Abstract
We hypothesized that regulatory T cells (Tregs) could play a beneficial role during HIV infection by controlling HIV replication in conventional T cells (Tcons). Purified Tregs and Tcons from healthy donors were activated separately. Tcons were infected with the X4 or R5 HIV strains and cultured with or without autologous Tregs. Coculture of Tcons and Tregs resulted in a dose-dependent inhibition of Tcon infection, which was significant when a 1:1 Treg:Tcon ratio was used. Treg suppression of HIV infection was largely mediated by contact-dependent mechanisms. Blockage of cytotoxic T-lymphocyte–associated antigen-4 did not significantly reduce Treg function. In contrast, Tregs acted through cAMP-dependent mechanisms, because the decrease of cAMP levels in Tregs, the blockade of gap junction formation between Tregs and Tcons, the blockage of CD39 activity, and the blockage of protein kinase A in Tcons all abolished Treg-mediated suppression of HIV replication. Our data suggest a complex role for Tregs during HIV infection. Although Tregs inhibit specific immune responses, their inhibition of HIV replication in Tcons may play a beneficial role, particularly during early HIV infection, when the effector immune cells are not yet activated. Such a protective role of Tregs could have a profound impact on infection outcome.
Introduction
Regulatory T cells (Tregs) are characterized by their ability to suppress effector responses to pathogens, particularly in the setting of chronic infections. Tregs accumulate in the lymphoid tissues of HIV-infected individuals with active viral replication.1-3 Interestingly, Treg frequency is higher in mucosal tissues than in the peripheral blood of untreated HIV-infected individuals,3 suggesting that Tregs could reduce the availability of target cells for HIV replication in these tissues. Supporting this hypothesis, Tregs are activated very early after Simian immunodeficiency virus (SIV) infection in African green monkeys, which could limit harmful generalized activation and allow for this infection to remain nonpathogenic.4 Similarly, in utero activation of Tregs in HIV-exposed, uninfected children appears to limit vertical transmission by reducing T-cell activation.5 Further supporting this hypothesis, cytotoxic T-lymphocyte–associated antigen-4 (CTLA-4) blockage during acute primary SIV infection in rhesus macaques reduced the numbers of Tregs and increased the viral replication at mucosal sites.6 These data suggest that the anti-inflammatory process mediated by Tregs could play a beneficial role during HIV/SIV infection, particularly during the early phases, when the effector immune cells are not yet activated. Tregs may also limit the infection in sites where there is high level of activation, such as the gut. However, the effect that Tregs exert on the HIV infection of CD4+ non-Treg cells has not yet been investigated and was the object of this study.
Tregs can exert their regulatory activity via a vast array of mechanisms, which include both contact-independent and -dependent mechanisms.7 The mechanisms underlying Treg-suppressive activity may vary depending on the experimental system. Contact-dependent mechanisms appear to be critical in vitro, because blockage of TGF-β and IL-10 signaling does not block Treg-suppressive activity, whereas physical separation between Tregs and conventional T cells (Tcons) does.8 Within these contact-dependent mechanisms, some experiments have implicated CTLA-4 as the major mediator of suppression,9 whereas other studies did not reach the same conclusion.10 Recently, Tregs were also shown to inhibit Tcon activation by a mechanism involving cAMP, a known inhibitor of T-cell growth, differentiation, and proliferation. Indeed, Tregs exhibit high intracellular concentrations of cAMP and can transfer cAMP to Tcons through the gap junctions that form between both cell types.11,12 Furthermore, it has been reported that the ectonucleotidases CD39 and CD73 are expressed on human Tregs and Tcons. CD39, a member of the ectonucleotidase triphosphate diphosphohydrolase family, is the rate-limiting enzyme that hydrolyzes ATP and ADP into AMP, whereas CD73, an ecto-5′-nucleotidase, exists in a soluble or membrane-bound form and catalyzes the dephosphorylation of AMP to adenosine.13 Such a pathway also contributes to Treg-suppressive functions14,15
In the present study, we show that Tregs decreased the level of HIV infection of Tcons in a dose-dependent manner. This activity did not depend on the type of HIV strain involved, because Tregs decreased infection by both X4 and R5 viruses. Our results also indicate that contact with Tcons was necessary for Treg sup-pression, whereas soluble mediators such as IL-10 were not critical. Furthermore, our data suggest that Tregs act through cAMP-dependent mechanisms activating protein kinase A (PKA) in Tcons.
Methods
Human subjects
Blood samples came from healthy, HIV-negative subjects recruited by the Hoxworth Blood Center (Cincinnati, OH). Because the samples were not collected for research purposes and no identifier was provided to us, the University of Cincinnati Institutional Review Board determined this activity to be exempted from IRB review and surveillance.
Cell isolation and culture
PBMCs were separated with a Ficoll-Hypaque gradient (GE Healthcare). Resting CD4+ T cells were purified by negative selection using a CD4 separation kit (Miltenyi Biotec) according to the manufacturer's instructions. Purified CD4+ T cells were stained with anti–CD8-FITC, anti–CD25-allophycocyanin (APC; BD Pharmingen), and anti–CD127-PE (Beckman Coulter), and Tregs and Tcons were separated by cell sorting using a FACSAria flow cytometer (BD Biosciences). The purity of Tregs (CD8negCD25hiCD127low) and Tcons (CD8negCD25lowCD127hi) was evaluated after sorting by intracellular staining of FOXP3 using the anti-FOXP3 Ab clone PCH101 (e-Bioscience) and analysis on an FACS LSR II flow cytometer (BD Biosciences). Purity of the sorted populations was ≥ 90% (data not shown).
Purified Tregs and Tcons were activated for 3 days using anti–CD3/CD28 beads (1 cell:3 beads; Invitrogen), incubated at 37°C and 5% CO2 in the presence of 100 U/mL IL-2 (Hoffmann La Roche; provided by the National Institutes of Health AIDS Research and Reference Reagent Program, Bethesda, MD) in RPMI medium supplemented with 100 U/mL penicillin, 10 μg/mL streptomycin, 2mM glutamine, 10mM HEPES, and 10% FCS (Life Technologies).
Virus production and infection
HIV was prepared from 293T cells transfected with plasmids encoding either R5-tropic (YK-JRCSF) or X4-tropic (NL4-3) HIV laboratory strains, using the FuGENE transfection technology (Roche) according to the manufacturer's protocol. After 2 days, supernatants were harvested and the viruses were precipitated using polyethyleneglycol. Virus titers were determined using TZM-bl indicator cells.
Coculture of Tregs and Tcons
Activated Tcons (3-4 × 105) were stained with 0.3μM CFSE (Molecular Probes) and infected with NL4-3 or YK-JRCSF for 2 hours at 37°C at a multiplicity of infection of 1, washed twice with complete RPMI medium, and cocultured for 3 days in the presence or absence of activated autologous Tregs in the presence of 100 U/mL of IL-2. Tregs were not labeled with CFSE.
Determination of HIV-p24Gag levels by ELISA and flow cytometry
Coculture supernatants were collected 3 days after infection, and HIV-p24Gag levels were quantified by ELISA (sensitivity limit of 150 pg/mL; SAIC). To evaluate intracellular HIV p24Gag, CD39, CD73, and cell proliferation, Tregs and Tcons were treated with 20 μg/mL of human IgG to block Fc receptors, and stained with anti–CD4-PB (clone RPA-T; BioLegend), anti–CD39-PECy7 (clone eBioA1e; eBiosciences), anti–CD73-APC (clone AD2; BioLegend), and Live/Dead Fixable Red Dead Cell Stain Kit (Invitrogen) for 30 minutes at room temperature in PBS containing 2% FCS and 0.1% sodium azide. Cells were washed, fixed with 2% formaldehyde for 30 minutes at 4°C, and stained with anti–HIV-p24Gag-PE (clone KC-57; Beckman Coulter), anti–Ki67-PercP-Cy5.5 (clone B56; BD Biosciences), or in some experiments with anti–cyclin B-AF647 (clone V152; Beckman Coulter) in 0.3% saponin buffer for 30 minutes at 4°C. To determine Tcon proliferation and cell cycle, the mean fluorescence intensity (MFI) of the CFSE, Ki67, and cyclin B staining was analyzed. At least 100 000 events were acquired for each sample and analyzed using FACSDiva software Version 6.1.2. Live cells were gated based on forward- and side-scatter properties and the absence of fluorescence in the Live/Dead Viability Assay.
Cytokine quantification in culture supernatants
IL-10, IL-13, TNF-α, IFN-γ, and GM-CSF levels were quantified in the 3-day coculture supernatants using the Luminex assay (Millipore) at a sensitivity limit of 3.2 pg/mL.
Blockade of Treg effect
In some experiments, Tregs and Tcons were cocultured in 3-μm pore size Transwell plates (Costar). In other experiments, cocultures were performed in the presence of anti–human CD152 (CTLA-4, 10 μg/mL, clone BNI3; Beckman Coulter), anti–human CD210 (IL-10R, 10 μg/mL, clone 3F9; BioLegend), or anti–human CD39 (10 μg/mL, clone A1; AdD Serotec), matched isotype control antibodies, or H89 dihydrochloride (H89), a PKA inhibitor (10uM; Tocris Bioscience). In some experiments, Tregs were treated with 2′,5′-dideoxyadenosine (ddADA), an inhibitor of adenyl cyclase (200uM; Sigma-Aldrich), for 24 hours or with connexin 43 mimetic peptide GAP27 (SRPTEKTIFII), a gap junction–blocking peptide (100uM; Tocris Bioscience) for 2 hours, after which Tregs were extensively washed to avoid a direct contact of Tcons with the reagents and cocultured with infected Tcons. In other experiments, Tcons were treated for 2 hours with forskolin (2μM; Sigma-Aldrich), an adenyl cyclase activator that increases intracellular cAMP content, and then infected with HIV. In some experiments, Tcons were infected and further cultured in the presence of N6-benzoyladenosine-3′,5′-cyclic monophosphate sodium salt (6-Bnz-cAMP), a cAMP analog that specifically activates PKA (500uM; Sigma-Aldrich).
Calcein transfer from Tregs to Tcons through gap junctions
To evaluate gap junction–mediated cell-cell communication, calcein transfer was evaluated by flow cytometry. Activated Tcons were stained with CFSE, infected with NL4-3, and cocultured for 3 days in the presence or absence of activated Tregs. Tregs were stained with calcein blue AM (Invitrogen) following the manufacturer's instructions. Briefly, Tregs were incubated in PBS containing 3μM calcein AM for 40 minutes at 37°C and 5% CO2. The cells were then washed with PBS plus FCS and cocultured with infected Tcons. The percentage of calcein-positive cells was measured in Tcons (gated CFSE-positive cells).
Intracellular cAMP quantification
Tregs and Tcons were collected and intracellular cAMP levels were quantified in cell lysates using a commercially available assay (cAMP Direct Biotrak EIA; GE Healthcare Biosciences) at a sensitivity limit of 12.5 fmol.
Statistical analysis
Statistical analysis was performed using Prism 5 software (GraphPad). HIV-p24Gag levels in supernatants and p24 intracellular stains were compared using paired t tests. P < .05 was considered to be significant.
Results
Culture of infected Tcons with Tregs decreases their level of HIV infection
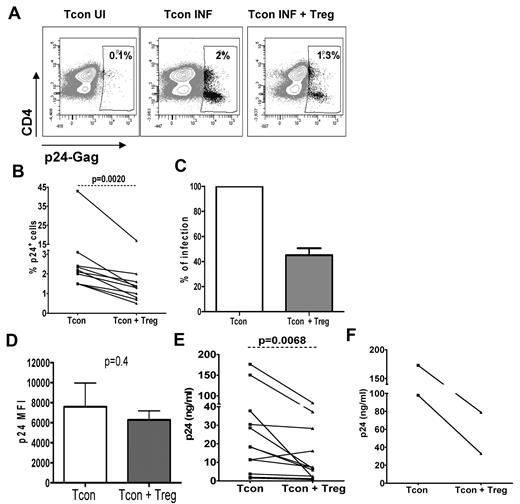
We used an in vitro infection model to test the hypothesis that Tregs can control HIV infection in activated T cells. Purified Tcons (CD4+CD25−CD127+) from healthy donors were stimulated and infected with HIV, then mixed with purified autologous Tregs (CD4+CD25+CD127−). To determine the level of infection in both populations, Tcons were labeled with CFSE at the time of infection. As shown in a representative experiment (Figure 1A), the percentage of infected Tcons decreased on average by 2-fold when cultured with Tregs at a 1:1 ratio (47% ± 5.5% of reduction, P < .01; Figure 1B-C). Similar results were found when the 2 donors with high levels of infection were excluded (P = .0039). Tregs did not affect the levels of p24Gag per cell, because p24 MFI was similar in both conditions (7599 ± 2365 in Tcons cultured alone vs 6283 ± 906 in Tcons in cocultures, P = .4; Figure 1D). As expected for cells that had been stimulated for 7 days, we observed a down-regulation of CD4, which was greater in the HIV-infected cultures, a usual feature of in vitro exposure to HIV.
Suppression of HIV infection in Tcons is mediated by Tregs. Tcons were infected with HIV and cocultured with Tregs for 3 days at a 1:1 ratio. HIV p24Gag levels were measured by flow cytometry and ELISA. (A) Flow cytometry data from 1 representative experiment. The percentage of HIV-p24Gag+ cells is indicated in each panel. UI indicates uninfected; INF, infected. (B) Graph showing the mean (± SEM) percentage of HIV-p24Gag+ Tcons cultured alone or in the presence of Tregs (n = 9). (C) Levels of infection in Tcons cultured alone were considered as 100% infection. The percentage of infection of Tcons cultured with Tregs was then calculated for each donor using the equation (X = percentage of HIVp24Gag+ Tcons cultured with Tregs × 100; percentage of HIV-p24Gag+ Tcons cultured alone; n = 9). (D) MFI of HIV-p24Gag determined in gated HIV-p24Gag+ Tcons. Graph shows the mean (± SEM) MFI of the HIV-p24Gag+ Tcons cultured alone and in the presence of Tregs (n = 6). (E-F) HIV-p24Gag+ levels were measured by ELISA in culture supernatants collected 3 days after infection with the X4 strain (E) or the R5 strain (F) of HIV.
Suppression of HIV infection in Tcons is mediated by Tregs. Tcons were infected with HIV and cocultured with Tregs for 3 days at a 1:1 ratio. HIV p24Gag levels were measured by flow cytometry and ELISA. (A) Flow cytometry data from 1 representative experiment. The percentage of HIV-p24Gag+ cells is indicated in each panel. UI indicates uninfected; INF, infected. (B) Graph showing the mean (± SEM) percentage of HIV-p24Gag+ Tcons cultured alone or in the presence of Tregs (n = 9). (C) Levels of infection in Tcons cultured alone were considered as 100% infection. The percentage of infection of Tcons cultured with Tregs was then calculated for each donor using the equation (X = percentage of HIVp24Gag+ Tcons cultured with Tregs × 100; percentage of HIV-p24Gag+ Tcons cultured alone; n = 9). (D) MFI of HIV-p24Gag determined in gated HIV-p24Gag+ Tcons. Graph shows the mean (± SEM) MFI of the HIV-p24Gag+ Tcons cultured alone and in the presence of Tregs (n = 6). (E-F) HIV-p24Gag+ levels were measured by ELISA in culture supernatants collected 3 days after infection with the X4 strain (E) or the R5 strain (F) of HIV.
Confirming the flow cytometry data, coculture of Tcons with Tregs led to a 2-fold decrease of the HIV-p24Gag levels measured by ELISA in coculture supernatants (P < .01; Figure 1E). This decrease in HIV-p24Gag release was observed in 10 of 12 donors analyzed. Because the HIV-p24Gag found in these supernatants could have also originated from Treg infection, we determined the cellular source of HIV-p24Gag by flow cytometry. HIV-p24Gag MFI was similar in Tcons and Tregs (data not shown), and the percentage of infected Tcons was 2-fold higher than that of infected Tregs (1.23% ± 0.16% vs 0.52% ± 0.14%, respectively), suggesting that the inhibitory effect of Tregs may have been underestimated in the ELISA data. The inhibition exerted by Tregs was not restricted to infections by an X4 virus, because a similar 50% reduction in HIV-p24Gag levels was observed when Tcons infected by the R5 strain YK-JRCSF were in contact with autologous Tregs (Figure 1F).
Treg-suppressor effect of HIV infection is dose dependent
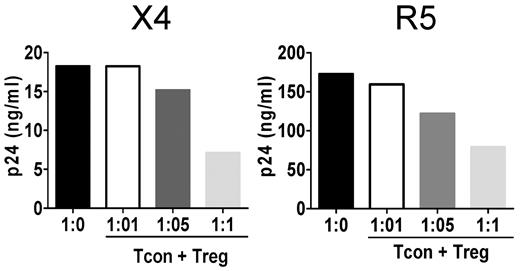
Treg-mediated suppression of T-cell proliferation has been shown to be dose dependent in vitro.16 Similarly, we found that Tregs were effective at a 1:1 ratio, but that their effect decreased with decreasing ratios (Figure 2A left). Equivalent dose-dependent inhibition was observed when R5 viruses were used (Figure 2A right). When the Tcon:Treg ratio was increased to 1:2, the Treg-mediated suppression reached 82% (data not shown).
Treg suppression of HIV infection is dose dependent. Tcons were infected with HIV and cocultured at Tcon:Treg ratios of 1:0, 1:0.1, and 1:1. Levels of HIV p24Gag measured by ELISA in culture supernatants 3 days after infection. One representative experiment for the X4 strain (left panel) and the R5 strain (right panel) of HIV are shown (n = 2 for each virus).
Treg suppression of HIV infection is dose dependent. Tcons were infected with HIV and cocultured at Tcon:Treg ratios of 1:0, 1:0.1, and 1:1. Levels of HIV p24Gag measured by ELISA in culture supernatants 3 days after infection. One representative experiment for the X4 strain (left panel) and the R5 strain (right panel) of HIV are shown (n = 2 for each virus).
Treg-suppressive activity of HIV infection is largely mediated by contact-dependent mechanisms
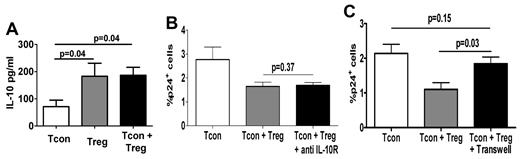
Secretion of suppressive cytokines such as IL-10 has been proposed as one of the mechanisms inducing Treg-mediated suppression.7 Because IL-10 levels were significantly increased in cocultures (P = .04; Figure 3A), we evaluated the role of IL-10 in the suppression of Tcon infection by Tregs using a blocking anti–IL-10R antibody.17 As shown in Figure 3B, similar levels of infection in Tcons were observed regardless of whether anti–IL-10R was present in the coculture. We therefore investigated whether the suppressive effect of Tregs on infection was contact dependent by coculturing Tregs and Tcons in Transwell plates. Blockade of the contact between Tcons and Tregs diminished Treg suppression by 80% in 5 independent experiments (P < .05; Figure 3C). It must be noted that the percentage of infected Tcons in Transwell experiments was always, albeit nonsignificantly, lower than that observed in Tcons cultured alone, a result that suggests the minor contribution of additional contact-independent mechanisms.
Treg-suppressive activity of HIV infection is mediated by contact. (A) IL-10 levels were measured in 3-day supernatants of cultured Tcons, Tregs, or Tcons + Tregs using the Luminex assay. (B) Tcons were HIV infected and cocultured with Tregs in the presence or absence of anti–IL-10R blocking antibody (10 μg/mL). Graph shows the mean (± SEM) percentage of HIV-p24Gag+ Tcons (n = 6). (C) Graph showing the mean (± SEM) percentage of HIV-p24Gag+ Tcons cultured alone, with Tregs, or with Tregs in a Transwell plate (n = 5).
Treg-suppressive activity of HIV infection is mediated by contact. (A) IL-10 levels were measured in 3-day supernatants of cultured Tcons, Tregs, or Tcons + Tregs using the Luminex assay. (B) Tcons were HIV infected and cocultured with Tregs in the presence or absence of anti–IL-10R blocking antibody (10 μg/mL). Graph shows the mean (± SEM) percentage of HIV-p24Gag+ Tcons (n = 6). (C) Graph showing the mean (± SEM) percentage of HIV-p24Gag+ Tcons cultured alone, with Tregs, or with Tregs in a Transwell plate (n = 5).
CTLA-4 does not play a major role in the Treg-suppressive activity of HIV infection
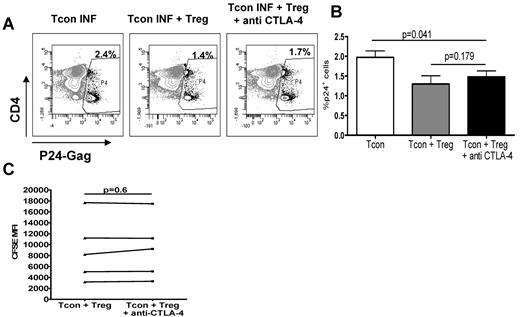
CTLA-4 is involved in Treg-contact–dependent suppression. We therefore addressed whether blockage of CTLA-4 affected Treg-mediated suppression. Although the results varied between donors, the presence of blocking anti–CTLA-4 antibody18 in the cocultures blocked the Treg-suppressive effect by only 28% ± 9%, which was not significant (Figure 4A-B). Blocking of CTLA-4 did not affect the levels of HIV-p24Gag per cell, because p24 MFI was similar whether anti–CTLA-4 was present or not (6034 ± 896 in Tcons + Tregs vs 6294 ± 1099 in Tcons + Tregs + anti–CTLA-4, P = .6). Blocking of CTLA-4 did not affect Tcon proliferation (Figure 4C) and was always less efficient at abolishing Treg-mediated suppression of HIV infection than blocking contact between Tregs and Tcons (25.7% ± 8.8% vs 4.9% ± 2.8% suppression, respectively, n = 3).
CTLA-4 in Tregs is not the main contact-dependent mechanism. (A) Representative dot plot showing the percentage of infection in Tcons cultured alone (left), with Tregs (middle), or with Tregs in the presence of anti–CTLA-4 blocking antibody (10 μg/mL; right). (B) Graph showing the mean (± SEM) percentage of HIV-p24Gag+ Tcons in these 3 conditions (n = 6). (C) CFSE MFI measured in Tcons cultured with Tregs in the presence or absence of anti–CTLA-4 blocking antibody (n = 5).
CTLA-4 in Tregs is not the main contact-dependent mechanism. (A) Representative dot plot showing the percentage of infection in Tcons cultured alone (left), with Tregs (middle), or with Tregs in the presence of anti–CTLA-4 blocking antibody (10 μg/mL; right). (B) Graph showing the mean (± SEM) percentage of HIV-p24Gag+ Tcons in these 3 conditions (n = 6). (C) CFSE MFI measured in Tcons cultured with Tregs in the presence or absence of anti–CTLA-4 blocking antibody (n = 5).
cAMP influx through gap junction formation plays an important role in Treg-mediated suppression of HIV infection
cAMP has been suggested as an alternative Treg-contact–dependent mechanism. In agreement with a previous report,11 Tregs contained high levels of cAMP compared with Tcons (234 ± 110 fmol/million cells and 38 ± 24 fmol/million cells, respectively). To determine whether cAMP can decrease HIV replication, Tcons were treated before infection with forskolin to increase their cAMP content. Forskolin-treated Tcons exhibited lower levels of infection than untreated cells (30% decrease of HIV-p24Gag+ cells in forskolin-treated cells). Therefore, we determined the role of cAMP in Treg suppression by treating Tregs with an adenyl cyclase inhibitor, ddADA, which decreases intracellular levels of cAMP in Tregs, before culturing them with infected Tcons. ddADA treatment decreased cAMP levels in treated Tregs (60.8 ± 39.0 vs 36.6 ± 25.8 fmol/million cells in untreated and ddADA-treated Tregs, respectively, n = 4). Strikingly, treatment of Tregs by this adenyl cyclase inhibitor completely abolished their suppressive activity (Figure 5A-B). To analyze whether Tregs decrease HIV infection through an effect on Tcon proliferation, we labeled Tcons with CFSE at the time of infection. Three days after coculture, we analyzed CFSE MFI and levels of the cell-cycle markers Ki67 and cyclin B in gated Tcons. Because Tcons had already been activated for 3 days when they were labeled, CFSE staining was homogeneous in this population, but decreased proliferation could be evidenced by a higher CFSE MFI. As expected, Tcon proliferation was significantly reduced when cells were cultured in the presence of Tregs, as evidenced by increased CFSE MFI. Decreased levels of expression of Ki67 and cyclin B were also found (Figure 5C). In addition, Tcon cytokine production was significantly reduced when cells were cultured in the presence of Tregs, as shown by decreased levels of IL-13, IFN-γ, TNF-α, and GM-CSF (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Treatment of Tregs with the adenylate cyclase inhibitor ddADA did not significantly reduce their capacity to inhibit Tcon proliferation, cell cycle, or cytokine production (Figure 5C and supplemental Figure 1).
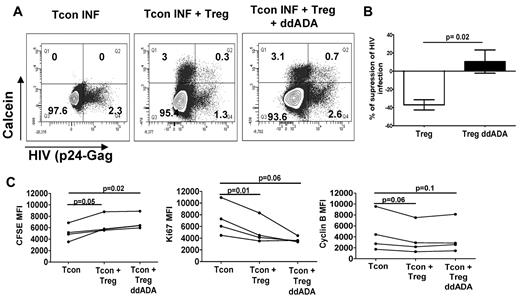
cAMP plays a role in the Treg-suppressive activity of HIV infection. (A) Infected Tcons cultured alone, with untreated Tregs, or with Tregs previously treated with an inhibitor of adenyl cyclase (200uM ddADA). Tregs were loaded with calcein AM and Tcons were stained by CFSE before culture. The formation of contact between Tregs and Tcons was evidenced by the transfer of calcein from Tregs to Tcons. Data represent the percentage of infected cells in gated (CFSE+) Tcons after 3 days of coculture. (B) Graph showing the mean (± SEM) percentage of suppression of HIV infection in these conditions (n = 4). (C) Graph showing the MFI of CFSE, Ki67, and cyclin B staining in gated Tcons after their culture with Tregs treated or not with ddADA (n = 4).
cAMP plays a role in the Treg-suppressive activity of HIV infection. (A) Infected Tcons cultured alone, with untreated Tregs, or with Tregs previously treated with an inhibitor of adenyl cyclase (200uM ddADA). Tregs were loaded with calcein AM and Tcons were stained by CFSE before culture. The formation of contact between Tregs and Tcons was evidenced by the transfer of calcein from Tregs to Tcons. Data represent the percentage of infected cells in gated (CFSE+) Tcons after 3 days of coculture. (B) Graph showing the mean (± SEM) percentage of suppression of HIV infection in these conditions (n = 4). (C) Graph showing the MFI of CFSE, Ki67, and cyclin B staining in gated Tcons after their culture with Tregs treated or not with ddADA (n = 4).
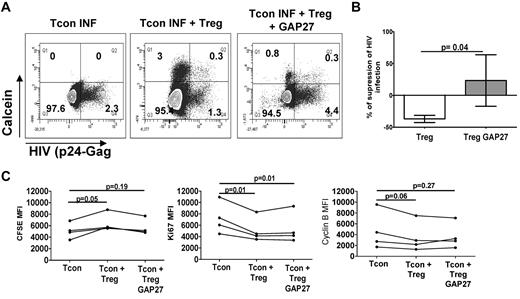
Tregs were shown to be able to transfer cAMP through gap junctions to their targets.11 To determine the contribution of such a mechanism in our model, we evaluated the transfer of a permeant dye, calcein AM, which can only be transferred from donor to recipient cells via gap junctions.11 Coculture of CFSE-labeled Tcons with calcein-loaded Tregs showed an influx of calcein into a small subset of Tcons (ranging from 2.9%-12%). Blocking gap junction formation with GAP27 (a connexin 43 mimetic peptide) decreased the percentage of calcein+ Tcons by 43% ± 6% and completely inhibited Treg suppression of Tcon infection (Figure 6A-B). Similar to our results with ddADA-treated Tregs, treatment of Tregs with GAP27 did not change their capacity to inhibit Tcon proliferation, cell cycle, or cytokine production (Figure 6C and supplemental Figure 1). These data support the hypothesis that Tregs can use gap junctions to infuse cAMP into Tcons, which then modulate Tcon levels of HIV infection. Our data also suggest that Treg-mediated inhibition of Tcon proliferation and cell cycling is not the only mechanism involved in their control of HIV infection. Treg viability was not decreased by the different treatments, indicating that the loss of their suppressive activity was not due to their death in vitro.
Gap junction formation plays a role in the Treg-suppressive activity of HIV infection. (A) Infected Tcons cultured alone, with untreated Tregs, or with Tregs previously treated with the gap junction inhibitor Gap27 (100uM). Tregs were loaded with calcein AM and Tcons were stained by CFSE before culture. The formation of contact between Tregs and Tcons was evidenced by the transfer of calcein from Tregs to Tcons. Data represent the percentage of infected cells in gated (CFSE+) Tcons after 3 days of coculture. (B). Graph showing the mean (± SEM) percentage of suppression of HIV infection in these conditions (n = 3). (C) Graph showing the MFI of CFSE, Ki67, and cyclin B staining in gated Tcons after their culture with Tregs treated or not with GAP27 (n = 4).
Gap junction formation plays a role in the Treg-suppressive activity of HIV infection. (A) Infected Tcons cultured alone, with untreated Tregs, or with Tregs previously treated with the gap junction inhibitor Gap27 (100uM). Tregs were loaded with calcein AM and Tcons were stained by CFSE before culture. The formation of contact between Tregs and Tcons was evidenced by the transfer of calcein from Tregs to Tcons. Data represent the percentage of infected cells in gated (CFSE+) Tcons after 3 days of coculture. (B). Graph showing the mean (± SEM) percentage of suppression of HIV infection in these conditions (n = 3). (C) Graph showing the MFI of CFSE, Ki67, and cyclin B staining in gated Tcons after their culture with Tregs treated or not with GAP27 (n = 4).
The ectonucleotidase CD39 play an important role in Treg-mediated suppression of HIV infection
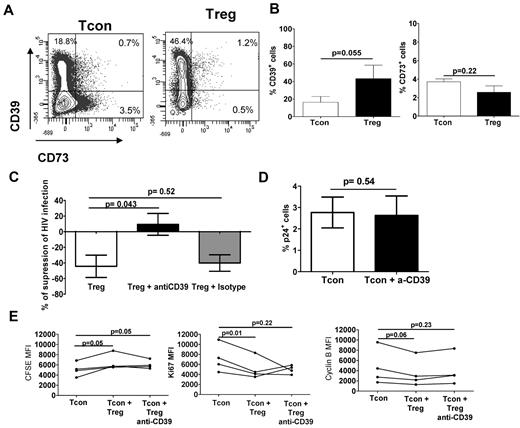
Transfer of cAMP only occurred in a small subset of Tcons, whereas HIV infection was reduced by 50% in cells cultured with Tregs, suggesting that a mechanism other than gap junction transfer of cAMP may also play a role. Therefore, we hypothesized that CD39-mediated adenosine production may also contribute to Treg HIV-suppressive capacity. We therefore evaluated the expression of CD39 and CD73 in the Tregs and Tcons after coculture. CD39 expression was significantly higher in Tregs compared with Tcons, whereas CD73 expression was similar in Tregs and Tcons (Figure 7A-B). We found very few CD39+CD73+ Tregs or Tcons. The addition of anti-CD39 blocking antibody19 to Treg:Tcon cocultures completely blocked Treg suppression (Figure 7C). The anti-CD39 blocking antibody acted on Tregs, because it had no effect on infected Tcons cultured alone (Figure 7D). Treatment of Tregs with anti-CD39 also did not abolish their capacity to inhibit Tcon proliferation, cell cycle, or cytokine production (Figure 7E and supplemental Figure 1).
CD39 activity plays a role in the Treg-suppressive activity of HIV infection. Tregs and Tcons were stained with anti–CD39PE-Cy7 and anti–CD73-APC after 3 days of coculture and analyzed by flow cytometry. (A) CD39 and CD73 expression of 1 representative individual. (B) Graph showing the mean (± SEM) percentage of CD39+ (left) and CD73+ (right) cells (n = 4). (C) Infected Tcons cultured alone or with Tregs in presence or absence of either an anti-CD39 blocking antibody or a matched isotype control. Tregs were loaded with calcein AM before culture. Graph shows the mean (± SEM) percentage of suppression of HIV infection after the addition of the anti-CD39 blocking antibody or the matched isotype control (n = 3). (D) Infected Tcons cultured in the presence or absence of anti-CD39 blocking antibody (10 μg/mL). Graph shows the mean (± SEM) percentage of HIV-p24Gag+ Tcons (n = 3). (E) Graph showing the MFI of CFSE, Ki67, and cyclin B staining in gated Tcons after their culture with Tregs treated or not with anti-CD39 (n = 4).
CD39 activity plays a role in the Treg-suppressive activity of HIV infection. Tregs and Tcons were stained with anti–CD39PE-Cy7 and anti–CD73-APC after 3 days of coculture and analyzed by flow cytometry. (A) CD39 and CD73 expression of 1 representative individual. (B) Graph showing the mean (± SEM) percentage of CD39+ (left) and CD73+ (right) cells (n = 4). (C) Infected Tcons cultured alone or with Tregs in presence or absence of either an anti-CD39 blocking antibody or a matched isotype control. Tregs were loaded with calcein AM before culture. Graph shows the mean (± SEM) percentage of suppression of HIV infection after the addition of the anti-CD39 blocking antibody or the matched isotype control (n = 3). (D) Infected Tcons cultured in the presence or absence of anti-CD39 blocking antibody (10 μg/mL). Graph shows the mean (± SEM) percentage of HIV-p24Gag+ Tcons (n = 3). (E) Graph showing the MFI of CFSE, Ki67, and cyclin B staining in gated Tcons after their culture with Tregs treated or not with anti-CD39 (n = 4).
PKA activation plays a significant role in Treg-mediated suppression of HIV infection
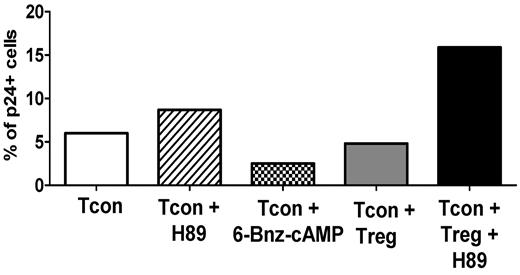
To elucidate the mechanism involved in cAMP-mediated suppression of HIV replication, we looked at the contribution of PKA as a downstream effector of cAMP activity. We performed experiments using the PKA inhibitor H89 and 6-Bnz-cAMP, a cAMP analog that specifically activates PKA. As shown in a representative experiment in Figure 8, H89 treatment increased the infection of Tcons, and the addition of H89 in the Tcon + Treg coculture abolished the capacity of Tregs to suppress HIV infection of Tcons. In support of a role of PKA, treatment of Tcons with 6-Bnz-cAMP decreased their level of infection. These results suggest that Treg-mediated activation of PKA through cAMP plays a pivotal role in their suppression of HIV infection
Treg-suppressive activity of HIV infection is PKA mediated. Infected Tcons were cultured alone, in the presence of the PKA inhibitor H89 (10uM), or in the presence of a cAMP analog that specifically activates PKA (6-Bnz-cAMP, 500uM). Tcon were also cultured with Tregs alone or in the presence of 10uM H89. Tregs were loaded with calcein AM and Tcons were stained with CFSE before culture. Data represent the percentage of infected cells in gated (CFSE+) Tcons after 3 days. One representative experiment of 2 is shown.
Treg-suppressive activity of HIV infection is PKA mediated. Infected Tcons were cultured alone, in the presence of the PKA inhibitor H89 (10uM), or in the presence of a cAMP analog that specifically activates PKA (6-Bnz-cAMP, 500uM). Tcon were also cultured with Tregs alone or in the presence of 10uM H89. Tregs were loaded with calcein AM and Tcons were stained with CFSE before culture. Data represent the percentage of infected cells in gated (CFSE+) Tcons after 3 days. One representative experiment of 2 is shown.
Discussion
The role played by Tregs in HIV pathogenesis remains controversial. Two seemingly opposing hypotheses have been proposed. A detrimental role of Tregs was suggested based on several lines of evidence showing that immune-mediated control of viral replication is associated with low frequency of lymphoid FOXP3+ T cells in lymphoid tissue, whereas uncontrolled active viral replication is associated with high frequency of these cells and Treg depletion increases specific anti-HIV and anti-SIV immune responses.1-3,20-23 In contrast, other data suggested that Tregs could be beneficial by controlling immune activation, which would reduce the size of the susceptible target cell pool and therefore HIV replication.5,6,21 The second hypothesis had not been thoroughly tested previously, but the results of our study strongly supports it. Tregs have recently been shown to decrease infection of murine macrophages by a pseudotyped HIV virus and consequently to attenuate HIV-associated neurodegeneration.24 These data broaden our hypothesis, because they suggest that Tregs may control HIV replication in several types of target cells. Furthermore, the presence of Tregs has also been reported to reduce viral loads in a murine model of herpes simplex virus infection, suggesting a more complex role for Tregs during viral infections than was recognized previously.25
The rationale underlying our experimental conditions involved the activation of Tregs and Tcons before they were cultured together. In this way, we tried to model the changes in the mucosa, in which a majority of Tcons are activated through exposure to commensal microbes or other foreign antigens, and are thus susceptible targets for HIV infection, as evidenced by their high expression of CCR5.26 Similarly, Tregs have been shown to have an activated phenotype during HIV infection,16 which is the reason that we activated them before culture with Tcons. Our previous data also suggested that tissue Tregs are more suppressive than resting circulating Tregs, because the depletion of Tregs from lymph node cells increased SIV-specific responses, whereas depletion of circulating Tregs had no effect.23 These data are in agreement with the fact that, in vitro, activated Tregs are more efficient at suppressing Tcon function than resting Tregs.27,28 During HIV/SIV infection, Treg frequency increases in the mucosa,1-3,20 suggesting that the Tcon:Treg ratio changes in these tissues after infection. Although we acknowledge that the 1:1 ratio used in our cocultures is higher than that achieved in vivo, this ratio has been routinely used in in vitro suppression assays performed with human cells because the variability between human donors requires using assays with a consistent and robust readout. Our in vitro studies therefore address the question of whether Tregs could control HIV infection of target cells independently of their effect on the initial activation of Tcons. Based on our data, it is possible to envision a model whereby the afflux of Tregs occurring in mucosa after HIV infection contributes to the control of Tcon infection. However, we acknowledge that, in vitro, infection of peripheral Tcons may not completely mimic infection of resident mucosal T cells, so this question will need to be addressed in future studies.
One important goal is to determine by which mechanism Tregs control HIV infection. Tregs can exert their regulatory activity via a vast array of mechanisms, which include both cell contact-independent and -dependent mechanisms. However, in our model, physical separation of the cells in Transwell plates abrogated Treg suppression. Furthermore, IL-10 did not appear to be critical. These data are in agreement with the fact that, in vitro, blockage of IL-10 did not affect the suppressive effect of Tregs on Tcon proliferation or cytokine production, either in our experiments or in previous studies.29 However, IL-10 could be involved in Treg-mediated suppression of HIV in vivo, because it has been shown to exhibit some antiviral properties30 and its levels increase during HIV infection (although Tregs are not the only cellular source of IL-10).31
Several studies have reported that target-cell suppression can occur through direct contact between Tregs and Tcons.32,33 FOXP3+ cells accumulate in the paracortical T-cell zones of secondary lymphatic tissues shortly after SIV infection, where they are found in close proximity to FoxP3−CD4+ T cells.20 Our previous immunohistochemistry studies of tonsils of chronically infected HIV+ patients also showed close proximity between FoxP3+ and activated CD69+FoxP3− T cells,1 suggesting that, in vivo, Tregs could directly act on Tcons through contact-dependent mechanisms. Possible mechanisms include CTLA-4 ligation and metabolic disruption mediated by cAMP transfer or CD39/CD73-mediated production of adenosine. CTLA-4 is constitutively expressed by Tregs, and CTLA-4 blockage decreases Treg activity both in vitro and in vivo.34 In addition, Treg-mediated suppression of Tcons was shown to be reduced in CTLA-4–deficient mice.35 Nonetheless, an anti–CTLA-4 blocking antibody had no significant effect on Treg suppression of HIV infection, leaving metabolic disruption as the most likely mechanism explaining our data.
cAMP was recently shown to be used by Tregs to suppress T-cell function. Indeed, blockage of cAMP degradation in Tregs using a selective inhibitor of phosphodiesterases such as rolipram strongly improved Treg-mediated suppression, leading to better control of tissue inflammation, goblet cell metaplasia, and allergic airway disease.12 Similarly, cholera toxin, which activates adenyl cyclase and thus increases cAMP levels, induced an anergic-like state in Tcons.36 We confirmed in the present study that Tregs contain higher intracellular levels of cAMP than do Tcons, as described previously.11 These increased cAMP levels could be explained by several mechanisms. First, in our experimental model, Tregs and Tcons were cultured with exogenous IL-2, which induces adenyl cyclase activity in Tregs, leading to cAMP accumulation, whereas it diminishes adenyl cyclase activity in Tcons.37 Alternatively, inhibition of miR-142-3p by FOXP3 could play a role, because miR-142-3p decreases adenyl cyclase and increases phosphodiesterase expression. Therefore, FOXP3-mediated inhibition of this microRNA would be expected to keep the cAMP pathway more active in Tregs than in Tcons.38
Our results clearly indicate a role for cAMP in the Treg-mediated suppression of HIV infection/replication in Tcons. Indeed, increased cAMP levels in Tcons caused a reduction in the frequency of HIV-p24Gag+ Tcons. In addition, inhibition of adenyl cyclase in Tregs, resulting in low levels of cAMP, abrogated Treg suppression, suggesting that elevated concentrations of endogenous cAMP in Tregs are necessary for them to suppress HIV replication. Furthermore, our data suggest that the mechanisms underlying Treg function involve the transfer of cAMP into Tcons through the formation of membrane gap junctions and/or the generation of adenosine mediated by CD39/CD79. Gap junctions allow for transfer of ions, metabolites, and molecules up to 1 kDa between cells,39 and are formed by the docking of 2 opposing hemichannels (connexons), each constructed from 6 connexins. Tregs and Tcons express different connexins, the expression of which is increased after activation.11 Using a dye that spreads from donor to recipient cells only via gap junctions,39 we found that gap junctions can form between Tregs and Tcons, as described previously.11 The fact that Treg treatment with a gap junction inhibitor reduced Treg function as much as the adenyl cyclase inhibitor strongly supports the hypothesis that transfer of cAMP through gap junctions from Tregs to Tcons plays an important role in Treg control of HIV infection. However, the percentage of infection in calcein− Tcons was also decreased when Tregs were treated with ddADA, suggesting several possibilities. First, it is possible that calcein staining underestimates the formation of gap junctions between Tregs and Tcons. Alternatively, and nonexclusively, cAMP has been shown to increase CTLA-4 expression,40 and therefore ddADA treatment may have further inhibited Treg function. More extensive studies of connexin regulation in Tregs will be needed in the future. Gap junctions also form between Tregs and dendritic cells, which results in the decreased stimulatory activity of dendritic cells,41 suggesting that this Treg-suppressive mechanism may also apply to other HIV cellular targets. Although gap junctions are involved in Treg suppression, our data also suggest that pericellular generation of adenosine by the CD39/CD73 ectoenzymes42 likely contributes to Treg-mediated suppression of HIV. Recently, it has been reported that CD39 and CD73 are expressed on human T cells,14,15 although the same cells rarely express both enzymes (Figure 7). CD73 also exists in a soluble form,13 so the low levels of expression detected by flow cytometry likely do not recapitulate the levels of CD73 present in the cultures. Adenosine is a signaling molecule that binds to the adenosine receptors (A1, A2a, A2b, and A3) present on many cell types, and it regulates multiple physiologic responses, including anti-inflammatory effects. The binding of adenosine to the A2A receptor activates inhibitory pathways through the elevation of cAMP.43 A significantly increased expression of CD39 on Tregs from HIV-1–infected patients was described previously in a study suggesting that the CD39-adenosine axis could be involved in Treg-mediated inhibition in these patients.44 The fact that treatment with an anti-CD39 blocking antibody reduced the capacity of Tregs to decrease HIV infection in Tcons strongly supports the hypothesis that cAMP induction in Tcons plays an important role in Treg control of HIV infection.
Downstream of cAMP, several signaling pathways could be activated in Tcons, including PKA and activation of small guanine nucleotide exchange factors.45 When bound to cAMP, an exchange protein directly activated by cAMP (Epac) can activate the small Ras-like GTPase Rap1 to elicit downstream responses such as T-cell anergy.46 In addition, cAMP is known to regulate Ca2+ levels both directly by opening cation channels and indirectly through PKA,47 which also regulates T-cell proliferation and cytokine production. However, our present results strongly implicate the involvement of the PKA pathway, because inhibition of PKA activation in Tcons abolished Treg suppression of HIV infection (Figure 8).
The exact mechanism involved in cAMP-mediated suppression of HIV replication is not yet known. Previous studies have demonstrated that cAMP has a negative effect on HIV replication/production and long-terminal repeat transcription in peripheral blood lymphocytes and human T-cell lines. Increasing cAMP by adenyl cyclase activation48 or blocking cAMP degradation with an inhibitor of phosphodiesterases such as rolipram prevented p24Gag antigen release in the supernatants from HIV-infected T cells.49 This effect was explained by activation of the CREB protein, which competes with phosphorylated NF-κB for limiting amounts of CBP/p300.48 cAMP also mediated suppression of the nuclear import of HIV DNA, indicating that the cAMP pathway can affect HIV infection at both the pre- and postintegration steps.50 Our data point toward these mechanisms, which are further supported by the fact that treatment of T cells with a phosphodiesterase inhibitor inhibited HIV transcription without affecting T-cell proliferation.49 However, an alternative explanation of our data is that a higher intracellular concentration of cAMP is required to affect HIV replication than to affect the cell cycle or proliferation, because ddADA treatment reduced cAMP levels in Tregs by only approximately one-half. Further work will be needed to precisely delineate the mechanism of action of Tregs, particularly their effect on HIV long-terminal repeat transcription and maturation.
In summary, our study describes a contact-dependent inhibition of HIV replication in Tcons that is mainly mediated by cAMP. Overall, Tregs play a complex role during HIV infection because they inhibit specific immune responses, thus decreasing immunologic control of HIV-infected cells and therefore uncontrolled viral replication. However, a Treg-mediated decrease of HIV infection could play a beneficial role, particularly during early HIV infection, when the effector immune cells are not yet activated, or by limiting the infection in sites where there is high level of activation, such as the gut. Furthermore, Treg-mediated control of HIV replication could also be beneficial by attenuating the toxicity exerted by HIV or HIV antigens on nonimmune cells such as neurons or liver cells. Such a protective role of Tregs could have a profound impact in infection outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank the National Institutes of Health AIDS Research and Reference Reagent Program for the IL-2, cell lines, and HIV laboratory strains, and the Cincinnati Children's Hospital Research Foundation Sorting Core and Drs Dave Hildeman, Gene Shearer, Celine Silva-Lages, Pietro Presicce, and Kris Orsborn for expert assistance and review of this manuscript.
This study was supported by grants from the Public Health Service (AI068524 to C.A.C.) and from the Departamento Administrativo de Ciencia, Tecnología e Innovación (Colciencias; 111540820490-1 to C.M.R.).
National Institutes of Health
Authorship
Contribution: M.E.M.-F., C.M.R., and L.K.R. performed experiments and analyzed data, and M.E.M.-F., C.M.R., and C.A.C. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Claire Chougnet, Division of Molecular Immunology (ML 7021), Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: Claire.Chougnet@cchmc.org.
References
Author notes
M.E.M.-F. and C.M.R. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal