Abstract
The immunomodulatory receptor CD300a is expressed on human B cells. Naive B cells express very low levels of this receptor, whereas memory B cells and plasmablasts/cells express variable levels of CD300a. Germinal center B cells are negative for CD300a expression. Stimulation of naive B cells via B-cell receptor (BCR) and Toll-like receptor 9, along with T-cell help, failed to up-regulate CD300a cell surface expression despite the increased expression of the memory marker CD27 and the down-regulation of CD305. However, Toll-like receptor 9 stimulation alone significantly increased CD300a expression on memory B cells, whereas interleukin-4 and transforming growth factor-β1 act as negative regulators of CD300a expression on memory B cells. Coligation of BCR and CD300a inhibits Ca2+ mobilization and nuclear factor of activated T cell transcriptional activity evoked by BCR ligation alone. Suppression of CD300a expression in primary B cells with siRNA resulted in increased BCR-mediated proliferation, thereby confirming the inhibitory capacity of CD300a. Finally, we show that CD300a expression levels are significantly down-regulated in the circulating B cells of HIV-infected patients. Altogether, these data demonstrate a novel mechanism for suppressing the activity of B cells and suggest a potential role for CD300a in the B-cell dysfunction observed in HIV-induced immunodeficiency.
Introduction
An adequate immune response is the result of a fine balance between a multitude of activating and inhibitory signals, and disruption of this delicate balance can lead to autoimmunity or immunodeficiency. Activation signals can be negatively regulated by cell surface receptors bearing immunoreceptor tyrosine-based inhibitory motifs (ITIMs) in their cytoplasmic tail.1 Examples of ITIM-containing receptors expressed on B cells include FcγRIIB, CD22, CD72, paired Ig-like receptor (PIR)-B, CD85j, Fc receptor-like (FCRL)4, and CD305.2-5 The coligation of the B-cell receptor (BCR) and ITIM-containing receptors results in the attenuation of BCR-mediated signals.3,5,6 Depending on the developmental stage or activation status, B cells express different sets of inhibitory receptors on their cell surface.5,7,8 For example, CD305 is highly expressed on naive human B cells, and its expression is low in memory B cells,5 whereas FCRL4 is mostly expressed on a subset of memory cells and is almost absent on naive B cells.7 The expression of certain ITIM-containing receptors, such as FCRL4 and CD85j, is increased in specific B-cell subsets that are substantially expanded in certain disease settings, such as in HIV-infected viremic patients with high viral loads9 and in persons exposed to Plasmodium falciparum.10 It is acknowledged that the deregulation of the expression of these receptors contributes to the B-cell dysfunctions observed in HIV and malarial chronic infections.11
CD300a belongs to the CD300 family of activating/inhibitory receptors whose genes are clustered in human chromosome 17.12 CD300a is a type I transmembrane receptor with an IgV-like extracellular domain and a cytoplasmic tail that has 3 classic ITIM motifs12,13 and is expressed on cells of both lymphoid and myeloid lineages.12 Its ligation is capable of inhibiting natural killer cell-mediated cytotoxicity,13,14 FcγRIIa-mediated reactive oxygen species production and Ca2+ flux in neutrophils,15 FcϵRI-mediated activation of mast cells,16 and eosinophil responses to eotaxin, granulocyte-macrophage colony-stimulating factor, and IL-5.17 In a murine model of asthma, treatment of mice with a bispecific antibody linking CD300a to CCR3 reversed remodeling and airway inflammation.18 In another in vivo study, a bispecific antibody fragment linking CD300a to IgE was able to abrogate allergic reactions.19 Finally, a recent study showed that a bispecific antibody that links kit with CD300a abrogates the allergic reaction induced by stem cell factor in a model of anaphylaxis.20 All these studies highlight the potential of specifically targeting CD300a for therapeutic purposes.
In this study, we demonstrate that CD300a is differentially expressed on human B-cell subsets and can function as a negative regulator of BCR-mediated signaling. Furthermore, we show that the expression of this receptor is deregulated in HIV-infected patients, suggesting a potential role for CD300a in HIV-associated disease progression.
Methods
Study population
Participants were recruited at the National Institutes of Health in Bethesda, MD, and at the Virgen del Rocío University Hospital in Seville, Spain. HIV-aviremic patients were receiving effective antiretroviral therapy (ART); all had plasma HIV-RNA less than 50 copies/mL. HIV-viremic patients had HIV-RNA more than 50 copies/mL and were not under ART. All study subjects provided informed consent, in accordance with the Declaration of Helsinki, with the approval of the Institutional Review Board of the National Institutes of Allergy and Infectious Diseases, National Institute of Health, the Food and Drug Administration, and Virgen del Rocío University Hospital. Tonsil cells were isolated from discarded samples after elective tonsillectomy. The study was performed according to specimen requirements of the National Institutes of Health Institutional Review Board.
Reagents
Antibodies and reagents used in this study were obtained from the following vendors: anti-CD300a and anti-CD21 from Beckman-Coulter; anti-CD19 and anti-CD27 from eBioscience; anti-IgD, anti-CD10, anti-CD19, anti-CD20, and anti-CD305 from BD Biosciences; anti-CD38 from Caltag; anti-chicken IgM (clone M1) and goat anti-human IgM from Southern Biotechnology; and goat anti-mouse, anti-human IgG, anti-human IgM, and anti-human IgM/A/G from Jackson ImmunoResearch Laboratories.
Recombinant IL-4 and transforming growth factor-β1 (TGF-β1) were purchased from R&D Systems. CpG (ODN 2006) was purchased from InvivoGen. Toxic shock syndrome toxin-1 was purchased from Toxin Technology. Carboxyfluorescein succinimidyl ester, Fluor-4, and Fura-Red were purchased from Invitrogen. The kits for cell purification were purchased from Miltenyi Biotec. The nuclear factor of activated T cell (NFAT) reporter plasmid was previously described.21 Human CD300a was cloned in pcDNA3.1 mammalian expression vector. Control nontargeting and CD300a On-target plus smart pool siRNAs, and also CD300a TaqMan probe/primer mix were obtained from Thermo Scientific.
Flow cytometric analyses
All phenotypic analyses were performed using mouse monoclonal antibodies (mAbs) specific for human markers conjugated with fluorochromes. The experiments performed in Table 1 and Figures 1, 2, and 3A were performed in a FACSCalibur (BD Biosciences), and experiments in Figures 3C, 4, 5, and 6 were performed in a FACSCanto (BD Biosciences).
Expression of CD300a on human B cell lines
| Cell line . | Origin . | MFI . | |
|---|---|---|---|
| Control IgG1 . | Anti-CD300a . | ||
| 721.221 | EBV+ B lymphoblastoid cell line | < 5 | 359.6 |
| BJAB | EBV− BL | < 5 | 28.2 |
| BL41 | EBV− BL | < 5 | < 5 |
| CA46 | EBV− BL | < 5 | < 5 |
| Daudi | EBV+ BL | < 5 | < 5 |
| JD38 | EBV− BL | < 5 | < 5 |
| Namalwa | EBV+ BL | < 5 | < 5 |
| Raji | EBV+ BL | < 5 | < 5 |
| Ramos | EBV− BL | < 5 | < 5 |
| REC-1 | EBV− mantle cell lymphoma | < 5 | 801.8 |
| Reh | EBV− acute lymphocytic leukemia | < 5 | 5.5 |
| SUDHL5 | EBV+ diffuse large B-cell lymphoma | < 5 | 233.3 |
| Cell line . | Origin . | MFI . | |
|---|---|---|---|
| Control IgG1 . | Anti-CD300a . | ||
| 721.221 | EBV+ B lymphoblastoid cell line | < 5 | 359.6 |
| BJAB | EBV− BL | < 5 | 28.2 |
| BL41 | EBV− BL | < 5 | < 5 |
| CA46 | EBV− BL | < 5 | < 5 |
| Daudi | EBV+ BL | < 5 | < 5 |
| JD38 | EBV− BL | < 5 | < 5 |
| Namalwa | EBV+ BL | < 5 | < 5 |
| Raji | EBV+ BL | < 5 | < 5 |
| Ramos | EBV− BL | < 5 | < 5 |
| REC-1 | EBV− mantle cell lymphoma | < 5 | 801.8 |
| Reh | EBV− acute lymphocytic leukemia | < 5 | 5.5 |
| SUDHL5 | EBV+ diffuse large B-cell lymphoma | < 5 | 233.3 |
EBV indicates Epstein-Barr virus; and BL, Burkitt lymphoma.
Cell cultures
Naive B cells (0.5 × 106/mL) and autologous irradiated CD4 T cells (1 × 106/mL) were purified by negative selection and seeded in U-bottom 96-well plates. Cells were stimulated with anti-IgM (2 μg/mL), CpG-ODN 2006 (3μM), IL-4 (10 ng/mL), and toxic shock syndrome toxin-1 (0.5 ng/mL) and cultured for 7 days. For CpG stimulation of CD27+ memory cells, whole B cells were purified by negative selection. The purified B cells were labeled with anti-CD19 and anti-CD27 mAb, and the CD19+CD27+ subset was sorted in a FACSAria (BD Biosciences). Then the sorted cells were seeded in U-bottom 96-well plates (0.5 × 106/mL) and cultured for 5 days in the presence of 3μM of CpG-ODN 2006. In other cultures, purified B cells were seeded in U-bottom 96-well plates at a density of 1 × 106/mL in the presence of the following stimuli: anti-IgG (20 μg/mL), anti-IgG (20 μg/mL) plus IL-4 (10 ng/mL), or anti-IgG (20 μg/mL) plus TGF-β1 (5 ng/mL). Cells were cultured for 4 days, and the expression of CD300a by the CD19+CD27+ cells was determined.
For cell proliferation studies, purified B cells were carboxyfluorescein succinimidyl ester labeled and nucleofected with 500nM of nontarget siRNA or CD300a siRNA using the 96-well plate system (Lonza Walkersville), following a protocol previously described.22 Then cells were treated with CpG-ODN 2006 (2.5 μg/mL) and goat antihuman IgM/A/G (10 μg/mL), goat antihuman IgM (10 μg/mL), or goat antihuman IgG (10 μg/mL). After 4 days, proliferating cells were determined in the CD22+ subset.
Quantitative polymerase chain reaction
CD300a knockdown at the mRNA level was assessed by quantitative RT-PCR. Total RNA was extracted from 0.5 to 1 × 106 cells using the RNeasy micro kit (QIAGEN) according to the manufacturer's recommendations. Total RNA was reverse transcribed and analyzed using CD300a TaqMan probe/primer mix by one-step quantitative polymerase chain reaction (ABI 7500 system). Data were normalized to the housekeeping gene POLRIIA by a comparative ΔΔCt method.
Calcium mobilization assays
Purified human B cells were resuspended in Hanks balanced salt solution with 1% fetal calf serum at 5 × 106 cells/mL. Next, cells were labeled with Fluo-4 (2 μg/mL) and Fura-Red (5 μg/mL) for 30 minutes at 30°C. After this, cells were washed twice with the Hanks buffer and resuspended at 2 × 106 cells/mL. Cells were incubated in a water bath at 37°C for 5 minutes, followed by acquisition in a flow cytometer. To establish a baseline, cells were first acquired for only 30 seconds, at which point the anti-CD300a mAb or isotype control IgG1 was added and acquisition of cells was continued for another 30 seconds. Then, anti-IgG and goat antimouse were added and fluorescence was measured in real time for 6 to 10 minutes. A similar protocol was followed with DT40 chicken cells transfected with CD300a, but instead the anti-IgM was added at the same time (30 seconds) as the anti-CD300a mAb or isotype control IgG1. Data were analyzed using the FlowJo software package Version 7.6.1.
NFAT luciferase reporter assay
DT40 chicken cells expressing human CD300a cells were transiently transfected with 5 μg of an NFAT luciferase reporter construct. After 16 hours, cells were distributed into duplicate wells of a 24-well plate containing medium alone, prebound goat antimouse plus mouse antichicken IgM and mouse isotype control IgG1, or mouse antichicken IgM and mouse antihuman CD300a. As a measure for maximal NFAT activity, 50 ng/mL phorbol myristate acetate plus 5μM ionomycin were added to cells. After 6 hours, cells were disrupted in lysis buffer (Promega) and assayed using luciferin (Promega).
Statistical analysis
Data were analyzed using GraphPad Prism software Version 5 and plotted as bar graphs or scatter plots. Comparisons were made with the 2-tailed unpaired t test with 99% of confidence interval. P < .05 was considered significant. Determination of whether there was a correlation between viremia or CD4 T-cell counts and CD300a expression on B cells was carried out using the nonparametric Spearman rank correlation test.
Results
CD300a is differentially expressed on human B-cell subsets
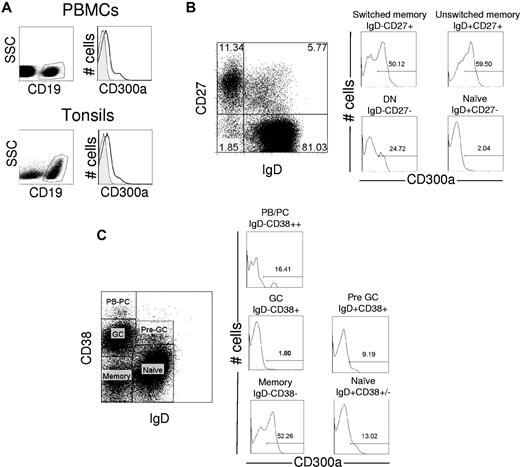
The CD300a receptor is broadly expressed on cells from both the lymphoid and myeloid lineages12 ; however, there is some controversy regarding its expression on human B cells.12,23 We looked at the expression of CD300a on human B cells from peripheral blood and tonsils using the specific anti-CD300a mAb, clone E59.126, which recognizes a unique epitope in CD300a.13,24 We performed multicolor flow cytometric analyses and showed that there is a significant proportion of peripheral blood and tonsil B cells, identified by the expression of CD19, which express CD300a (Figure 1A).
Flow cytometric analysis of CD300a expression on human peripheral blood and tonsil B cells. (A) Freshly isolated PBMCs were labeled with anti-CD19 and anti-CD300a mAb. The lymphocyte gate was determined according to the forward and side scatter parameters. The expression of CD300a was assessed on B cells (CD19+). Empty histogram represents binding of anti-CD300a mAb; and gray histogram, the binding of isotype control Ig. (B) Freshly isolated PBMCs were labeled with anti-CD19, anti-IgD, anti-CD27, and anti-CD300a mAb. The expression of CD300a for a representative donor was measured for the 4 subsets within the CD19+ gate (B cells) defined by the expression of IgD and CD27. The number in the histograms indicates the percentage of CD300a+ cells. (C) Single-cell suspensions from tonsils were labeled with anti-CD19, anti-IgD, anti-CD38, and anti-CD300a mAb. The expression of CD300a for a representative donor was measured in the 5 subsets of the CD19+ gate (B cells) defined by the expression of IgD and CD38. The number in the histograms indicates the percentage of CD300a+ cells.
Flow cytometric analysis of CD300a expression on human peripheral blood and tonsil B cells. (A) Freshly isolated PBMCs were labeled with anti-CD19 and anti-CD300a mAb. The lymphocyte gate was determined according to the forward and side scatter parameters. The expression of CD300a was assessed on B cells (CD19+). Empty histogram represents binding of anti-CD300a mAb; and gray histogram, the binding of isotype control Ig. (B) Freshly isolated PBMCs were labeled with anti-CD19, anti-IgD, anti-CD27, and anti-CD300a mAb. The expression of CD300a for a representative donor was measured for the 4 subsets within the CD19+ gate (B cells) defined by the expression of IgD and CD27. The number in the histograms indicates the percentage of CD300a+ cells. (C) Single-cell suspensions from tonsils were labeled with anti-CD19, anti-IgD, anti-CD38, and anti-CD300a mAb. The expression of CD300a for a representative donor was measured in the 5 subsets of the CD19+ gate (B cells) defined by the expression of IgD and CD38. The number in the histograms indicates the percentage of CD300a+ cells.
To determine which specific subpopulations of human B cells express the CD300a receptor, we analyzed human B-cell subsets from peripheral blood using the IgD/CD27 classification. Four major B-cell subpopulations are distinguished according to this classification: naive cells (IgD+CD27−), unswitched memory cells (IgD+CD27+), switched memory cells (IgD−CD27+), and double-negative cells (IgD−CD27−).8 In healthy donors, immature/transitional B cells that represent less than 2% to 3% of circulating total B cells would also be IgD+CD27−. The double-negative cells are largely memory cells that represent less than 5% of all B cells in the blood of healthy donors, but they are significantly expanded in certain diseases, such as systemic lupus erythematosus, and HIV, and malaria infections.8,10,25,26 We show that naive B cells have almost undetectable levels of CD300a receptor on their cell surface. However, both CD27+ unswitched and switched memory B cells have variable levels of CD300a expression depending on the donor. Although the double-negative cells express CD300a, it is at lower levels than on CD27+ memory cells (Figure 1B; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). In addition to the IgD/CD27 classification, we measured the expression of CD300a on peripheral blood B-cell subsets based on the expression of the CD21 and CD27 markers. The results obtained with this labeling strategy are very similar to those obtained with the IgD/CD27 classification. Naive cells (CD21+CD27−) are mostly negative for CD300a expression, whereas the classic memory B cells (CD21low/+CD27+) and the atypical memory (exhausted) B cells (CD21lowCD27−) are positive for CD300a at variable levels depending on the donor (data not shown; see following paragraphs).
We next examined the expression of CD300a on B cells from single-cell preparations of human tonsil. Five major B-cell subsets can be identified based on the expression of IgD and CD38. Naive cells, identified by the IgD+CD38+/− phenotype, express low levels of CD300a, although at higher levels than peripheral blood naive B cells; pre-germinal center (pre-GC) (IgD+CD38+) cells also express relatively low levels of CD300a, whereas GC (IgD−CD38+) cells are mostly negative for this receptor. Memory B cells (IgD−CD38−) as well as plasmablasts/plasma cells (IgD−CD38++) express CD300a at variable levels depending on the donor (Figure 1C; supplemental Figure 1B).
Finally, we examined the expression of CD300a on a panel of human B-cell lines (Table 1). Burkitt lymphoma is the prototypic GC-like lymphoma27 and, in agreement with our results on CD300a expression by GC cells, we found that, except for the BJAB cell line that expresses low amounts of CD300a, the rest of the Burkitt lymphoma cell lines (n = 7) are negative for CD300a expression. On the other hand, the non-Hodgkin lymphoma cell lines REC-1 (mantle cell lymphoma) and SUDHL5 (diffuse large B-cell lymphoma with ABC-like phenotype) are positive for CD300a expression, which is in agreement with the origin of these lymphomas, namely, pre-GC B cells and activated B cells, respectively.28,29 We did not find a correlation between Epstein-Barr virus transformation and expression of CD300a.
Taken together, these results indicate that the immunomodulatory receptor CD300a is differentially expressed on mature B cells. Whereas naive cells are mostly negative, or express very low levels, memory and plasmablasts/plasma cells are clearly CD300a+. The finding that memory B cells can be divided into 2 populations based on their level of CD300a expression raises the possibility of a functional dichotomy related to the level of this expression.
Regulation of the expression of CD300a on human B cells
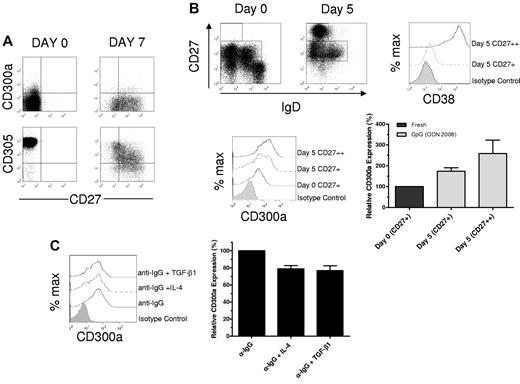
We and others have shown that the expression of CD300a is modulated on T cells, neutrophils and plasmacytoid dendritic cells, and other cell types in response to different stimuli.12,15,30,31 As the range of expression on B-cell subsets varied from low levels on naive B cells to substantial on a significant percentage of memory B cells, we considered what might dictate expression. First, we isolated naive B cells and stimulated them according to the protocol described by Ruprecht and Lanzavecchia.32 As expected, the purified naive B cells were CD27−, expressed very high levels of CD305,5 and were mostly CD300a-negative (Figure 2A). Then, cells were cultured in the presence of anti-IgM, autologous CD4+ T cells in the presence of the bacterial superantigen toxic shock syndrome toxin-1 as a source of cognate T-cell help, IL-4, and the Toll-like receptor 9 (TLR9) agonist CpG 2006. After 7 days of culture, cells expressed CD27 and down-regulated CD305 expression, indicating that naive B cells had been activated; however, no change in the level of CD300a cell surface expression was observed in these cultures (Figure 2A). We tested other conditions, such as adding soluble CD40L as the only source of T-cell help along with BCR stimulation. We also tested the effect of adding several cytokines to the cultures, namely, IL-2, IL-6, and IL-12, plus TLR ligands, LPS, R848, or Pam3CSK4, along with irradiated autologous peripheral blood mononuclear cells (PBMCs). None of these conditions was able to up-regulate CD300a expression (data not shown).
Regulation of the expression of CD300a on human B cells. (A) Purified naive B cells were activated and cultured according to the protocol described in “Cell cultures.” The expression of CD27, CD300a, and CD305 was assessed at day 0 and day 7 of the cultures. Results are representative of 3 independent experiments. (B) Memory CD27+ B cells were purified and sorted according to the protocol described in “Cell cultures” and “Flow cytometric analyses” and stimulated with CpG 2006 for 5 days. Dot plots of IgD and CD27 expression at day 0 (before sorting) and day 5, and a histogram of CD38 expression for a representative culture is also shown (upper panels). Histogram of CD300a expression in the CD27+ and CD27++ cells and graphic representation of the average relative MFI ± SEM of CD300a expression (n = 4) (lower panels). (C) Purified B cells were stimulated with anti-IgG in the presence of IL-4 or TGF-β1 for 4 days according to the protocol described in “Cell cultures.” Flow cytometric analyses were performed to assess CD300a expression by the CD27+ cells. Histograms from a representative donor are shown (left panel) along with the graphic representation of the average relative MFI plus or minus SEM of CD300a expression (n = 4; right panel).
Regulation of the expression of CD300a on human B cells. (A) Purified naive B cells were activated and cultured according to the protocol described in “Cell cultures.” The expression of CD27, CD300a, and CD305 was assessed at day 0 and day 7 of the cultures. Results are representative of 3 independent experiments. (B) Memory CD27+ B cells were purified and sorted according to the protocol described in “Cell cultures” and “Flow cytometric analyses” and stimulated with CpG 2006 for 5 days. Dot plots of IgD and CD27 expression at day 0 (before sorting) and day 5, and a histogram of CD38 expression for a representative culture is also shown (upper panels). Histogram of CD300a expression in the CD27+ and CD27++ cells and graphic representation of the average relative MFI ± SEM of CD300a expression (n = 4) (lower panels). (C) Purified B cells were stimulated with anti-IgG in the presence of IL-4 or TGF-β1 for 4 days according to the protocol described in “Cell cultures.” Flow cytometric analyses were performed to assess CD300a expression by the CD27+ cells. Histograms from a representative donor are shown (left panel) along with the graphic representation of the average relative MFI plus or minus SEM of CD300a expression (n = 4; right panel).
We next looked for signals capable of modulating CD300a expression on memory B cells. TLR9 is constitutively expressed on human memory B cells, and its triggering leads to proliferation and differentiation into Ab-secreting cells.33 We purified CD27+ memory (switched and unswitched) B cells and stimulated them with CpG 2006. After 5 days of culture, a very significant portion of cells dramatically increased the expression of CD27 and CD38 (Figure 2B). These CD27++CD38++ cells are plasmablasts/cells. Analyses showed that both CD27+CD38+/− (activated memory) cells and CD27++CD38++ (plasmablasts) cells have an increased expression of CD300a compared with unstimulated CD27+ memory B cells (Figure 2B). These results indicate that there is a differential effect by TLR9 stimulation that depends on the developmental stage of the B cells. Whereas naive cells do not up-regulate CD300a expression in response to CpG 2006 stimulation, the memory cell subset significantly up-regulates CD300a after TLR9 triggering with higher levels of CD27 expression correlating with greater CD300a expression.
Others have shown that IL-4 is able to down-regulate the expression of the inhibitory receptors CD22, FcγRIIB, CD72, and PIR-B on B cells.4 We have also shown that TGF-β1 down-regulates CD300a expression on TCR-stimulated human CD4 T cells.31 We then tested the ability of these 2 cytokines to modulate CD300a expression on B cells. Results reported in Figure 2C show that BCR-activated, CD27+-switched memory B cells, in the presence of IL-4 or TGF-β1, down-regulate the expression of CD300a. These results extend previous findings showing that these 2 cytokines have a broad range of effects on the expression of inhibitory receptors.4,31 Taken together, these results indicate that CD300a expression on human B cells is modulated by multiple factors that include IL-4, TGF-β1, and TLR9 agonists.
CD300a functions as a negative regulator of BCR signaling
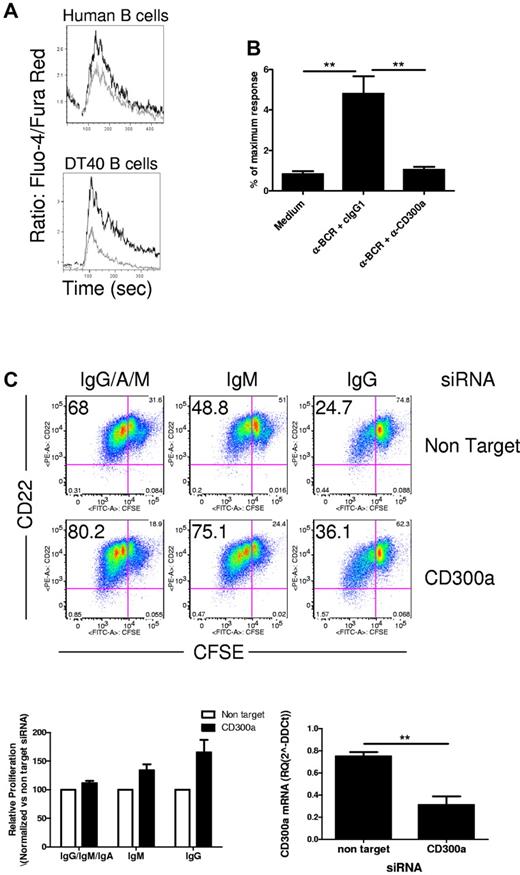
The fact that CD300a is variably expressed on B-cell subsets suggests that this receptor regulates B cells at different stages of maturation. To verify that CD300a ligation is capable of inhibiting B-cell functions, we examined whether ligation of CD300a resulted in inhibition of BCR-mediated Ca2+ mobilization. First, we showed that coligation of CD300a with surface IgG on peripheral blood B cells results in a modest reduction in the rise of intracellular Ca2+ concentration compared with cross-linking of the BCR alone (Figure 3A). The modest CD300a-mediated inhibition probably reflects the fact that not all IgG+ switched memory B cells express CD300a (Figure 1; supplemental Figure 1). To further prove that CD300a inhibits BCR-mediated Ca2+ mobilization, we stably transfected the chicken B-cell line DT40 with a plasmid encoding human CD300a. In Figure 3A, we show that coligation of CD300a with BCR resulted in a dramatic reduction in the rise of intracellular Ca2+ concentration compared with ligation of the BCR alone. Then, we transiently transfected the CD300a-expressing DT40 cells with a plasmid encoding the luciferase reporter gene under the control of NFAT-dependent promoters. Cells were stimulated through the BCR with specific Ab in the presence of the anti-CD300a mAb or an isotypic Ig control. Cross-linking of the BCR induced cell activation as revealed by the increase in luciferase activity, and the activity of NFAT was dramatically decreased when both BCR and CD300a were simultaneously engaged (Figure 3B).
CD300a down-regulates BCR-mediated activation signals. (A) Purified human B cells (upper panel) or DT40 cells expressing CD300a (lower panel) were loaded with Fluo-4 and Fura-Red. Then, cells were acquired in a flow cytometer and stimulated with anti-BCR plus anti-CD300a mAb (gray line) or anti-BCR plus isotype control (black line), according to the protocol described in “Calcium mobilization assays.” Intracellular Ca2+ concentration was measured by the ratio of Fluo-4/Fura-Red as a function of time. Results are representative of 2 (human B cells) and 4 (DT40 cells) independent experiments. (B) DT40 cells expressing CD300a were transiently transfected with a NFAT luciferase reporter plasmid and stimulated with medium, antichicken BCR plus isotype control, or antichicken BCR plus anti-CD300a mAb as indicated. The measured luciferase activity was normalized to the activity obtained for the treatment with phorbol myristate acetate plus ionomycin. The data presented are the mean plus or minus SEM for 6 separate experiments. (C) Purified human B cells were transfected with nontarget or CD300a siRNA. Then, cells were stimulated with anti-Ig (IgG/A/M, IgM, or IgG) and CpG, and the proliferation was measured after 4 days of culture as described in “Cell cultures.” A representative donor is shown. Numbers in the upper left quadrant indicate the percentage of CD22+ cells that have divided one or more times (upper panel). Graphic representation of proliferating cells treated with CD300a siRNA normalized to the nontarget siRNA-treated cells (lower panel, left side) and the CD300a mRNA relative levels measured 4 days after transfection with siRNA (n = 3) are shown (lower panel, right side).
CD300a down-regulates BCR-mediated activation signals. (A) Purified human B cells (upper panel) or DT40 cells expressing CD300a (lower panel) were loaded with Fluo-4 and Fura-Red. Then, cells were acquired in a flow cytometer and stimulated with anti-BCR plus anti-CD300a mAb (gray line) or anti-BCR plus isotype control (black line), according to the protocol described in “Calcium mobilization assays.” Intracellular Ca2+ concentration was measured by the ratio of Fluo-4/Fura-Red as a function of time. Results are representative of 2 (human B cells) and 4 (DT40 cells) independent experiments. (B) DT40 cells expressing CD300a were transiently transfected with a NFAT luciferase reporter plasmid and stimulated with medium, antichicken BCR plus isotype control, or antichicken BCR plus anti-CD300a mAb as indicated. The measured luciferase activity was normalized to the activity obtained for the treatment with phorbol myristate acetate plus ionomycin. The data presented are the mean plus or minus SEM for 6 separate experiments. (C) Purified human B cells were transfected with nontarget or CD300a siRNA. Then, cells were stimulated with anti-Ig (IgG/A/M, IgM, or IgG) and CpG, and the proliferation was measured after 4 days of culture as described in “Cell cultures.” A representative donor is shown. Numbers in the upper left quadrant indicate the percentage of CD22+ cells that have divided one or more times (upper panel). Graphic representation of proliferating cells treated with CD300a siRNA normalized to the nontarget siRNA-treated cells (lower panel, left side) and the CD300a mRNA relative levels measured 4 days after transfection with siRNA (n = 3) are shown (lower panel, right side).
We next examined the role of CD300a on B-cell proliferation. To do this, we suppressed CD300a expression with RNA interference technology according to a protocol that we have recently described.22 We were able to down-regulate CD300a mRNA expression to one-third of the levels normally expressed by peripheral blood B cells (Figure 3C). Then, we stimulated the cells with anti-Ig plus CpG and measured proliferation using the carboxyfluorescein succinimidyl ester method. We observed an increase in the number of proliferating cells in CD300a siRNA-treated cells, indicating a role of CD300a in down-modulating B-cell proliferation (Figure 3C). This effect was more apparent when only surface IgG was engaged, reflecting the fact that switched memory B cells have a higher expression of CD300a than naive and unswitched memory cells combined (Figure 1; supplemental Figure 1). The modest increase in the proliferation of CD300a knockdown cells stimulated with anti-IgM, anti-IgG, and anti-IgA plus CpG may reflect the difficulty of CD300a in inhibiting B cells activated with a strong stimuli and/or the fact that there are proportionally less CD300a+ cells when all B cells are taken into account compared with switched memory B cells. Similar results were obtained when we used purified CD27+ and CD27− B cells instead of whole peripheral blood B cells (supplemental Figure 2).
CD300a expression is deregulated on B cells from HIV-infected patients
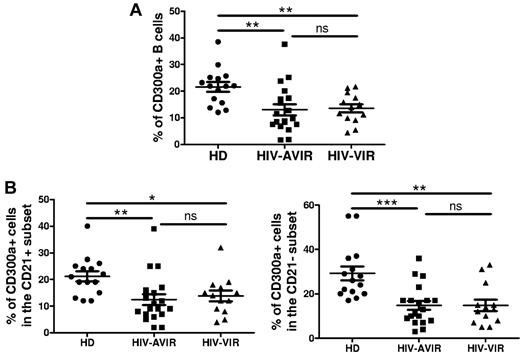
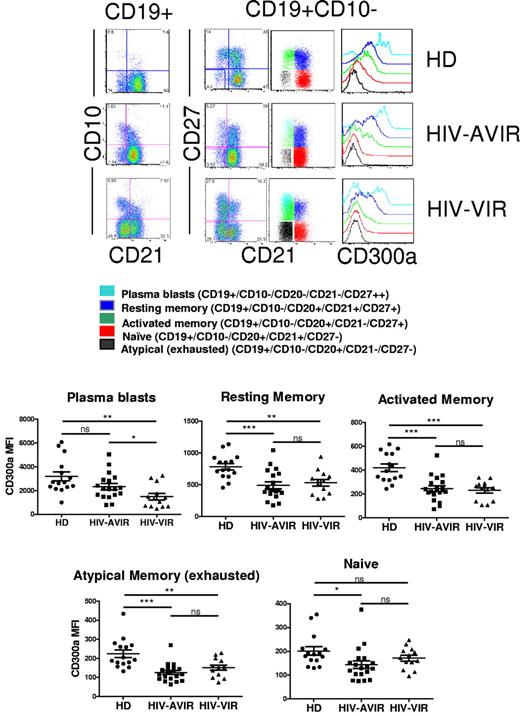
B-cell hyperactivation and dysfunction, as well as hypergammaglobulinemia, are all hallmarks of HIV infection.25 One of the features observed in HIV-viremic patients is the altered expression of multiple inhibitory receptors in certain B-cell subsets.9 It is thought that this deregulation of the expression of inhibitory receptors on B cells has an important role in the pathogenesis of the disease.11 Therefore, we examined whether CD300a expression is deregulated on B cells from HIV-infected patients. In a preliminary study, using peripheral blood from a small cohort of patients from Spain, we found that the percentage of CD300a+ cells is decreased in memory B cells from both viremic and aviremic patients compared with healthy donors (supplemental Figure 3). This encouraged us to study a cross-sectional cohort of HIV-infected patients from the National Institutes of Health. We confirmed previously published results11 showing that in HIV infection there is an increase in the number of circulating plasmablasts, activated memory B cells, and atypical memory (exhausted) B cells, along with a decrease in naive and resting memory B cells. As expected, these alterations were more pronounced in HIV-viremic patients (data not shown; and supplemental Figure 4). Then, we looked at of the expression of CD300a by peripheral blood B cells and found, as with the Spanish cohort, a decrease in the frequency of CD300a+ B cells (Figure 4A). This decrease in the frequency of CD300a+ cells was observed in both the CD21+ and CD21− subsets compared with healthy donors (Figure 4B); however, it should be noted that generally the frequency of CD21− B cells is low in the peripheral blood of healthy donors and HIV-aviremic persons (Figure 5 top panel). Accordingly, further analysis showed that there is a decrease in the frequency of CD300a+CD21+ B cells in the blood of HIV-infected patients, for both aviremic and viremic groups compared with healthy donors, and a significant increase in the CD300a+CD21− subset for the group of HIV-viremic patients (supplemental Figure 4A). This increase in the percentage of circulating CD300a+CD21− B cells correlates with the increased frequency of activated memory B cells (CD27+CD21−) observed in HIV-viremic patients (supplemental Figure 4B).
Decreased frequency of CD300a+ B cells in HIV-infected patients. PBMCs from healthy donors (HD), HIV-aviremic (HIV-AVIR) patients, and HIV-viremic (HIV-VIR) patients were labeled with anti-CD10, anti-CD19, anti-CD20, anti-CD21, anti-CD27, and anti-CD300a mAb. The lymphocyte gate was determined according to the forward and side scatter parameters. (A) The percentage of CD300a+ cells in the CD19+ gate (B cells) was determined. Each symbol represents a different donor. (B) The percentage of CD300a+ cells among CD21+ B cells (left panel) and CD21− B cells (right panel) was determined.
Decreased frequency of CD300a+ B cells in HIV-infected patients. PBMCs from healthy donors (HD), HIV-aviremic (HIV-AVIR) patients, and HIV-viremic (HIV-VIR) patients were labeled with anti-CD10, anti-CD19, anti-CD20, anti-CD21, anti-CD27, and anti-CD300a mAb. The lymphocyte gate was determined according to the forward and side scatter parameters. (A) The percentage of CD300a+ cells in the CD19+ gate (B cells) was determined. Each symbol represents a different donor. (B) The percentage of CD300a+ cells among CD21+ B cells (left panel) and CD21− B cells (right panel) was determined.
Decreased CD300a expression on circulating mature B cells from HIV-infected patients. For the healthy donors (HD), HIV-aviremic (HIV-AVIR) patients, and HIV-viremic (HIV-VIR) patients reported in Figure 5, CD300a expression was determined for each of the following mature (CD19+CD10−) circulating B-cell subsets: naive, resting memory, activated memory, atypical memory (exhausted), and plasmablasts. (Top panel) Dot plots and histograms from a representative person within the HD, HIV-AVIR, and HIV-VIR groups. (Bottom panel) The MFI for CD300a for each of the 5 B-cell subsets. Each symbol represents a different donor.
Decreased CD300a expression on circulating mature B cells from HIV-infected patients. For the healthy donors (HD), HIV-aviremic (HIV-AVIR) patients, and HIV-viremic (HIV-VIR) patients reported in Figure 5, CD300a expression was determined for each of the following mature (CD19+CD10−) circulating B-cell subsets: naive, resting memory, activated memory, atypical memory (exhausted), and plasmablasts. (Top panel) Dot plots and histograms from a representative person within the HD, HIV-AVIR, and HIV-VIR groups. (Bottom panel) The MFI for CD300a for each of the 5 B-cell subsets. Each symbol represents a different donor.
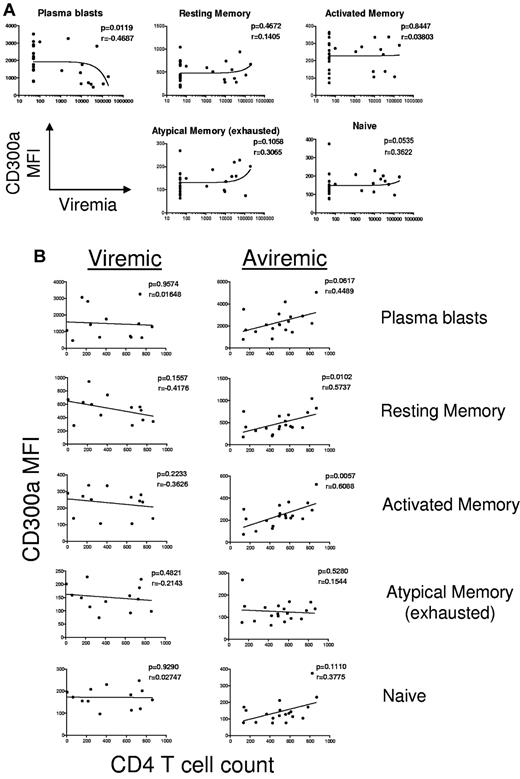
Some of the B-cell subsets that are expanded in the peripheral blood of HIV-infected, particularly in viremic patients, include immature/transitional B cells (CD10+CD21low/+CD27−), activated memory B cells (CD10−CD21lowCD27+), short-lived plasmablasts (CD10−CD21lowCD27++), and atypical (exhausted) memory B cells (CD10−CD21lowCD27−).9,11,34-36 Because the immature/transitional B cells can represent a considerable fraction of circulating B cells in HIV-infected patients, we performed flow cytometric analysis on PBMCs gated on the CD19+/CD10− population. Results presented in Figure 5 clearly show that CD300a expression is significantly down-regulated, as measured by the mean fluorescence intensity (MFI), in circulating mature B cells of HIV-infected patients, both in viremic and aviremic patients compared with healthy donors. With the exception of plasmablasts, there was no correlation between the viremic status of the patient and the down-modulation of CD300a expression (Figure 6A). On the other hand, we observed a significant positive correlation between CD4 T-cell count and CD300a expression on memory B cells, both resting and activated, in patients whose viremia was controlled by undergoing ART (Figure 6B). Altogether, these results indicate that the deregulation of CD300a expression during HIV infection is a complex process that may involve several factors.
Correlation of CD300a expression on B cells with HIV plasma viremia and CD4+ T-cell counts in HIV-infected patients. (A) The correlation between HIV plasma viremia and CD300a expression on the mature (CD19+CD10−) circulating B-cell subsets from the HIV (combined aviremic and viremic) patients. (B) The correlation between CD4+ T-cell counts and CD300a expression on the mature (CD19+CD10−) circulating B-cell subsets from the HIV-aviremic and HIV-viremic patients.
Correlation of CD300a expression on B cells with HIV plasma viremia and CD4+ T-cell counts in HIV-infected patients. (A) The correlation between HIV plasma viremia and CD300a expression on the mature (CD19+CD10−) circulating B-cell subsets from the HIV (combined aviremic and viremic) patients. (B) The correlation between CD4+ T-cell counts and CD300a expression on the mature (CD19+CD10−) circulating B-cell subsets from the HIV-aviremic and HIV-viremic patients.
Discussion
To fine-tune BCR-mediated signals, B cells express a variety of cell surface receptors that are able to tweak BCR-evoked signaling pathways. These receptors include the FcγRIIB receptor, CD22, PIR-B, FCRL4, and CD305.3-6 We now add to the list the immunomodulatory receptor CD300a. We show that this receptor is predominantly expressed on a subset of memory B cells and plasmablasts/plasma cells and is capable of down-modulating BCR-mediated signals. Furthermore, we show that the expression of this receptor is down-regulated on B-cell subsets during HIV infection, which suggests that it contributes to the B-cell dysfunction observed in HIV-infected patients.
The expression of CD300a on human B cells has been a matter of controversy. Daish et al23 showed that a small fraction of circulating B cells were reactive with the CMRF-35 mAb that recognizes both CD300a and CD300c. However, Clark et al indicated that CD300a and CD300c are not expressed by B lymphocytes.12 Cantoni et al,13 using the E59.126 clone, showed that a very small subset of mature B cells is positive for CD300a, although they suggested that this expression was insignificant. Here, using the same E59.126 clone, we unequivocally show that, for all donors tested, a subset of memory B cells express CD300a. The expression of this receptor on a subpopulation of memory B cells resembles the expression of FCRL4, another ITIM-containing receptor7 ; however, the expression patterns of CD300a and FCRL4 do not overlap. FCRL4 is mostly expressed on a CD27− subset of memory B cells,7 whereas CD300a is expressed by both the CD27+ and CD27− subsets of B cells. Additional studies are required to further define the CD300a+ and CD300a− memory B cells. Nonetheless, our observations add another layer of complexity to the growing field of human memory B-cell subsets.8,37
We could not induce CD300a expression on cultured naive B cells, which contrasts with our ability to increase the expression of this receptor after stimulating memory B cells with CpG (Figure 2B). This may be because of the very low expression of TLR9 on naive cells and/or to the fact that is inefficiently coupled to signal transduction pathways at this stage of development.33 Moreover, the modulation of CD300a expression may be an indirect consequence of TLR9 stimulation of human B cells, as indicated by the fact that, when we stimulate memory B cells for one day with only CpG, the expression of CD300a is essentially unchanged, whereas CD69 expression is induced, indicating that the cells were activated (data not shown). In this context, it has been shown that TLR9-mediated interferon-α (IFN-α) production modulates the expression of CD300a in plasmacytoid dendritic cells.30 Nonetheless, the increased expression of CD300a in CpG-stimulated memory B cells may serve to fine-tune B-cell activation induced by microbial products.
We demonstrate that CD300a functions as a negative regulator for BCR-mediated signaling. We used antibodies to cross-link CD300a and BCR in freshly isolated peripheral blood B cells and in CD300a-expressing DT40 chicken cells. Results showed that CD300a was able to inhibit early and crucial events on the BCR-mediated signaling cascade, such as Ca++ mobilization, which starts 20 to 30 seconds after BCR ligation (Figure 3A), and later events that happen 6 hours after BCR ligation, such as NFAT-mediated transcription (Figure 3B). In addition, we showed that down-regulation of CD300a by siRNA enhances B-cell proliferation. This suggests that the unknown ligand of CD300a is expressed by at least a subset of the proliferating B cells and that its interaction with CD300a is able to attenuate B-cell proliferation. Indeed, a CD300a-Fc chimeric protein binds to a subset of proliferating B cells, whereas a control LAIR-1-Fc chimeric protein does not (data not shown), strongly indicating the presence of the CD300a ligand on those cells. The freshly purified B cells from blood and DT40 chicken cells used for the experiments shown in Figure 3A-B do not bind the CD300a-Fc chimeric protein, indicating that they do not express the CD300a ligand. This suggests that activation and proliferation for a long period of time (the proliferation assays are performed during 4 days) are required to induce the expression of the CD300a ligand on B cells.
Others have published that, after pervanadate treatment, the CD300a cytoplasmic tail is phosphorylated and recruits phosphatases, namely, SHP-1, SHP-2, and SHIP depending on the cell type.13,14,16,17 We have found that CD300a is capable of inhibiting the early steps in the activation signaling cascade and that the phosphatase SHP-1 has a dominant role in the transmission of the CD300a-mediated inhibitory signal (manuscript in preparation).
B-cell abnormalities observed during the course of HIV infection are probably the result of indirect effects of the virus on B cells. Many of the B-cell defects that are associated with HIV replication can be reversed by ART, such as increased levels of IgG, whereas other abnormalities persist during effective ART, particularly in memory B cells.11 Our results suggest that the down-regulation of CD300a expression in HIV infection is probably a defect that is not corrected by effective ART, as shown by similar decrease in the CD300a expression in viremic and aviremic patients compared with healthy donors. It may be possible that residual effects of HIV infection are sufficient to maintain the low levels of CD300a expression on B cells, as evidenced by the correlation with CD4+ T-cell count (Figure 6). Preliminary data suggest that there is no correlation between CD300a expression and hypergammaglobulinemia (data not shown). The down-regulation of CD300a expression contrasts with the increase in the expression of multiple inhibitory receptors on a subset of atypical (exhausted) memory B cells that are significantly expanded in HIV viremic patients.9 Quite the opposite, we observed a decrease in CD300a expression on all circulating mature B-cell subsets, which may in part explain the B-cell hyperactivation observed in HIV-infected patients. Thus, whereas atypical (exhausted) memory B cells may reflect efforts of the immune system to dampen the deleterious effects of HIV-induced immune activation, the down-regulation of CD300a may be associated with the HIV-induced polyclonal effects themselves. Our results showing the ability of CD300a to down-regulate BCR-mediated signals (Figure 3) support this conclusion.
We do not know the mechanism(s) that is(are) responsible for the down-regulation of CD300a on B cells of HIV-infected patients. Here, we have shown that IL-4 and TGF-β1 are capable of down-regulating CD300a expression. It may well be that during HIV infection there are elevated levels of these cytokines that in part explain the CD300a down-regulation. In support of this, it has been proposed that during HIV infection there is an imbalance in cytokine production that favors immune activation.38-41 This imbalance is characterized by increased levels of IL-4 during progression to AIDS, along with a decrease of IFN-γ production,38,40-42 a cytokine that in CD4 T cells is associated with CD300a expression.31 In addition, several authors have reported an increase in TGF-β1 levels in HIV-infected patients.43-45 Other factors may also contribute to the down-regulation of CD300a expression. For example, it has been shown that in HIV viremic persons there are increased levels of the B-cell activation factor belonging to the TNF family, IL-6, IFN-α, and other cytokines.11 Although there are no data about the effect of these cytokines on the regulation of CD300a expression by B cells, it has been demonstrated that IFN-α is able to down-modulate the expression of this receptor on plasmacytoid dendritic cells.30
In addition to cytokines, viral-derived proteins, such as Nef, may be direct or indirect activators of B cells.11 It has been shown that Nef accumulates in B cells and inhibits immunoglobulin class switching by interfering with CD40-ligand mediated signals.46-48 Nevertheless, we do not think that accumulation of Nef in B cells has a role in down-modulating CD300a cell surface levels because transfection of CD300a+ B-cell lines with a plasmid encoding Nef does not affect the levels of this receptor (data not shown). However, Nef can indirectly promote B-cell dysfunction, which could include CD300a down-regulation, through a mechanism that promotes the secretion of proinflammatory cytokines, including type I interferon,49 or the acute phase protein ferritin by HIV-infected macrophages.50
In conclusion, we describe the expression of CD300a on human B cells, particularly on memory B cells, and its role in down-modulating BCR-mediated signals. Furthermore, we show that the levels of CD300a are modulated by a variety of stimuli and that during HIV infection B cells express low levels of CD300a. This striking down-regulation of CD300a expression, in contrast with other inhibitory receptors, may contribute to the B-cell dysfunction, particularly B-cell hyperactivity, observed in HIV-infected patients.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the personnel from the Research Technologies Branch, National Institute of Allergy and Infectious Diseases/National Institutes of Health, and flow cytometry facility for helping with cell sorting; the National Institutes of Health Clinical Center Department of Transfusion Medicine and the Service of Infectious Diseases of the Virgen del Rocío University Hospital for the blood samples; all the patients for their gracious willingness to participate in this study; and Giovanna Peruzzi for critically reading the manuscript.
This work was supported by the Food and Drug Administration (intramural program), the National Institute of Allergy and Infectious Diseases (intramural program), the Redes Temáticas de Investigación en SIDA (grant ISCIII RETIC RD06/0006/0021), Spain, and Fondo de Investigaciones Sanitarias (PS09/00424), Spain.
National Institutes of Health
Authorship
Contribution: R.S., L.K., K.D., V.R.S., S.F.-M., and F.B. performed the experiments; R.S., S.M., L.K., S.F.-M., M.L., J.P., and F.B. analyzed results; S.M. and F.B. designed the research; and J.E.C. and F.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francisco Borrego, Laboratory of Molecular and Developmental Immunology, Division of Monoclonal Antibodies, OBP, Center for Drug Evaluation and Research, Food and Drug Administration, Bldg 29B, Rm 3NN18, 29 Lincoln Dr, Bethesda, MD, 20892; e-mail: Francisco.Borrego@fda.hhs.gov.