Abstract
The median age of chronic myeloid leukemia (CML) patients is ∼ 60 years, and age is still considered an important prognostic factor, included in Sokal and EURO risk scores. However, few data are available about the long-term outcome of older patients treated with imatinib (IM) frontline. We analyzed the relationship between age and outcome in 559 early chronic-phase CML patients enrolled in 3 prospective clinical trials of Gruppo Italiano Malattie Ematologiche dell'Adulto CML Working Party, treated frontline with IM, with a median follow-up of 60 months. There were 115 older patients (≥ 65 years; 21%). The complete cytogenetic and major molecular response rates were similar in the 2 age groups. In older patients, event-free survival (55% vs 67%), failure-free survival (78% vs 92%), progression-free survival (62% vs 78%), and overall survival (75% vs 89%) were significantly inferior (all P < .01) because of a higher proportion of deaths that occurred in complete hematologic response, therefore unrelated to CML progression (15% vs 3%, P < .0001). The outcome was similar once those deaths were censored. These data show that response to IM was not affected by age and that the mortality rate linked to CML is similar in both age groups. This trial was registered at www.clinicaltrials.gov as #NCT00514488 and #NCT00510926.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test and/or complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 5784.
Disclosures
Giovanni Martinelli serves on the speakers' bureaus of Novartis, Bristol-Myers Squibb, and Pfizer. F. Pane receives research support from Novartis; is a consultant for Novartis and Bristol-Myers Squibb; and serves on the Novartis speakers' bureau. Giuseppe Saglio is a consultant for Novartis and Bristol-Myers Squibb and serves on the speakers' bureaus of Novartis and Bristol-Myers Squibb. Michele Baccarani receives research support from Novartis, Bristol-Myers Squibb, and Pfizer; is a consultant for Novartis, Bristol-Myers Squibb, and Pfizer; and serves on the Novartis speakers' bureau. Gianantonio Rosti is a consultant with Novartis and serves on the speakers' bureaus of Novartis and Bristol-Myers Squibb. The remaining authors; the Associate Editor Martin S. Tallman; and the CME questions author Charles P. Vega, University of California, Irvine, CA, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Analyze how age affects outcomes of CML
Compare treatment response to imatinib among younger and older adults
Evaluate survival outcomes among older and younger adults treated with imatinib
Release date: May 26, 2011; Expiration date: May 26, 2012
Introduction
The incidence of Philadelphia-positive (Ph+) chronic myeloid leukemia (CML) increases with age: the reported median age at diagnosis is more than 60 years in epidemiologic registries1,2 and of approximately 50 to 55 years in clinical trials.3-5 Following the most widely accepted recommendations that set at 65 years the boundary between young and old persons, a relevant proportion of CML patients at diagnosis are “elderly.” However, it should be remembered that no definition of elderly based purely on age in the context of oncohematology is satisfactory: a different “scoring system” would help the proper allocation of patients to an effective, albeit expensive and potentially toxic, treatment.6
Older age has been considered a poor prognostic factor in patients with CML.7,8 The negative impact of age on response rates and long-term survival was observed regardless of the treatment strategy: busulfan, hydroxyurea (HU), interferon-α (IFN-α), and allogeneic stem cell transplantation (SCT).9-11 The 2 more widely used prognostic scores for CML, namely, the Sokal12 and EURO13 risk scores, proposed and validated before the advent of imatinib (IM), identified older age as a variable predicting lower response rates and worse outcome. The reasons that underlie the adverse impact of older age on outcome in CML are poorly understood: it is a common notion that toxicities of SCT and IFN-α increase with age; for other forms of treatment, such as busulfan and HU, the explanation is much more difficult and elusive. Moreover, it was thought that different biologic features of CML, comorbidities, worse medical care (which leads to delayed diagnosis and/or inadequate follow-up), and other factors in addition to the therapy given may contribute to the negative impact of age in older CML patients.14,15
The introduction of IM, an orally taken and well-tolerated tyrosine kinase inhibitor, changed dramatically the prognosis of CML patients, especially in late and early chronic phase (CP).16-20 Therefore, well-established prognostic factors may have lost or reduced their relevance after the advent of IM. Currently, few data are available reporting the long-term outcome of older IM-treated CML patients. The 2 largest published series15,21 analyzed mainly late-CP patients after IFN-α failure or intolerance. Rosti et al21 reported on 284 patients in late CP, of whom 58 (20%) were more than 65 years of age: lower rates of response (hematologic and cytogenetic) in older versus younger patients were observed; however, overall survival (OS), with a median follow-up of 36 months, was similar. Of 747 patients treated in a single institution (M. D. Anderson Cancer Center) and reported by Cortes et al, 187 were in early-CP and 49 (26%) of them were more than 60 years old at starting IM: with a short follow-up (median, 16 months), similar rates of complete cytogenetic response (CCyR) and OS were observed between the 2 groups of age. Recently, in a small single-center cohort of 40 older patients (≥ 65 years) of 117 patients in early CP treated frontline with IM, no differences in response rates were found between older and younger patients.22
Therefore, we analyzed the impact of age on the outcome of a large series of 559 patients with early-CP CML, treated with IM frontline, enrolled in 3 different, concurrent trials of the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) CML Working Party.
Methods
Patients
We analyzed 559 patients with Ph+ CML in early CP, enrolled between May 2003 and April 2007 in 3 clinical trials of the GIMEMA CML Working Party: CML/021 (#NCT00514488), a phase 2 trial exploring IM 800 mg in intermediate Sokal risk CP CML (82 patients); CML/022 (#NCT00510926), a phase 3 trial comparing IM 400 mg versus IM 800 mg in high Sokal risk CP CML (112 patients); and CML/023, an observational trial of IM 400 mg in CP CML (365 patients).
Patients enrolled were required to be at least 18 years old and to have Ph+ and BCR-ABL+ CML in early CP (6 months or less from diagnosis to IM start, HU only allowed). Women of childbearing potential were required to have a negative pregnancy test before starting IM, and all fertile patients were asked to use an acceptable method to avoid pregnancy. No upper age limit was set for any of the 3 studies. For the studies CML/021 and CML/022, patients were required to have an adequate performance status (Eastern Cooperative Oncology Group 0-2), serum creatinine < 2 mg/dL, total bilirubin, aspartate transaminase, and alanine transaminase < 3 times upper limit of normal (detailed inclusion criteria have been published previously).23,24 All the patients provided written informed consent before enrollment. The studies were reviewed and approved by the Internal Review Board of all the participating institutions and performed in accordance with the Declaration of Helsinki.
Treatment monitoring and definition of response
Hematologic, cytogenetic, and molecular responses have been defined according to the updated European LeukemiaNet (ELN) recommendations (2009).25 Details regarding monitoring have been published previously.23 Briefly, a conventional bone marrow cytogenetic (chromosome banding analysis) evaluation was requested baseline, after 3 (only high Sokal risk patients, trial CML/022), 6, and 12 months on treatment and every 6 months thereafter. Peripheral blood fluorescence in situ hybridization was allowed to confirm a CCyR if < 20 metaphases scored in chromosome banding analysis.26
The molecular monitoring was based on peripheral blood samples for real-time quantitative polymerase chain reaction, collected before therapy, after 3, 6, 12 months, and every 6 months thereafter. All the evaluations have been performed in the same molecular biology laboratory (Bologna University). The molecular biology methodology has been published previously.23,24 The molecular response was defined as major (MMR) if the BCR-ABL/ABL ratio was ≤ 0.1% according to the International Scale.27,28
Definition of progression, failure, and events
Progression to accelerated/blast phase (AP/BP) was defined according to ELN criteria29 : myeloblasts in blood or bone marrow of at least 15%; myeloblasts plus promyelocytes in blood or bone marrow more than 30%; basophils in blood 20% or more; persistent thrombocytopenia (platelet count < 100 × 109/L) unrelated to therapy; or by any extramedullary blast involvement, excluding spleen and liver. Treatment failures were defined according to the updated ELN recommendations25 : no complete hematologic response (CHR) at 3 months, no cytogenetic response (CyR) at 6 months, < partial CyR (PCyR) at 12 months, < CCyR at 18 months, loss of CHR or CCyR or progression to AP/BP, occurrence of clonal cytogenetic abnormalities in Ph+ cells (CCA/Ph+), occurrence of mutation poorly sensitive to IM at any time, and death. Events were defined as: treatment failure or permanent discontinuation of IM for any reason (including toxicity, patient refusal, or loss to follow-up). For the patients treated within the 2 prospective clinical trials CML/021 and CML/022 (194 of 559; 35%), detailed analyses of adverse events and IM dosing are available and already published23,24 ; for the patients registered in the observational trial CML/023 (365 of 559; 65%), only data about response and severe adverse events occurrence have been retrieved.
Statistical methods
Means were compared with the t test and frequencies with the χ2 test or Fisher exact test, as appropriate. OS, progression-free survival (PFS), failure-free survival (FFS), and event-free survival (EFS) were calculated from the date of the first IM dose until death (OS), until progression to AP or BP or death (PFS), until failure (FFS), and until any event (EFS). Curves of CCyR, MMR, OS, PFS, FFS, and EFS were plotted using the Kaplan-Meier method. Survival functions were compared by the log-rank test. The analyses have been performed according to the intention-to-treat principle.
Results
Patients
The database for this analysis has been locked in October 2010, when the median follow-up was 60 months (range, 1-83 months). Baseline characteristics of the patients are detailed in Table 1. The median age of the whole population was 52 years (range, 18-84 years). For the purpose of this evaluation, we divided the patients population in 2 groups of older (≥ 65 years old, n = 115, 21%) and younger patients (< 65 years, n = 444, 79%), according to the commonly accepted cut-off to define an “old” person.
Patients
| . | Total . | ≥ 65 y . | < 65 y . | P . |
|---|---|---|---|---|
| Patients, n (%) | 559 | 115 (21) | 444 (79) | |
| Males, n (%) | 336 (60) | 71 (61) | 265 (59) | .75 |
| Age, y* | 52 (18-84) | 71 (65-84) | 46 (18-64) | |
| Prior HU* | 254 (45) | 48 (42) | 206 (46) | .4 |
| Hemoglobin, g/dL* | 12.2 (6.4-17.5) | 12.8 (7.5-16.3) | 12.1 (6.4-17.5) | .02 |
| White blood cells, ×109/L* | 54.8 (1-500) | 42 (4-481) | 61 (1-500) | .01 |
| Blast cells, %* | 1 (0-10) | 0 (0-8) | 1 (0-10) | < .0001 |
| Eosinophils, %* | 2 (0-15) | 2 (0-11) | 2 (0-15) | .53 |
| Basophils, %* | 1 (0-19) | 2 (0-16) | 2 (0-19) | .47 |
| Platelets, ×109/L* | 352 (74-4920) | 337 (74-1520) | 355 (90-4920) | .11 |
| Spleen, cm below costal margin* | 2 (0-24) | 0 (0-15) | 2 (0-24) | < .0001 |
| Sokal, n (%) | ||||
| Low | 219 (39) | 10 (9) | 209 (47) | < .0001 |
| Intermediate | 216 (39) | 83 (72) | 133 (30) | |
| High | 124 (22) | 22 (19) | 102 (23) | |
| ECOG performance status, n (%) | ||||
| 0 | 0 | 0 | 0 | .37 |
| 1 | 441(79) | 87 (76) | 354 (80) | |
| 2 | 118 (21) | 28 (24) | 90 (20) | |
| High-dose imatinib, n (%) | 136 (24) | 26 (23) | 110 (25) | .71 |
| . | Total . | ≥ 65 y . | < 65 y . | P . |
|---|---|---|---|---|
| Patients, n (%) | 559 | 115 (21) | 444 (79) | |
| Males, n (%) | 336 (60) | 71 (61) | 265 (59) | .75 |
| Age, y* | 52 (18-84) | 71 (65-84) | 46 (18-64) | |
| Prior HU* | 254 (45) | 48 (42) | 206 (46) | .4 |
| Hemoglobin, g/dL* | 12.2 (6.4-17.5) | 12.8 (7.5-16.3) | 12.1 (6.4-17.5) | .02 |
| White blood cells, ×109/L* | 54.8 (1-500) | 42 (4-481) | 61 (1-500) | .01 |
| Blast cells, %* | 1 (0-10) | 0 (0-8) | 1 (0-10) | < .0001 |
| Eosinophils, %* | 2 (0-15) | 2 (0-11) | 2 (0-15) | .53 |
| Basophils, %* | 1 (0-19) | 2 (0-16) | 2 (0-19) | .47 |
| Platelets, ×109/L* | 352 (74-4920) | 337 (74-1520) | 355 (90-4920) | .11 |
| Spleen, cm below costal margin* | 2 (0-24) | 0 (0-15) | 2 (0-24) | < .0001 |
| Sokal, n (%) | ||||
| Low | 219 (39) | 10 (9) | 209 (47) | < .0001 |
| Intermediate | 216 (39) | 83 (72) | 133 (30) | |
| High | 124 (22) | 22 (19) | 102 (23) | |
| ECOG performance status, n (%) | ||||
| 0 | 0 | 0 | 0 | .37 |
| 1 | 441(79) | 87 (76) | 354 (80) | |
| 2 | 118 (21) | 28 (24) | 90 (20) | |
| High-dose imatinib, n (%) | 136 (24) | 26 (23) | 110 (25) | .71 |
No relevant baseline differences were evident between the 2 groups of patients, apart from the risk distribution: a larger proportion of younger patients were low Sokal risk versus older ones; high Sokal risk was equally represented in the 2 cohorts.
HU indicates hydroxyurea; and ECOG, Eastern Cooperative Oncology Group.
Median (range).
No relevant differences, as far as baseline hematologic variables, were evident between the 2 groups, apart from the risk distribution: a larger proportion of younger patients were low Sokal risk versus older ones (47% vs 9%), whereas high Sokal risk patients were equally represented in the 2 cohorts.
Response and outcome
The actual response rates observed in the 2 age groups were similar: CHR at 3 months was 111 of 115 (97%) and 426 of 444 (96%), in older and younger patients, respectively; CCyR at 6, 12, and 18 months was 79 of 115 (69%), 90 of 115 (78%), and 85 of 115 (74%) in older patients, respectively, and 299 of 444 (67%), 343 of 444 (77%), and 346 of 444 (78%) in younger ones, respectively; MMR at 6, 12, and 18 months was 54 of 115 (47%), 67 of 115 (58%), and 65 of 115 (57%) in older patients, respectively, and 215 of 444 (48%), 262 of 444 (59%), and 278 of 444 (63%) in younger ones, respectively; all the differences were not significant (Table 2; Figure 1). Median time to CCyR and median time to MMR were 6 and 12 months, respectively, in both groups of patients. The cumulative incidence of CCyR and MMR was 87% (100 of 115) and 85% (98 of 115) vs 88% (391 of 444) and 85% (377 of 444), in older and younger patients, respectively (all the differences were not significant; Table 2; Figure 1). Moreover, with a median observation of 52 months after the first CCyR, no significant difference in its stability was observed: CCyR was lost by 14 of 100 (14%) and 32 of 391 (8%) older and younger patients, respectively (P = .084); of these patients, 3 older (3%; after 7-35 months) and 9 younger ones (2%; after 4-54 months) subsequently progressed to AP/BP (P = .7). Similarly, the rate of progression to AP/BP for patients obtaining a MMR was not different between older and younger ones (3 of 95, 3.1% vs 6 of 371, 1.6%; P = .4).
Response rates at each time point*
| . | ≥ 65 y (115 pts), n (%) . | < 65 y (444 pts), n (%) . | P . |
|---|---|---|---|
| 3 mo | |||
| CHR | 111 (97) | 426 (96) | > .999 |
| 6 mo | |||
| CCyR | 79 (69) | 299 (67) | .82 |
| MMR | 54 (47) | 215 (48) | .83 |
| 12 mo | |||
| CCyR | 90 (78) | 343 (77) | .9 |
| MMR | 67 (58) | 262 (59) | .92 |
| 18 mo | |||
| CCyR | 85 (74) | 346 (78) | .38 |
| MMR | 65 (57) | 278 (63) | .24 |
| Cumulative incidence | |||
| CCyR | 100 (87) | 391 (88) | .75 |
| MMR | 98 (85) | 377 (85) | > .999 |
| . | ≥ 65 y (115 pts), n (%) . | < 65 y (444 pts), n (%) . | P . |
|---|---|---|---|
| 3 mo | |||
| CHR | 111 (97) | 426 (96) | > .999 |
| 6 mo | |||
| CCyR | 79 (69) | 299 (67) | .82 |
| MMR | 54 (47) | 215 (48) | .83 |
| 12 mo | |||
| CCyR | 90 (78) | 343 (77) | .9 |
| MMR | 67 (58) | 262 (59) | .92 |
| 18 mo | |||
| CCyR | 85 (74) | 346 (78) | .38 |
| MMR | 65 (57) | 278 (63) | .24 |
| Cumulative incidence | |||
| CCyR | 100 (87) | 391 (88) | .75 |
| MMR | 98 (85) | 377 (85) | > .999 |
No significant difference between older and younger patients was observed at any time point for complete hematologic response (CHR; time points after 3 months not shown), complete cytogenetic response (CCyR), and major molecular response (MMR) rates. The cumulative incidence of CCyR and MMR were similar in the 2 groups of age.
All analyses were performed according to the intention-to-treat principle.
CCyR and MMR rates at 6, 12, and 18 months (actual response rates). Cumulative response rates were similar in older and younger patients (all differences were not significant). All analyses were performed according to the intention-to-treat principle.
CCyR and MMR rates at 6, 12, and 18 months (actual response rates). Cumulative response rates were similar in older and younger patients (all differences were not significant). All analyses were performed according to the intention-to-treat principle.
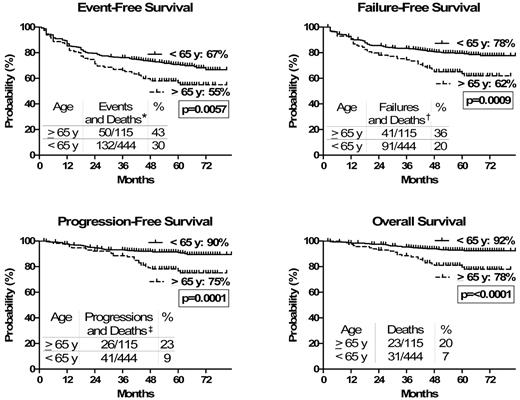
Regarding the long-term outcome, the estimated 6-year EFS (55% vs 67%, P = .006), FFS (62% vs 78%, P = .009), PFS (75% vs 90%, P = .0001), and OS (78% vs 92%, P < .0001) were all significantly worse in the older age group (Table 3; Figure 2).
Outcome*
| . | ≥ 65 y (115 pts), % (95% CI) . | < 65 y (444 pts), % (95% CI) . | P . |
|---|---|---|---|
| EFS† | 55 (45-64) | 67 (61-72) | .006 |
| FFS† | 62 (52-70) | 78 (73-82) | .0009 |
| PFS† | 75 (65-83) | 90 (86-92) | .0001 |
| OS† | 78 (68-85) | 92 (89-95) | < .0001 |
| Censoring deaths unrelated to CML progression | |||
| EFS† | 68 (58-76) | 70 (64-75) | .27 |
| FFS† | 76 (67-83) | 81 (77-85) | .14 |
| PFS† | 91 (84-95) | 93 (90-95) | .40 |
| OS† | 94 (87-98) | 96 (94-98) | .38 |
| . | ≥ 65 y (115 pts), % (95% CI) . | < 65 y (444 pts), % (95% CI) . | P . |
|---|---|---|---|
| EFS† | 55 (45-64) | 67 (61-72) | .006 |
| FFS† | 62 (52-70) | 78 (73-82) | .0009 |
| PFS† | 75 (65-83) | 90 (86-92) | .0001 |
| OS† | 78 (68-85) | 92 (89-95) | < .0001 |
| Censoring deaths unrelated to CML progression | |||
| EFS† | 68 (58-76) | 70 (64-75) | .27 |
| FFS† | 76 (67-83) | 81 (77-85) | .14 |
| PFS† | 91 (84-95) | 93 (90-95) | .40 |
| OS† | 94 (87-98) | 96 (94-98) | .38 |
Considering all events, with a median observation time of 60 (1-83) months, the estimated 6-year outcome resulted inferior for older patients (Kaplan-Meier method). Censoring the deaths unrelated to CML progression (in chronic phase and in complete hematologic response at the time of death) no difference was observed in the estimated 6-year outcome between older and younger patients (Kaplan-Meier method).
EFS indicates event-free survival; FFS, failure-free survival; PFS, progression-free survival; OS, overall survival; CML, chronic myeloid leukemia; ELN, European LeukemiaNet; CHR, complete hematologic response; CyR, cytogenetic response; PCyR, partial CyR; CCyR, complete CyR; AP/BP, accelerated phase/blast phase; CCA, clonal cytogenetic abnormalities; Ph+, Philadelphia positive; and IM, imatinib.
All analyses were performed according to the intention-to-treat principle
EFS, FFS, PFS, and OS were calculated from the date of the first imatinib dose until any event (EFS), until failure (FFS), until progression to AP/BP or death (PFS), and until death (OS). Treatment failures were defined according to the updated ELN recommendations25 : No CHR at 3 months, no CyR at 6 months, < PCyR at 12 months, < CCyR at 18 months, loss of CHR or CCyR or progression to AP/BP, occurrence of CCA in Ph+ cells (CCA/Ph+), occurrence of mutation poorly sensitive to imatinib at any time, and death. Events were defined as: treatment failure or permanent discontinuation of IM for any reason (including toxicity, patient refusal or loss to follow-up, and death).
Outcome. Considering all events, including deaths unrelated to CML progression, outcome was inferior for older patients. All analyses were performed according to the intention-to-treat principle. EFS, FFS, PFS, and OS were calculated from the date of the first IM dose until any event (EFS), failure (FFS), progression to AP/BP or death (PFS), and death (OS). *Events and deaths: treatment failure or permanent discontinuation of IM for any reason, including toxicity, patient refusal, or loss to follow-up and deaths of any cause. †Failures (updated European LeukemiaNet recommendations25 ) indicate no CHR at 3 months, no CyR at 6 months, < PCyR at 12 months, < CCyR at 18 months, loss of CHR or CCyR or progression to AP/BP, occurrence of CCA/Ph+, and occurrence of mutation poorly sensitive to IM at any time. Deaths indicate deaths of any cause. ‡Progressions indicate progressions to AB/BP. Deaths indicate deaths of any cause.
Outcome. Considering all events, including deaths unrelated to CML progression, outcome was inferior for older patients. All analyses were performed according to the intention-to-treat principle. EFS, FFS, PFS, and OS were calculated from the date of the first IM dose until any event (EFS), failure (FFS), progression to AP/BP or death (PFS), and death (OS). *Events and deaths: treatment failure or permanent discontinuation of IM for any reason, including toxicity, patient refusal, or loss to follow-up and deaths of any cause. †Failures (updated European LeukemiaNet recommendations25 ) indicate no CHR at 3 months, no CyR at 6 months, < PCyR at 12 months, < CCyR at 18 months, loss of CHR or CCyR or progression to AP/BP, occurrence of CCA/Ph+, and occurrence of mutation poorly sensitive to IM at any time. Deaths indicate deaths of any cause. ‡Progressions indicate progressions to AB/BP. Deaths indicate deaths of any cause.
The differences in term of outcome between older and younger patients, giving the same rates of CCyR and MMR, prompted a further analysis on the treatment failures in the 2 groups of patients. Failures because of primary and secondary resistance, detailed in Table 4, were 12 of 115 (10%) and 14 of 115 (12%), respectively, in older patients and 34 of 444 (8%) and 42 of 444 (10%), respectively, in younger ones (all differences were not significant); however, the proportion of patients not primary or secondary resistant that died was significantly higher among older patients. The analysis of the causes of death (Table 5) showed that in older patients more deaths in CHR (unrelated to CML progression) have been recorded: 17 of 115 (15%) and 15 of 444 (3%) for older and younger patients, respectively (P < .0001). On the other hand, deaths resulting from progression of CML were 6 of 115 (5%) and 15 of 444 (3%), for older and younger patients, respectively (P = .4). Regarding the deaths unrelated to CML progression, older patients died more frequently of: dementia (2 patients vs 0); pulmonary embolism (2 patients, after major surgery, vs 0); hemorrhagic event (2 patients, central nervous system [CNS] hemorrhage, vs 0); all patients, including the patients with pulmonary embolism and CNS hemorrhage, had a normal blood count at the time of death. In addition, deaths for cardiac disease were more frequent in older patients; however, this difference was not statistically significant (3, 2.6% vs 2, 0.5%). In particular, in 1 male patient (73 years old at CML diagnosis), a preexistent congestive heart failure worsened during 10 months of IM therapy; in 1 male patient of 66 years, in which coexisted chronic renal failure, congestive heart failure was the consequence of an acute coronary syndrome that occurred after 2 years of IM therapy and for which the patient was subsequently shifted to HU; the last patient (76 years old at CML diagnosis) died of a cardiac event not otherwise specified after 2.5 years in IM therapy. Deaths for second malignancies were more frequent in older patients: 5 of 115 (4%; 1 breast carcinoma, 1 pancreas carcinoma, 1 bile duct carcinoma, 1 renal carcinoma, and 1 multiple myeloma) versus 10 of 444 (2%; 4 colon carcinomas, 2 non-Hodgkin lymphomas, 2 CNS cancers, 1 esophageal carcinoma, and 1 lung carcinoma) in younger ones, albeit this difference was not statistically significant (P = .2). Three other older patients died of: a bacterial infection occurred in CCyR; a reactivation of a chronic pulmonary disease, preexistent to IM therapy; an acute renal failure secondary to nonsteroidal anti-inflammatory drug abuse. All patients, including the 2 patients with infection and reactivation of chronic pulmonary disease, had a normal blood count at the time of death.
| . | ≥ 65 y (115 pts), n (%) . | < 65 y (444 pts), n (%) . | P . |
|---|---|---|---|
| Primary resistance‡ | 12 (10) | 34 (8) | .34 |
| Secondary resistance§ | 14 (12) | 42 (10) | .38 |
| Deaths‖ | 15 (13) | 15 (3) | .0003 |
| Total | 41 (35) | 91 (21) | .0012 |
| . | ≥ 65 y (115 pts), n (%) . | < 65 y (444 pts), n (%) . | P . |
|---|---|---|---|
| Primary resistance‡ | 12 (10) | 34 (8) | .34 |
| Secondary resistance§ | 14 (12) | 42 (10) | .38 |
| Deaths‖ | 15 (13) | 15 (3) | .0003 |
| Total | 41 (35) | 91 (21) | .0012 |
No difference in terms of Primary or Secondary resistance was observed between older and younger patients; on the contrary, failures due to death unrelated to CML progression were significantly more in the older cohort.
European LeukemiaNet (ELN) criteria 25
All analysis were performed according to the intention-to-treat principle.
Primary resistance: no CHR within 3 months; no CyR at 6 months; no PCyR at 12 months; no CCyR at 18 months.
Secondary resistance: loss of CHR; loss of CCyR; progression to accelerated or blast phase; additional chromosomal abnormalities in Ph+ cells; new mutations.
Deaths: unrelated to CML progression.
Causes of death
| . | ≥ 65 y (115 pts), n (%) . | < 65 y (444 pts), n (%) . | P . |
|---|---|---|---|
| Progression to AP/BP | 6 (5.2) | 15 (3.4) | .41 |
| Transplantation-related mortality | 0 | 1 (0.2) | > .999 |
| Unrelated to CML progression* | 17 (14.8) | 15 (3.4) | < .0001 |
| Other malignancy | 5 (4.3) | 10 (2.3) | .21 |
| Cardiac disease | 3 (2.6) | 2 (0.5) | .06 |
| Pulmonary embolism | 2 (1.7) | 0 | .04 |
| CNS hemorrhage | 2 (1.7) | 0 | .04 |
| Dementia | 2 (1.7) | 0 | .04 |
| Acute renal failure | 1 (0.9) | 0 | .2 |
| Chronic pulmonary disease | 1 (0.9) | 1 (0.2) | .36 |
| Infection | 1 (0.9) | 2 (0.5) | .50 |
| Total | 23 (20) | 31 (7) | .0001 |
| . | ≥ 65 y (115 pts), n (%) . | < 65 y (444 pts), n (%) . | P . |
|---|---|---|---|
| Progression to AP/BP | 6 (5.2) | 15 (3.4) | .41 |
| Transplantation-related mortality | 0 | 1 (0.2) | > .999 |
| Unrelated to CML progression* | 17 (14.8) | 15 (3.4) | < .0001 |
| Other malignancy | 5 (4.3) | 10 (2.3) | .21 |
| Cardiac disease | 3 (2.6) | 2 (0.5) | .06 |
| Pulmonary embolism | 2 (1.7) | 0 | .04 |
| CNS hemorrhage | 2 (1.7) | 0 | .04 |
| Dementia | 2 (1.7) | 0 | .04 |
| Acute renal failure | 1 (0.9) | 0 | .2 |
| Chronic pulmonary disease | 1 (0.9) | 1 (0.2) | .36 |
| Infection | 1 (0.9) | 2 (0.5) | .50 |
| Total | 23 (20) | 31 (7) | .0001 |
The proportion of patients who died as a direct consequence of CML, either for progression to AP/BP or for transplantation related mortality, was similar between older and younger patients. On the other hand, older patients died more frequently of other causes, in CP and in CHR at the time of death, with respect to younger ones.
CNS indicates central nervous system.
See “Results” for details.
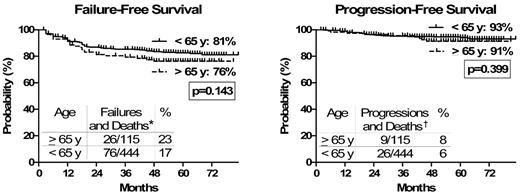
The analysis of the estimated 6-year EFS, FFS, PFS, and OS, censoring deaths unrelated to CML progression, showed that there was no longer any significant difference between the 2 age groups (OS, 94% vs 96; PFS, 91% vs 93%; FFS, 76% vs 81%; EFS, 68% vs 70%, in older and younger cohort, respectively; all P > .1; Table 3; Figure 3).
Outcome (deaths unrelated to CML progression censored). Censoring the deaths unrelated to CML progression (in CP and in CHR at the time of death), no difference was observed in the outcome between older and younger patients. FFS and PFS were calculated, censoring deaths unrelated to CML progression, from the date of the first IM dose until failure or death (FFS), and progression to AP/BP or death (PFS). *Failures (updated European LeukemiaNet recommendations25 ) indicate no CHR at 3 months, no CyR at 6 months, < PCyR at 12 months, < CCyR at 18 months, loss of CHR or CCyR or progression to AP/BP, occurrence of CCA/Ph+, and occurrence of mutation poorly sensitive to IM at any time. Deaths indicate deaths resulting from CML progression (other deaths censored). †Progressions indicate progressions to AB/BP. Deaths indicate deaths resulting from CML progression (other deaths censored).
Outcome (deaths unrelated to CML progression censored). Censoring the deaths unrelated to CML progression (in CP and in CHR at the time of death), no difference was observed in the outcome between older and younger patients. FFS and PFS were calculated, censoring deaths unrelated to CML progression, from the date of the first IM dose until failure or death (FFS), and progression to AP/BP or death (PFS). *Failures (updated European LeukemiaNet recommendations25 ) indicate no CHR at 3 months, no CyR at 6 months, < PCyR at 12 months, < CCyR at 18 months, loss of CHR or CCyR or progression to AP/BP, occurrence of CCA/Ph+, and occurrence of mutation poorly sensitive to IM at any time. Deaths indicate deaths resulting from CML progression (other deaths censored). †Progressions indicate progressions to AB/BP. Deaths indicate deaths resulting from CML progression (other deaths censored).
At last follow-up, the majority of patients were still on IM treatment: 75 of 115 (65%) and 327 of 444 (74%) of older and younger patients, respectively (P = .08). Among the patients who discontinued IM, 8 of 115 (7%) versus 51 of 444 (11%) were on second generation tyrosine kinase inhibitors (P = .008); 3 of 115 (3%) and 18 of 444 (4%) were on other treatment (SCT; HU; IFN-α) (P = .28); 23 of 115 (20%) versus 31 of 444 (7%) patients have died (P = .009); the disposition of 6 of 115 (5%) and 17 of 444 (4%) patients (P = 1) was unknown (Table 6).
Patient disposition at last contact
| . | ≥ 65 y (115 pts), n (%) . | < 65 y (444 pts), n (%) . | P . |
|---|---|---|---|
| Still on imatinib | 75 (65) | 327 (74) | .08 |
| Off imatinib | 40 (35) | 117 (26) | |
| Second-generation TKIs | 8 (7) | 51 (11) | .008 |
| Other (SCT; HU; IFNα) | 3 (3) | 18 (4) | .28 |
| Deaths | 23 (20) | 31 (7) | .0009 |
| Unknown | 6 (5) | 17 (4) | > .999 |
| . | ≥ 65 y (115 pts), n (%) . | < 65 y (444 pts), n (%) . | P . |
|---|---|---|---|
| Still on imatinib | 75 (65) | 327 (74) | .08 |
| Off imatinib | 40 (35) | 117 (26) | |
| Second-generation TKIs | 8 (7) | 51 (11) | .008 |
| Other (SCT; HU; IFNα) | 3 (3) | 18 (4) | .28 |
| Deaths | 23 (20) | 31 (7) | .0009 |
| Unknown | 6 (5) | 17 (4) | > .999 |
At last contact, the proportion of patients still on imatinib, although higher for younger ones, was not statistically different among the 2 groups of patients. A higher proportion of younger patients received a second generation TKIs, while deaths were more frequent in the older cohort.
TKIs indicates tyrosine kinase inhibitors; SCT, stem cell transplantation; HU, hydroxyurea; and IFNα, interferon alpha
Discussion
The most relevant limitations to conventional chemotherapy for oncohematologic diseases in elderly persons are the high incidence of drug-related adverse events, hematologic, biochemical, or clinical.6 Therefore, even tailored treatments in elderly persons usually allow inferior results with respect to those obtained in younger patients.
IM is a clear example of “ideal” drug treatment for a disease, such as CML: it is given orally; the incidence of relevant, severe toxicities is relatively low; and a proper dose management allows long-term treatment and responses in most patients.17,18
Aging has been associated with a poorer outcome of CML: both Sokal12 and EURO13 scores include age among parameters significantly impacting on outcome. Consequently, a higher proportion of older patients are at intermediate or high risk (either Sokal or EURO) with respect to younger ones. Moreover, in the past, effective treatments for CML were restricted to younger patients (allogeneic stem cell transplantation) or characterized by low compliance and higher toxicity in elderly ones (IFN-α).30-34 Right now, with IM therapy in late CP, this negative impact of age has been, at least partially, reappraised.15,21 However, only limited data are available about the outcome of elderly early CP CML patients treated frontline with IM.22,35,36 Indeed, an important issue is represented by the allocation of elderly patients to IM treatment. An epidemiologic survey in southeast of Germany showed that the chance for an elderly CML patient (≥ 65 years) to be enrolled in a clinical study was 3.8 times lower than for a younger CML patient, suggesting that a not negligible part of elderly patients are excluded from clinical trials.37 Although this analysis refers to the years 1998 to 2000; thus, before IM marketing, a more recent survey conducted in the same area of Germany (2006) reported that of all CML patients treated outside clinical studies (median age, 64 years), only 59% received IM frontline.1 In a recently published study of 423 CML patients diagnosed in 2003 and randomly selected from cancer registries in the Surveillance, Epidemiology, and End Results Program, IM use was inversely associated with age: 90%, 75%, and 46% for patient ages 20 to 59 years, 60 to 79 years, and 80 years or more, respectively; this different approach negatively impacted on survival of older patients.38 According to these data, allocation of elderly patients to IM is not, apparently, a widely accepted practice, even in Western countries.
The results of our analysis should add significant information in this field, based on the high number of patients analyzed and treated in 61 Italian centers over a period of 7 years (May 2003 to October 2010) and not in single, highly experienced, referral center. We cannot give an estimation of the proportion of patients not allocated to IM during the enrollment period because of the presence of relevant comorbidities, which generally are more frequent in elderly patients, or because of age per se: a population-based registry (EUTOS), under the auspices of ELN, is currently retrieving extensive information about the general “intention to tyrosine kinase inhibitor treatment” all over Europe.
Currently, the most powerful surrogate endpoints of long-term outcome are the CCyR and the MMR.39-43 In our experience, the actual CCyR and MMR rates at 6, 12, and 18 months; thus, the rapidity of achieving a given response at each milestone and the cumulative incidence of CCyR and MMR were similar in the 2 groups of age (Table 2; Figure 1). Moreover, even the rates of progression to AP/BP for patients obtaining a CCyR or a MMR were almost the same in older and younger patients. Previously, Cortes et al15 reported a CCyR rate of 87% for the 46 patients more than 60 years old at diagnosis with respect to 79% for the 128 patients 60 years of age or younger (P = not significant). The median follow-up at the time of reporting was 16 months, and no information about the stability of response was available.
The evaluation of the long-term outcome is particularly relevant for the older age group where a number of jeopardizing factors (eg, lower compliance, drug-to-drug interactions, higher rates of clinical and biochemical toxicity) could affect negatively the result of the treatment. To the best of our knowledge, the long-term outcome of elderly CML patients in early CP IM-treated is presented for the first time in this analysis because no large multicenter study until now addressed it specifically.
The OS, PFS, FFS, and EFS, considering all the events (Table 3; Figure 2), were significantly better for the younger cohort. However, analyzing the causes of death (Table 5), it resulted that they were differently distributed between the 2 cohorts: the proportion of deaths resulting from progression to AP/BP, as probably expected considering the same rates and the comparable deepness of the response, was similar. On the other hand, more patients died in chronic phase and in CHR (at the time of death), therefore for reasons unrelated to CML progression, in the older cohort (17 of 115, 15%) versus the younger one (15 of 444, 3%) (P < .0001). This was not surprising, considering that increasing age is associated in the general population to higher mortality. In particular, in our analysis, typical age-related causes of death, such as thromboembolic events, SNC hemorrhages, and dementias, were significantly more frequent in older patients with respect to younger ones; all patients, including those with pulmonary embolism and CNS hemorrhage, had a normal blood count at the time of death. When considering the causes of death, it is important to evaluate the potential role played by IM, especially in elderly patients. Available data from the literature suggest that IM has no relevant long-term side effects44 able to impact significantly on mortality unrelated to CML progression. However, specific subset of patients may have higher grades of toxicity and side effects; for example, although the potential cardiotoxicity of IM has been already suspected45 and substantially excluded by different groups of investigators and by the company marketing IM, older patients are considered at higher risk of such complications.46,47 Interestingly, in our study, cardiovascular deaths were few, and even if they were more frequent in elderly patients, this was not statistically significant. Another relevant aspect is the influence that IM may have on the immune system: suppressive as well stimulating effects on CD4+ and CD8+ T lymphocytes or dendritic cells have been reported48 ; it is still unknown whether this determines an increased susceptibility to infections (especially opportunistic ones) in humans; however, this aspect may be of particular concern in older patients, generally considered at higher risk for infections. Despite that, in our study only one older patient died of an infection (of bacterial etiology) while in CCyR (normal blood count). Finally, deaths resulting from secondary malignancies were more frequent in elderly patients, although this was not statistically significant; of note, they represented the second cause of death, after progression to AB/BP, both in the older and younger cohorts.
Recalculating the OS, PFS, FFS, and FFS curves censoring death unrelated to CML progression, no significant difference in the 6-year outcome between older and younger patients was evident (OS, 94% vs 96; PFS, 91% vs 93%; FFS, 76% vs 81%; EFS, 68% vs 70%, respectively; all P > .1; Table 6).
In conclusion, our analysis, the largest one presented so far and with a proper follow-up, confirmed and reinforced the concept that IM is a very effective treatment also for older patients with early CP CML, allowing high response rates and survival; therefore, older age per se must not be a limitation for treating patients with IM and, in particular, for enrolling them in clinical trials. The challenge for the next decade will be the further improvement of CML outcome in the long term. Second-generation tyrosine kinase inhibitors are being implemented for frontline CML treatment: with a relatively short follow-up, both dasatinib and nilotinib have been demonstrated to be superior to IM.49,50 This improved outcome is expected to be maintained in the setting of older patients, as it is for IM. In the meantime, a superior activity of second-generation tyrosine kinase inhibitors must be or should be joined with long term safety similar to the, still unrivalled, IM one.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mrs Katia Vecchi for her valuable assistance.
This study was sponsored by the GIMEMA CML Working Party (formerly Italian Cooperative Study Group on Chronic Myeloid Leukemia). This work was supported by COFIN 2003, FIRB 2001, Associazione Italiana per la Ricerca sul Cancro, Consiglio Nazionale delle Ricerche, Fondazione del Monte di Bologna e Ravenna, European LeukemiaNet, Bologna AIL.
Authorship
Contribution: G.G. and G.R. wrote the manuscript; G.G., F.C., and G.R. analyzed data; M.A. performed molecular analysis; N.T. and G. Marzocchi performed cytogenetic analysis; G.G., F.C., F. Palandri, M. Breccia, T.I., A.C., B.M., P.P., S.R., D.F., F. Gherlinzoni, E.M., M. Bocchia, M.T., I.P., F. Grifoni, G. Martinelli, G.A., F. Pane, G.S., and G.R. enrolled the study patients and collected clinical data; M. Baccarani and G.R. supervised the study; and all authors gave final approval for submission.
Conflict-of-interest disclosure: G. Martinelli serves on the speakers' bureaus of Novartis, Bristol-Myers Squibb, and Pfizer. F. Pane receives research support from Novartis; is a consultant for Novartis and Bristol-Myers Squibb; and serves on the Novartis speakers' bureau. G.S. is a consultant for Novartis and Bristol-Myers Squibb and serves on the speakers' bureaus of Novartis and Bristol-Myers Squibb. M. Baccarani receives research support from Novartis, Bristol-Myers Squibb, and Pfizer; is a consultant for Novartis, Bristol-Myers Squibb, and Pfizer; and serves on the Novartis speakers' bureau. G.R. is a consultant with Novartis and serves on the speakers' bureaus of Novartis and Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Gianantonio Rosti, Department of Hematol ogy and Oncology Seràgnoli, S. Orsola-Malpighi Hospital, University of Bologna, Via Massarenti 9, 40138 Bologna, Italy; e-mail: totorosti@gmail.com.