Abstract
Norovirus (NV) infections are a frequent cause of gastroenteritis (GE), but data on this disease in immunocompromised patients are limited. We analyzed an NV outbreak, which affected immunosuppressed patients in the context of chemotherapy or HSCT. On recognition, 7 days after admission of the index patient, preventive measures were implemented. Attack rates were only 3% (11/334) and 10% (11/105) among patients and staff members, respectively. The median duration of symptoms was 7 days in patients compared with only 3 days in staff members (P = .02). Three patients died of the NV infection. Commonly used clinical diagnostic criteria (Kaplan-criteria) were unsuitable because they applied to 11 patients with proven NV-GE but also to 15 patients without NV-GE. With respect to the therapeutic management, it is important to differentiate intestinal GVHD from NV-GE. Therefore, we analyzed the histopathologic patterns in duodenal biopsies, which were distinctive in both conditions. Stool specimens in patients remained positive for NV-RNA for a median of 30 days, but no transmission was observed beyond an asymptomatic interval of 48 hours. NV-GE is a major threat to patients with chemotherapy or HSCT, and meticulous measures are warranted to prevent transmission of NV to these patients.
Introduction
Infections with norovirus (NV) are a dominating cause of gastroenteritis (GE) worldwide, with great impact on public health. The clinical disease and its association with the winter season has been first described in 1929.1 In 1945, the transmissibility of NV-GE from patients to healthy volunteers was recognized, but it was not until 1972 when virus particles were discovered in fecal specimens obtained during an outbreak in Norwalk, Ohio.2 Until now, the NV is found to be refractory to replication in cell cultures or animal models, except for a study in which the authors used a sophisticated, highly differentiated 3-dimensional cell culture model and another study in which the authors investigated gnotobiotic animals.3,4 Thus, the understanding about NV infections is largely derived from volunteer studies and outbreak investigations. Traditionally, outbreaks or cases of NV-GE are diagnosed by clinical criteria (Kaplan criteria), which include a short 24- to 48-hour incubation period, ≥ 50% of affected patients with vomiting, an average duration of illness of only 12-60 hours, negative stool cultures for bacteria and parasites for outbreaks, or ≥ 2 episodes of vomiting and/or ≥ 3 episodes of diarrhea for case-defining criteria.5,6 Detection of NV-specific RNA in stool specimens by PCR is currently considered the standard to confirm the diagnosis of NV-GE.7
Outbreaks of NV-GE display notoriously high rates of attack, which is at least in part explained by a very low infectious dose (< 20 viral particles) and easy transmission not only by contaminated food or the fecal-oral route but also through infectious aerosols, which could be inhaled while another infected person is vomiting nearby.8,9 Attack rates exceeding 50% have been reported during NV-GE outbreaks, resulting in ward closures and major disruption of hospital services.10,11 Symptoms of NV-GE typically appear with abrupt onset and are mostly limited to 1-2 days in healthy patients, but surveillance data indicate that the duration of symptoms is prolonged in hospitalized patients.6,12 In addition, a growing number of reports suggest that NV-GE could result in severe and, occasionally, life-threatening complications in immunocompromised patients associated with prolonged viral shedding.13-17 However, clinical and virologic characteristics of otherwise-healthy patients and immunocompromised patients have yet not been compared during a single outbreak caused by a particular NV strain.
During an outbreak of GE in our hematologic and transplantation unit, which was caused by the predominant NV genotype II.4 variant strain, we recorded the pattern of disease among patients and staff members, the disease duration and severity, virologic surveillance data, the attack rates, and outbreak management. Histo-blood group antigens on gastroduodenal epithelium have been identified as receptors for NV.18 A low-to-absent susceptibility to NV infections has been recognized among patients with particular histo-blood group characteristics, ie, reduced susceptibility to NV genogroup I in patients with histo-blood group B and resistance against NV genogroup II among nonsecretors.19,20 Thus, we evaluated histo-blood group characteristics of all infected patients, including those with allogeneic HSCT. A subtle onset or prolonged illness in patients with NV infection after allogeneic HSCT may be undistinguishable from gastrointestinal GVHD. Because both conditions trigger completely different therapeutic consequences (increase of immunosuppression in patients with GVHD vs decrease of immunosuppression in NV infection), we compared the histopathologic patterns in biopsies from the gastrointestinal tract in patients with GVHD or NV-GE after allogeneic HSCT.
Methods
Hospital structure and staffing patterns
The outbreak occurred in the department of hematology and oncology of a 1057-bed tertiary center, which consisted in a closed transplantation unit and a 50-bed ward for patients with cancer or hematologic diseases, both separated from facilities for general medical patients. None of the patients nursed in the transplant unit was affected, but some patients with autologous HSCT and patients with stable engraftment and without intensified immunosuppressive therapies after allogeneic HSCT, who were treated outside the transplant unit, together with patients with cancer or hematologic diseases, acquired NV-GE. Outside the transplant unit, lavatories were separately available in 2 single rooms but were otherwise shared by patients from 2 neighboring double rooms.
Except for a single physician during night shift duty and trained cleaning staff, there were no common staff responsibilities inside and outside the transplant unit. However, staff member duties outside the transplant unit included care for patients with cancer, hematologic diseases, or autologous HSCT as well as stable patients after allogeneic HSCT.
Hygiene measures and case definitions
Before this outbreak, standard hygiene practices included reverse isolation of patients with neutropenia or intensified immunosuppressive therapies (eg, high-dose steroids) with the use of face masks by any visitor, strict avoidance of handshaking, hand disinfection before entering and after leaving any patient room, and limited access of visitors, excluding visitors younger than 16 years and visitors with symptoms of infection or with recent contact to another person with symptoms of infection. In addition, extra gowns, shoes, and face masks were used in the transplant unit with a limitation of visitors (usually < 2) per patient.
On recognition of the NV outbreak, any patient or staff member with nausea, vomiting, or diarrhea was considered as potentially harboring a NV infection. Patients with suspected NV infection were immediately transferred to an isolation ward and nursed in single rooms. Preventive measures always included the use of surgical face masks, single-use coats, gloves, and hand disinfectants with enhanced (75.1%) ethanol content. In individual patients, preventive measures were continued until negative results from stool specimen testing for NV-specific RNA were available. In patients with NV-GE confirmed by PCR testing, isolation and preventive measures were continued for at least 48 hours beyond complete resolution of symptoms of GE. All contact patients were also transferred to the isolation ward and observed in a similar fashion for at least 48 hours with screening for NV RNA in stool specimens. Staff members with symptoms of GE were immediately set off duty until they were without symptoms for at least 48 hours. All patients with symptoms of GE were classified according to established outbreak and case-defining criteria.5,6
Data collection and statistical analyses
Patient and staff member data were collected prospectively. Staff members were requested to complete questionnaire forms about symptoms and clinical signs. In all patients with suspected NV infection, clinical signs and symptoms of GE were recorded. In addition, patient files were reviewed to obtain clinical (eg, underlying diseases, ongoing and previous therapeutic interventions, immunosuppressive therapy, or medication) and laboratory data (eg, neutrophil count, histo-blood group typing, microbiologic findings in stool specimens). Patient and staff member data were made anonymous and were filed after approval from the Charité ethical committee was obtained.
Categorical and continuous variables were compared by the Fisher exact and Mann-Whitney U test, respectively. Kaplan-Meier plots were also produced, and time-dependent variables were analyzed by use of the Log-rank test. Calculations were performed with Prism statistical software (GraphPad Software Inc).
NV detection
Stool samples were screened for NV-specific RNA by real-time PCR located in the junction between ORF 1 and ORF 2 of the NV genome as previously described.21 Identification of the NV genotype was performed by a nested RT-PCR approach targeting the RNA polymerase gene within the open reading frame 1 with subsequent sequencing of PCR products.22 Stool specimens from all symptomatic patients were screened at least once for NV-specific RNA by real-time PCR. To investigate the duration of viral shedding in patients with confirmed NV-GE, sequential PCR testing of stool specimens was scheduled at 2-week intervals.
Histo-blood group and secretor status typing
Phenotyping of ABO histo-blood group characteristics in patients were available from previously performed standard serologic testing by the use of red blood cells and serum. In patients with allogeneic HSCT, full information on ABO histo-blood group characteristics of donors and recipients were available. In staff members without previous information on ABO histo-blood group characteristics (eg, from previous blood donation), ABO phenotyping was performed with informed consent by the use of red blood cells and bed-side testing cards containing anti-A and anti-B monoclonal antibodies (Medtro GmbH).
The secretor gene status in patients was identified by the use of stored peripheral blood, BM cells, or isolated DNA, which were in patients with allogeneic HSCT always obtained before transplantation. The embryonic epithelial cell line INT-407 harboring FUT2 polymorphism associated with a nonsecretor phenotype served as a control. FUT2 genotyping that predicts the secretor status was performed by PCR with sequence specific priming as described previously.23
Results
Outbreak description
The index patient was a 55-year-old female patient with high-risk B-CLL, carrying a del17p13, who had received an allogeneic HSCT 7 month before admission. After allogeneic HSCT, this patient experienced acute stage IV intestinal GVHD and, subsequently, protracted intestinal GVHD (1-2 bowel movements per day) requiring chronic immunosuppressive therapy with cyclosporine and methylprednisolone (8 mg per day). The current hospital admission was required because of disorientation, which was caused by dehydration and cyclosporine accumulation. Three days after admission, symptoms of GE, ie, emesis and diarrhea, occurred. Stool specimens were negative for bacterial and parasitic agents, and symptoms were first considered to be caused by intestinal GVHD. Within 6 days after admission of the index patient and before the NV outbreak was confirmed, 4 other patients developed vomiting and diarrhea with abrupt onset, which prompted laboratory screening for NV-GE.
On day 5 after admission of the index patient, her roommate (secondary patient) developed emesis and diarrhea, after being referred on day 4 to another room. The roommate of this secondary patient (tertiary patient) developed symptoms of GE on day 6. Another 2 patients nursed in the adjacent room and who shared the lavatory with the secondary and tertiary patient became symptomatic also on day 6. On day 7, results from PCR testing were available and confirmed presence of a NV outbreak. Overall, the likely modes of transmission were inhalation of infectious aerosols during nearby vomiting in 5 patients and 5 staff members and fecal-oral through lavatory sharing in 2 patients. The routes of transmission in the remaining 4 patients and 6 staff members remained less clear, but person-to-person transmission via fecal-oral route appeared likely. We were unable to identify obvious breaks in hygiene practices. However, 6 of 11 staff members with NV-GE developed their first symptoms 3 days or later after NV was identified by PCR, which makes at least occasional breaks or less strict adherence to the modified hygiene policies likely.
Further investigations revealed that the index patient was previously treated in a rehabilitation center at which an outbreak of NV-GE was recorded, indicating the likely source of the infection. During this outbreak no other hospital facilities recorded cases of NV-GE, making a food- or water-borne source of this outbreak from our hospital facilities unlikely.
On recognition that this cluster of GE was caused by NV, additional hygiene measures were implemented. Patients with nausea, vomiting, or diarrhea and contact patients (roommates or those who had shared lavatories with symptomatic patients) were immediately transferred to an isolation ward and nursed in single rooms until negative results from stool specimen testing for NV-specific RNA were available. In patients with NV-GE confirmed by PCR, isolation and preventive measures were continued for at least 48 hours beyond complete resolution of symptoms. The total duration of this outbreak, defined by admission of the index patient to the occurrence of the last documented infection, was 54 days (April 10, 2005 to June 3, 2005). During this period, a total of 334 inpatients were treated, and 105 staff members were involved into patient care. Thus, the attack rates for patients and staff members were 3.3% (11 of 334) and 10.5% (11 of 105), respectively (P = .008).
Clinical characteristics of patients and staff members
Overall, 26 patients and 11 staff members experienced vomiting, diarrhea, or both during the outbreak. Of these, NV-GE was confirmed by PCR in 11 patients and 11 staff members. Affected staff members were younger and otherwise healthy compared with patients (Table 1). The underlying diseases among the 11 patients with confirmed NV-GE were acute leukemia (n = 5) and malignant lymphoma (n = 6: B-cell chronic lymphocytic leukemia, 3; diffuse large cell lymphoma, 2; lymphoblastic B-cell lymphoma, 1). A single patient with B-cell chronic lymphocytic leukemia had been treated only with rituximab monotherapy until 4 months before the occurrence of NV-GE (Table 1). Polychemotherapy had been administered to 5 patients 4-56 days (median, 19 days) before the onset of NV-GE. Two patients with autologous HSCT were roommates and experienced vomiting and diarrhea during conditioning BEAM chemotherapy or shortly thereafter (days −1 and −7). Another 3 patients had received allogeneic HSCT 71, 72, and 210 days before onset of NV-GE. Overall, 7 patients had neutropenia (neutrophil count < 0.5/nL) at the onset of symptoms (Table 1).
Characteristics and symptoms of patients and staff members with norovirus gastroenteritis
| . | Patients (n = 11) . | Staff members (n = 11) . | P . |
|---|---|---|---|
| Median age, y (range) | 56 (17-71) | 38 (20-56) | .045 |
| Sex, male/female | 4/7 | 4/7 | ns |
| Secretor status | |||
| Se/se | 7 | nd | |
| Se/Se | 4 | nd | |
| Underlying diseases | |||
| Acute leukemia | 5 | ||
| Lymphoma | 6 | ||
| Therapy | |||
| Rituximab monotherapy | 1 | ||
| Chemotherapy | 5 | ||
| Autologous HSCT | 2 | ||
| Allogeneic HSCT | 3 | ||
| First symptoms after chemotherapy (n = 5) median days (range) | 19 (4-56) | ||
| First symptoms relative to HSCT (n = 5), median days (range) | 71 (−6 to 210) | ||
| Neutrophils, < 500/μL* | 7 | ||
| Symptoms, median days (range) | 7 (2-36) | 3 (1-13) | .02 |
| Nausea | 4 | 9 | ns |
| Vomiting | 8 | 7 | ns |
| Diarrhea | 11 | 9 | ns |
| Fever (≥ 38.5°C) | 6 | 1 | ns |
| Complications | |||
| Fatal aspiration | 1 | ||
| Septicemia | 2 |
| . | Patients (n = 11) . | Staff members (n = 11) . | P . |
|---|---|---|---|
| Median age, y (range) | 56 (17-71) | 38 (20-56) | .045 |
| Sex, male/female | 4/7 | 4/7 | ns |
| Secretor status | |||
| Se/se | 7 | nd | |
| Se/Se | 4 | nd | |
| Underlying diseases | |||
| Acute leukemia | 5 | ||
| Lymphoma | 6 | ||
| Therapy | |||
| Rituximab monotherapy | 1 | ||
| Chemotherapy | 5 | ||
| Autologous HSCT | 2 | ||
| Allogeneic HSCT | 3 | ||
| First symptoms after chemotherapy (n = 5) median days (range) | 19 (4-56) | ||
| First symptoms relative to HSCT (n = 5), median days (range) | 71 (−6 to 210) | ||
| Neutrophils, < 500/μL* | 7 | ||
| Symptoms, median days (range) | 7 (2-36) | 3 (1-13) | .02 |
| Nausea | 4 | 9 | ns |
| Vomiting | 8 | 7 | ns |
| Diarrhea | 11 | 9 | ns |
| Fever (≥ 38.5°C) | 6 | 1 | ns |
| Complications | |||
| Fatal aspiration | 1 | ||
| Septicemia | 2 |
Numbers of patients are given, if not stated otherwise.
nd indicates not determined; and ns, not significant.
At onset of symptoms.
There was no difference in ABO types between patients and donors (O 10, A 9, B 2, AB 1). Genotyping for the Secretor status in the 11 patients with confirmed NV-GE revealed always a homozygous Se/Se (n = 4) or heterozygous Se/se (n = 7) FUT2 polymorphism, predicting a Secretor-positive phenotype (Table 1).
Outbreak and case-defining criteria
Clinical outbreak criteria were fulfilled with a ≥ 50% frequency of vomiting not only in patients with confirmed NV-GE but also in patients without NV-GE (73% of patients with vomiting in both groups; Table 2).5 The median duration of illness was longer in patients with confirmed NV-GE (7 days) than the median 12- to 60-hour disease duration proposed by commonly accepted outbreak-defining criteria.5 Microbiologic testing of stool specimens was performed in 8 patients with confirmed NV-GE and 11 patients without NV-GE, which gave always negative results except in 4 patients without NV-GE (Table 2). Case-defining criteria, designed for hospital patients, were always fulfilled in patients with confirmed NV-GE.6 However, these case-defining criteria were also fulfilled in up to 10 of 15 (67%) patients with symptoms of GE not caused by NV (Table 2).
Outbreak and case-defining criteria in all patients who experienced diarrhea and/or vomiting during the outbreak with norovirus gastroenteritis
| . | Norovirus PCR . | |
|---|---|---|
| Positive (n = 11) . | Negative (n = 15) . | |
| Outbreak criteria5 | ||
| Duration of symptoms, days, median (range)* | 7 (2-36) | 6 (1-58) |
| Patients with vomiting, n (%) | 8/11 (73) | 11/15 (73) |
| Patients with stool specimens negative for bacterial pathogens, n (%) | 8/11 (73)† | 7/15 (47)‡ |
| Case-defining criteria6 | ||
| ≥ 2 episodes of vomiting, n (%)* | 8/11 (73) | 6/15 (40) |
| ≥ 3 episodes of diarrhea, n (%)§ | 11/11 (100) | 10/15 (67) |
| Both criteria fulfilled during a 24-hour period, n (%)§ | 7/11 (64) | 3/15 (20) |
| . | Norovirus PCR . | |
|---|---|---|
| Positive (n = 11) . | Negative (n = 15) . | |
| Outbreak criteria5 | ||
| Duration of symptoms, days, median (range)* | 7 (2-36) | 6 (1-58) |
| Patients with vomiting, n (%) | 8/11 (73) | 11/15 (73) |
| Patients with stool specimens negative for bacterial pathogens, n (%) | 8/11 (73)† | 7/15 (47)‡ |
| Case-defining criteria6 | ||
| ≥ 2 episodes of vomiting, n (%)* | 8/11 (73) | 6/15 (40) |
| ≥ 3 episodes of diarrhea, n (%)§ | 11/11 (100) | 10/15 (67) |
| Both criteria fulfilled during a 24-hour period, n (%)§ | 7/11 (64) | 3/15 (20) |
Not significant.
Three patients without microbiological testing.
Four patients without microbiological testing, 3 patients with a positive test result for Clostridium difficile toxin, and 1 patient with enhanced number of yeasts in a stool specimen.
P ≤ .05.
Symptoms and complications
Diarrhea and vomiting occurred in the majority of patients and staff members with confirmed NV-GE. However, in 6 patients compared with only 1 staff member fever was recorded (Table 1). The median duration of symptoms in affected patients was significantly longer compared with staff members (7 vs 3 days, P = .02; Table 1 and Figure 1).
Kaplan-Meier curves showing the disease duration in NV GE. The symptom durations in 11 patients and 11 staff members with NV gastroenteritis are displayed.
Kaplan-Meier curves showing the disease duration in NV GE. The symptom durations in 11 patients and 11 staff members with NV gastroenteritis are displayed.
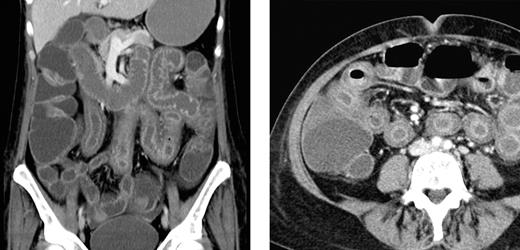
The severity of symptoms in patients varied largely and ranged from subtle and slowly progressive in the index patient to severe in 2 patients with high-dose carmustine, etoposide, cytarabine, and melphalan (BEAM) chemotherapy and autologous HSCT. In 1 of these latter 2 patients, bowel perforation was suspected because of severe abdominal pain and tenderness. In this particular patient, an abdominal computed tomography (CT) scan disclosed extensive and pronounced edema of the small bowel sparing other parts of the gastrointestinal tract (Figure 2). A similar small bowel edema was seen in a CT series in another patient who developed NV-GE with acute abdomen after high-dose methotrexate-based polychemotherapy. The other patient with BEAM chemotherapy and autologous HSCT experienced continuous retching, which was refractory to intensified antiemetic therapy. Unfortunately, this patient experienced severe aspiration resulting in hypoxic brain damage and death 11 days after onset of NV-GE. Another 2 patients experienced fatal sepsis 17 and 49 days after onset of symptoms of NV-GE. The first of these latter 2 patients showed profound neutropenia (neutrophil count, 0.03/nL) after intensified chemotherapy for acute myeloid leukemia and at the onset of NV-GE. Subsequently, this patient experienced fatal sepsis despite broad-spectrum antibacterial and antifungal therapy (repetitive blood cultures were negative). In the other patient, who had received allogeneic HSCT 2 months before the NV infection, Escherichia coli was repeatedly recovered from the blood stream without any clinical site of infection, suggesting that bacterial translocation from the intestine was promoted by the NV infection.
Abdominal CT scan of a patient with NV GE. Extensive bowel edema in a patient with NV GE acquired during BEAM conditioning chemotherapy for autologous HSCT. The bowel wall edema was restricted to the small intestine, and the colon showed only a moderate dilation.
Abdominal CT scan of a patient with NV GE. Extensive bowel edema in a patient with NV GE acquired during BEAM conditioning chemotherapy for autologous HSCT. The bowel wall edema was restricted to the small intestine, and the colon showed only a moderate dilation.
Serial RT-PCR analyses of stool specimens from patients with confirmed NV-GE was scheduled. Three patients already had negative RT-PCR test results at the first follow-up investigation, 2 patients showed positive RT-PCR test results in serial stool specimens without further follow-up specimens, and in 1 patient only a single stool specimen was available. The minimal duration of viral shedding (onset of symptoms to last positive RT-PCR) ranged from 11 to 87 days (median, 30 days). The median interval between onset of symptoms until documented cessation of viral shedding (first negative RT-PCR) was 36 days (range, 9-118 days).
Endoscopic findings
Endoscopy of the upper gastrointestinal tract was performed in 3 patients with allogeneic HSCT and showed a normal appearance of the gastric mucosa in 2 patients and a pangastric erythema in the remaining patient. In all patients, the endoscopic appearance of the duodenal mucosa was unremarkable. Two patients with allogeneic HSCT underwent additional sigmoidoscopy, which showed petechial lesions in one patient (low platelet counts, no biopsy taken) and a normal appearance of the mucosa in the other patient.
Histopathology findings
Duodenal biopsies obtained during intestinal GVHD and after onset of the NV infection were available from 3 patients with allogeneic HSCT. A detailed analysis revealed a distinct histopathologic pattern during GVHD and NV-GE. Intestinal GVHD was characterized by a partial loss of epithelial cells, increased numbers of apoptotic crypt cells pronounced at the base of the crypts, and increased numbers of CD8+ T cells within the lamina propria with only focal infiltration of the crypt epithelium (Figure 3A-B). In contrast, biopsies during NV-GE showed villous blunting, only slightly elevated numbers of apoptotic epithelial cells predominantly at the luminal surface, and a massive increase in the numbers of intraepithelial lymphocytes consisting of CD8+ T cells (Figure 3C-D).
Sequential duodenal biopsies from a patient with allogeneic HSCT. (A) Duodenal biopsy with severe GVHD showing partial loss of surface epithelium (H&E) and increased numbers of apoptotic crypt epithelial cells (inset: cleaved caspase-3 staining showing apoptotic epithelial cells at the base of crypts). (B) Predominance of CD8+ T cells in the lamina propria with focal infiltration of the crypt epithelium (immunohistochemistry, APAAP method). (C) In contrast, duodenal biopsy in the same patient during NV infection shows villous blunting (H&E) and only a slight increase of apoptotic epithelial cells at the tip of the villi (inset: cleaved caspase-3) and (D) a massive increase of intraepithelial CD8+ T cells pronounced in the surface epithelium. Original magnifications, ×200 and ×600 (inset). Microscope: Olympus AX70 (Olympus); numeric aperture of objective lenses: ×20, 0.70 mm; ×60, 1.40. Stains: H&E (panels A and C); APAAP and streptavidin AP (panels B and D, inset). Camera: JVC KY-F70 (JVC); acquisition software: DISKUS; and software used for image processing: Adobe Photoshop 7.0 (Adobe Systems).
Sequential duodenal biopsies from a patient with allogeneic HSCT. (A) Duodenal biopsy with severe GVHD showing partial loss of surface epithelium (H&E) and increased numbers of apoptotic crypt epithelial cells (inset: cleaved caspase-3 staining showing apoptotic epithelial cells at the base of crypts). (B) Predominance of CD8+ T cells in the lamina propria with focal infiltration of the crypt epithelium (immunohistochemistry, APAAP method). (C) In contrast, duodenal biopsy in the same patient during NV infection shows villous blunting (H&E) and only a slight increase of apoptotic epithelial cells at the tip of the villi (inset: cleaved caspase-3) and (D) a massive increase of intraepithelial CD8+ T cells pronounced in the surface epithelium. Original magnifications, ×200 and ×600 (inset). Microscope: Olympus AX70 (Olympus); numeric aperture of objective lenses: ×20, 0.70 mm; ×60, 1.40. Stains: H&E (panels A and C); APAAP and streptavidin AP (panels B and D, inset). Camera: JVC KY-F70 (JVC); acquisition software: DISKUS; and software used for image processing: Adobe Photoshop 7.0 (Adobe Systems).
The gastric biopsies from 2 of these patients showed a mild chronic gastritis with no signs of GVHD and were normal in the remaining patient. The rectosigmoidal biopsies obtained from one patient showed increased numbers of apoptotic crypt cells with an edematous lamina propria consistent with stage I GVHD (data not shown).
Discussion
NV infection is among the most frequent causes of infectious GE worldwide, and outbreaks could result in major disruption of hospital services. The disease in otherwise-healthy patients is self-limiting and of short duration, but growing numbers of case reports and retrospective analyses indicate that immunocompromised patients with NV-GE experience a prolonged and more severe illness.13-17 However, a study in which the authors documented the disease severity in adult patients after chemotherapy or allogeneic HSCT in comparison with healthy patients was missing.
NV-GE is very easily transmitted with a very low infectious dose (< 20 viral particles), including transmission through inhalation of infectious aerosols during nearby vomiting.8 Noteworthy, we recorded a low overall attack rate, which was significantly lower among patients compared with staff members (3% vs 10%). These low attack rates contrast results from a recently published analysis, which demonstrated a median > 32% patient attack rate recorded during 21 hospital outbreaks.24 This large difference might be explained by the early identification of NV in this study and the more carefully applied standard hygiene procedures established in the care of severely immunocompromised patients. We recorded prolonged viral shedding in patients beyond the resolution of symptoms, which is in line with previous observations.13,14,17 However, we did not observe further transmission of NV from any patient beyond a 48-hour asymptomatic interval, which suggests that transmission from immunocompromised hosts with prolonged shedding is uncommon. All NV infected patients had a Secretor-positive phenotype, which expresses the carbohydrate H type 1 known as a target structure for NV binding. Thus, it remains unclear whether severely immunocompromised patients with Secretor-negative phenotype display any enhanced susceptibility to infection by NV genogroup II.
In a recently published analysis of 12 patients with allogeneic HSCT and epidemiologically unrelated NV-GE, the median duration of illness was remarkably longer compared with our study (3 months vs 7 days).14 In this previously published series of patients with allogeneic HSCT, one patient died because of malnutrition after more than 1 year with unresolved NV-GE compared with 3 patients in our study with NV-related deaths, which occurred within 49 days after onset of symptoms. In our study, only 3 patients had received allogeneic HSCT, and most patients acquired the NV infection during chemotherapy-induced neutropenia. It appears therefore likely that T-cell–directed immunosuppressive therapies after allogeneic HSCT promote chronic NV infections with prolonged diarrhea and wasting, whereas recent chemotherapy and neutropenia enhance the disease severity and the likelihood of life-threatening complications.
In the previously published series of patients with NV-GE after allogeneic HSCT, the median time from onset of symptoms until the diagnosis was established widely ranged from 0.25 to 6 months,14 which illustrates to some extent the diagnostic dilemma in patients with diarrhea after allogeneic HSCT. Diarrhea is a frequent complication after HSCT and might be caused by the conditioning therapy, intestinal GVHD, or infectious agents, or it could also be drug-related (eg, mycophenolate mofetil). In a previously published study with HSCT patients, 22 (79%) of 28 patients with allogeneic HSCT experienced diarrhea sometime after the transplantation procedure. Most diarrheal episodes occurred early (ie, within 20 days after transplantation) and in none of these episodes an infectious agent was identified.25 Four of these 22 allogeneic HSCT recipients experienced diarrhea later than 20 days after transplantation, which was always caused by viral infections (rotavirus 3, adenovirus 1). However, screening did not include NV in this study.25
We found a significantly prolonged duration of symptoms in patients compared with staff members, which clearly confirms findings from previously published, noncomparative reports on patients with various immunocompromising conditions and NV-GE.13-17 In this outbreak, the maximum duration of symptoms among patients was limited to 36 days, but profuse diarrhea with wasting lasting up to more than 1 year has been previously observed in allogeneic HSCT patients with delayed diagnosis of NV-GE.14 Of importance, we observed severe-to-fatal complications, which were clearly attributable or likely caused by the NV infection. Two patients with BEAM chemotherapy developed symptoms of an acute abdomen with peritonitis, which prompted abdominal CT scans showing extensive and pronounced bowel wall edema restricted to the small intestine.
This radiologic pattern is identical to previous observations in a small number of nonimmunocompromised patients with NV-GE.26,27 However, BEAM chemotherapy, gastrointestinal CMV infection, or GVHD might also cause small bowel edema, but exclusive involvement of the small intestine has been observed infrequently in intestinal CMV infection or GVHD, and radiologic studies in patients with gastrointestinal complications after BEAM chemotherapy (eg, colitis and stomatitis) are missing.28,29 The coexistence of NV infection and intestinal GVHD could represent a diagnostic and therapeutic challenge. We found evidence of simultaneous GVHD in large bowel biopsies in a single patient with NV-GE. It appears less likely that the GVHD-like pattern in this particular patient was directly caused by the NV as studies in healthy volunteers with experimental NV-GE showed unremarkable rectal biopsies.30 However, we found no evidence of small intestine GVHD coexisting with NV infection in any of our patients.
Three patients finally died in close association with the NV-GE. One patient experienced fatal aspiration. The other 2 patients died as a consequence of sepsis, which was likely promoted by the mucosal barrier defect induced by the NV infection and consecutive translocation of intestinal bacteria.
Infections with widespread enteric viral pathogens other than NV have been repeatedly reported in patients with hematologic diseases or HSCT patients. Two larger studies in which the authors evaluated rotavirus or astrovirus infections in patients with HSCT or hematologic diseases demonstrated a pronounced illness and a prolonged duration of illness (median > 10 days).31,32 In contrast to NV, rotavirus and astrovirus infections appear to some extent less severe as lethal complications have not been reported from these studies. Interestingly, asymptomatic shedding of astrovirus was found in 8 of 21 pediatric patients investigated during an outbreak in a transplant unit,32 which further indicates that this small round structured virus is less virulent than NV.
As shown in this study, established clinical outbreak and case-defining criteria are unsuitable to recognize NV infections in immunocompromised patients because side effects of chemotherapy or HSCT may mimic symptoms of NV infection. In addition, symptoms of intestinal GVHD may be undistinguishable from NV-GE, and the therapeutic strategies in these 2 conditions are greatly divergent. Comparison of duodenal biopsies from patients with GVHD before and after onset of the NV infection exhibited distinct histopathologic patterns, which allow segregation of GVHD from NV infection. During the NV infection, histopathologic findings in immunocompromised patients were similar to those described in nonimmunocompromised hosts with NV-GE, which clearly contrasts the characteristic histopathologic pattern in the same patients during acute GVHD and before the onset of NV-GE.33
Our data demonstrate that NV infections cause severe disease and fatal outcomes in patients after chemotherapy and HSCT. Meticulous isolation may reduce transmission of NV and such enhanced hygiene measures should always be implemented at first suspicion of an NV outbreak in health care facilities with immunocompromised patients, while the laboratory screening for NV in risk patients is pending. On the basis of these experiences, we maintained our modified hygiene strategy in suspicious cases and incorporated routine testing for NV and other enteric viral pathogens into the diagnostic algorithm.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank M. Höhne and K. Stanossek for NV genotyping and skillful technical assistance.
M.V. has received a research grant from the Berliner Krebsgesellschaft.
Authorship
Contribution: S.S., E.T., and T.S. designed the study, analyzed/interpreted the data and wrote the paper; M.V., M.R., and M.S.-H. collected data and performed data analyses; E.S. and W.A.F. contributed vital analytical tools (norovirus genotyping and detection; molecular Histo-blood group typing), analyzed data, and wrote the paper; and C.L. performed and analyzed histopathology and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stefan Schwartz, MD, Medizinische Klinik III, Charité–Universitätsmedizin Berlin, Campus Benjamin Franklin, Hindenburgdamm 30, 12200 Berlin, Germany; e-mail: stefan.schwartz@charite.de.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal