are not required for clinical or histologic GVHD in the B6bm12→B6 model. Tg+ and Tg− hosts were irradiated and reconstituted with B6bm12 BM with or without B6bm12 splenocytes containing 106 CD4 cells. (A) Percentage weight change in 2 independent experiments. P > .07 comparing Tg+ and Tg− CD4 recipients at all time points; P < .05 comparing each CD4 group to its respective BM-alone control from day +13 onward. (B) Pathology scores from the 2 experiments combined; P values are shown in the figure. Serum samples collected from predesignated mice on day +7 after BMT were analyzed for cytokine levels (C; 3 samples per group; *P = .05 comparing BM-alone to CD4 recipients; P = .7 comparing CD4-recipient groups; data from 1 of 2 experiments with similar results).
Recipient Langerhans cells are not required for clinical or histologic GVHD in the B6bm12→B6 model. Tg+ and Tg− hosts were irradiated and reconstituted with B6bm12 BM with or without B6bm12 splenocytes containing 106 CD4 cells. (A) Percentage weight change in 2 independent experiments. P > .07 comparing Tg+ and Tg− CD4 recipients at all time points; P < .05 comparing each CD4 group to its respective BM-alone control from day +13 onward. (B) Pathology scores from the 2 experiments combined; P values are shown in the figure. Serum samples collected from predesignated mice on day +7 after BMT were analyzed for cytokine levels (C; 3 samples per group; *P = .05 comparing BM-alone to CD4 recipients; P = .7 comparing CD4-recipient groups; data from 1 of 2 experiments with similar results).
Host Langerhans cell turnover
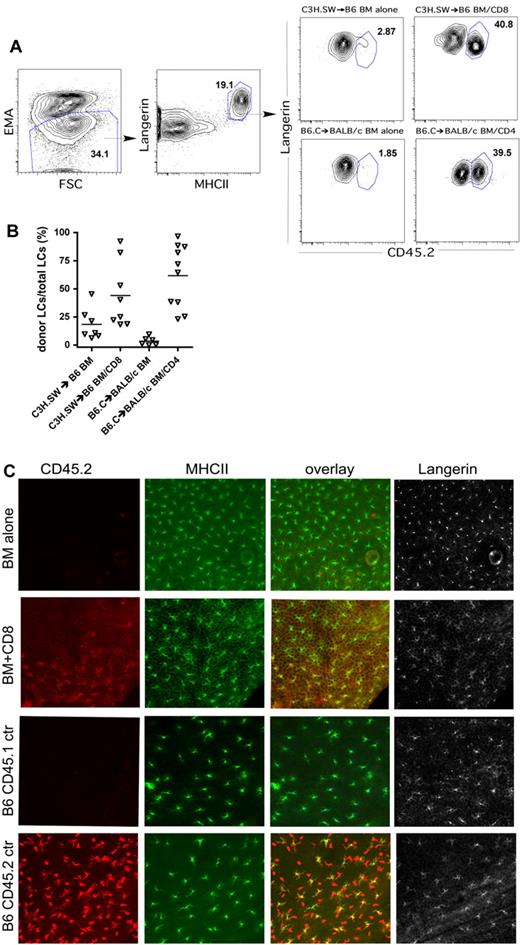
It was previously reported that LCs remain host in origin in irradiated recipients of MHC-mismatched, T cell–depleted BM44 or if the transplantation is performed across only miHAs, even with donor T cells.46 The mechanism behind resistance to LC turnover is not understood, and therefore we were interested whether the absence of recipient LCs would affect donor LC engraftment after BMT. We began by examining LC turnover in LC-intact mice. To do so, we created C3H.SW (CD45.2)→B6 (CD45.1) and B6.C (CD45.2; H-2d)→BALB/c (CD45.1; H-2d) chimeras using T cell–depleted BM. We analyzed LC turnover, both by flow cytometry of LCs induced to migrate from skin after incubation with granulocyte-macrophage colony-stimulating factor in an LC-migration assay45 and by immunofluorescence of epidermal sheets. Consistent with prior reports,44,46 LCs remained mostly recipient in origin even though other hematopoietic cells were primarily of the donor type (Figure 3A, representative flow cytometry, and 3B, scatter plot of percentage of LCs that are of donor origin). Taken in the context of our prior finding that identically prepared C3H.SW→B6 BM chimeras are resistant to GVHD when reirradiated and reconstituted with C3H.SW BM and CD8 cells,7 we can conclude that recipient LCs are not sufficient to induce CD8-mediated GVHD in this model system. In B6 mice transplanted with C3H.SW BM and CD8 cells and BALB/c mice transplanted with B6.C BM and CD4 cells, LC turnover was variable, ranging from 18%-97% donor when analyzed 40-50 days after transplantation (Figure 3A-B). By immunofluorescence staining of epidermal sheets, LC engraftment in CD8 recipients (Figure 3C) and CD4 recipients (not shown) was focal, with islands of donor-derived LCs. This differs from the complete turnover of LCs in an MHC-mismatched system44 and the lack of replacement of host with donor LCs in an MHC-matched model.46
Donor T cells induce variable turnover of Langerhans cells. Ears from transplanted mice were analyzed for LC turnover by the LC-migration assay (A-B) and by immunofluorescence (C). To identify LCs (A), we gated on ethidium monoazide-negative cells (left panel) that were Langerin+MHCII+ (second panel) Note that nearly all MHCII+ cells express Langerin. We gated on MHCII+Langerin+ cells and determined the fractions that were host- or donor-derived based on expression of CD45.1 or CD45.2 (right panels). (B) Scatter plot of data analyzed as per panel A; each symbol represents data from an individual mouse. P < .03 comparing T-cell recipients to their respective BM-alone controls (data combined from 2 independent experiments with similar results). (C) Immunofluorescence staining. Epidermal preps were costained for expression of CD45.2 (red), MHCII (green), and Langerin (gray). Langerhans cells are present in recipients of BM only, but they are host-derived (CD45.2−; top row). With donor CD8 cells, donor CD45.2+ LCs engraft (second row), but engraftment is focal. Shown is an area of donor LCs abutting residual host LCs (see overlay of CD45.2 and MHCII staining in the third panel). The bottom 2 rows are staining of samples from CD45.1 and CD45.2 control mice. Images were originally captured with 200× magnification.
Donor T cells induce variable turnover of Langerhans cells. Ears from transplanted mice were analyzed for LC turnover by the LC-migration assay (A-B) and by immunofluorescence (C). To identify LCs (A), we gated on ethidium monoazide-negative cells (left panel) that were Langerin+MHCII+ (second panel) Note that nearly all MHCII+ cells express Langerin. We gated on MHCII+Langerin+ cells and determined the fractions that were host- or donor-derived based on expression of CD45.1 or CD45.2 (right panels). (B) Scatter plot of data analyzed as per panel A; each symbol represents data from an individual mouse. P < .03 comparing T-cell recipients to their respective BM-alone controls (data combined from 2 independent experiments with similar results). (C) Immunofluorescence staining. Epidermal preps were costained for expression of CD45.2 (red), MHCII (green), and Langerin (gray). Langerhans cells are present in recipients of BM only, but they are host-derived (CD45.2−; top row). With donor CD8 cells, donor CD45.2+ LCs engraft (second row), but engraftment is focal. Shown is an area of donor LCs abutting residual host LCs (see overlay of CD45.2 and MHCII staining in the third panel). The bottom 2 rows are staining of samples from CD45.1 and CD45.2 control mice. Images were originally captured with 200× magnification.
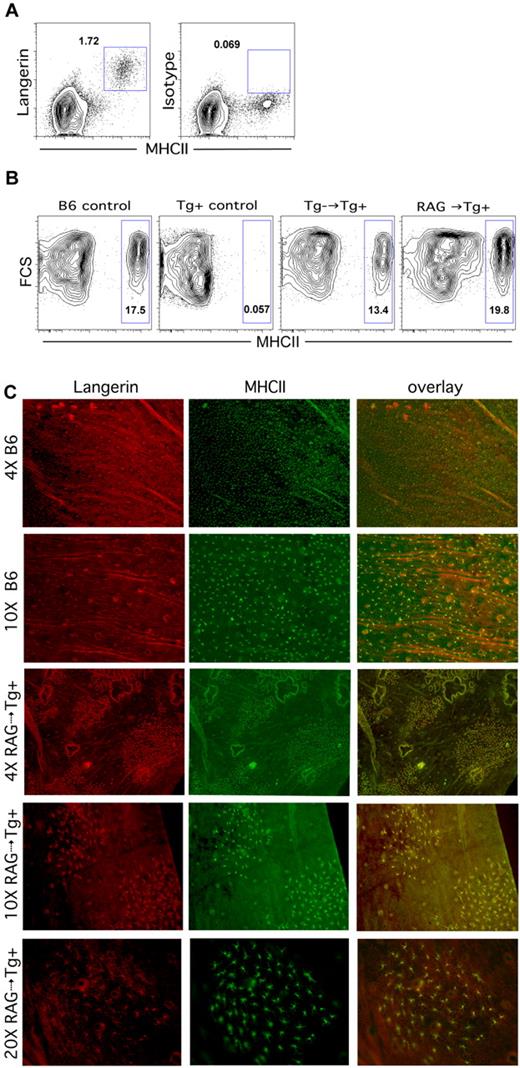
We next analyzed B6 Tg+ LC-deficient recipients of C3H.SW BM for donor LC engraftment. To our surprise, the epidermis in these mice, which was previously devoid of LCs, now had a clear population of MHCII+Langerin+ cells, which could only be donor derived (Figure 4A). Tg+ recipients of B6bm12 BM also developed donor-derived LCs (data not shown). Thus, in contrast to the situation in which host LCs are intact, donor-derived LCs engraft in irradiated LC-deficient recipients of T cell–depleted BM.
Donor Langerhans cells engraft in Langerin-DTA mice independent of donor T cells. (A-B) Flow cytometric analysis of LCs. LCs are present in ears of Langerin-DTA mice transplanted with T cell–depleted C3H.SW BM (A) (representative flow cytometry). Donor LCs engraft in Langerin-DTA recipients of Tg− BM (B third panel) or RAG1−/− BM (B fourth panel). The first 2 panels show data from unmanipulated B6 and Tg+ control mice. Data are representative of 3-6 mice per group from at least 2 independent experiments with similar results. (C) Epidermal preparations from B6 controls or RAG1−/−→Tg+ transplantation recipients were stained for Langerin (red, first column) and MHCII (green, second column); the overlay of Langerin and MHCII expression is shown in the third column. Note the patchy engraftment of donor LCs in RAG1−/−→Tg+ recipients.
Donor Langerhans cells engraft in Langerin-DTA mice independent of donor T cells. (A-B) Flow cytometric analysis of LCs. LCs are present in ears of Langerin-DTA mice transplanted with T cell–depleted C3H.SW BM (A) (representative flow cytometry). Donor LCs engraft in Langerin-DTA recipients of Tg− BM (B third panel) or RAG1−/− BM (B fourth panel). The first 2 panels show data from unmanipulated B6 and Tg+ control mice. Data are representative of 3-6 mice per group from at least 2 independent experiments with similar results. (C) Epidermal preparations from B6 controls or RAG1−/−→Tg+ transplantation recipients were stained for Langerin (red, first column) and MHCII (green, second column); the overlay of Langerin and MHCII expression is shown in the third column. Note the patchy engraftment of donor LCs in RAG1−/−→Tg+ recipients.
Donor LCs engraft in LC-deficient mice independently of donor T cells
These results suggested that donor LC engraftment in LC-deficient hosts does not require alloreactive T cells. However, it was possible that such T cells were required, but that in the absence of host Langerin+ cells, the small number of T cells remaining after BM T-cell depletion (< 1 × 104 per recipient) was sufficient to promote donor LC engraftment. To test this, we first analyzed LC turnover in Tg+ recipients of BM from Tg− littermates, reasoning that contaminating T cells from these mice would not be alloreactive. As was the case with C3H.SW BM donors, 6 weeks after BMT, Tg+ recipients of Tg− BM had LCs (Figure 4B, second panel), suggesting that alloreactive T cells were not required. To definitively exclude a role for T cells at all, we analyzed LC turnover in B6 RAG1−/−→Tg+ CD45.1 recipients. Even in the complete absence of donor T cells, donor-derived LCs developed in Tg+ recipients (Figure 4B, flow cytometry, and 4C, immunofluorescence). LC engraftment in Tg+ recipients of RAG1−/− BM was focal, with islands of LCs suggestive of seeding by a progenitor followed by local proliferation (Figure 4C). Similar patchy engraftment was also present when donor BM was from B6bm12 or Tg− littermate controls (data not shown). Thus, in the absence of host LCs, donor-derived LCs engraft without alloreactive T cells.
Donor Langerhans cells in GVHD
That donor LCs engraft in T-cell recipients in both the C3H.SW→B6 and B6.C→BALB/c models raised the possibility that donor LCs could influence subsequent ongoing GVHD in either a positive or negative way. LCs could affect systemic GVHD or they might only affect cutaneous GVHD by acting in draining LNs, the epidermis, or both. Donor-derived APCs participate in GVHD in both the B6.C→BALB/c and C3H.SW→B6 strain pairings.5,6 Notably, skin disease was reduced in the C3H.SW→B6 model when donor BM was beta-2-microglobulin−/− and therefore unable to cross-present antigens to donor CD8 cells.5 We therefore compared GVHD in recipients of Langerin-DTA donor BM with GVHD in recipients of BM from Tg− littermates in both the C3H.SW→B6 CD8-dependent and the B6.C→BALB/c CD4-dependent GVHD models.
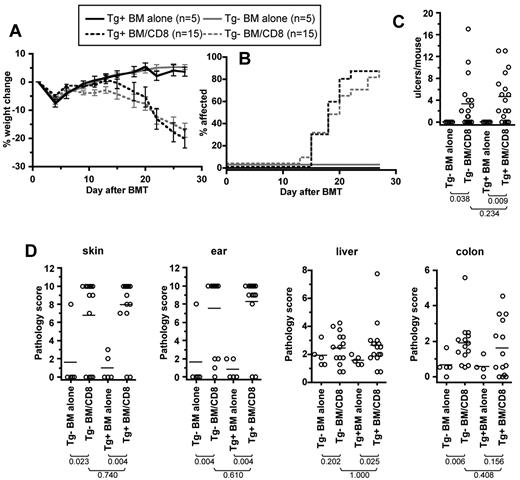
B6 mice were irradiated and reconstituted with BM from C3H.SW Tg+ or Tg− littermates, with or without 2-3 × 106 CD8 cells from Tg+ mice. We used T cells from Tg+ mice to ensure that there was no response against DTA, because T cells from these mice should be tolerant to DTA. As measured by weight loss, incidence of skin disease, and the number of skin ulcers, GVHD was similar in recipients of CD8 cells and Tg+ or Tg− C3H.SW BM (Figure 5). Histopathologic skin and ear GVHD developed in both Tg+ and Tg− CD8 recipients compared with their respective BM-alone groups, and scores were not significantly different between the CD8 groups. GVHD of the liver and colon developed only in Tg+ and Tg− CD8 recipients, respectively, although scores in CD8-recipient groups were not significantly different. Thus, the absence of donor-derived LCs did not significantly affect clinical or histopathologic GVHD.
Donor Langerhans cells are not required for GVHD in the C3H.SW→B6 strain pairing. B6 mice were irradiated and reconstituted with Tg+ or Tg− C3H.SW BM with or without Tg+ C3H.SW CD8+ T cells. Clinical GVHD was similar in both CD8-recipient groups as measured by percentage weight loss (A; P > .146 comparing CD8 recipients of Tg+ or Tg− BM from day 14 onward; P < .021 comparing each CD8 group to its respective BM alone control from day 22 onward), incidence of skin disease (B; P = .514 comparing CD8 recipients of Tg+ and Tg− BM; P < .004 comparing each CD8 recipient to its BM-alone control) and the number of skin ulcers (C; P = .234 comparing CD8 recipients of Tg+ and Tg− BM; P < .04 comparing each CD8 recipient to its BM-alone control). (D) Histopathology scores. P values are noted in the figure.
Donor Langerhans cells are not required for GVHD in the C3H.SW→B6 strain pairing. B6 mice were irradiated and reconstituted with Tg+ or Tg− C3H.SW BM with or without Tg+ C3H.SW CD8+ T cells. Clinical GVHD was similar in both CD8-recipient groups as measured by percentage weight loss (A; P > .146 comparing CD8 recipients of Tg+ or Tg− BM from day 14 onward; P < .021 comparing each CD8 group to its respective BM alone control from day 22 onward), incidence of skin disease (B; P = .514 comparing CD8 recipients of Tg+ and Tg− BM; P < .004 comparing each CD8 recipient to its BM-alone control) and the number of skin ulcers (C; P = .234 comparing CD8 recipients of Tg+ and Tg− BM; P < .04 comparing each CD8 recipient to its BM-alone control). (D) Histopathology scores. P values are noted in the figure.
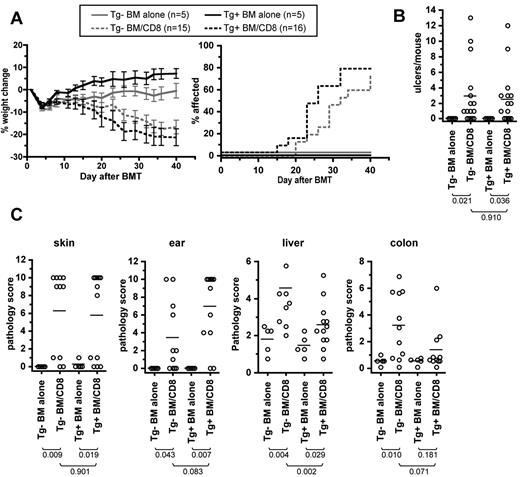
We next determined whether GVHD in the C3H.SW→B6 model would differ if mice lacked all LCs. We compared CD8-mediated GVHD in Tg+ C3H.SW→Tg+ B6 mice with that in Tg− C3H.SW→Tg− B6 mice. As measured by weight loss, incidence of skin disease, and number of skin ulcers, similar clinical GVHD developed in both CD8-recipient groups (Figure 6). Histopathologic ear, skin, and liver GVHD developed in both CD8-recipient groups. Thus, even in the complete absence of donor or recipient LCs, GVHD was largely unaffected.
Similar GVHD in C3H.SW Tg−→B6 Tg− and C3H.SW Tg+→B6 Tg+ CD8 recipients. GVHD in Tg−→Tg− and Tg+→Tg+ transplantations was similar as measured by percentage weight change (A; P > .13 comparing Tg+ and Tg− CD8 recipients from day 8 onward; P < .02 comparing each CD8 group to its respective BM-alone control from day 26 onward), incidence of skin disease (B; P = .41 comparing Tg+ and Tg− CD8 recipients; P < .014 comparing each CD8 recipient to its BM-alone control), and number of skin ulcers (C; P = .91 comparing Tg+ and Tg− CD8 recipients; P < .036 comparing each CD8 recipient to its BM-alone control). (D) Pathology scores. P values are noted in the figure.
Similar GVHD in C3H.SW Tg−→B6 Tg− and C3H.SW Tg+→B6 Tg+ CD8 recipients. GVHD in Tg−→Tg− and Tg+→Tg+ transplantations was similar as measured by percentage weight change (A; P > .13 comparing Tg+ and Tg− CD8 recipients from day 8 onward; P < .02 comparing each CD8 group to its respective BM-alone control from day 26 onward), incidence of skin disease (B; P = .41 comparing Tg+ and Tg− CD8 recipients; P < .014 comparing each CD8 recipient to its BM-alone control), and number of skin ulcers (C; P = .91 comparing Tg+ and Tg− CD8 recipients; P < .036 comparing each CD8 recipient to its BM-alone control). (D) Pathology scores. P values are noted in the figure.
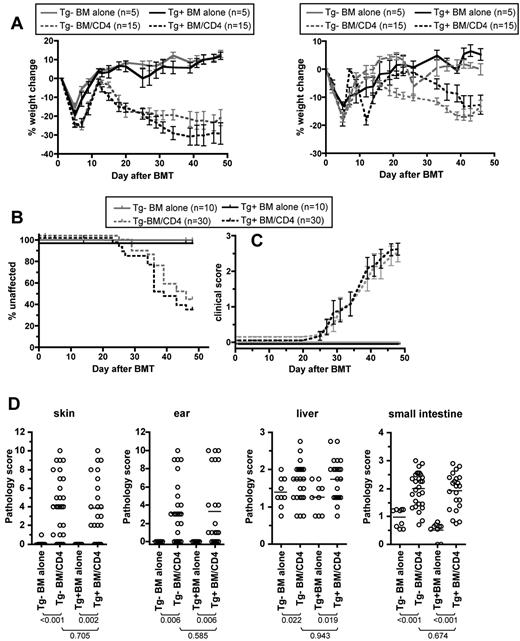
To determine the role of donor LCs in CD4-mediated GVHD, we used Langerin-DTA B6.C mice (H-2d) as BM donors in the B6.C→BALB/c CD4-mediated GVHD model. BALB/c mice were irradiated and reconstituted with T cell–depleted BM from Tg+ or Tg− B6.C mice and purified 1 × 106 CD4 cells isolated from Tg+ donors. Recipients of CD4 cells and Tg+ or Tg− donor BM developed similar weight loss, incidence of skin disease, and severity of skin disease in affected mice (Figure 7A-C, data from 2 experiments with similar results). Histopathologic GVHD developed in skin, ear, liver, and small intestine in both CD4-recipient groups relative to the respective control groups that did not receive CD4 cells (Figure 7D). Importantly, pathology scores were also similar in both CD4-recipient groups.
Donor Langerhans cells are not required for GVHD in the B6.C→BALB/c strain pairing. BALB/c mice were irradiated and reconstituted with Tg+ or Tg− B6.C BM with or without Tg+ B6.C CD4 cells. (A) Percentage weight loss. Two independent experiments are shown as left and right panels. Data were not combined due to differences in the kinetics and magnitude of weight loss in the 2 experiments. Experiment 1: P < .028 comparing each CD4 recipient group to its respective BM-alone group at all measurements; P > .05 comparing Tg+ and Tg− CD4 recipients at all measurements except days 13, 15, and 34). Experiment 2: P < .026 comparing each CD4-recipient group to its respective BM-alone group from day +33; P > .05 comparing percentage weight loss of Tg+ and Tg− CD4 on all days except day +2 and days +16 to +23. (B-C) Combined data from 2 experiments shown in A. (B) Percentage unaffected by skin disease (P < .003 comparing each CD4 recipient group to its respective BM-alone groups; P > .384 comparing Tg+ and Tg− CD4-recipient groups). (C) Clinical scores of mice affected by skin GVHD (P > .179 comparing Tg+ and Tg− CD4 recipient groups at all time points). (D) Pathology scores. P values are noted in the figure.
Donor Langerhans cells are not required for GVHD in the B6.C→BALB/c strain pairing. BALB/c mice were irradiated and reconstituted with Tg+ or Tg− B6.C BM with or without Tg+ B6.C CD4 cells. (A) Percentage weight loss. Two independent experiments are shown as left and right panels. Data were not combined due to differences in the kinetics and magnitude of weight loss in the 2 experiments. Experiment 1: P < .028 comparing each CD4 recipient group to its respective BM-alone group at all measurements; P > .05 comparing Tg+ and Tg− CD4 recipients at all measurements except days 13, 15, and 34). Experiment 2: P < .026 comparing each CD4-recipient group to its respective BM-alone group from day +33; P > .05 comparing percentage weight loss of Tg+ and Tg− CD4 on all days except day +2 and days +16 to +23. (B-C) Combined data from 2 experiments shown in A. (B) Percentage unaffected by skin disease (P < .003 comparing each CD4 recipient group to its respective BM-alone groups; P > .384 comparing Tg+ and Tg− CD4-recipient groups). (C) Clinical scores of mice affected by skin GVHD (P > .179 comparing Tg+ and Tg− CD4 recipient groups at all time points). (D) Pathology scores. P values are noted in the figure.
Discussion
Current interventions to both prevent and treat GVHD mostly target donor T cells. Pharmacologic approaches are incompletely effective, whereas T cell–depleting strategies cause a prolonged deficiency of T cells and result in an increased susceptibility to infections. In contrast, targeting host APCs to mitigate GVHD would not cause a permanent loss of pathogen-specific immunity, because donor-derived APCs would be able to prime donor T cells to respond to invading pathogens. Because APCs are diverse in phenotype, location, and function, optimal approaches for targeting APCs will require a clear understanding of their functions in vivo in alloBMT models. Thus far, studies on DC subsets have largely relied on exposing mice to an antigenic challenge, harvesting DC subsets, and testing their ability to prime T cells reactive against that antigen ex vivo.14-16,18,19 While this is a reasonable assay for whether a DC subset has access to antigen, it may not predict whether that population has an essential role in priming T cells in vivo. Our studies were directed at determining the necessary and sufficient roles of LCs, a key subset of DCs, in the genesis and severity of GVHD using systems that did not require artificial cell transfer.
LCs were of interest, particularly for skin GVHD, because a long-standing view has been that they have an essential role in priming T cells against cutaneous antigens.47 LCs were previously shown to be sufficient for cutaneous GVHD induction when other host APCs were replaced by donor APCs,45 and T cells primed by skin-draining LNs, which contain migratory LCs, are more likely to accumulate in the skin.32 We therefore hypothesized that host LCs would contribute to GVHD even when other APCs were present. However, counter to the hypothesis, we found that GVHD was similar in LC-deficient and LC-sufficient recipients in both MHC-matched and MHC-mismatched alloBMT models. Notably, in the strain pairings we chose, host-derived APCs were essential for GVHD induction.
The absence of host LCs, if anything, promoted cutaneous GVHD. Histopathology scores of the skin and ear were similar in both Tg+ and Tg− CD8-recipient groups, although there was a trend toward worse skin pathology in Tg+ recipients (P = .094). In the B6bm12→B6 MHCII-mismatched GVHD model, CD4 cells induced similar clinical and histopathologic GVHD and serum inflammatory cytokines in LC-deficient or LC-sufficient recipients. Thus, when all other host APCs were intact and only host LCs were missing, GVHD was not impaired. If these models accurately represent HLA-matched and HLA-mismatched alloSCT in humans, then our data indicate that specific ablation of epidermal LCs before transplantation would not be an effective means of preventing GVHD.
Our studies also shed light on the regulation of host LC turnover in alloBMT. As in prior reports, we found that LCs remained host in origin in wild-type recipients of T cell–depleted allogeneic BM.44,46 We previously found that C3H.SW→B6 chimeras, which we show in the present work to have residual host LCs, do not develop GVHD.7 From this we can conclude that host LCs are not sufficient for GVHD induction in this MHC-matched model, and therefore the sufficiency of host LCs to initiate GVHD45 is at most model dependent. It is likely that LCs are more effective in initiating GVHD in MHC-mismatched transplantations wherein a relatively small number of competent host APCs are sufficient for GVHD induction.
In both the C3H.SW→B6 and B6.C→BALB/c models, donor T cells only partially induced replacement of host LCs by donor LCs. This is different from the complete turnover reported in a fully MHC-mismatched system44 and the absence of any turnover in the B6→C3H.SW MHC-matched strain pairing.46 Thus, the ability of donor T cells to induce LC turnover is also model specific and perhaps depends on the magnitude of the allogeneic response and whether cutaneous GVHD develops.
Immunofluorescence of epidermal sheets from transplanted mice revealed that LC turnover in T-cell recipients was focal, with islands of donor-derived LCs. This suggests that the conditions that induce LC migration and engraftment by blood-derived LC precursors occur locally. This LC geography parallels observations we made in mice with LC-specific ablation of transforming growth factor-β1 (TGF-β1) or the TGF-β receptor.48 In these mice, LCs were generally absent; however, over time, due to some inefficiency of Cre-mediated gene deletion, foci of LCs with intact TGF-β1 or TGF-βR genes developed.
Because GVHD itself promotes donor LC engraftment, it follows that GVHD ongoing after the first few weeks of transplantation would have to be supported by donor-type LCs, if indeed LCs were involved in ongoing GVHD. We therefore tested whether donor LCs promote or inhibit GVHD. As was the case with recipient LCs, the absence of donor LCs did not have a major effect on clinical or histopathologic GVHD in either the C3H.SW→B6 or B6.C→BALB/c models. Even when both donors and hosts lacked LCs (C3H.SW→B6 model), GVHD was similar to that in LC-intact recipients of LC-intact donor BM.
The mechanisms whereby donor T cells and ultraviolet (UV) irradiation induce LC turnover have not been fully elucidated.44,45 Merad et al speculated that a combination of an empty niche, inflammation, and chemokine induction contributed to LC turnover.44,45 Indeed, the effects of both donor T cells and UV irradiation are pleiotropic. UV irradiation causes acute cell death and local inflammation,49-52 and alloreactive T cells induce systemic cytokines and local inflammation in skin and can directly target nonhematopoietic and hematopoietic cells in addition to LCs. Our finding that donor LCs engrafted in Langerin-DTA recipients, which have a very specific loss of only LCs,23 without the need for allogeneic T cells or UV irradiation indicates that skin inflammation per se is not required for donor LC engraftment. Rather, the data suggest that the status of the LC compartment is regulated by a Langerin+ cell in the LC lineage, and that in the absence of this cell type, the compartment is receptive to LC repopulation by circulating cells53 regardless of inflammation or induction of MCP-1 and CCL20.44,45 Therefore, it is very likely that the role of donor allogeneic T cells in promoting LC engraftment in the setting of GVHD is to eliminate resident LCs specifically, creating an empty compartment that is receptive to engraftment in the same manner as the skin of Langerin-DTA mice. This scenario may be similar to how the LC compartment is populated during embryonic and fetal development.54
The finding that host LCs were radiation resistant and sufficient to promote GVHD has garnered significant attention. However, from a clinical perspective, it is important to know whether such cells are necessary rather than just sufficient, and we found that they are not. Moreover, even engrafting donor LCs in a GVHD setting was not required to sustain GVHD. In the course of these studies, we also found that host LCs are not sufficient to cause GVHD in a miHA-mismatched system, further defining and restricting the roles of LCs in GVHD. A final contribution of these studies is further insight into the basic biology of LC turnover, in that we found that an empty niche is sufficient to promote donor LCs. Prior studies using allogeneic T cells to target host LCs also had very considerable associated tissue destruction and inflammation, and thus could not distinguish these factors from whether the elimination of host LCs was required for donor LC engraftment. Niches are thus normally filled and are limiting for LC colonization of epidermis. Overall, the major clinical implication of our studies is that the ablation of any single DC subset, such as LCs, may not have a decisive effect because alloreactive T cells would be productively primed by other subsets, and thus a comprehensive host APC depletion strategy is likely to be required for clinical impact.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the Yale Animal Resources Center for expert animal care.
This work was supported National Institutes of Health Grants HL-0183072 and P01 AI064343. W.D.S is a recipient of a Clinical Scholar Award from the Leukemia & Lymphoma Society.
National Institutes of Health
Authorship
Contribution: H.L. designed and executed experiments, analyzed results, and cowrote the paper; H.S.T., S.V., and K.J. assisted in experiments; J.M. and A.J.D. analyzed pathology; D.H.K. created the Langerin-DTA mice and edited the manuscript; C.M.-M. assisted in experiments and analyzed data; M.J.S. created the Langerin-DTA mice, designed experiments, and cowrote the manuscript; and W.D.S. created the Langerin-DTA mice, designed experiments, analyzed results, and cowrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Warren D. Shlomchik, Section of Hematology, Yale Comprehensive Cancer Center, PO Box 208032, Yale University School of Medicine, New Haven, CT 06520-8032; e-mail: warren.shlomchik@yale.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal