Abstract
Although hematopoietic stem cell transplantation and gene therapy have the potential to cure β-thalassemia and sickle cell disease, they are not currently available to most people with these diseases. In the near term, pharmacologic induction of fetal hemoglobin (HbF) may offer the best possibility for safe, effective, and widely available therapy. In an effort to define new pathways for targeted drug development for HbF induction, we evaluated the nuclear factor erythroid 2–related factor 2 (NRF2) antioxidant response element signaling pathway. We found that 3 well-known activators of this pathway increased γ-globin mRNA at nontoxic doses in K562 cells. Tert-butylhydroquinone (tBHQ), the most active of these compounds, increased cellular levels and nuclear translocation of NRF2 and binding of NRF2 to the γ-globin promoter. siRNA knockdown of NRF2 inhibited γ-globin induction by tBHQ. When tested in human primary erythroid cells, tBHQ induced NRF2 binding to the γ-globin promoter, increased γ-globin mRNA and HbF, and suppressed β-globin mRNA and HbA, resulting in a > 3-fold increase in the percentage of HbF. These results suggest that drugs that activate the NRF2/antioxidant response element signaling pathway have the potential to induce therapeutic levels of HbF in people with β-hemoglobinopathies.
Introduction
Sickle cell disease (SCD) results from a single amino acid substitution at position 6 of the β-globin protein.1 Under conditions of low oxygen saturation, this mutation causes sickle hemoglobin (HbS) to polymerize, forming long fibers that deform red blood cells, leading to vascular obstruction and acute and chronic complications that affect nearly every organ system.2 In the United States, the median survival for people with SCD has improved recently to approximately 50 years.3 In sub-Saharan Africa, where an estimated 180 000 infants are born with SCD each year,4 the survival rate is much lower, with only one-half of children with SCD living longer than 5 years of age.5
β-thalassemia is caused by > 200 different mutations that result in decreased or absent β-chain synthesis, leading to ineffective erythropoiesis.6 In its most severe form, this produces transfusion-dependent anemia, skeletal abnormalities, splenomegaly, and life-threatening iron overload. Despite advances in iron chelation therapy, median survival has been estimated to be 49 years for patients who use optimal chelation regimens, whereas a median survival of only 28 years was estimated for “typical” compliance to chelation schedules.7 Greenberg et al,8 in recent studies in Southeast Asia, estimate life expectancy in β-thalassemia to be < 10 years for patients without access to transfusion and chelators.
Although hematopoietic stem cell transplantation9 and gene therapy10 have the potential to cure the β-hemoglobinopathies, neither is currently applicable to most patients with these diseases because of technical issues, cost, and a lack of the highly sophisticated medical care necessary to provide these therapies in the areas of the world in which most patients live. An alternative approach is pharmacologic induction of fetal Hb (HbF). More than 50 agents have been described that increase γ-globin expression and/or HbF in primary human erythroid cell cultures or when administered to humans. However, most inducing agents, including cancer chemotherapy drugs, DNA methyltransferase inhibitors, and histone deacetylase inhibitors, are cytotoxic, damage DNA, alter epigenetic marks on a genome-wide basis, or suppress erythropoiesis.11 Only hydroxyurea (HU) has been approved for use in humans and although it provided a major advance in the care of people with SCD, it is only effective in approximately one-half of SCD patients,12 largely ineffective for β-thalassemia,13 and can produce significant suppression of blood counts. Because of these and other issues, HU has low rates of compliance in the United States14 and Africa.15 Given these shortcomings of current HbF-inducing agents, there are no drugs that are safe, effective, and have the ease of use that would make them applicable to most patients with β-hemoglobinopathies.
Recently, we proposed that most HbF-inducing agents act through the p38 MAPK or integrated stress response cell stress signaling pathways.11 However, 2 agents reported to induce γ-globin gene expression, angelicin16 and resveratrol,17 have been shown to induce cell signaling through the nuclear factor erythroid–related factor 2/antioxidant response element (NRF2/ARE) pathway.18,19 On the basis of their ability to activate antioxidant response genes, these and several other agents are currently undergoing preclinical and clinical testing for cancer chemoprevention, cardiovascular disease, and neurodegenerative disorders.20-22 Because members of this class of drugs are already in clinical trials, are relatively well tolerated, and have adequate oral availability, they could offer a novel approach to HbF induction with wide applicability. We investigated the ability of NRF2 activating compounds to stimulate γ-globin gene expression and HbF production and the mechanism of action in K562 and primary human erythroid cells.
Methods
Cells and reagents
K562 cells were cultured in RPMI 1640 with l-glutamine (Mediatech) supplemented with 10% FBS and 1% penicillin/streptomycin. G-CSF–primed peripheral blood CD34+ cells were obtained from the Hematopoietic Cell Processing Core of the University of Washington via an institutional review board–approved protocol. Our in vitro differentiation system has been previously described.23 Tert-butylhydroquinone (tBHQ), curcumin, epigallocatechin 3-gallate, and hemin were all purchased from Sigma-Aldrich. Oltipraz, D3T, and ADT were generous gifts from Dr Bill Roebuck, Dartmouth Medical School. All compounds were dissolved in DMSO except epigallocatechin 3-gallate, which was dissolved in RPMI.
RNA and hemoglobin analysis
Total RNA was isolated from cells by the use of RNeasy columns (QIAGEN), according to manufacturer's instructions. Equal amounts of RNA were reverse transcribed by the use of iScript cDNA Synthesis Kit (BioRad). A total of 2 μL of cDNA was used in each real-time PCR with iQ Syber Green Super Mix (BioRad). Gene expression levels were calculated by the method of Larionov et al24 relative to β-actin and GAPDH mRNA levels. Real-time PCR primers for mRNA quantification were GAPDH: 5′-TCCCATCACCATCTTCCA-3′(S), 5′-CATCACGCCACAGTTTCC-3′(AS); NQO1: 5′-CTGGTTTGAGCGAGTGTTCA-3′(S), 5′-AGGCTGCTTGGAGCAAAATA-3′(AS). Primer sequences for γ-globin, β-globin, and β-actin have been previously published.23 Hb HPLC analysis was performed as described by Ou and Rognerud25 by the use of a PolyCAT A cation exchange column (The Nest Group).
siRNA transfection
K562 cells were transfected with 50nM siGENOME SMART pool siRNA for NRF2 (M-003755-02; Dharmacon), 100nM siGENOME SMART pool siRNA for KEAP1 (M-012453-00-0005; Dharmacon), or 50nM or 100nM of a nontargeting control siRNA (sc-37 007, Santa Cruz Biotechnology, Inc; or siGENOME D-001210-02-05, Dharmacon) for 36-48 hours. Transfection was performed by the use of HiPerfect transfection reagent (QIAGEN) according to manufacturer's instructions.
Luciferase constructs
The reporter construct AγLuc wild-type (WT) was a generous gift from Dr Joyce Lloyd, Virginia Commonwealth University.26 Mutant reporter constructs were made by mutating the ARE in the AγLuc vector with the Quickchange XL kit (Stratagene). Forty micrograms of WT, Mutant 1, or ΔARE and 10 μg of pCMVGLuc control vector (NEB) were electroporated into 1 × 107 K562 cells by the use of BioRad Gene Pulser II at 200V and 950 μF. Cells were treated with tBHQ for 24 hours and harvested at 48 hours after transfection. Cell lysis and luciferase detection was performed by use of the Luciferase Assay System (Promega) and Gaussia Luciferase Assay (NEB) kits. Luciferase activity of test constructs were normalized to pCMVGLuc signal.
EMSA
Nuclear extracts were prepared as described in Tanigawa et al.27 Nuclear extracts from K562 cells treated with 25μM tBHQ were incubated with probes from the γ-globin promoter, the ARE region of the NQO1 promoter,28 or a previously characterized GATA1 biding site29 as a negative control. EMSAs were performed by use of the Lightshift Chemiluminescent kit (Pierce). Binding reactions contained 5 μg of protein, 20 fmol of biotinylated DNA probe, binding buffer, 5% glycerol, 1 μg of poly (dI.dC), 25mM MgCl2, and 0.05% NP-40. Reactions were incubated for 20 minutes at room temperature. Cold competitor reactions contained 4 pmol of unlabeled probe. For supershift assays, 1 μg of NRF2 or IgG control antibody was added after the binding reaction and incubated for an additional hour at 4°C. Complexes were run on a 5% polyacyrlamide/TBE nondenaturing gel, transferred to a nylon membrane, and cross-linked at 120 mJ/cm2. γ-globin probe oligonucleotide: 5′-CCAATAGCCTTGACAAGGCAAACTTGACC-3′, NQO1,28 and GATA29 probes have been previously published. Antibodies used were NRF2 (sc-722; Santa Cruz Biotechnology, Inc) and AffiniPure rabbit antirat IgG (Jackson ImmunoResearch Laboratories).
Western blotting
Cells were centrifuged at 470g for 5 minutes at 4°C, washed in ice-cold PBS, and the pellet lysed in Laemmli buffer supplemented with 100mM DTT, protease (Roche, 11836170001), and phosphatase (Sigma-Aldrich, P2580) inhibitors to obtain total lysates. Nuclear extracts were isolated by resuspending washed cell pellets in RSB (10mM Tris, pH7.5; 10mM NaCl; 3mM MgCl2) plus inhibitors, and then adding 10% NP40 dropwise to a final concentration of 0.25%. Nuclear pellets were resuspended in Laemmli buffer plus 100mM DTT and inhibitors. Lysates were loaded onto SDS-polyacrylamide gels and electrophoresed for 1 hour at 200 V. Protein was transferred onto polyvinylidene fluoride membranes at 100 V for 1 hour. Membranes were blocked in 5% milk in TBST. The following primary antibodies were used and diluted in blocking agent: NRF2 (sc-13 032; Santa Cruz Biotechnology, Inc), KEAP1 (sc-15 246; Santa Cruz Biotechnology, Inc), β-actin (AC-15; Sigma-Aldrich), TATA binding protein (ab818; Abcam), and Histone H3 (05-499; Upstate Biotechnology Inc). Densitometric analysis of blots was performed with the use of Image J software.
ChIP analysis
ChIP assays were performed in triplicate by the use of 1.2 × 107 cells per IP. Formaldehyde was added to cells at a final concentration of 1.42%. The cross-linking reaction was quenched with the addition of 125mM glycine for 5 minutes. Cells were washed twice at 4°C with PBS. The remainder of the assay was performed with the use of the Fast ChIP protocol of Nelson et al30 with the following specific parameters. Sonication was performed by the use of a tip sonicator (Vibra-cell) at 4°C with 10 pulses for 30 seconds at amplitude 40. Immunoprecipitations were performed with 5 μg of anti-NRF2 (sc-13 032; Santa Cruz Biotechnology, Inc) or 5 μg of AffiniPure rabbit antirat IgG (Jackson ImmunoResearch Laboratories) for a negative control. The immunoprecipited DNA was quantified in triplicate by real-time PCR by use of the following primers: γ-globin: 5′-AACGGTCCCTGGCTAAACTC-3′(S), GCTGAAGGGTGCTTCCTTTT-3′(AS); necdin: 5′-GTGTTATGTGCGTGCAAACC-3′(S), 5′-CTCTTCCCGGGTTTCTTCTC-3′(AS); and NQO1: 5′-CAGTGGCATGCACCCAGGGAA-3′(S), and 5′-GCATGCCCCTTTTAGCCTTGGCA-3′(AS). Results were calculated as using an input DNA standard curve. The hemin ChIP results were calculated as fold over IgG with an input DNA standard curve and normalized to necdin.
Statistical analysis
P values were determined by the use of an unpaired Student t test (*P < .05, **P < .01). Data are presented as mean ± SEM, unless otherwise noted.
Results
NRF2 activators induce γ-globin gene expression in K562 cells
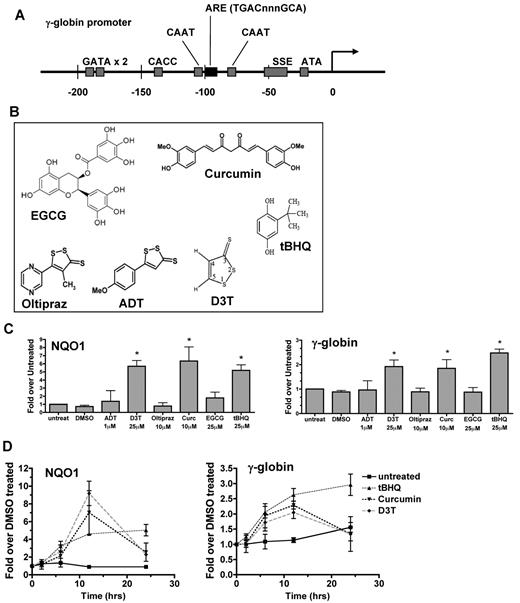
NRF2 binds to a specific ARE sequence (TGACnnnGCA) in the promoters of target genes such as NADPH-quinone oxireductase 1 (NQO1), glutamate-cysteine ligase (GCL), and glutathione S-transferase (GST). As shown in Figure 1A, a 7/7-bp match of the ARE sequence was found in the proximal γ-globin gene promoters between 2 well-characterized CAAT boxes. To begin to evaluate the hypothesis that NRF2 might directly activate γ-globin gene expression, we selected 6 compounds that have been shown to induce NRF2 signaling in nonerythroid cells (Figure 1B).20,31,32 These agents were chosen because each is active in cancer chemoprevention20,31,32 and at least one other disease model,20,32-35 and with one exception (D3T), are either currently in human trials20,33,34 or already approved as a food additive (tBHQ).
Induction of γ-globin gene expression by activators of the NRF2 antioxidant response element signaling pathway. (A) The human γ-globin gene promoter contains an ARE consensus sequence. (B) Compounds that activate NRF2/ARE pathway signaling. (C) Effect of NRF2 activators on NQO1 and γ-globin mRNA levels. K562 cells were treated with nontoxic doses of each compound for 12 hours before RNA isolation. (D) Time course of tBHQ, curcumin, and D3T induction of NQO1 and γ-globin mRNA in K562 cells. Real-time PCR was performed in triplicate. Results are presented as relative mRNA expression normalized to untreated (C) or DMSO treated (D) samples. Error bars represent ± SEM, *P < .05, compared with DMSO.
Induction of γ-globin gene expression by activators of the NRF2 antioxidant response element signaling pathway. (A) The human γ-globin gene promoter contains an ARE consensus sequence. (B) Compounds that activate NRF2/ARE pathway signaling. (C) Effect of NRF2 activators on NQO1 and γ-globin mRNA levels. K562 cells were treated with nontoxic doses of each compound for 12 hours before RNA isolation. (D) Time course of tBHQ, curcumin, and D3T induction of NQO1 and γ-globin mRNA in K562 cells. Real-time PCR was performed in triplicate. Results are presented as relative mRNA expression normalized to untreated (C) or DMSO treated (D) samples. Error bars represent ± SEM, *P < .05, compared with DMSO.
After determining the maximum dose of each drug that did not inhibit cell growth or induce differentiation (data not shown), we treated K562 cells at these doses for 12 hours and then determined the levels of mRNA for the γ-globin genes and, as a positive control, the NRF2 target gene, NQO1.20 At the doses tested, D3T, curcumin, and tBHQ each significantly increased levels of RNA from both genes at 12 hours (Figure 1C). To further characterize these responses, we monitored mRNA levels of the NQO1 and γ-globin genes for 24 hours after drug treatment (Figure 1D). Initial responses occur by 6 hours and peak between 12 and 24 hours. These results suggest that similar to NQO1, γ-globin gene expression may also be induced by NRF2 signaling. Because tBHQ induced the greatest levels of γ-globin in our model system, we used this compound at a dose of 25μM for further mechanistic studies.
tBHQ induces NRF2 nuclear translocation and binding to the γ-globin promoter
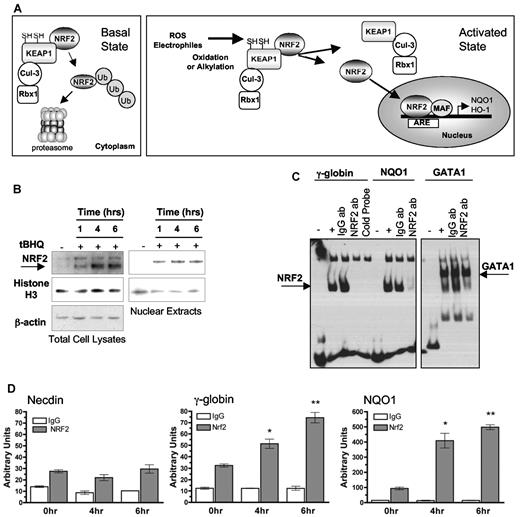
Our hypothesis predicts that tBHQ and similar agents activate γ-globin gene expression via the NRF2 antioxidant signaling pathway. This pathway is depicted in Figure 2A. In the basal state, NRF2 is bound to a cytoplasmic complex through interaction with KEAP1. KEAP1 acts as an E3 ubiquitin ligase substrate adaptor for the Cul3/Rbx1 complex, which ubiquinates NRF2, targeting it for proteasomal degradation. When present, reactive oxygen species or electrophiles chemically alter the structure of KEAP1, releasing NRF2 from the complex. Because newly translated NRF2 is no longer ubiquinated and sequestered in the cytoplasm, NRF2 accumulates and translocates to the nucleus, where it heterodimerizes with small Maf proteins and activates antioxidant genes by binding to ARE sequences in their promoters (reviewed in Niture et al36 ). Consistent with this model, tBHQ treatment of K562 cells results in increased total and nuclear levels of NRF2 as early as 1 hour after drug treatment. This response persists for at least 6 hours (Figure 2B).
tBHQ induces NRF2 nuclear translocation and binding of NRF2 to the γ-globin promoter in K562 cells. (A) Simplified schematic of the NRF2/ARE pathway. Under basal conditions, NRF2 is sequestered in the cytoplasm by KEAP1, which is an E3 ligase substrate adaptor for the Cul3/Rbx1 complex that ubiquitinates NRF2, targeting it to the proteasome. Reactive oxygen species or electrophiles alter KEAP1 structure so that newly translated NRF2 is no longer ubiquinated and can accumulate and translocate to the nucleus where it heterodimerizes with small Maf proteins and binds the ARE in target gene promoters. (B) Western blot analysis of NRF2 protein in total cell and nuclear lysates after tBHQ treatment. (C) EMSA shows NRF2 binding to the γ-globin ARE region in vitro. The ARE region of the NQO1 promoter, and known GATA1 binding site are used as positive and negative controls for NRF2 binding. Unlabeled γ-globin promoter probe was used as in competition reactions. One microgram of IgG or NRF2 antibody was added and incubated for an additional hour after the binding reaction. The reactions were all run on the same gel. Different exposures are presented for clarity. (D) ChIP analysis of cells treated with tBHQ. Quantitative real-time PCR was performed on immunoprecipitated DNA with the use of primers that flank the ARE region in the γ-globin promoter. A total of 25μM tBHQ was used in these experiments. The NQO1 promoter was used as a positive control and necdin promoter was used as a negative control. *P < .05, **P < .01 compared with untreated control.
tBHQ induces NRF2 nuclear translocation and binding of NRF2 to the γ-globin promoter in K562 cells. (A) Simplified schematic of the NRF2/ARE pathway. Under basal conditions, NRF2 is sequestered in the cytoplasm by KEAP1, which is an E3 ligase substrate adaptor for the Cul3/Rbx1 complex that ubiquitinates NRF2, targeting it to the proteasome. Reactive oxygen species or electrophiles alter KEAP1 structure so that newly translated NRF2 is no longer ubiquinated and can accumulate and translocate to the nucleus where it heterodimerizes with small Maf proteins and binds the ARE in target gene promoters. (B) Western blot analysis of NRF2 protein in total cell and nuclear lysates after tBHQ treatment. (C) EMSA shows NRF2 binding to the γ-globin ARE region in vitro. The ARE region of the NQO1 promoter, and known GATA1 binding site are used as positive and negative controls for NRF2 binding. Unlabeled γ-globin promoter probe was used as in competition reactions. One microgram of IgG or NRF2 antibody was added and incubated for an additional hour after the binding reaction. The reactions were all run on the same gel. Different exposures are presented for clarity. (D) ChIP analysis of cells treated with tBHQ. Quantitative real-time PCR was performed on immunoprecipitated DNA with the use of primers that flank the ARE region in the γ-globin promoter. A total of 25μM tBHQ was used in these experiments. The NQO1 promoter was used as a positive control and necdin promoter was used as a negative control. *P < .05, **P < .01 compared with untreated control.
To determine whether NRF2 binds to the ARE sequence in the γ-globin promoter, we performed EMSAs. Nuclear extracts from tBHQ-treated K562 cells were incubated with a biotinylated DNA probe specific for either the γ-globin ARE, or as a positive control, the NQO1 ARE. Our results show a specific complex that is the same size with either probe (Figure 2C). Excess unlabeled γ-globin ARE probe competes off this band. An NRF2 antibody, which binds to the DNA-binding portion of NRF2, eliminates the shifted band, whereas an equal amount of IgG does not. The NRF2 antibody does not inhibit binding to a GATA1 probe. These results show that NRF2 specifically binds the γ-globin ARE region in vitro.
To determine whether tBHQ induces NRF2 binding at the ARE sequence of the proximal γ-globin promoter in vivo, ChIP analysis with the use of K562 cells treated with tBHQ for 0, 4, and 6 hours was performed. Immunoprecipitated DNA was quantified by the use of real-time PCR with primers that amplify an approximately 150-bp region centered at the ARE. As shown in Figure 2D, NRF2 binding to the γ-globin promoter progressively increased at 4 and 6 hours after tBHQ treatment. Binding also was detected at the same time points at the NQO1-positive control promoter but not at the human necdin gene promoter, the negative control. Taken together, these data demonstrate that tBHQ treatment induces nuclear translocation and subsequent binding of NRF2 to the ARE region of the γ-globin promoter.
NRF2 knockdown or mutation of the ARE element inhibits tBHQ-induced γ-globin gene expression
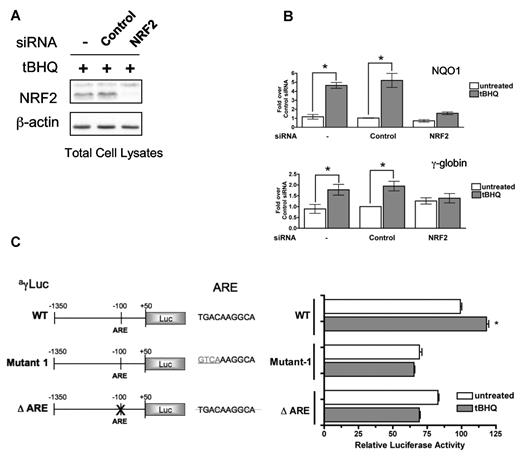
To determine whether NRF2 is required for tBHQ induction of γ-globin gene expression, we transfected K562 cells with siRNA specific for NRF2 mRNA 24 hours before tBHQ treatment. An siRNA sequence that does not target any known cellular mRNA served as a negative control. NRF2-specific siRNA treatment resulted in a > 75% suppression of both NRF2 mRNA (data not shown) and protein levels (Figure 3A) by 24 hours after transfection. Cells were then treated with 25μM tBHQ for 24 hours. As shown in Figure 3B, NRF2 knockdown prevented tBHQ induction of both NQO1 and γ-globin mRNA. These results suggest that NRF2 is necessary for tBHQ induction of γ-globin expression.
siRNA knockdown of NRF2 or mutation of the ARE element inhibit tBHQ induction of γ-globin gene expression. siRNA specific for NRF2 was transiently transfected into K562 cells. At 24 hours later, cells were treated with tBHQ for 12 hours before RNA isolation. Control siRNA and mock transfection (−) were used as controls. (A) Western blot analysis of total cellular NRF2 protein after siRNA transfection and tBHQ treatment. (B) Effect of NRF2 siRNA on tBHQ induction of NQO1 and γ-globin mRNA. Results of real-time PCR are presented as relative mRNA expression normalized to untreated control siRNA. (C) ARE element is required for tBHQ induction. Forty micrograms of WT or mutant ARE constructs were electroporated with 10 μg of pCMVGLuc control vector into K562 cells. tBHQ was added to a final concentration of 25μM at 24 hours after transfection and harvested at 48 hours. WT-untreated vector was set to 100%. Transfection efficiency was controlled for by normalizing to pCMVGLuc signal. Error bars represent ± SEM. P values are calculated relative to untreated controls. *P < .05.
siRNA knockdown of NRF2 or mutation of the ARE element inhibit tBHQ induction of γ-globin gene expression. siRNA specific for NRF2 was transiently transfected into K562 cells. At 24 hours later, cells were treated with tBHQ for 12 hours before RNA isolation. Control siRNA and mock transfection (−) were used as controls. (A) Western blot analysis of total cellular NRF2 protein after siRNA transfection and tBHQ treatment. (B) Effect of NRF2 siRNA on tBHQ induction of NQO1 and γ-globin mRNA. Results of real-time PCR are presented as relative mRNA expression normalized to untreated control siRNA. (C) ARE element is required for tBHQ induction. Forty micrograms of WT or mutant ARE constructs were electroporated with 10 μg of pCMVGLuc control vector into K562 cells. tBHQ was added to a final concentration of 25μM at 24 hours after transfection and harvested at 48 hours. WT-untreated vector was set to 100%. Transfection efficiency was controlled for by normalizing to pCMVGLuc signal. Error bars represent ± SEM. P values are calculated relative to untreated controls. *P < .05.
It is well established that NRF2 binds to an ARE element in target gene promoters.36 To determine the role of the γ-globin ARE consensus sequence in tBHQ induced γ-globin expression, we used a luciferase reporter gene under the control of sequences from −1350 to +50 of the Aγ-globin promoter (WT). Two ARE mutants of this construct were created. Mutant 1 changes the first 4 base pairs of the ARE sequence from TGAC to GTCA. These residues have been shown to be essential for basal and induced expression of NQO1.37 The second mutant vector, ΔARE, has the entire ARE deleted. Figure 3C shows that tBHQ induces the WT vector > 1.25-fold. Mutating the first 4 base pairs or deleting the entire ARE causes 25% and 20% reductions in basal activity of the γ-globin promoter, respectively. Both mutants are unresponsive to tBHQ stimulation. Although the induction of the WT construct by tBHQ is modest, it is reproducible and statistically significant. These results indicate that the ARE sequence in the γ-globin promoter is a functional element and is required for induction with tBHQ.
Nuclear accumulation of NRF2 by suppression of KEAP1 is not sufficient to induce γ-globin mRNA expression
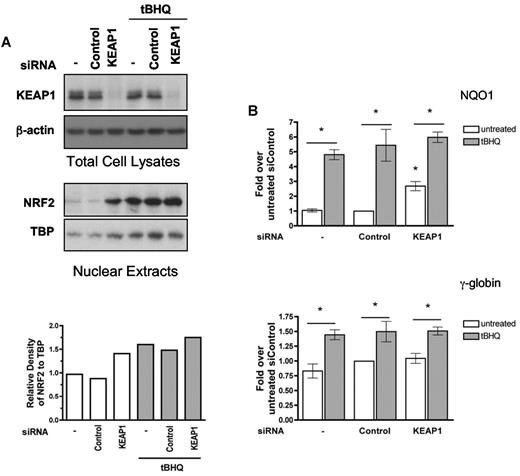
Reducing the amount of KEAP1, a component of the complex that targets NRF2 to the proteasome, is a strategy others have used to induce NRF2 signaling.38 We took advantage of this approach to determine whether NRF2 nuclear translocation is sufficient for induction of γ-globin expression in K562 cells. We transiently transfected K562 cells with KEAP1 siRNA or a nontargeting control siRNA. After 24 hours, cells were treated with tBHQ. Protein was isolated 24 hours later to determine total cellular KEAP1 and nuclear NRF2 levels. As seen in Figure 4A, suppression of KEAP1 induced nuclear translocation of NRF2 to nearly the same levels as treatment with tBHQ. This was associated with a significant increase in NQO1 mRNA levels in cells not treated with tBHQ, but to a level less than that seen with tBHQ alone (Figure 4B). However, KEAP1 suppression alone did not increase γ-globin mRNA. Combining KEAP1 knockdown with tBHQ treatment did not further enhance tBHQ-mediated induction of NQO1 or γ-globin. These results suggest that although suppressing KEAP1 levels can induce NRF2 nuclear translocation and NQO1 expression, it is insufficient for γ-globin induction and for full NQO1 induction.
siRNA suppression of KEAP1 levels enhance NRF2 nuclear translocation and tBHQ induced γ-globin mRNA expression. siRNA specific for KEAP1 was transfected into K562 cells. At 24 hours later, cells were treated with tBHQ at 25μM for 24 hours before protein and RNA isolation. Control siRNA and mock transfection (−) were used as controls. (A) Western blot analysis of total cellular KEAP1 protein and nuclear NRF2 protein levels. Bottom: Densitometric analysis of NRF2 nuclear protein relative to TATA binding protein (TBP) normalized to the untreated control. (B) Effect of KEAP1 siRNA on tBHQ induction of NQO1 and γ-globin mRNA levels. Results of real-time PCR are expressed as relative mRNA levels normalized to the untreated mock transfected control. Densitometric analysis was performed with Image J software. P values compare tBHQ treated control siRNA with tBHQ treated KEAP1 siRNA. *P < .05.
siRNA suppression of KEAP1 levels enhance NRF2 nuclear translocation and tBHQ induced γ-globin mRNA expression. siRNA specific for KEAP1 was transfected into K562 cells. At 24 hours later, cells were treated with tBHQ at 25μM for 24 hours before protein and RNA isolation. Control siRNA and mock transfection (−) were used as controls. (A) Western blot analysis of total cellular KEAP1 protein and nuclear NRF2 protein levels. Bottom: Densitometric analysis of NRF2 nuclear protein relative to TATA binding protein (TBP) normalized to the untreated control. (B) Effect of KEAP1 siRNA on tBHQ induction of NQO1 and γ-globin mRNA levels. Results of real-time PCR are expressed as relative mRNA levels normalized to the untreated mock transfected control. Densitometric analysis was performed with Image J software. P values compare tBHQ treated control siRNA with tBHQ treated KEAP1 siRNA. *P < .05.
tBHQ treatment of differentiating primary human erythroid cells induces γ-globin mRNA and HbF production
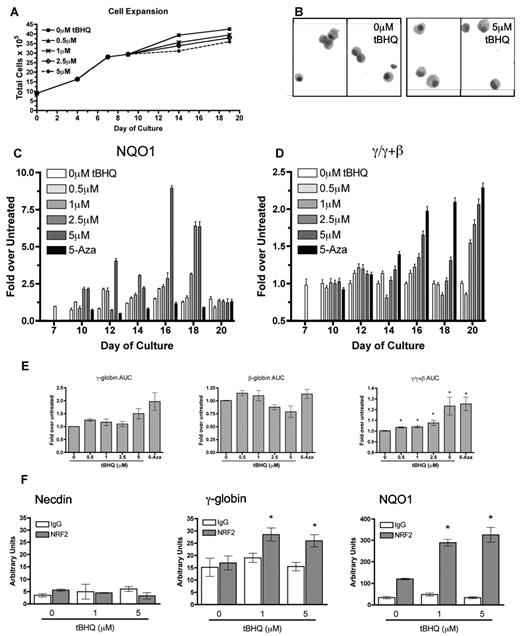
To determine whether NRF2-mediated induction of γ-globin gene expression can be applied to primary human erythroid cells, G-CSF–mobilized peripheral blood CD34+ cells were treated with tBHQ during in vitro erythroid differentiation. In this 2-phase system, cells are grown in media containing stem cell factor, IL-3, and Fms-like tyrosine kinase-3 ligand for the first 7 days and then erythropoietin for an additional 14 days.39 During the erythroid phase of the culture (days 9-17) tBHQ was added every 2 days at concentrations from 0 to 5μM. This dosing schedule was chosen because NQO1 mRNA levels peak and then return to baseline by 48 hours after a single tBHQ treatment. 5-Aza was added daily during this period as a positive control for γ-globin mRNA and HbF induction.
As seen in Figure 5A, tBHQ treatment had a minor effect on cell proliferation with the greatest concentration of tBHQ (5μM), causing a 14% decrease in total cells produced by day 20 of differentiation. Figure 5B displays cytospins of untreated and 5μM tBHQ-treated cells at time of harvest (day 20) during differentiation, illustrating no obvious histologic difference between untreated and tBHQ-treated cells. We next evaluated the effect of tBHQ on NQO1 and γ/γ + β-globin mRNA levels during differentiation in 3 separate experiments. As shown in Figure 5C, NQO1 mRNA is normally expressed at low levels during erythroid differentiation but is strongly induced in a dose-dependent fashion by tBHQ treatment, indicating that NRF2 signaling is active in primary human erythroid cells. 5-Aza, as shown in Figure 5C, does not induce NQO1 expression indicating that 5-Aza and tBHQ are working through different mechanisms.
tBHQ increases γ-globin mRNA and NRF2 binding to the γ-globin promoter in primary human erythroid cells. Human CD34+ peripheral blood cells were treated with tBHQ during in vitro erythroid differentiation. The effects of increasing concentrations of tBHQ were determined for (A) cell expansion and (B) cytospins from untreated and cells treated with 5μM tBHQ at end of differentiation (day 20). (C) NQO1 gene expression at specific time points during differentiation relative to untreated n = 3, (D) γ/γ+β mRNA at specific time points during differentiation relative to untreated, n = 3. (E) tBHQ dose response on γ, β and γ/(γ + β) mRNA expressed as the AUC for each dose n = 3, 5-Aza was used as a positive control for γ-globin mRNA induction (panels C and D). Error bars represent ± 1 SD. (F) ChIP analysis of CD34+ cells on day 13 of differentiation treated with 0, 1, and 5μM tBHQ. Quantitative real-time PCR was performed on immunoprecipitated DNA with the use of primers that flank the ARE region in the γ-globin promoter. The NQO1 promoter was used as a positive control and necdin promoter was used as a negative control. n = 2, *P < .05 compared with untreated. Photomicrographs were taken using a Nikon eclipse 80i camera at 10× magnification and Qcapture v2.7.3 software.
tBHQ increases γ-globin mRNA and NRF2 binding to the γ-globin promoter in primary human erythroid cells. Human CD34+ peripheral blood cells were treated with tBHQ during in vitro erythroid differentiation. The effects of increasing concentrations of tBHQ were determined for (A) cell expansion and (B) cytospins from untreated and cells treated with 5μM tBHQ at end of differentiation (day 20). (C) NQO1 gene expression at specific time points during differentiation relative to untreated n = 3, (D) γ/γ+β mRNA at specific time points during differentiation relative to untreated, n = 3. (E) tBHQ dose response on γ, β and γ/(γ + β) mRNA expressed as the AUC for each dose n = 3, 5-Aza was used as a positive control for γ-globin mRNA induction (panels C and D). Error bars represent ± 1 SD. (F) ChIP analysis of CD34+ cells on day 13 of differentiation treated with 0, 1, and 5μM tBHQ. Quantitative real-time PCR was performed on immunoprecipitated DNA with the use of primers that flank the ARE region in the γ-globin promoter. The NQO1 promoter was used as a positive control and necdin promoter was used as a negative control. n = 2, *P < .05 compared with untreated. Photomicrographs were taken using a Nikon eclipse 80i camera at 10× magnification and Qcapture v2.7.3 software.
Similar to our previous results with 5-Aza,39 the major effect of tBHQ on γ/γ + β mRNA expression is not on peak levels but on prolonging expression into the later stages of differentiation (Figure 5D). Figure 5E displays the area under the curve (AUC) averaged from 3 experiments. AUC is quantified by calculating the area under the mRNA curves from days 7 to 20 for each drug treatment for γ, β, and γ/γ + β. This provides an estimate of the total mRNA from each gene that is available for translation throughout differentiation. Our results indicate that 5μM tBHQ increases γ-globin AUC approximately 50% while decreasing β-globin AUC approximately 20%. This resulted in an approximately 25% increase in γ/γ + β AUC. These results demonstrate that tBHQ induces NRF2 signaling and γ-globin mRNA and decreases β-globin mRNA in differentiating primary human erythroid cells.
To determine whether tBHQ induces NRF2 to bind to the ARE region of the γ-globin promoter during differentiation, we performed a ChIP analysis on day 13 of differentiation with tBHQ. As shown in Figure 5F, NRF2 binding to the γ-globin promoter was only detected after treatment with either 1 or 5μM tBHQ. This finding is significant compared with untreated cells, which showed no difference from IgG. Binding to the NQO1-positive control promoter was also detected for both concentrations of tBHQ. No binding was detected at the negative control necdin gene promoter. These data are consistent with results obtained in K562 cells and further support our model that tBHQ induces NRF2 binding to the ARE region of the γ-globin promoter.
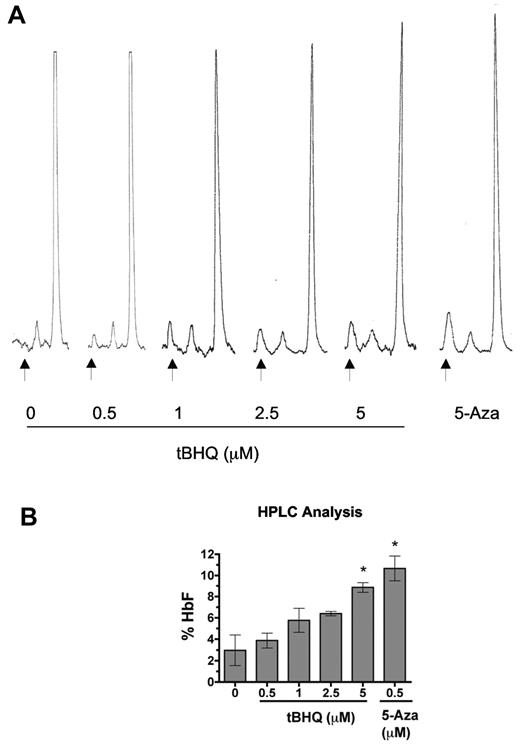
We next performed HPLC analysis to determine the effect of tBHQ on HbF levels in erythroid cells at the end of the culture period (day 20). Figure 6A displays representative HPLC traces, and Figure 6B shows the mean results of 3 separate experiments. As shown in Figure 6B, there was a dose-dependent increase in the proportion of HbF with 5μM tBHQ, producing an increase in HbF from 3% to 9%, compared with 11% with 500nM 5-Aza. Both increases were statistically significant compared with untreated cells. These results demonstrate that tBHQ increases the proportion of HbF in differentiating primary human erythroid cells.
tBHQ increases HbF levels in primary human erythroid cells. Human CD34+ peripheral blood cells were treated with tBHQ during in vitro erythroid differentiation. At the end of differentiation on day 20, cells were lysed, and the proportions of HbA and HbF were determined by ion exchange HPLC. (A) Representative HPLC traces. Arrows indicate HbF peak. (B) Quantitation of HbF from 3 experiments. A total of 500nM 5-Aza was used as a positive control for HbF induction. Error bars represent ± 1 SEM. *P < .05.
tBHQ increases HbF levels in primary human erythroid cells. Human CD34+ peripheral blood cells were treated with tBHQ during in vitro erythroid differentiation. At the end of differentiation on day 20, cells were lysed, and the proportions of HbA and HbF were determined by ion exchange HPLC. (A) Representative HPLC traces. Arrows indicate HbF peak. (B) Quantitation of HbF from 3 experiments. A total of 500nM 5-Aza was used as a positive control for HbF induction. Error bars represent ± 1 SEM. *P < .05.
Hemin induces NRF2 binding to the γ-globin promoter in vivo and requires NRF2 for full γ-globin induction in K562 cells
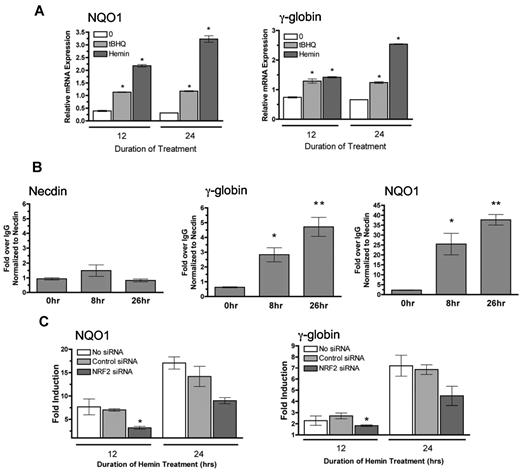
We next investigated whether hemin, a conventional inducer of γ-globin gene expression used the NRF2 pathway.40 In support of this idea, hemin has been shown to induce thioredoxin through NRF2.41 We first confirmed that hemin induces both NQO1 and γ-globin expression in K562 cells after 12 and 24 hours of treatment (Figure 7A). Results from ChIP assays in Figure 7B show increasing NRF2 binding to the γ-globin promoter after 8 and 26 hours of hemin treatment. Hemin also induces NRF2 to bind to the positive control NQO1 promoter but not the negative control necdin gene promoter.
Hemin induces NRF2 to bind to the γ-globin promoter and requires NRF2 for full induction of γ-globin. (A) Effect of tBHQ and hemin on NQO1 and γ-globin mRNA levels. K562 cells were treated with 25μM of tBHQ and 20μM of hemin for 12 and 24 hours before RNA isolation. (B) ChIP analysis of K562 cells treated with hemin for 0, 8, and 26 hours. Quantitative real-time PCR was performed on immunoprecipitated DNA with the use of primers that flank the ARE region in the γ-globin promoter. The NQO1 promoter was used as a positive control, and necdin promoter was used as a negative control. *P < .05 compared with untreated. Results presented as Enrichment over IgG, normalized to necdin. (C) Effect of NRF2 siRNA on hemin fold induction of NQO1 and γ-globin mRNA. siRNA specific for NRF2 was transiently transfected into K562 cells. At 24 hours later, cells were treated with tBHQ for 12 or 24 hours before RNA isolation. Control siRNA and a mock transfection (−) were used as controls. Results of real-time PCR are presented as fold induction. *P < .05 compared with control siRNA.
Hemin induces NRF2 to bind to the γ-globin promoter and requires NRF2 for full induction of γ-globin. (A) Effect of tBHQ and hemin on NQO1 and γ-globin mRNA levels. K562 cells were treated with 25μM of tBHQ and 20μM of hemin for 12 and 24 hours before RNA isolation. (B) ChIP analysis of K562 cells treated with hemin for 0, 8, and 26 hours. Quantitative real-time PCR was performed on immunoprecipitated DNA with the use of primers that flank the ARE region in the γ-globin promoter. The NQO1 promoter was used as a positive control, and necdin promoter was used as a negative control. *P < .05 compared with untreated. Results presented as Enrichment over IgG, normalized to necdin. (C) Effect of NRF2 siRNA on hemin fold induction of NQO1 and γ-globin mRNA. siRNA specific for NRF2 was transiently transfected into K562 cells. At 24 hours later, cells were treated with tBHQ for 12 or 24 hours before RNA isolation. Control siRNA and a mock transfection (−) were used as controls. Results of real-time PCR are presented as fold induction. *P < .05 compared with control siRNA.
Finally, to determine whetherNRF2 is necessary for hemin induction of γ-globin and NQO1 mRNA, we used siRNA to knockdown NRF2, then treated with hemin for 12 and 24 hours. As shown in Figure 7C, cells transfected with NRF2 siRNA have attenuated induction of both NQO1 and γ-globin after 12 and 24 hours of hemin treatment compared with untransfected cells or cells transfected with control siRNA. These results indicate that NRF2 signaling is required for full γ-globin gene induction by hemin in K562 cells, making this mechanism applicable to more than just classic NRF2 inducers.
Discussion
Although allogeneic hematopoietic stem cell transplantation, gene transfer therapy, and gene correction in induced pluripotent stem cells all have the potential to cure the β-hemoglobinopathies, technical challenges and the requirement for highly sophisticated medical care make it unlikely that these therapies will be available to most people with these diseases in the near future. Until these treatments become widely available, pharmacologic induction of HbF may offer the best opportunity for a safe, effective, and widely available therapy. However, currently available inducing agents, including the DNA methyltransferase inhibitors, cytotoxic drugs, and histone deacetylase inhibitors, damage DNA, alter global epigenetic marks, or suppress hematopoiesis. Although such treatments can be effective in the short term, their long-term side effects are unknown and may be significant.
Given recent successes in targeted drug development for cancer and other diseases, a similar approach to HbF induction should be possible. Such strategies have, however, been limited by an incomplete understanding of the process of HbF induction and a lack of “drugable” molecular targets. On the basis of the work of many other investigators and our own studies, we have proposed that pharmacologic induction of HbF by most agents can be explained by activation of cell stress signaling pathways. Because these same pathways are under investigation for the development of targeted agents for cancer and many other diseases, and because most targeted drug development has focused on modulating signaling pathways, if specific targets can be identified, they should be addressable using current drug development technology.
In this work we report our investigations of a novel target for HbF induction, the NRF2/ARE signaling pathway. Although the Nrf2 gene was originally cloned by Moi et al,42 on the basis of in vitro binding of NRF2 to the β-globin locus control region hypersensitive site 2 core enhancer, no role in globin gene regulation or erythroid biology was found.43,44 NRF2 was subsequently shown to be the primary transcriptional activator of a large battery of genes involved in cellular responses to oxidants and electrophiles.45 The possibility that these agents may be protective against cancer and may modulate oxidation-related inflammatory responses has led to their evaluation for cancer chemoprevention and treatment of cardiovascular and inflammatory diseases.20,21
This is the first report of γ-globin induction through the NRF2/ARE signaling pathway. In support of this mechanism, we confirmed that 3 compounds that activate this pathway induce γ-globin mRNA production in K562 cells, that the Food and Drug Administration-approved food additive tBHQ induces NRF2 nuclear translocation and NRF2 binding to the ARE region of the γ-globin promoter, and that NRF2 and the ARE element are required for tBHQ induction of γ-globin expression. We also showed that the conventional inducer, hemin, uses the NRF2 pathway to induce γ-globin expression.
Our experiments used the well-characterized NRF2 target gene NQO1 as a positive control. We observed 2 significant differences in the regulation of this gene and γ-globin. First, in all experiments, NQO1 mRNA was induced to greater levels than γ-globin mRNA. A large number of studies over the course of approximately 30 years have shown that the γ-globin promoters have a complex structure with several cis-acting elements that bind many important trans-acting factors and that the most important aspects of transcriptional regulation of the γ-globin genes are related to erythroid development and differentiation. In contrast, NRF2 is the primary factor regulating expression of the NQO1 gene, and its function is to mediate acute responses to specific chemical toxins.36
On the basis of these considerations, it is not surprising that manipulation of a single transcription factor (NRF2) has a lesser effect on γ-globin gene expression compared with NQO1. The second difference we noted was in experiments involving siRNA knockdown of KEAP1 protein levels. This led to nuclear accumulation of NRF2 and activation of NQO1 expression but not to increased γ-globin gene expression. A similar observation has been previously described in vascular endothelial cells.46 One possibility is that activation of γ-globin gene expression may require specific posttranscriptional modifications of NRF2 that do not occur with KEAP1 knockdown alone. Known NRF2 modifications include phosphorylation20 and acetylation47 in response to various stimuli. Another possibility is that γ-globin gene induction may require interaction of NRF2 with other factors such as CAAT binding protein (p300/CBP), a binding partner of NRF2.48 Finally, in some settings the NRF2 pathway exhibits cross-talk with the MAPK pathways.36 Coactivation of this or other stress related signaling, that does not occur with KEAP1 knockdown, may be necessary for γ-globin induction by NRF2 pathway agonists.
To begin to determine whether induction of γ-globin gene expression through NFR2/ARE signaling might be of clinical relevance, we tested tBHQ in an in vitro model of erythroid differentiation. At a dose of 5μM in 3 separate experiments, we observed an average 1.25-fold increase in the γ/(γ + β) globin mRNA ratio when measured as the AUC across the course of erythroid differentiation. This finding was associated with an increase in HbF in terminally differentiated cells, from 3% in the untreated control cells to 9%. This increase in HbF is similar to those reported in similar culture systems for HU,49 CysNO,49 mithramycin,50 and for 5-Aza, our positive control. Consistent with our previous results with 5-Aza,39 tBHQ not only increased γ-globin mRNA and HbF but also decreased β-globin mRNA and HbA. The mechanisms underlying β-globin mRNA suppression by tBHQ and 5-Aza are not yet known. Also consistent with 5-Aza experiments, we saw a proportional increase in HbF that was much greater than the modest increase in γ-globin mRNA.
A major rationale for investigating the NRF2/ARE pathway is that some of the agents that activate it are already in clinical trials. This suggests that doses that activate the pathway may be achievable in patients. tBHQ has been given orally to adult volunteers at a dose of 125 mg that resulted in plasma levels of 31 mg/L,32 which translates to approximately 186μM, more than 30 times our effective dose in primary human peripheral blood cells. We have used tBHQ for proof of principle testing of our hypothesis. There are currently many potent inducers of NRF2 signaling under development for therapeutic use. Some of these could prove effective for therapeutic induction of HbF in people with β-hemoglobinopathies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dr Lionel Lewis and Bernie Beaulieu for assistance with Hb HPLC; Dr Joyce Lloyd for the γ-globin promoter construct; Dr Bill Roebuck for NRF2 inducing compounds; and Dr Rachel West, Dr Rodwell Mabaera, Emily Schaeffer, and Cynthia Hahn for their helpful insights and discussion.
This work was supported by National Institutes of Health grant HL73442 to C.H.L. and from funding provided by the Knights of the York Cross of Honour. Ms Macari is supported by National Cancer Institute T32 training grant CA009658.
National Institutes of Health
Authorship
E.R.M. designed, performed, and analyzed experiments and wrote the paper; and C.H.L. designed and oversaw the project, analyzed experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christopher H. Lowrey, MD, Section of Hematology/Oncology, Dartmouth-Hitchcock Medical Center, One Medical Center Dr, Lebanon, NH 03756; e-mail: c.lowrey@dartmouth.edu.