Abstract
Controversy persists regarding the role of histopathology in the distinction between essential thrombocythemia (ET) and early-prefibrotic primary myelofi-brosis (PMF) presenting with thrombocythemia. To investigate the impact and reproducibility of bone marrow (BM) morphology according to the World Health Organization classification, 295 patients with the presumptive clinical diagnosis of either ET or early PMF were studied. Data of this cohort (Vienna group) were compared with 732 corresponding patients (Cologne group). Evaluating blindly (only age and gender known) BM specimens, the 2 groups of pathologists achieved an overall consensus of 78% regarding the total series and 88% concerning the discrimination between ET versus PMF. In 126 ET and 81 early PMF patients without pretreatment and complete documentation, a 90% concordance with the independently established clinical diagnosis was found. In 12 patients, overlapping of histopathology and some clinical findings between ET and polycythemia vera occurred. Contrasting ET, early PMF showed significant differences of presenting hematologic data and an unfavorable prognosis (estimated mean survival, 14 vs 21 years). Comparison of clinical and survival data of the Vienna cohort with the historical Cologne series revealed an overall congruence. This study highlights the impact of BM morphology for the differentiation between true vs false ET.
Introduction
Regarding the revised 2008 World Health Organization (WHO) criteria1 that were defined by a panel of expert hematopathologists and clinicians,2 serious concern has been repeatedly expressed. These controversies were essentially focused on the inclusion of histologic bone marrow (BM) features as diagnostic parameters for the characterization of distinctive entities of chronic myeloproliferative neoplasms (MPNs).3 It has been argued that these criteria show poor interobserver reliability and are not sufficiently robust enough to allow a clear-cut identification of MPN subgroups.4 In this context, it was stated that “MPN marrow morphology is not only a moving target, but also nonspecific with respect to phenotype, in contrast to lymphomas and acute myeloid malignancies.”3 Particularly, controversy and discussion persist regarding the discrimination of essential thrombocythemia (ET) from early-prefibrotic stages of primary myelofibrosis (PMF) frequently presenting with thrombocythemia (false ET) using BM histopathology as diagnostic yardstick.4-9 This distinction was claimed to be important because of an only minimal tendency to myelofibrotic progression in patients with (true) ET contrasting the development of BM fibrosis in long-term follow-up in early PMF.10-13 Moreover, a reduction of life expectancy was recorded in patients with early PMF contrasting true ET.14,15 Finally, another argument was that the body of published literature, including the cohorts of patients, was predominantly originating from the Cologne group and focused on histologic features.4
The first aim of this investigation was to address the role of BM morphology in a totally blinded evaluation of patients with the presumptive clinical findings of ET and early PMF, including the reproducibility of the WHO morphologic criteria. The second aim was to test the concordance of morphology with the independently obtained clinical findings; and finally, we tried to compare clinical data derived from the Vienna series of patients with a corresponding historical cohort from Cologne.
Methods
Study population
A cohort of 295 patients (132 males, 163 females; median age, 60 years) with the presumptive clinical diagnosis of either ET or early PMF were primarily recruited from the files of the University Clinic of Vienna covering a period between 1982 and 2009. For comparison corresponding data from a historical group of 732 patients (320 males, 412 females; median age, 61 years) were collected from our database in Cologne. In this context, it has to be underscored that contrasting the Vienna series the Cologne group was predominantly recruited based on BM histopathologic findings.
The study protocol was approved by institutional research ethics committees in both centers, and written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
BM biopsies
Representative BM trephine biopsies were available at presentation of all patients, including in 52 patients with sequential examinations (up to 3) during follow-up in the Vienna group. The majority of specimens were formalin fixed, decalcified in EDTA, and paraffin embedded; approximately 30 samples recruited before 1991 were plastic embedded. For assessment, trephine biopsy sections were stained with hematoxylin and eosin, Giemsa, periodic acid-Schiff reagent (PAS), ASD-naphthol-chloroacetate esterase, Prussian Blue, and Gomori silver impregnation to identify reticulin/collagen fibers.
Evaluation of BM slides and clinical data
Assessment of BM morphology consisted of a 3-step procedure and included a strict application of the original 2001 and 2008 revised WHO criteria,1,16 including characteristic histologic patterns for the recognition of different MPN subgroups.8,9,17 Consequently, to enhance objectivity in this study, the entered diagnostic criteria of distinctive value concerning WHO-defined ET versus early-prefibrotic PMF regarded more than 20 standardized morphologic features (Table 1). As explicitly outlined in Figure 1, specific changes of 4 major diagnostic parameters applied in our study allowed the generation of characteristic histologic BM patterns that enabled an accurate discrimination between both MPN entities. In this context, it is important to emphasize that no isolated BM feature characterizes any subtype of MPNs2,20 but only a distinctive histologic pattern. Evaluation of slides was done in a block and very rapidly (duration ∼ 1 week) to ensure consistency of diagnosis and avoid the unwanted bias of a learning effect. Following this study design, self-assessment was automatically ascertained because of the repeatedly distributed sets of slides at the beginning of each session provided in a blinded fashion. Regarding intraobserver assessment, results proved to be overall consistent. The multistep procedure of BM slide and subsequent clinical evaluation was conducted as follows: (1) A totally blinded (only ID, date of biopsy, and age known) and independently performed evaluation of the slide material derived from Vienna by the 2 groups of pathologists: L.M. (Vienna) and J.T. and H.M.K. (Cologne), respectively. (2) A subsequent consensus panel meeting of the pathologists at a multiheaded microscope to discuss the discrepant cases, but especially to specify so-called MPN-unclassifiable (MPN-U) cases with the attempt to reach a concordance of histologic diagnosis, again without any knowledge of clinical data. It is imperative in this context to emphasize that the morphologic diagnoses of all panel members were equally regarded, and consensus was only documented when all reached the same diagnosis. (3) A panel meeting of pathologists with the clinicians in Vienna to conduct a comparative evaluation of the hematologic data at presentation, follow-up, and survival by a critical review of the original clinical records.
Semiquantitative evaluation of prominent morphologic features exerting a discriminating impact between ET and early-prefibrotic stage of PMF applied to generate histologic patterns in representative treatment-naive bone marrow biopsies
| Feature* . | ET, % . | PMF, % . |
|---|---|---|
| Increased cellularity (age-matched) | 10-20 | 80-100 |
| Neutrophil granulopoiesis | ||
| Increased quantity | ≤ 10 | 50-80 |
| Left-shifting | ≤ 10 | 20-50 |
| Erythropoiesis | ||
| Increased quantity | ≤ 10 | ≤ 10 |
| Left-shifting | ≤ 10 | 10-20 |
| Megakaryopoiesis | ||
| Increased quantity | 80-100 | 50-80 |
| Size | ||
| Small | 0 | 20-50 |
| Medium | 10-20 | 10-20 |
| Large | 20-50 | 20-50 |
| Giant | 20-50 | 10-20 |
| Histotopography | ||
| Endosteal translocation | 10-20 | 20-50 |
| Cluster formation: size | ||
| Small (≥ 3) | 10-20 | 50-80 |
| Large (> 7) | 0 | 20-50 |
| Cluster formation: quality | ||
| Dense | 0 | 20-50 |
| Loose | 20-50 | 50-80 |
| Nuclear features | ||
| Hypolobulation (bulbous/cloud-like) | ≤ 10 | 50-80 |
| Hyperlobulation (staghorn-like) | 50-80 | ≤ 10 |
| Maturation defects | 0 | 50-80 |
| Naked nuclei | 20-50 | 50-80 |
| Fibers | ||
| Increased reticulin (minor fibrosis, grade 1)18,19 | 0 | 20-50 |
| Increased collagen | 0 | 0 |
| Osteosclerosis | 0 | 0 |
| Feature* . | ET, % . | PMF, % . |
|---|---|---|
| Increased cellularity (age-matched) | 10-20 | 80-100 |
| Neutrophil granulopoiesis | ||
| Increased quantity | ≤ 10 | 50-80 |
| Left-shifting | ≤ 10 | 20-50 |
| Erythropoiesis | ||
| Increased quantity | ≤ 10 | ≤ 10 |
| Left-shifting | ≤ 10 | 10-20 |
| Megakaryopoiesis | ||
| Increased quantity | 80-100 | 50-80 |
| Size | ||
| Small | 0 | 20-50 |
| Medium | 10-20 | 10-20 |
| Large | 20-50 | 20-50 |
| Giant | 20-50 | 10-20 |
| Histotopography | ||
| Endosteal translocation | 10-20 | 20-50 |
| Cluster formation: size | ||
| Small (≥ 3) | 10-20 | 50-80 |
| Large (> 7) | 0 | 20-50 |
| Cluster formation: quality | ||
| Dense | 0 | 20-50 |
| Loose | 20-50 | 50-80 |
| Nuclear features | ||
| Hypolobulation (bulbous/cloud-like) | ≤ 10 | 50-80 |
| Hyperlobulation (staghorn-like) | 50-80 | ≤ 10 |
| Maturation defects | 0 | 50-80 |
| Naked nuclei | 20-50 | 50-80 |
| Fibers | ||
| Increased reticulin (minor fibrosis, grade 1)18,19 | 0 | 20-50 |
| Increased collagen | 0 | 0 |
| Osteosclerosis | 0 | 0 |
Semiquantitative evaluation (relative incidence, %): 0 indicates usually absent; ≤ 10, rare; 10-20, slight; 20-50, moderate; 50-80, manifest; and 80-100, overt.
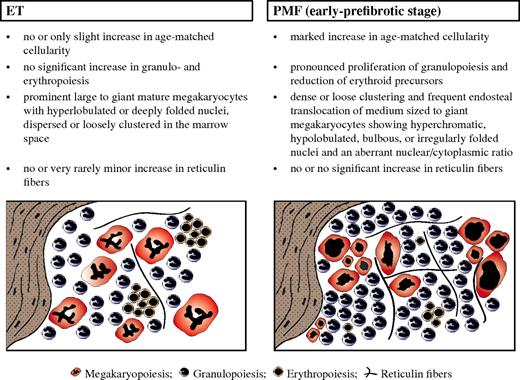
Diagnostic criteria of distinctive value regarding WHO-defined ET (left) versus early-prefibrotic stage of PMF (right), including standardized morphologic features (Table 1 contains more details), allowing the generation of characteristic histologic BM patterns.
Diagnostic criteria of distinctive value regarding WHO-defined ET (left) versus early-prefibrotic stage of PMF (right), including standardized morphologic features (Table 1 contains more details), allowing the generation of characteristic histologic BM patterns.
Statistical analysis
To determine consistency among pathologists, an inter-rater reliability analysis using the κ statistic was performed.21 Furthermore, corresponding κ values were calculated for the agreement between morphologic and clinical diagnosis. Descriptive statistics, including a nonparametric Mann-Whitney U test (significance level .05), were conducted to compare mean ranks of clinical-laboratory data between ET and PMF. Differences in observed survival rates were tested with a 2-sided log-rank test. All analyses were performed with SPSS (SPSS for Windows, Rel. 18; SPSS Inc).
Results
The first and main part of this study was to investigate the impact of BM histology as strictly isolated parameter in the more complex setting of MPN diagnosis, as is required by the WHO classification, and to test the reproducibility of characteristic morphologic features. The second part was devoted to a comparative assessment of corresponding data obtained from a large historical cohort of ET and early PMF patients from the Cologne archive material to validate these issues.
Following a totally blinded fashion of evaluation between the pathologists from Vienna and Cologne concerning the total cohort of 295 patients under study, an overall concordance of 78% could be reached (Table 2). The inter-rater reliability for the pathologists was found to be κ = 0.626 (P < .001), 95% confidence interval (0.550-0.702) consistent with substantial agreement. Regarding the presumptive clinical diagnosis of ET and early PMF without prior reference to BM morphology, the consensus rate was 215 of 295 (73%). Contrasting these calculations, the critical morphologic differentiation of the BM specimens revealing either histologic features of ET (Figure 2) or early PMF (Figure 3) has to take different cohorts into account. These consisted not only of the 215 concordant patients (Table 2), but of 29 cases as well (total 244 patients with either ET or PMF diagnosed by one of the panelists), where no consensus could be reached. Consequently, a concordance of 88% was revealed between both groups of hematopathologists regarding the blinded histopathologic discrimination of ET and early PMF with κ = 0.739 (P < .001), 95% confidence interval (0.651-0.827) compatible with substantial agreement. Remarkable were the few BM samples that displayed some morphologic features on the one side characteristic of ET (like a predominance of many large to giant megakaryocytes with hyperlobulated nuclei) and on the other side a striking proliferation of neutrophil granulopoiesis and particularly erythropoiesis (panmyelosis) resembling initial pre-polycythemic polycythemia vera (PV; Figure 4). These peculiar cases exhibiting a chimeric appearance concerning their histopathologic features were listed as ET/PV. There was only a small number of patients with morphologic diagnoses other than ET or early PMF (Table 2) in this primarily clinically generated series. A more detailed analysis of the discrepant cases showed some difficulties concerning the histologic distinction between the main MPN entities versus the 32 discordant cases of MPN-U from the Vienna group (Table 2). On the other hand, the consensus conference between the involved pathologists in Vienna, including particularly a critical discussion of these specimens at a multiheaded microscope, resulted in a specification of these MPN-U entities in 23 patients (mostly ET/PV, PV, and unclassifiable cases). However, there were still 9 cases left where no final agreement on morphology could be reached. Altogether, by following this procedure, an overall histopathologic concordance of 97% was achieved. The final clinicopathologic consensus meeting was strictly focused on a scrutinized review of clinical records and a comparison with the independently established morphologic diagnosis. Regarding explicitly the subset of cases in which a morphologic consensus was noted, the overall agreement with the final clinical diagnosis was 82% in the total cohort (including particularly the MPN-U and ET/PV cases) and 93% concerning the crucial differentiation between ET and early PMF. The overall agreement between morphologic and clinical diagnosis was found to be κ = 0.740 (P < .001), 95% confidence interval (0.658-0.822), again consistent with a substantial agreement.
Results of the blinded and independently performed evaluation of 295 bone marrow biopsies according to WHO criteria by a panel of pathologists from Vienna and Cologne
| . | Vienna . | Cologne . | Concordance . |
|---|---|---|---|
| ET | 160 | 179 | 144 |
| ET/PV | 2 | 8 | 2 |
| PV | 1 | 2 | 1 |
| Early PMF (prodromal) | 87 | 93 | 71 |
| Advanced PMF (classic) | 6 | 6 | 6 |
| MPN-U | 37 | 5 | 5 |
| UC | 2 | 2 | 1 |
| . | Vienna . | Cologne . | Concordance . |
|---|---|---|---|
| ET | 160 | 179 | 144 |
| ET/PV | 2 | 8 | 2 |
| PV | 1 | 2 | 1 |
| Early PMF (prodromal) | 87 | 93 | 71 |
| Advanced PMF (classic) | 6 | 6 | 6 |
| MPN-U | 37 | 5 | 5 |
| UC | 2 | 2 | 1 |
A concordant histologic diagnosis was revealed in 230 patients (78%) concerning the total cohort. Regarding the patients that were primarily diagnosed by the clinicians as presumptive ET or early PMF, consensus was 115 of 295 (73%).
ET/PV indicates overlapping BM features between ET and PV; and UC, unclassifiable.
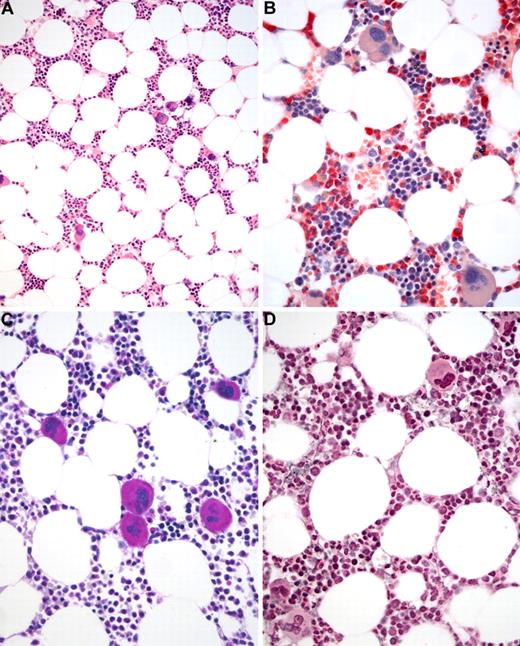
BM biopsy histology in ET in overview (×70) and low-power (×210) field. (A) Survey with hematoxylin and eosin-stained section shows an age-matched cellularity but prominent large to giant mature megakaryocytes in random distribution or loose clusters. (B) Chloroacetate esterase reaction demonstrates a normal amount and distribution of neutrophil granulopoiesis (red) and erythropoiesis besides the prominent megakaryocytes. (C) PAS staining reveals a hyperlobulation of megakaryocyte nuclei (almost staghorn-like) and mature cytoplasm without evidence of significant abnormalities. (D) Reticulin stain discloses no increase in fibers but large megakaryocytes. Images were acquired using Zeiss Axioplan 2, 10×/0.50 and 20×/0.50 EC PlanNeofluar.
BM biopsy histology in ET in overview (×70) and low-power (×210) field. (A) Survey with hematoxylin and eosin-stained section shows an age-matched cellularity but prominent large to giant mature megakaryocytes in random distribution or loose clusters. (B) Chloroacetate esterase reaction demonstrates a normal amount and distribution of neutrophil granulopoiesis (red) and erythropoiesis besides the prominent megakaryocytes. (C) PAS staining reveals a hyperlobulation of megakaryocyte nuclei (almost staghorn-like) and mature cytoplasm without evidence of significant abnormalities. (D) Reticulin stain discloses no increase in fibers but large megakaryocytes. Images were acquired using Zeiss Axioplan 2, 10×/0.50 and 20×/0.50 EC PlanNeofluar.
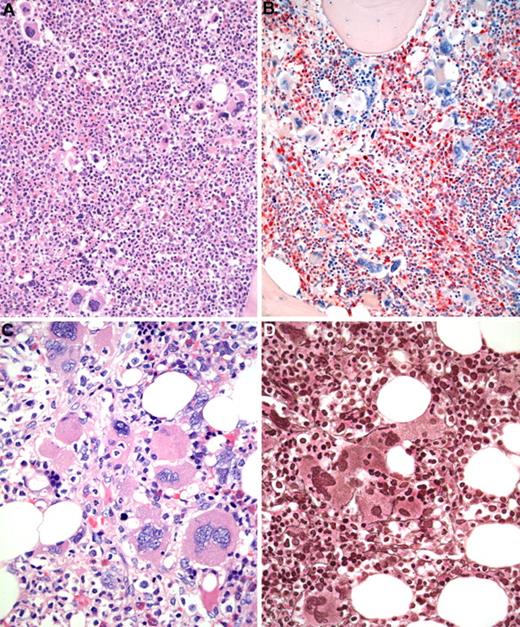
BM biopsy histology in early PMF in low-power (×170) or medium-power (×350) field. (A) Survey with hematoxylin and eosin-stained section shows increase in age-matched cellularity and conspicuous clusters of atypical megakaryocytes. (B) Chloroacetate esterase reaction discloses that increased cellularity is mostly the result of neutrophil granulopoiesis (red) and prominent clustering of large, abnormal megakaryocytes with paratrabecular dislocation. (C) PAS staining reveals not only loose and tight clustering but also striking abnormalities of megakaryocytes showing large hyperchromatic-hypolobulated, cloud-like nuclei. (D) Reticulin stain displays minimal increase with a fine network of fibers between the clustered abnormal megakaryocytes. Images were acquired using Zeiss Axioplan 2, 20×/0.50 and 40×/0.60 EC PlanNeofluar.
BM biopsy histology in early PMF in low-power (×170) or medium-power (×350) field. (A) Survey with hematoxylin and eosin-stained section shows increase in age-matched cellularity and conspicuous clusters of atypical megakaryocytes. (B) Chloroacetate esterase reaction discloses that increased cellularity is mostly the result of neutrophil granulopoiesis (red) and prominent clustering of large, abnormal megakaryocytes with paratrabecular dislocation. (C) PAS staining reveals not only loose and tight clustering but also striking abnormalities of megakaryocytes showing large hyperchromatic-hypolobulated, cloud-like nuclei. (D) Reticulin stain displays minimal increase with a fine network of fibers between the clustered abnormal megakaryocytes. Images were acquired using Zeiss Axioplan 2, 20×/0.50 and 40×/0.60 EC PlanNeofluar.
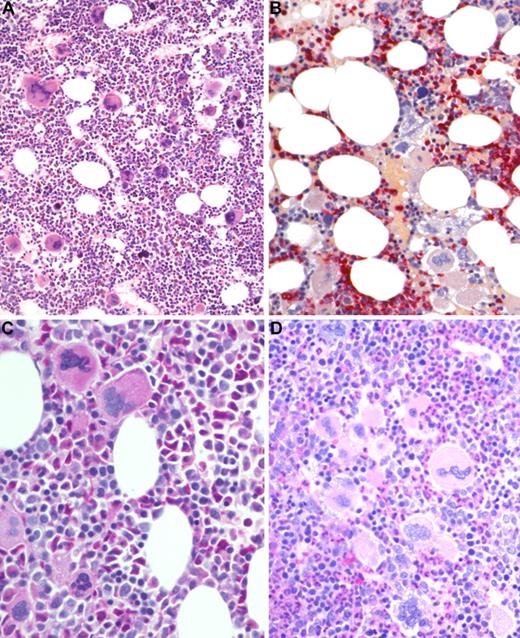
BM biopsy histology in ET/PV in low-power (×170) or medium-power (×350) field. (A) Survey with hematoxylin and eosin-stained section shows hypercellularity with conspicuous small to giant megakaryocytes and increased erythropoiesis and granulopoiesis. (B) Chloroacetate esterase reaction indicates panmyelosis or a PV-like picture: proliferation of neutrophil granulopoiesis (red) and large inlets of nucleated erythroid precursors intermingled with small to large megakaryocytes. (C) PAS staining demonstrates large to giant megakaryocytes, some with hyperlobulated (staghorn-like) nuclei surrounded by mature cytoplasms (resembling ET; see Figure 1C). (D) PAS staining with grouping of small to giant megakaryocytes with moderate nuclear lobulation consistent with a PV-like appearance. Images were acquired using Zeiss Axioplan 2, 20×/0.50 and 40×/0.60 EC PlanNeofluar.
BM biopsy histology in ET/PV in low-power (×170) or medium-power (×350) field. (A) Survey with hematoxylin and eosin-stained section shows hypercellularity with conspicuous small to giant megakaryocytes and increased erythropoiesis and granulopoiesis. (B) Chloroacetate esterase reaction indicates panmyelosis or a PV-like picture: proliferation of neutrophil granulopoiesis (red) and large inlets of nucleated erythroid precursors intermingled with small to large megakaryocytes. (C) PAS staining demonstrates large to giant megakaryocytes, some with hyperlobulated (staghorn-like) nuclei surrounded by mature cytoplasms (resembling ET; see Figure 1C). (D) PAS staining with grouping of small to giant megakaryocytes with moderate nuclear lobulation consistent with a PV-like appearance. Images were acquired using Zeiss Axioplan 2, 20×/0.50 and 40×/0.60 EC PlanNeofluar.
In the course of this clinicopathologic review, we had to exclude 76 patients from the original series of 295 cases. A variety of reasons were responsible for this exclusion from further clinical investigation in these MPN patients: a histologic diagnosis other than ET, early-prefibrotic PMF, or ET/PV (18 patients); no consensus either on morphologic diagnosis between the panelists (9 patients) or a discordance between clinical and morphologic diagnosis regarding ET versus early PMF (15 patients); a history of short-term therapy (except aspirin derivates) before BM biopsy found on critical reevaluation of the original clinical records (4 patients); and finally, patients missing one of the clinical parameters listed in the tables (8 patients) or incomplete data strictly at time of biopsy without any compromise (15 patients) or during follow-up (7 patients). According to this very restrictive procedure, 207 patients representing the main disease entities under consideration (ET and early PMF) were eligible for further clinical workup. Clinical data of both cohorts are detailed in Tables 3 and 4. Moreover, differences in some of the presenting hematologic findings between ET and early PMF patients are listed in Table 5.
Comparison of clinical data of ET patients between the Vienna and the historical Cologne series
| . | Vienna (126 patients) . | Cologne (167 patients) . | P* . |
|---|---|---|---|
| Age, y | 58.1 | 56 | NS |
| Sex, male/female | 46/80 | 67/100 | NS |
| Hemoglobin, g/dL† | |||
| Males | 14.2 | 14.3 | NS |
| Females | 14.1 | 14.0 | NS |
| Hematocrit, % | |||
| Males | 42.2 | 42.1 | NS |
| Females | 42.5 | 41.7 | NS |
| Leukocytes, × 109/L† | 8.8 | 11.0 | .012 |
| Myeloblasts, % | 0 | 0 | NS |
| Erythroblasts, % | 0 | 0 | NS |
| Platelets, × 109/L† | 758 | 1,000 | .001 |
| Palpable spleen, no. (%) | 22 (19) | 36 (22) | .001 |
| LDH, U/L† | 212 | 244 | NS |
| . | Vienna (126 patients) . | Cologne (167 patients) . | P* . |
|---|---|---|---|
| Age, y | 58.1 | 56 | NS |
| Sex, male/female | 46/80 | 67/100 | NS |
| Hemoglobin, g/dL† | |||
| Males | 14.2 | 14.3 | NS |
| Females | 14.1 | 14.0 | NS |
| Hematocrit, % | |||
| Males | 42.2 | 42.1 | NS |
| Females | 42.5 | 41.7 | NS |
| Leukocytes, × 109/L† | 8.8 | 11.0 | .012 |
| Myeloblasts, % | 0 | 0 | NS |
| Erythroblasts, % | 0 | 0 | NS |
| Platelets, × 109/L† | 758 | 1,000 | .001 |
| Palpable spleen, no. (%) | 22 (19) | 36 (22) | .001 |
| LDH, U/L† | 212 | 244 | NS |
NS indicates not significant; and LDH, lactate dehydrogenase.
Two-tailed Mann-Whitney U test.
Median value.
Comparison of clinical data of early PMF patients between the Vienna and the historical Cologne series
| . | Vienna (81 patients) . | Cologne (565 patients) . | P* . |
|---|---|---|---|
| Age, y† | 64.9 | 66 | NS |
| Sex, male/female | 33/48 | 253/312 | NS |
| Hemoglobin, g/dL† | |||
| Males | 14.2 | 14.0 | NS |
| Females | 12.9 | 13.5 | NS |
| Hematocrit, % | |||
| Males | 41.2 | 41.4 | NS |
| Females | 39.2 | 41.0 | NS |
| Leukocytes, × 109/L† | 10.0 | 12.7 | .023 |
| Myeloblasts, % | 0 | 0 | NS |
| Erythroblasts, % | 0 | 0 | NS |
| Platelets, × 109/L† | 784 | 891 | .001 |
| Palpable spleen, no. (%) | 45 (59) | 300 (53) | NS |
| LDH, U/L† | 321 | 289 | NS |
| . | Vienna (81 patients) . | Cologne (565 patients) . | P* . |
|---|---|---|---|
| Age, y† | 64.9 | 66 | NS |
| Sex, male/female | 33/48 | 253/312 | NS |
| Hemoglobin, g/dL† | |||
| Males | 14.2 | 14.0 | NS |
| Females | 12.9 | 13.5 | NS |
| Hematocrit, % | |||
| Males | 41.2 | 41.4 | NS |
| Females | 39.2 | 41.0 | NS |
| Leukocytes, × 109/L† | 10.0 | 12.7 | .023 |
| Myeloblasts, % | 0 | 0 | NS |
| Erythroblasts, % | 0 | 0 | NS |
| Platelets, × 109/L† | 784 | 891 | .001 |
| Palpable spleen, no. (%) | 45 (59) | 300 (53) | NS |
| LDH, U/L† | 321 | 289 | NS |
NS indicates not significant; and LDH, lactate dehydrogenase.
Two-tailed Mann-Whitney U test.
Median value.
Differences of clinical data between ET and early PMF in the Vienna cohort of patients
| . | ET (126 patients) . | Early PMF (81 patients) . | Z value . | P (2-tailed)* . |
|---|---|---|---|---|
| Age, y | 58.1 (18.7-85.4) | 64.9 (26.9-88.1) | −2.084 | .037 |
| Hemoglobin, g/dL | 14.2 (11.4-17.3) | 13.2 (7.9-16.6) | −2.622 | .009 |
| Hematocrit, % | 42.5 (33.0-52.0) | 39.6 (25.0-51.0) | −2.930 | .003 |
| Leukocytes, × 109/L | 8.8 (4.0-21.8) | 10.0 (5.5-24.5) | −2.827 | .005 |
| Erythroblasts, % | 0 (0-0) | 0 (0-2) | −3.293 | .001 |
| Myeloblasts, % | 0 (0-1) | 0 (0-2) | −3.163 | .002 |
| Platelets, × 109/L | 758 (326-2204) | 784 (112-1960) | −0.198 | .843 |
| Spleen size, cm† | 0 (0-11) | 1.5 (0-15) | −5.388 | < .001 |
| LDH, U/L | 212 (118-534) | 321 (164-667) | −7.456 | < .001 |
| . | ET (126 patients) . | Early PMF (81 patients) . | Z value . | P (2-tailed)* . |
|---|---|---|---|---|
| Age, y | 58.1 (18.7-85.4) | 64.9 (26.9-88.1) | −2.084 | .037 |
| Hemoglobin, g/dL | 14.2 (11.4-17.3) | 13.2 (7.9-16.6) | −2.622 | .009 |
| Hematocrit, % | 42.5 (33.0-52.0) | 39.6 (25.0-51.0) | −2.930 | .003 |
| Leukocytes, × 109/L | 8.8 (4.0-21.8) | 10.0 (5.5-24.5) | −2.827 | .005 |
| Erythroblasts, % | 0 (0-0) | 0 (0-2) | −3.293 | .001 |
| Myeloblasts, % | 0 (0-1) | 0 (0-2) | −3.163 | .002 |
| Platelets, × 109/L | 758 (326-2204) | 784 (112-1960) | −0.198 | .843 |
| Spleen size, cm† | 0 (0-11) | 1.5 (0-15) | −5.388 | < .001 |
| LDH, U/L | 212 (118-534) | 321 (164-667) | −7.456 | < .001 |
Values are median (range).
LDH indicates lactate dehydrogenase.
Mann-Whitney U test.
Below left costal margin.
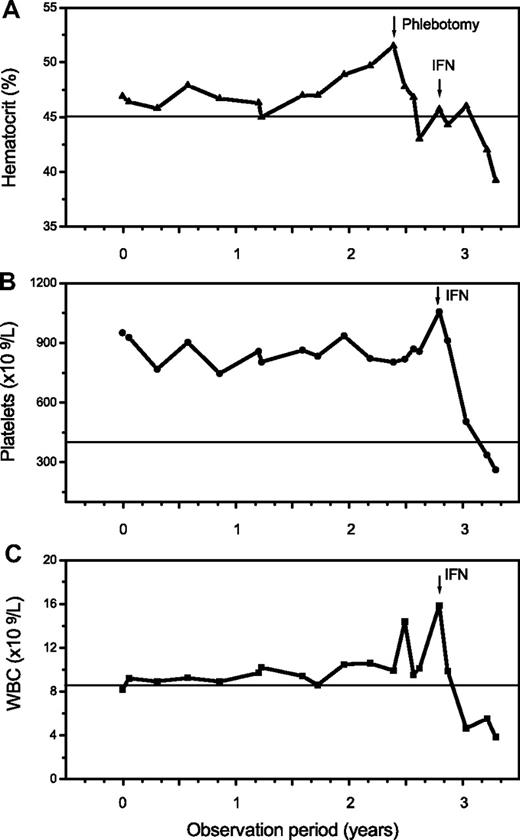
The additional small group of 12 patients with overlapping morphologic as well as clinical features of ET/PV displayed a sustained thrombocythemia (685 × 109/L) and borderline increased hemoglobin (male, 15.8 g/dL; female, 15.3 g/dL) values. Contrasting the other entities, this cohort had a serum erythropoietin level at the lower range (1.2-4.8 U/L), and all showed a positive JAK2 mutation status. Consistent with histologic BM features, an overlapping morphology between ET and PV was noted (Figure 4). Accordingly, clinical findings and particularly follow-up were suspicious in this hybrid group for occult-initial (prepolycythemic) PV mimicking ET at presentation, at least in a few patients. During the observation period, 4 patients evolved into manifest PV (Figure 5). However, most of these cases should be diagnosed as presenting with MPN-U.
Follow-up in a 46-year-old patient clinically presenting as ET with a platelet count of 951 × 109/L, a hemoglobin/hematocrit value at the upper limit, a positive JAK2 mutation status, and an erythropoietin level of 2.0 U/L. There was an increase of the hematocrit and white blood cells (WBC) starting after more than 2 years of observation with manifestation of overt PV requiring treatment with interferon (IFN).
Follow-up in a 46-year-old patient clinically presenting as ET with a platelet count of 951 × 109/L, a hemoglobin/hematocrit value at the upper limit, a positive JAK2 mutation status, and an erythropoietin level of 2.0 U/L. There was an increase of the hematocrit and white blood cells (WBC) starting after more than 2 years of observation with manifestation of overt PV requiring treatment with interferon (IFN).
Because of the time entering the patients into this study and the retrospective nature of this investigation, in 84% of the total cohort the JAK2 mutation status was available and showed an approximately 50% positivity in ET and early PMF.
Concerning the second aim of this investigation, the comparative evaluation of clinical data of the Vienna series of patients with the historical cohort from Cologne revealed a high level of concordance (Tables 3, 4). Although overlaps do occur, contrasting ET with early PMF showed a higher incidence of a borderline reduced hemoglobin/hematocrit level, slight increase in the leukocyte count and peripheral blood precursors (erythroblasts and myeloblasts), and higher lactate dehydrogenase levels and more patients with palpable spleen up to 5 cm below costal margin.
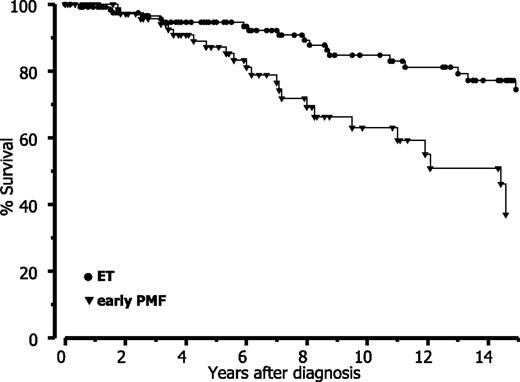
Finally, when strictly applying the WHO criteria, our data revealed that prognosis is significantly reduced in early PMF with thrombocythemia compared with (true) ET. Based on a long-term follow-up (Table 6), estimated mean survival data are comparable with the Cologne cohort for both entities if the differences in follow-up and amount of censored patients are considered. Accordingly, this analysis shows a significant (P < .0001) difference of approximately 7 years between ET and early PMF and consequently also a different relative survival (Figure 6).
Follow-up status and survival according to WHO diagnosis of patients in the Vienna series compared with the historical cohort from Cologne
| . | Vienna . | Cologne . |
|---|---|---|
| ET | ||
| Follow-up, y (median) | 7.3 | 9.5 |
| Person-years | 1162 | 1621 |
| Censored patients, % | 83 | 73 |
| Survival, y* | 21.2 | 16.1 |
| 95% CI | 19.1-23.3 | 14.6-17.4 |
| Early PMF | ||
| Follow-up, y (median) | 6.9 | 7.0 |
| Person-years | 565 | 3,932 |
| Censored patients, % | 68 | 56 |
| Survival, y* | 14.4 | 10.8 |
| 95% CI | 11.8-17.0 | 9.4-12.2 |
| . | Vienna . | Cologne . |
|---|---|---|
| ET | ||
| Follow-up, y (median) | 7.3 | 9.5 |
| Person-years | 1162 | 1621 |
| Censored patients, % | 83 | 73 |
| Survival, y* | 21.2 | 16.1 |
| 95% CI | 19.1-23.3 | 14.6-17.4 |
| Early PMF | ||
| Follow-up, y (median) | 6.9 | 7.0 |
| Person-years | 565 | 3,932 |
| Censored patients, % | 68 | 56 |
| Survival, y* | 14.4 | 10.8 |
| 95% CI | 11.8-17.0 | 9.4-12.2 |
Estimated mean survival.
Relative survival in ET versus early-prefibrotic PMF according to WHO diagnosis in the Vienna cohort.
Relative survival in ET versus early-prefibrotic PMF according to WHO diagnosis in the Vienna cohort.
Discussion
When addressing the criteria for diagnosing the different MPN entities, it is essential to emphasize that the WHO classification does not claim that a single histologic parameter characterizes a subgroup,2 but that morphologic BM patterns are very important, however, only in context with clinical and laboratory findings.20 Consequently, the revised WHO classification continues to advocate the need for defining operational procedures by regrading BM morphology explicitly as a first step toward a clinical and molecular-genetic characterization of MPNs.22 This basic issue can be easily demonstrated on the small group of patients with prodromal (prepolycythemic) stages of PV that mimic ET at presentation and later on evolve into an overt manifestation of disease. There are clinical observations in keeping with a transition of so-called ET into PV.23,24 On the other hand, a scrutinized evaluation of BM morphology enables in these patients, with the hybrid-overlapping features, a differentiation between a PV versus ET-like histologic pattern.25,26 As has been recently emphasized in addition to morphology, clinical-laboratory findings (borderline increase in red cell parameters, palpable splenomegaly, low erythropoietin level) and JAK2 mutation status are most important to differentiate early or prepolycythemic phases of PV with marked thrombocythemia from ET.26
Regarding clinical data of distinctive value in ET versus early-prefibrotic PMF, an important issue has to be underscored: According to our study design, the Vienna group of patients were primarily selected by the clinicians, independently of morphologic findings or absolutely complete documentation of all data strictly at BM biopsy date. Contrasting this study design in the Cologne series, BM histopathology was the basis for recruitment with a subsequently performed clinical evaluation. Although we are aware that we lost several of the originally recruited patients with ET and PMF in the Vienna cohort, it seemed for us more important to gain a very homogeneous series based on a stringent selection of those cases presenting with a complete set of all clinical data matching with the date of the BM biopsy. It is noteworthy that laboratory findings revealed no significant differences in both groups of patients (Tables 3–4), thus validating these data and therefore the impact of the WHO criteria. As has been explicitly emphasized, although always in context with clinical and, if possible, molecular genetic findings, BM morphology remains an integral and most important issue to the WHO classification.2,20,22 The consensus rate of 82% between histologic and clinical diagnosis of the total cohort of patients and a concordant result of 90% concerning the crucial discrimination of ET versus early PMF would have increased significantly if the pathologists were not blinded according to the study design. In this context, it should be underscored that, regarding the level of concordance among pathologists evaluating complex hematopoietic tissues, such as malignant T- and B-cell lymphomas, an agreement ranging between 41% and 71%27 (71%-86%, respectively)28 could be reached depending on the application of immunophenotyping. Conforming data were reported about subtyping of Hodgkin lymphoma.29 These results, derived from large series of international study groups, are in line with our rates showing a more than 80% reliability of diagnosis/reproducibility. Moreover, the peculiar double-blinded approach in this study, including 2 different series of patients evaluated in an independent as well as reverse fashion concerning clinical data and morphology, yielded results that are particularly novel and supportive for the WHO classification.
A conflict of opinion still persists concerning the reproducibility of the WHO histologic criteria by 2 groups of hematopathologists. Controversy is especially focused on the crucial discrimination between ET and early-stage PMF presenting with an elevated platelet count (false ET). Apart from the Cologne group, an increasing number of authorities were able to confirm the validity and reproducibility of morphologic BM features as proposed by the WHO.6,7,10,26,30,31 In addition, an international clinicopathologic study on 1104 patients with ET derived from 7 institutions, including a central totally blinded rereview strictly according to WHO criteria, revealed a concordance rate of 83% between the pathologists.32 Contrasting these confirmative results, a blinded evaluation among 3 hematologists/pathologists questions explicitly the reproducibility of the applied WHO criteria.4 In this context, it has to be noted that the BM biopsy specimens investigated were derived from the UK-PT1 trial33 and that study design and performance were unfortunately impaired by a number of inconsistencies: There was no clear-cut standardization of the 16 evaluated BM parameters strictly according to WHO (dysplastic and pyknotic megakaryocytes not included in Table 1) as has been previously detailed.8,9,17 A failing intraobserver evaluation (self-assessment) during the long period of examination may generate the unwanted bias of a learning effect. Moreover, the small size of a fraction of biopsy specimens (only ≥ 0.5 cm), contrasting the minimally requested length of 1.5 cm,20 precludes an accurate recognition of localized features, such as clusters, or a more exact grading of fibrosis and age-matched cellularity. Finally, the very poor reproducibility of basic BM features that may have served as controls for reliability, such as erythropoiesis (interobserver reliability only 3.9 with score 1 indicating no agreement beyond chance contrasting 10.1 for the conspicuous feature of new bone formation) or the wide range of a 37% to 76% incidence between the panelists concerning higher grades of BM fibrosis, are very disturbing. Further details regarding this study and critical interpretation of results have been already reported.34 Although this series of patients was explicitly defined to be consistent with ET4 according to the diagnostic criteria of the Polycythemia Vera Study Group,35 in a considerable fraction of samples moderate to overt BM fibrosis (grades 3 and 4) on a 4-graded scale36 and even new bone formation (osteosclerosis) were found.4 These unusual features, obviously present also in the BM trephines of ET patients enrolled in the UK-PT1 trial,33 were reiterated in a recent paper reporting that approximately 60% of the patients showed an increased BM fibrosis at disease onset, including more than 20% with moderate to overt myelofibrosis.5 According to a number of experts, ET patients present rarely with minimal to mild reticulin fibrosis but no collagen and, contrasting PMF, progression into overt myelofibrosis is a rather rare event occurring after many years.10-13,18,19,32,37,38 For this reason, it should be discussed that a considerable number of cases reported in all 3 papers, including overlapping cohorts of patients,4,5,33 are more probably consistent with thrombocythemic manifestations of PMF.39 In a recently published paper, 2 pathologists tried to reproduce the WHO diagnostic criteria16 on 127 BM biopsy specimens with ET according to Polycythemia Vera Study Group,35 resulting in a discordance of 35%.40 The overall conclusion of these authors that a discrimination between ET and early-prefibrotic PMF is impaired by subjectivity and of questionable clinical relevance has to be refuted considering a number of inconsistencies characterizing this study. Among others, these include the selection of the BM samples up to 3 years after clinical diagnosis (pretreatment unknown) and particularly the conspicuous finding that 54% of the ET patients showed a minor to moderate reticulin BM fibrosis. The latter feature is a very rare event when following strictly the WHO classification.1,39 Moreover, statistical analysis of morphologic features (scoring system) and clinical data including outcome did not take the significant disparity in the number of cases in both groups (ET 102 patients vs PMF 18 patients) into account. For this reason, no convincing evidence has been produced by the authors to discredit the validity of the WHO morphologic criteria.
An accurate distinction between ET and early PMF with excess in platelets (false ET) is not a matter of semantics but may exert an influence on decisions for therapeutic strategies and, most important, complications and outcome. Concerning prognostic differences under standard therapy, former studies from Cologne14,15 demonstrated a significant worsening of survival in early PMF contrasting ET diagnosed according to the WHO criteria.1,16 This result is confirmed and extended by this study, and it cannot be overemphasized that in this regard comparative data from the Vienna cohort of patients are matching with the large historical series of Cologne characterized by a very long follow-up. All this provides persuasive evidence that strict adherence to the WHO-defined ET diagnosis, survival appears to be significantly more favorable than previously reported.32,41-43 In this regard, it may be speculated that these striking features have been partially and involuntarily unveiled in an indirect fashion by the already discussed paper on “reticulin” (ie, correct reticulin and/or collagen) accumulation in so-called ET regarding laboratory data as well as complications but especially prognostic significance.5 After statistical analysis, patients presenting with moderate to overt BM fibrosis (fiber grades 3 to 4)36 at onset. This finding is comparable with survival data describing prognosis in early-prefibrotic versus fibro-osteosclerotic stages of PMF.14,15,19,30
In conclusion, an independently and blindly performed evaluation of BM specimens by 2 groups of pathologists has validated the reproducibility of morphologic parameters proposed by the WHO in the setting of MPN classification, particularly concerning ET versus early-stage PMF. As has been explicitly required, by regarding clinical data always in context with BM morphology a very high level of consensus was achieved. A comparison between clinical data and survival analysis, including a large historical series of patients derived from Cologne with the present cohort from Vienna revealed a striking congruence. Consequently, our findings highlight the clinical characteristics of the 2 distinctive entities ET and early-prefibrotic PMF.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Central European Myeloproliferative Neoplasm Study Organization.
Authorship
Contribution: J.T., H.M.K., L.M., V.B.-A., and H.G. designed the study; J.T., L.M., and H.M.K. evaluated BM morphology; V.B.-A., B.G., and H.G. evaluated clinical and laboratory data; H.M.K. performed the statistical analysis and provided Figures 1 and 6; H.M.K. and L.M. provided Figures 2 to 4; B.G. provided Figure 5; V.B.-A. and B.G. collected all clinical data, including outcome, under the oversight of H.G.; J.T. drafted the paper; and all other authors had the opportunity to contribute to the composition of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jürgen Thiele, Institute for Pathology, University of Cologne, Kerpener Strasse 62, D-50924 Cologne, Germany; e-mail: j.thiele@uni-koeln.de.
References
Author notes
J.T., H.M.K., and H.G. contributed equally to this study.