Abstract
TLR7 and TLR8 are intracellular sensors activated by single-stranded RNA species generated during viral infections. Various synthetic small molecules can also activate TLR7 or TLR8 or both through an unknown mechanism. Notably, direct interaction between small molecules and TLR7 or TLR8 has never been shown. To shed light on how small molecule agonists target TLRs, we labeled 2 imidazoquinolines, resiquimod and imiquimod, and one adenine-based compound, SM360320, with 2 different fluorophores [5(6) carboxytetramethylrhodamine and Alexa Fluor 488] and monitored their intracellular localization in human plasmacytoid dendritic cells (pDCs). All fluorescent compounds induced the production of IFN-α, TNF-α, and IL-6 and the up-regulation of CD80 and CD86 by pDCs showing they retained TLR7-stimulating activity. Confocal imaging of pDCs showed that, similar to CpG-B, all compounds concentrated in the MHC class II loading compartment (MIIC), identified as lysosome-associated membrane protein 1+, CD63, and HLA-DR+ endosomes. Treatment of pDCs with bafilomycin A, an antagonist of the vacuolar-type proton ATPase controlling endosomal acidification, prevented the accumulation of small molecule TLR7 agonists, but not of CpG-B, in the MIIC. These results indicate that a pH-driven concentration of small molecule TLR7 agonists in the MIIC is required for pDC activation.
Introduction
Since the discovery of type I IFN (IFN-I) in 1957 and its potential for treatment of viral infections and cancer,1 several small molecule inducers of this factor have been identified. Many of these compounds are now known to be TLR7 or TLR8 or both agonists. Among them are imidazoquinolines,2,3 such as resiquimod (R848) that activates both TLR7 and TLR8 in humans, and imiquimod (R837) that activates TLR7 only.4,5 Imidazoquinolines have been largely tested in humans, and R837, formulated as a cream (Aldara), is licensed for topical treatment of genital warts, basal cell carcinoma, and actinic keratosis.6 Similar to R837, purine-like molecules, such as 9-benzyl-8-hydroxy-2-(2-methoxyethoxy) adenine (SM360320 or 1V136), are TLR7 agonists.7 TLR7 and TLR8 are phylogenetically related, located on the X chromosome in most mammals, and probably derive from a gene duplication event. TLR7 and TLR8 are both activated by various single-stranded RNAs (ssRNAs) from viruses,8 synthetic guanosine- or uridine-rich ssRNAs,9 and synthetic small molecules. TLR9, together with TLR7 and TLR8, form a subfamily of TLRs that is based on genomic structure and sequence homology.10 The natural agonist of TLR9 is unmethylated, CpG-containing DNA of bacterial or viral origin that can be mimicked by synthetic ssCpG oligonucleotides that can be classified as A-type (CpG-A), B-type (CpG-B), or C-type (CpG-C) on the basis of their different sequence motifs and biologic activities.11 Several reports have suggested that TLR7, TLR8, and TLR9 can interact directly with nucleic acids12-15 ; however, structural evidence confirming this hypothesis is not available yet. No convincing evidence of direct binding of imidazoquinolines or purine-like molecules to TLR7 or TLR8 exists either.
TLR7 and TLR9 are the only TLRs expressed by plasmacytoid dendritic cells (pDCs), which are a rare subset of circulating DCs that are considered as a frontline defense against viral infections. pDCs produce most of the systemic IFN-I (ie, IFN-α and -β) after viral infection and rapidly activate T cells with the use of presynthesized MHC class I and II molecules stored in their early and late endosomal compartments, respectively.16,17
Although nucleic acid sensing provides protection from intracellular infection, nucleic acids are not exclusively from pathogens; therefore, a balance between self versus foreign responsiveness is necessary. Indeed, recognition of self nucleic acids by TLR7 and TLR9 and the resultant IFN-I production have been linked to autoimmune disorders such as lupus.18 To avoid autoimmunity driven by recognition of self nucleic acids, TLR3, which binds double-stranded RNA,19 TLR7, TLR8, and TLR9 are sequestered in intracellular compartments. In particular, TLR7 and TLR9 localize to the endoplasmic reticulum and the endolysosomal compartment, which is a still incompletely understood vesiculotubular network of sorting and degradative organelles that accept, redirect, and process molecules from the exterior or interior of the cell.20 TLR7 and TLR9 translocation from the endoplasmic reticulum to the endolysosomal compartment depends on the chaperone protein UNC93B and is required for TLR7 and TLR9 signaling.21,22 Recent evidence shows that TLR9 becomes competent for signaling on proteolytic cleavage of its ectodomain in the endolysosomal compartment.23-26 The need for proteases in intracellular TLR activation may be a common phenomenon because cleaved TLR7 has been found in RAW264.7 cells,23 and Sepulveda et al25 claimed that human TLR7 is cleaved in DCs on R837 stimulation, possibly by asparagine endopeptidase, such as TLR9. Furthermore, several reports have shown that CpG concentrates in the endolysosomal compartment,13,27,28 supporting the idea that the productive encounter between TLR9 and its ligand occurs there. In particular, localization of CpG-A to transferrin receptor–positive (TfR+) endosomes was associated with IFN-α production, whereas localization of CpG-B in lysosome-associated membrane protein 1–positive (LAMP-1+) endosomes was associated with costimulatory molecule (eg, CD80 and CD86) up-regulation in human pDCs.28
Unlike CpG, the intracellular localization of small molecule TLR7/8 agonists has not been characterized. Thus, we labeled R848, R837, and SM360320 with fluorophores to track them in human pDCs. All 3 fluorescently labeled compounds were TLR7 agonists and induced IFN-α, TNF-α, and IL-6 production as well as CD80 and CD86 up-regulation by human pDCs, like their unlabeled parent compounds and CpG-B, as described.28-30 Fluorescently labeled compounds colocalized with CpG-B in LAMP-1+, CD63+, and HLA-DR+ endosomes, which belong to the MHC class II loading compartment (MIIC) of pDCs, independently of their chemotype and of the fluorophore used to label them. Bafilomycin A (BafA), an antagonist of the vacuolar type proton ATPase responsible for endosomal acidification,31 strongly inhibited the localization of the TLR7 agonist small molecules while leaving unaltered the localization of CpG-B. We conclude that the acidic pH present in the MIIC is the driving force for small molecule TLR7 agonist localization and a key requirement for their immunostimulatory activity.
Methods
Fluorescently labeled TLR7 agonist small molecules and CpGs
Synthesis of R848, R837, and SM360320, fluorescently labeled or not, is described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). CpG-B (2006): 5′-tcg tcg ttt tgt cgt ttt gtc gtt-3′ and CpG-A (D19): 5′-ggTGCATCGATGCAGggggg-3′, labeled or not at the 3′-end with 5(6) carboxytetramethylrhodamine (TAMRA), were from Primm. CpG-B–FITC was from Invivogen. Bases shown in capital letters are phosphodiester and those in lowercase letters are phosphorothioate.
Antibodies and chemicals
The following mouse mAbs were from BD PharMingen: FITC-conjugated anti–LAMP-1, FITC-conjugated anti-CD63, allophycocyanin (APC)–conjugated anti–HLA-DR, BD–Horizon V450–conjugated anti-CD80, APC–conjugated anti-CD86, APC-conjugated anti–BDCA-2, FITC-conjugated anti-CD123 (IL-3R), biotin-conjugated anti–HLA-DR, and purified anti-TfR. Purified rabbit mAb anti-EEA1 was from Cell Signaling Technology. Purified rabbit polyclonal anti–LAMP-1 Ab was from Abcam. Alexa Fluor 488 (AF488)–, AF568-, and AF647-conjugated goat anti–rabbit F(ab′)2 IgG, AF488-conjugated goat anti–mouse F(ab′)2 IgG, and APC-conjugated streptavidin were from Molecular Probes (Invitrogen). Live-Dead Fixable Aqua Dead Cell Stain kit was from Invitrogen. BafA was from Sigma-Aldrich.
Luciferase assay
HEK293T cells stably transfected with the firefly luciferase gene under the transcriptional control of NF-κB together with human TLR7, TLR8, or TLR9 (TLR-DST) were made in our laboratory. TLR-DST cells were cultured overnight at 5 × 104 cells/well in microclear 96-well plates (Greiner Bio-One). Cells were stimulated with serial dilutions of compounds in duplicate for 6 hours and then lysed with cell culture lysis reagent (Promega), according to the manufacturer's instructions. Luciferase assay substrate (Promega) was added, and luciferase activity was measured by SpectraMax L microplate reader (Molecular Devices). Luciferase activity was expressed as fold induction over nonstimulated cells.
Donors
Buffy coats from healthy HIV-, hepatitis B virus–, and hepatitis C virus–negative donors were obtained from the Blood Transfusion Section, Alta Val D'Elsa Hospital, Poggibonsi, Italy. Informed consent was obtained before all blood donations. The study protocol was approved by the Novartis Research Center Ethical Committee and conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Purification and stimulation of pDCs
PBMCs were isolated from fresh buffy coats by Ficoll-Hypaque (Amersham Biosciences) density gradient centrifugation, following standard procedures. Highly purified pDCs (≥ 98% BDCA-2+CD123+) were obtained with Diamond Plasmacytoid Dendritic Cell Isolation Kit (Miltenyi Biotec), which consists of magnetic depletion of non-pDCs followed by positive selection of pDCs with anti–BDCA-4 Ab. pDCs were cultured at 5 × 104 per well in 96-roundbottom plates in RPMI 1640 medium (Gibco) supplemented with 10% FCS (HyClone), glutamine, penicillin, and streptomycin. Human rIL-3 (100 ng/mL; R&D Systems) was added to the cultures for 16-24 hours before stimulation with TLR7 agonist small molecules or CpGs to keep pDCs alive.32
Cytokine production, CD80 and CD86 up-regulation, and compound uptake
pDCs were stimulated with different concentrations of TLR7 agonist small molecules or CpGs overnight. IFN-α was quantified with human IFN-α multiple subtype ELISA kit (PBL Biomedical Laboratories). TNF-α and IL-6 were measured with the human proinflammatory 7-plex (TNF-α, IL-6, IFN-γ, IL-10, IL-12p70, IL-1β, IL-8) tissue culture kit (MSD Technology). Other cytokines were produced at low concentrations, except IL-8 that was produced constitutively and poorly induced on stimulation (data not shown). pDCs were washed twice with ice-cold PBS, stained with anti-CD80 HorizonV450 and anti–CD86-APC, and analyzed for CD80 and CD86 expression as well as for the fluorescence compound resulting from uptake by FACS LSRII instrument (Becton Dickinson) with the use of the BD FACSDiva v6.1.3 software (Becton Dickinson). Data were analyzed with FlowJo software (TreeStar Inc).
Confocal immunofluorescence microscopy
pDCs were incubated with fluorescent compounds (TLR7 agonist small molecules or CpG) for 90 minutes. Cells were washed, fixed with 1% paraformaldehyde in PBS at room temperature for 15 minutes, and permeabilized with saponin buffer (0.5% saponin, 1% BSA in PBS). Samples were blocked for 30 minutes with Image-iT FX signal enhancer (Invitrogen) and stained with the indicated antibodies in saponin buffer. Cells were cytocentrifuged on glass slides and mounted with SlowFade Gold antifade reagent with 4,6 diamidino-2-phenylindole (DAPI; Invitrogen), as described.28 Images were acquired sequentially with separate laser excitations to avoid cross talks between different fluorophores with the use of either a Nikon Eclipse TE2000, Radiance 2100 Confocal System (Bio-Rad) with the acquisition software Lasersharp 2000 (Zeiss) and processed with Volocity 3.6 (Improvision Inc) or with a LSM 710 confocal microscope System (Zeiss) and processed with Zen2008 software (Zeiss). An oil-immersion objective (63×/1.4 numerical aperture [NA]), with the pinhole set for a section thickness of 0.8 μm (pinhole set to 1 airy unit in each channel) was used. Unless otherwise stated, the images show one representative cell, as checked by DAPI staining of the nucleus, of ≥ 30 cells analyzed for each sample.
Results
Fluorescently labeled R848, R837, and SM360320 are TLR7 agonists
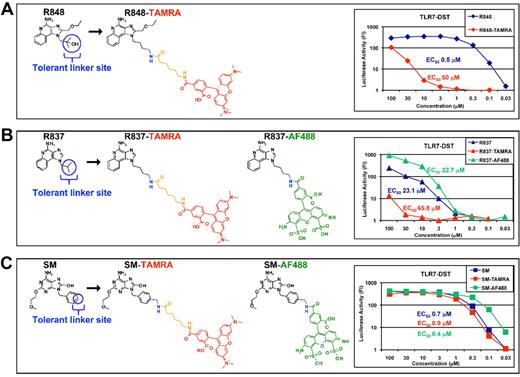
Structure-activity relationship analysis of R848 led us to the identification of a “tolerant” linker site. We used this site to conjugate R848, R837, and SM360320 (SM) with TAMRA or AF488 fluorophores (Figure 1). We then assessed the biologic activity of these fluorescent molecules on HEK293T cells stably transfected with the luciferase gene under the transcriptional control of NF-κB and TLR7 (TLR7-DST) and compared them with their unlabeled parent molecules. R848 (Figure 1A) activity on TLR7-DST was strongly decreased on conjugation with TAMRA (EC50, 50 μm for R848-TAMRA vs 0.5 μm for R848), R837 (Figure 1B) activity was slightly decreased on TAMRA conjugation (EC50, 65.8 μm vs 23.1 μm for R837) and unaltered on AF488 conjugation (EC50, 22.7 μm vs 23.1 μm for R837), whereas SM (Figure 1C) activity was not affected by conjugation with either fluorophore (EC50, 0.9 μm for SM-TAMRA, 0.4 μm for SM-AF488 vs 0.7 μm for SM). All fluorescent compounds were inactive on TLR8-DST (supplemental Figure 1).
Fluorescently labeled R848, R837, and SM360320 are TLR7 agonists. R848 (A), R837 (B), and SM (C) were conjugated with TAMRA (in red) or with Alexa Fluor 488 (AF488; in green) fluorophores at the indicated tolerant linker site. A spacer was introduced together with TAMRA (in orange). Both fluorophores were a mixture of 5-6 regioisomers. Structures depicted here represent the 5-regioisomer. The biologic activity of these fluorescently labeled molecules was assessed on HEK293T cells stably transfected with TLR7 and the luciferase gene under the transcriptional control of NF-κB and (TLR7-DST) in parallel with their nonfluorescent parent molecules. Luciferase activity was expressed as fold induction (FI) over nonstimulated cells. EC50 values are reported.
Fluorescently labeled R848, R837, and SM360320 are TLR7 agonists. R848 (A), R837 (B), and SM (C) were conjugated with TAMRA (in red) or with Alexa Fluor 488 (AF488; in green) fluorophores at the indicated tolerant linker site. A spacer was introduced together with TAMRA (in orange). Both fluorophores were a mixture of 5-6 regioisomers. Structures depicted here represent the 5-regioisomer. The biologic activity of these fluorescently labeled molecules was assessed on HEK293T cells stably transfected with TLR7 and the luciferase gene under the transcriptional control of NF-κB and (TLR7-DST) in parallel with their nonfluorescent parent molecules. Luciferase activity was expressed as fold induction (FI) over nonstimulated cells. EC50 values are reported.
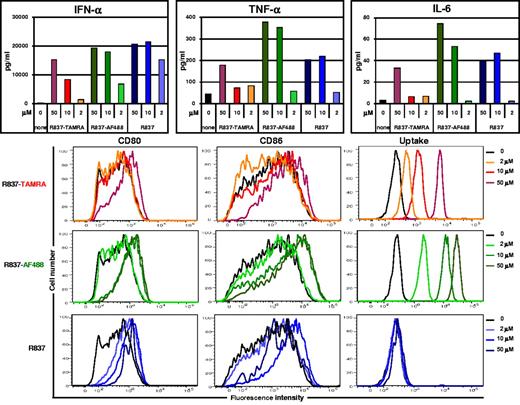
The activity of the fluorescently labeled TLR7 agonists was tested on human pDCs that express TLR7 and TLR9. For practical reasons in this study pDCs were cultured with IL-3 for 18-24 hours before stimulation with TLR7 agonist small molecules or CpG to prevent them from dying.32 pDCs were stimulated overnight with labeled or unlabeled R848, R837, or SM360320. Cytokine concentrations in the supernatants were measured while cells were stained for CD80 and CD86 and analyzed by flow cytometry for costimulatory molecule expression and for compound uptake (TAMRA or AF488 fluorescence). R837-TAMRA and R837-AF488 induced the production of IFN-α, TNF-α, and IL-6 and the up-regulation of CD80 and CD86 in a dose-dependent manner (Figure 2). Internalization of R837-TAMRA and R837-AF488 was readily detected by flow cytometry. Similar experiments were performed with fluorescent R848 and SM (supplemental Figure 2A-B). All the fluorescent compounds tested induced mainly IFN-α, although production of TNF-α and IL-6 and up-regulation of CD80 and CD86 were also observed, similar to the parent compounds and CpG-B (supplemental Figure 2C). The ability of each fluorescent compound to activate human pDCs with respect to its parent compound was in good agreement with the EC50 measured on TLR7-DST (Figure 1).
Fluorescently labeled R837 activates human pDCs. Human pDCs, purified from fresh buffy coats and precultured with IL-3 for 24 hours, were stimulated with different concentrations of R837, labeled or not with TAMRA or AF488. After overnight incubation, cytokine concentrations in the culture supernatant fluids were assayed, and cells were analyzed by flow cytometry for CD80 and CD86 expression and for compound uptake (TAMRA or AF488 fluorescence). Data shown are from one donor and are representative of ≥ 3 independent donors.
Fluorescently labeled R837 activates human pDCs. Human pDCs, purified from fresh buffy coats and precultured with IL-3 for 24 hours, were stimulated with different concentrations of R837, labeled or not with TAMRA or AF488. After overnight incubation, cytokine concentrations in the culture supernatant fluids were assayed, and cells were analyzed by flow cytometry for CD80 and CD86 expression and for compound uptake (TAMRA or AF488 fluorescence). Data shown are from one donor and are representative of ≥ 3 independent donors.
Imidazoquinolines localize to the MIIC of human pDCs
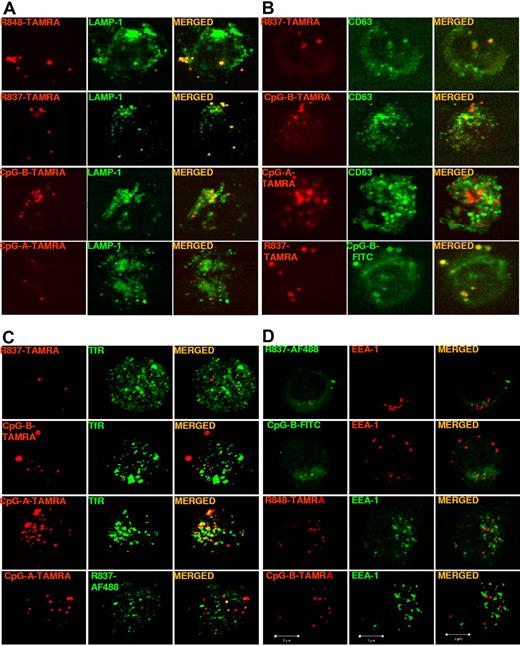
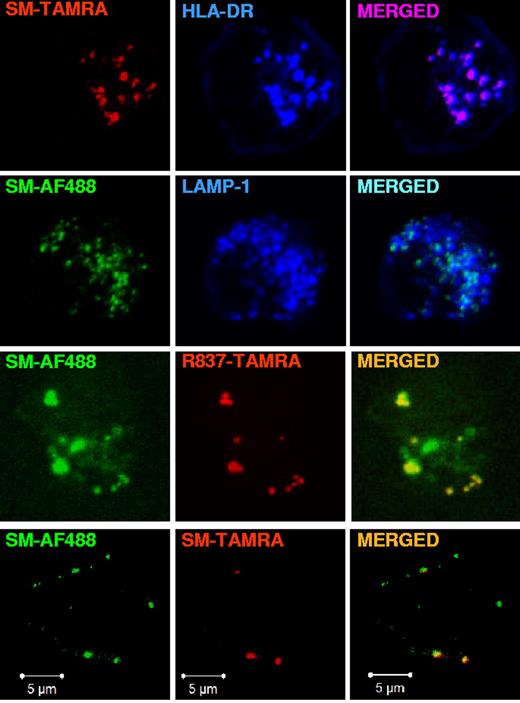
The localization of fluorescent TLR7 agonists in pDCs was determined with the protocol applied by Guiducci et al28 to assess the localization of CpG-A and CpG-B in these cells. pDCs were incubated with fluorescent TLR7 agonists or CpGs and stained for LAMP-1, CD63, or TfR to identify distinct classes of endosomes.33,34 As shown by confocal microscopy (Figure 3A-B), both imidazoquinolines labeled with TAMRA colocalized with CpG-B in LAMP-1+CD63+ endosomes but not with CpG-A. As a control, the ethanol amine-capped TAMRA fluorophore when used alone did not localize in any intracellular compartment (data not shown). However, neither R837-TAMRA nor CpG-B–TAMRA localized to TfR+ endosomes, whereas CpG-A–TAMRA showed a partial colocalization with TfR and almost no colocalization with R837-AF488 (Figure 3C). The ability of CpGs to induce IFN-α production by freshly isolated human pDCs has been correlated with their localization in TfR+ endosomes (early/recycling endosomes).28 However, we found that fluorescently labeled TLR7 agonist small molecules, like their parent compounds and CpG-B, induced pDCs precultured in IL-3 to produce high amounts of IFN-α, yet neither R837-TAMRA nor CpG-B–TAMRA colocalized with TfR. To confirm the absence of localization in early endosomes we analyzed fluorescent TLR7 agonist small molecules and CpG-B for colocalization with EEA1. None of the fluorescent TLR7 agonist small molecules and CpG-B localized to EEA1+ endosomes (Figure 3D; data not shown).
Imidazoquinolines localize to LAMP1+CD63+ endosomes but not to TfR+EEA-1+ endosomes in pDCs. Human pDCs, precultured overnight with IL-3, were stimulated for 90 minutes with (A) R848-TAMRA (20 μm), R837-TAMRA (50 μm), CpG-B–TAMRA (10 μm), or CpG-A–TAMRA (10 μm); (B) R837-TAMRA (50 μm), CpG-B–TAMRA (10 μm), CpG-A–TAMRA (10 μm), or R837-TAMRA (50 μm) together with CpG-B–FITC (10 μm); (C) R837-TAMRA (50 μm), CpG-B–TAMRA (10 μm), CpG-A–TAMRA (10 μm) alone or together with R837-AF488 (20 μm); (D) R837-AF488 (20 μm), CpG-B–FITC (10 μm), R848-TAMRA (20 μm), or CpG-B–TAMRA (10 μm). Cells were fixed, permeabilized, and stained with anti–LAMP-1-FITC Ab (A), anti–CD63-FITC Ab (B), purified mouse mAb anti-TfR followed by anti–mouse Ab labeled with AF488 (C), or purified rabbit mAb anti-EEA1 followed by anti–rabbit Ab labeled with AF568 (D, top and upper middle) or AF488 (D, lower middle and bottom). Images were acquired with a Bio-Rad TE2000-U (A-B) or a ZEISS LSM 710 (C-D) confocal microscope and an oil-immersion objective (63×/1.4 NA), with the pinhole set for a section thickness of 0.8 μm (pinhole set to 1 airy unit in each channel). Images were acquired sequentially with separate laser excitations to avoid cross talks between different fluorophores and processed with Zen2008 software. Images show single cells from 1 representative donor of 3.
Imidazoquinolines localize to LAMP1+CD63+ endosomes but not to TfR+EEA-1+ endosomes in pDCs. Human pDCs, precultured overnight with IL-3, were stimulated for 90 minutes with (A) R848-TAMRA (20 μm), R837-TAMRA (50 μm), CpG-B–TAMRA (10 μm), or CpG-A–TAMRA (10 μm); (B) R837-TAMRA (50 μm), CpG-B–TAMRA (10 μm), CpG-A–TAMRA (10 μm), or R837-TAMRA (50 μm) together with CpG-B–FITC (10 μm); (C) R837-TAMRA (50 μm), CpG-B–TAMRA (10 μm), CpG-A–TAMRA (10 μm) alone or together with R837-AF488 (20 μm); (D) R837-AF488 (20 μm), CpG-B–FITC (10 μm), R848-TAMRA (20 μm), or CpG-B–TAMRA (10 μm). Cells were fixed, permeabilized, and stained with anti–LAMP-1-FITC Ab (A), anti–CD63-FITC Ab (B), purified mouse mAb anti-TfR followed by anti–mouse Ab labeled with AF488 (C), or purified rabbit mAb anti-EEA1 followed by anti–rabbit Ab labeled with AF568 (D, top and upper middle) or AF488 (D, lower middle and bottom). Images were acquired with a Bio-Rad TE2000-U (A-B) or a ZEISS LSM 710 (C-D) confocal microscope and an oil-immersion objective (63×/1.4 NA), with the pinhole set for a section thickness of 0.8 μm (pinhole set to 1 airy unit in each channel). Images were acquired sequentially with separate laser excitations to avoid cross talks between different fluorophores and processed with Zen2008 software. Images show single cells from 1 representative donor of 3.
Collectively, these data show that imidazoquinolines do not diffuse randomly inside pDCs but concentrate in LAMP1+CD63+ endosomes despite that they are cell permeable and thus able to spread readily throughout the cell.
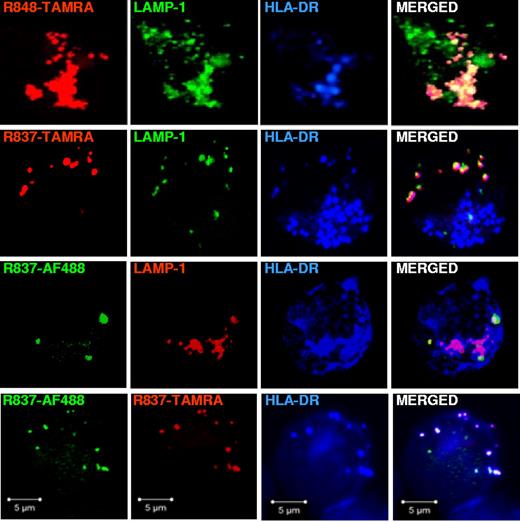
LAMP-1 and CD63 are markers of late endosomes and lysosomes, which are characterized by an acidic pH.34 Because in pDCs, like in other antigen-presenting cells, the endolysosomal compartment is specialized in antigen presentation onto MHC class II molecules (MHC II),16 we stained for HLA-DR, which is a MHC II receptor, and LAMP-1 to check whether the endosomes where imidazoquinolines concentrated belonged to the MIIC. As shown in Figure 4, we found that R848-TAMRA, R837-TAMRA, and R837-AF488 localized to endosomes that were positive not only for LAMP-1 but also for HLA-DR. In addition, R837-TAMRA and R837-AF488 colocalized with each other in HLA-DR+ endosomes. As a control, the ethanol amine-capped AF488 fluorophore alone did not localize in any intracellular compartment (data not shown).
Fluorescently labeled R848 and R837 localize to LAMP1+HLA-DR+ endosomes in pDCs. pDCs, precultured overnight with IL-3, were stimulated with R848-TAMRA (20 μm), R837-TAMRA (50 μm), R837-AF488 (20 μm) alone or together with R837-TAMRA (50 μm) for 90 minutes. Cells were fixed, permeabilized, and stained with purified rabbit polyclonal anti-LAMP1 Ab followed by anti–rabbit Ab labeled with AF488 (top and upper middle) or AF568 (lower middle) and anti–HLA-DR-APC. Images were acquired with a ZEISS LSM 710 confocal microscope and an oil-immersion objective (63×/1.4 NA), with the pinhole set for a section thickness of 0.8 μm (pinhole set to 1 airy unit in each channel). Images were acquired sequentially with separate laser excitations to avoid cross talks between different fluorophores and processed with Zen2008 software. Images show single cells from 1 representative donor of 3.
Fluorescently labeled R848 and R837 localize to LAMP1+HLA-DR+ endosomes in pDCs. pDCs, precultured overnight with IL-3, were stimulated with R848-TAMRA (20 μm), R837-TAMRA (50 μm), R837-AF488 (20 μm) alone or together with R837-TAMRA (50 μm) for 90 minutes. Cells were fixed, permeabilized, and stained with purified rabbit polyclonal anti-LAMP1 Ab followed by anti–rabbit Ab labeled with AF488 (top and upper middle) or AF568 (lower middle) and anti–HLA-DR-APC. Images were acquired with a ZEISS LSM 710 confocal microscope and an oil-immersion objective (63×/1.4 NA), with the pinhole set for a section thickness of 0.8 μm (pinhole set to 1 airy unit in each channel). Images were acquired sequentially with separate laser excitations to avoid cross talks between different fluorophores and processed with Zen2008 software. Images show single cells from 1 representative donor of 3.
Purine-like TLR7 agonist SM colocalizes with imidazoquinolines in the MIIC
To assess if localization in the MIIC is a general feature of TLR7 agonists, we looked at fluorescent SM, a purine-like compound that is structurally distinct from imidazoquinolines. As shown in Figure 5, SM labeled with TAMRA colocalized with HLA-DR and SM-AF488 colocalized with LAMP-1. Moreover, SM-AF488 colocalized with SM-TAMRA and with R837-TAMRA. Thus, TLR7 agonists concentrated in LAMP-1+CD63+HLA-DR+ endosomes of pDCs independently of the compound chemical scaffold and the fluorophore used for labeling. While we were writing this article, Shukla et al35 described the synthesis of yet another imidazoquinoline conjugated with different fluorophores. Those investigators observed an intracellular localization of these compounds in the murine J774 macrophage cell line that, although not characterized further, was compatible with our observations.
Purine-like TLR7 agonist SM360320 colocalizes with imiquimod in the MIIC. pDCs, precultured overnight with IL-3, were stimulated with SM-TAMRA (20 μm) or with SM-AF488 (3 μm), alone or together with SM-TAMRA (20 μm) or R837-TAMRA (20 μm), for 90 minutes. Cells were fixed, permeabilized, and stained with anti–HLA-DR-APC or purified rabbit polyclonal anti-LAMP1 Ab followed by anti–rabbit Ab labeled with AF647. Images were acquired with a ZEISS LSM 710 confocal microscope and an oil-immersion objective (63×/1.4 NA), with the pinhole set for a section thickness of 0.8 μm (pinhole set to 1 airy unit in each channel). Images were acquired sequentially with separate laser excitations to avoid cross talks between different fluorophores and processed with Zen2008 software. Images show single cells from 1 representative donor of 3.
Purine-like TLR7 agonist SM360320 colocalizes with imiquimod in the MIIC. pDCs, precultured overnight with IL-3, were stimulated with SM-TAMRA (20 μm) or with SM-AF488 (3 μm), alone or together with SM-TAMRA (20 μm) or R837-TAMRA (20 μm), for 90 minutes. Cells were fixed, permeabilized, and stained with anti–HLA-DR-APC or purified rabbit polyclonal anti-LAMP1 Ab followed by anti–rabbit Ab labeled with AF647. Images were acquired with a ZEISS LSM 710 confocal microscope and an oil-immersion objective (63×/1.4 NA), with the pinhole set for a section thickness of 0.8 μm (pinhole set to 1 airy unit in each channel). Images were acquired sequentially with separate laser excitations to avoid cross talks between different fluorophores and processed with Zen2008 software. Images show single cells from 1 representative donor of 3.
BafA prevents the concentration of small molecule TLR7 agonists in the MIIC
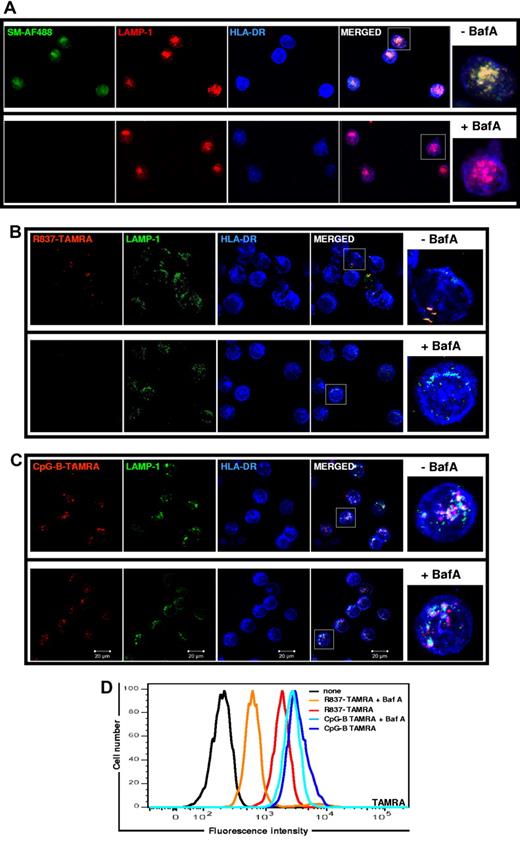
The pH of the MIIC is acidic for optimal protease activity necessary for the generation of peptides to be complexed with MHC II molecules.36 The acidic luminal environment is maintained by v-ATPase, which pumps protons into the MIIC lumen. To investigate whether the acidic pH plays a role in the concentration of TLR7 agonist small molecules in the MIIC we used BafA to block v-ATPase and raise the MIIC pH.31 As shown in Figure 6, pretreatment of pDCs with BafA resulted in the near complete inhibition of SM-AF488 (Figure 6A) and R837-TAMRA (Figure 6B) localization in LAMP1+HLA-DR+ endosomes. By contrast, CpG-B–TAMRA localization in the BafA-treated and untreated cells were indistinguishable (Figure 6C). Moreover, BafA strongly inhibited R837-TAMRA uptake while leaving CpG-B–TAMRA uptake unaltered (Figure 6D), confirming what we observed by confocal microscopy (Figure 6B-C). In line with the fact that BafA is a well-known inhibitor of TLR7- and TLR9-induced signaling,26 in our experiments BafA blocked the production of IFN-α, TNF-α, and IL-6 in response to SM-AF488, R837-TAMRA, or CpG-B–TAMRA by pDCs (data not shown). Blocking of TLR9 signaling by BafA was probably because of inhibition of proteases responsible for TLR9 cleavage.23,25 Although this mechanism could contribute also to the blockade of TLR7 signaling,23,25 our findings highlighted that the acidic pH of the MIIC is required for the localization of TLR7 agonist small molecules in there.
BafA prevents the localization of small molecule TLR7 agonists to the MIIC. pDCs, precultured overnight with IL-3, were treated with BafA (100nM) for 2 hours before stimulation with SM-AF488 at 3 μm (A), R837-TAMRA at 50 μm (B), or CpG-B–TAMRA at 10 μm (C). After 90 minutes of stimulation, cells were fixed, permeabilized, and stained with anti–HLA-DR-APC and purified rabbit polyclonal anti–LAMP-1 Ab followed by anti–rabbit Ab labeled with AF568 (A) or with AF488 (B-C). Images were acquired with a ZEISS LSM 710 confocal microscope and an oil-immersion objective (63×/1.4 NA), with the pinhole set for a section thickness of 0.8 μm (pinhole set to 1 airy unit in each channel). Images were acquired sequentially with separate laser excitations to avoid cross talks between different fluorophores and processed with Zen2008 software. Images show several cells from 1 representative donor of 3. Cells in squares are shown in higher magnification on the right. (D) After 18 hours of stimulation with R837-TAMRA (50 μm) or CpG-B–TAMRA (10 μm), compound uptake by pDCs was evaluated by flow cytometry.
BafA prevents the localization of small molecule TLR7 agonists to the MIIC. pDCs, precultured overnight with IL-3, were treated with BafA (100nM) for 2 hours before stimulation with SM-AF488 at 3 μm (A), R837-TAMRA at 50 μm (B), or CpG-B–TAMRA at 10 μm (C). After 90 minutes of stimulation, cells were fixed, permeabilized, and stained with anti–HLA-DR-APC and purified rabbit polyclonal anti–LAMP-1 Ab followed by anti–rabbit Ab labeled with AF568 (A) or with AF488 (B-C). Images were acquired with a ZEISS LSM 710 confocal microscope and an oil-immersion objective (63×/1.4 NA), with the pinhole set for a section thickness of 0.8 μm (pinhole set to 1 airy unit in each channel). Images were acquired sequentially with separate laser excitations to avoid cross talks between different fluorophores and processed with Zen2008 software. Images show several cells from 1 representative donor of 3. Cells in squares are shown in higher magnification on the right. (D) After 18 hours of stimulation with R837-TAMRA (50 μm) or CpG-B–TAMRA (10 μm), compound uptake by pDCs was evaluated by flow cytometry.
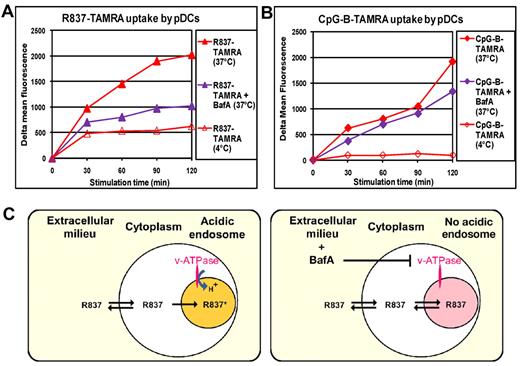
To get further insights on the mechanism of entry and accumulation of small molecule TLR7 agonists we studied the effect of BafA on R837-TAMRA uptake over time. As shown in Figure 7A, although BafA had little effect on the R837-TAMRA uptake at 30 minutes, it blocked further uptake of the compound almost completely at later time points. As expected, CpG-B–TAMRA uptake was almost unaffected by BafA (Figure 7B). To understand whether the internalization of TLR7 agonist small molecules was the result of passive diffusion or an active mechanism, we compared R837-TAMRA uptake at 4°C with uptake at 37°C (standard culture condition). Although the R837-TAMRA uptake after 30 minutes of incubation at 4°C was only marginally reduced compared with 37°C, further accumulation of the compound was blocked at 4°C. These results showed that an early passive diffusion of R837 is followed by an active mechanism that dramatically increases the accumulation of the TLR7 agonist in the MIIC (Figure 7A). No CpG-B–TAMRA internalization occurred at 4°C (Figure 7B), in agreement with previous data showing that CpG-B enters cells by an active mechanism involving rapid translocation to tubular lysosomal compartment (LAMP-1+) from early endosomes (EEA1+TfR+).13 On the basis of these results we propose that R837, which is a cell-permeable weak base, passively diffuses inside the cells (fast phase, v-ATPase independent, insensitive to 4°C) and then starts to accumulate in the acidic vesicles of the MIIC because it gets protonated and therefore trapped (slow phase, v-ATPase dependent, blocked at 4°C; Figure 7C).37
BafA prevents the concentration of imiquimod in the MIIC. pDCs, precultured with IL-3 for 24 hours, were treated or not with BafA (100nM) for 2 hours. Then, cells were stimulated with R837-TAMRA at 50 μm (A) or CpG-B–TAMRA at 10 μm (B) for the indicated times at 37°C or at 4°C. Cells were stained with Live/Dead and analyzed by flow cytometry for compound uptake gating on live cells. The mean fluorescence of cells alone cultured under the same conditions was subtracted (δ mean fluorescence). Cell viability was not affected by BafA treatment. Data were obtained with pDCs purified from a single donor and were reproduced with pDCs from 2 other donors. (C) Model for the concentration of TLR7 agonist small molecules in acidic endosomes on the basis of the previous data. R837 is a cell-permeable weak base that passively diffuses everywhere inside the cell (fast phase, v-ATPase-independent) and then starts to accumulate in the class II loading compartment where it gets protonated and therefore trapped (slow phase, v-ATPase-dependent). In the presence of BafA, v-ATPase is blocked, and endosomal pH is no longer acidic. Therefore, R837 does not get protonated in endosomes and does not accumulate there.
BafA prevents the concentration of imiquimod in the MIIC. pDCs, precultured with IL-3 for 24 hours, were treated or not with BafA (100nM) for 2 hours. Then, cells were stimulated with R837-TAMRA at 50 μm (A) or CpG-B–TAMRA at 10 μm (B) for the indicated times at 37°C or at 4°C. Cells were stained with Live/Dead and analyzed by flow cytometry for compound uptake gating on live cells. The mean fluorescence of cells alone cultured under the same conditions was subtracted (δ mean fluorescence). Cell viability was not affected by BafA treatment. Data were obtained with pDCs purified from a single donor and were reproduced with pDCs from 2 other donors. (C) Model for the concentration of TLR7 agonist small molecules in acidic endosomes on the basis of the previous data. R837 is a cell-permeable weak base that passively diffuses everywhere inside the cell (fast phase, v-ATPase-independent) and then starts to accumulate in the class II loading compartment where it gets protonated and therefore trapped (slow phase, v-ATPase-dependent). In the presence of BafA, v-ATPase is blocked, and endosomal pH is no longer acidic. Therefore, R837 does not get protonated in endosomes and does not accumulate there.
Discussion
To understand the mechanism of action of small molecule TLR7 agonists more completely, we labeled R848, R837, and SM36020 with TAMRA or AF488 fluorophore and studied their subcellular localization. All of the fluorescent compounds retained TLR7 agonist activity and induced human pDCs to produce cytokines and IFN-α and to increase expression of costimulatory molecules. Examination of pDCs by confocal microscopy showed that all compounds colocalized with CpG-B in LAMP-1+CD63+HLA-DR+ endosomes, independently of their chemotype or labeling fluorophore. Our observations are in line with an elegant study by Sadaka et al38 showing that MHC II molecules converge with LAMP-1 and CD63 (but not with TfR and EEA1 markers) in human pDCs treated with IL-3. LAMP-1 and CD63 are markers of late endosomes and lysosomes, whereas HLA-DR identifies MHC II molecules. Therefore, the vesicles where small molecule TLR7 agonists accumulate appear to belong to the MIIC.20,38,39 Indeed, in pDCs, like in other antigen-presenting cells, the endolysosomal compartment is specialized for MHC II antigen presentation and is considered as a lysosome-related organelle.39 The function of MHC II molecules is to access the endolysosomal network, bind peptides derived from the antigens, and display them on the cell surface to CD4+ T cells. Notably, the regulation of MHC II expression and transport is completely different in pDCs compared with conventional DCs (cDCs) from both humans38 and mice.40,41 In particular, cDCs halt the synthesis and degradation of MHC II after activation, whereas pDCs maintain synthesis and turnover of MHC II. As a result pDCs are less efficient at presenting antigens than cDCs, but their more dynamic mode of antigen presentation should be advantageous in counteracting viruses that exhibit mutation rates.40,41 Peptide binding by MHC II molecules requires the degradation of the invariant chain by endolysosomal proteases. These proteases have been implicated in the activation of TLR7 and TLR9.23-25 Our data showing that small molecule TLR7 agonists and CpG-B localize to MIIC suggest that this compartment is the crossroad where antigens and MHC II molecules are assembled and nucleic acid–sensing TLRs signal on encounter with their ligands. The commonality of proteolytic cleavage to form functionally relevant forms of both MHC II and TLRs and their colocalization could affect the way pDCs inform T cells as to the specific nature of distinct pathogenic threats.
Small molecule TLR7 agonists and CpG-B did not localize to early endosomes (TfR+ or EEA1+), yet they induced high amounts of IFN-α production. These results are in contrast with the proposed model that in human pDCs TLR9 signaling from early endosomes (TfR+) leads to IFN-α production, whereas signaling from late endosomes (LAMP-1+) leads to up-regulation of costimulatory molecules.28 However, our observations are in line with a recent report from Sasai et al42 which showed that in mouse BM-derived macrophages TLR9 signals leading to IFN-I production are generated in acidic endosomes (LAMP-2+LysoTracker+).It is unclear why CpG-B is a poor inducer of IFN-α by freshly isolated pDCs28 but a good inducer for IL-3 pretreated pDCs.43 In both pDC populations CpG-B localizes to LAMP-1+ endosomes, suggesting that IL-3 treatment leads to changes in the constituents of these vesicles. Indeed, IL-3 treatment induced the relocalization of MHC II to LAMP-1+ endosomes, and it is tempting to speculate that key IFN-I–signaling components, such as IFN regulatory factor 7, are recruited to the MIIC in response to IL-3 treatment.
A key feature of the MIIC is low pH. Blockade of vesicle acidification by BafA or NH4Cl (data not shown) abolished the accumulation of R837-TAMRA in the MIIC, suggesting that the electrochemical proton gradient is the driving force for the accumulation of small molecule TLR7 agonists in this compartment. Interestingly, a similar mechanism has been shown to cause the accumulation of neurotransmitters and hormones into secretory vesicles.44 At the acidic pH found in the MIIC of pDCs (pH 5-628 ), small molecule TLR7 agonists are predicted to be positively charged and protonated and are expected to reach concentrations in the acidic compartment ∼ 100-fold higher than the rest of the cell.37 This substantial accumulation of compound is consistent with what we observed in live pDCs (data not shown) and could serve to drive low-affinity interactions of small molecule TLR7 agonists with TLR7 or a coreceptor molecule or both above the threshold necessary for signaling.
The online version of this article contains a data supplement.
Presented in abstract form at the DC2010: Forum on Vaccine Science, Lugano, Switzerland, September 26-30, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Michela Brazzoli for her initial support in confocal microscopy analysis; Susanna Aprea, Sara Valentini, and Ugo D'Oro for providing TLR-DST cell lines; Diego Piccioli for his advice on pDCs; Giorgio Corsi for artwork; Alberto Visintin, Qian Huang, Xinming Cai, Tom Wu, and Alex Cortez for discussion; and Sylvie Bertholet for careful revision of the manuscript.
This work was supported in part by the Transformational Medical Technologies program (contract HDTRA1-07-9-0001) from the Department of Defense Chemical and Biologic Defense program through the Defense Threat Reduction Agency (DTRA).
This work is part of the PhD thesis for C.R.
DTRA was founded in 1998 to integrate and focus the capabilities of the Department of Defense (DOD) that address the threat by weapons of mass destruction (WMDSs). DTRA's mission is to safeguard the United States and its allies from chemical, biologic, radiologic, nuclear, and high-yield explosive WMDSs by providing capabilities to reduce, eliminate, and counter the threat and mitigate its effect. DTRA combines DOD resources, expertise, and capabilities to ensure the United States remains ready and able to address present and future WMDS threats. For more information on DTRA, visit www.dtra.mil. Transformational Medical Technologies (TMT) was created by DOD to protect the warfighter from emerging and genetically engineered biologic threats by discovering and developing a wide range of medical countermeasures through enhanced medical research, development, and test and evaluation programs. The TMT program office is matrixed from the joint science and technology office—DTRA and joint program executive office—chemical and biologic defense with oversight from the office of the secretary of defense. For more information on TMT, visit www.tmti-cbdefense.org.
Authorship
Contribution: C.R. performed experiments, analyzed data, and edited the manuscript; I.C.-T. and R.J. supervised the synthesis of small molecule TLR7 agonists; L.G.-S. performed experiments; E.H. and Y.I. synthesized small molecule TLR7 agonists; S.T., C.S., and S.N. advised on flow cytometric experiments and analysis; M.L.M. advised on study design; J.T. designed and supervised the synthesis of small molecule TLR7 agonists; N.M.V. and E.D.G. advised on study design and wrote the paper; and E.S. designed, supervised and performed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: All authors are employees of Novartis.
The current affiliation for I.C.-T. is Sanofi-Aventis Oncology, Cambridge, MA.
Correspondence: Elisabetta Soldaini, Novartis Vaccines & Diagnostics, via Fiorentina, 1, 53 100 Siena, Italy; e-mail: elisabetta.soldaini@novartis.com; and Ennio De Gregorio, Novartis Vaccines & Diagnostics, via Fiorentina, 1, 53 100 Siena, Italy; e-mail: ennio.de_gregorio@novartis.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal