Abstract
The cytoplasmic domain of band 3 serves as a center of erythrocyte membrane organization and constitutes the major substrate of erythrocyte tyrosine kinases. Tyrosine phosphorylation of band 3 is induced by several physiologic stimuli, including malaria parasite invasion, cell shrinkage, normal cell aging, and oxidant stress (thalassemias, sickle cell disease, glucose-6-phosphate dehydrogenase deficiency, etc). In an effort to characterize the biologic sequelae of band 3 tyrosine phosphorylation, we looked for changes in the polypeptide's function that accompany its phosphorylation. We report that tyrosine phosphorylation promotes dissociation of band 3 from the spectrin-actin skeleton as evidenced by: (1) a decrease in ankyrin affinity in direct binding studies, (2) an increase in detergent extractability of band 3 from ghosts, (3) a rise in band 3 cross-linkability by bis-sulfosuccinimidyl-suberate, (4) significant changes in erythrocyte morphology, and (5) elevation of the rate of band 3 diffusion in intact cells. Because release of band 3 from its ankyrin and adducin linkages to the cytoskeleton can facilitate changes in multiple membrane properties, tyrosine phosphorylation of band 3 is argued to enable adaptive changes in erythrocyte biology that permit the cell to respond to the above stresses.
Introduction
Early views of the human erythrocyte argued that the cell was inert to external stimuli and that its complement of protein kinases, phospholipases, G proteins, phosphatases, and hormone receptors simply constituted nonfunctional vestiges of signaling pathways that were once operational in erythroid precursor cells. More recent evidence, however, has revealed that the human erythrocyte is highly responsive to its environment and that the cell's rich ensemble of signaling proteins likely comprise critical components in the cell's communication with its extracellular milieu. Classic hormones/signaling molecules such as prostaglandin E2, insulin, epinephrine, endothelin, ADP, and NO are now known to modulate erythrocyte properties in an adaptive manner, and the functional activities of many intracellular signaling intermediates have been demonstrated to regulate erythrocyte behavior.1-5
One of the major targets of erythrocyte signaling appears to be the predominant membrane-spanning protein, band 3. Band 3 (AE1) catalyzes the exchange of anions (primarily HCO3− for Cl−) across the erythrocyte membrane,6 anchors the spectrin/actin cytoskeleton to the lipid bilayer,7 organizes and regulates a complex of glycolytic enzymes,8,9 participates in control of erythrocyte lifespan,10,11 nucleates several important membrane-spanning proteins,12 and serves as a docking site for multiple peripheral membrane proteins, including protein 4.1, protein 4.2, and several kinases and phosphatases.13-16 Not surprisingly, mutations in band 3 are frequently associated with various hemolytic diseases.17 Perhaps because of its many important functions, band 3 is also a prominent substrate of Ser/Thr kinases,18,19 and is the major substrate of the cell's protein tyrosine kinases.20,21
In response to physiologic stimuli such as hypertonic conditions or oxidative stress, and in severe hematologic disorders such as thalassemias, sickle cell anemia, and glucose-6-phosphate dehydrogenase deficiency,22,23 phosphorylation of band 3 on tyrosine residues can increase by several orders of magnitude.20,22-26 Although the protein tyrosine kinase Lyn has been shown to participate in this phosphorylation,21 the protein tyrosine kinase Syk can be argued to play the more prominent role, because it has been reported to phosphorylate tyrosines 8 and 21 of band 3,20 which in turn generates a binding site for other protein tyrosine kinases.21 Of more direct relevance to erythrocyte signal transduction, Syk may also mediate the effects of oxidant stress on band 3 tyrosine phosphorylation, because it strongly prefers to phosphorylate a reversibly oxidized conformation of AE1.20 Except for displacement of glycolytic enzymes from their binding sites on band 3,27,28 the functional consequences of its tyrosine phosphorylation remain largely unexplored. However, because the sites of band 3 tyrosine phosphorylation are distributed throughout the protein (ie, at least tyrosines 8, 21, 359, and 90419,29 ), it can be hypothesized that the biologic impact of tyrosine phosphorylation should extend beyond its regulation of glycolysis. The only suggestion that other functions of band 3 might also be affected by tyrosine phosphorylation lay in the observation that tyrosine phosphorylation of the polypeptide's cytoplasmic domain is somehow inhibited by ankyrin binding30 ; however, no connection between this observation and the regulation of any membrane-cytoskeletal interaction has ever been examined. Therefore, the purpose of this study was to evaluate whether tyrosine phosphorylation of band 3 might alter its interaction with the spectrin/actin skeleton. We report here that tyrosine phosphorylation of band 3 markedly reduces its affinity for ankyrin, leading to release of band 3 from the spectrin/actin membrane skeleton, enhancement of the lateral mobility of band 3 in the bilayer, and progressive vesiculation and loss of plasma membrane from stimulated cells.
Methods
Unless otherwise stated, all materials were obtained from Sigma-Aldrich.
Treatment of erythrocytes
Venous blood was drawn from healthy volunteers after informed consent and treated as described previously.20 To induce tyrosine phosphorylation, erythrocytes were suspended at 30% hematocrit in PBS (137mM NaCl, 2.7mM KCl, 8.1mM K2HPO4, and 1.5mM KH2PO4, pH 7.4) in the presence of 5mM glucose (PBS-glucose) to obtain packed cells, and incubated at different times (up to 10 hours) and concentrations of o-vanadate at 37°C. For controls, erythrocytes were either left untreated or pretreated (before addition of o-vanadate) with 1μM concentrations of Syk inhibitors II and IV (Calbiochem) for 1 hour at 37°C in the dark. Each reaction was stopped by washing 3 times in PBS-glucose, and membranes were prepared as described in “Erythrocyte membrane and KI-ION preparation”.
The ability of chemical cross-linkers to cross-link band 3 in the aforementioned cells was assessed by incubation with increasing concentrations of bis-sulfosuccinimidyl-suberate (BS3) (Thermo Scientific) for 10 minutes at 20°C. Erythrocytes were then washed 3 times in PBS containing 40mM Tris buffer, pH 7.4, to inactivate residual cross-linking agent, and membranes were prepared and analyzed in the standard manner.
To simulate oxidative stress, erythrocytes were incubated for 45 minutes at 37°C in PBS-glucose containing increasing concentrations of diamide, after which the reaction was stopped by washing 3 times in PBS-glucose. When desired, treated erythrocytes were further processed to prepare membranes and KI-stripped inside-out vesicles (KI-IOVs). For all protocols described, untreated controls were processed identically except the stimulant/inducer was omitted from the incubation.
Erythrocyte membrane and KI-IOV preparation
Standard hypotonic membranes were prepared on ice as described previously.20 Prepared membranes were stored at −20°C if used within 24 hours or at −80°C if stored for a longer time. Membrane protein content was quantified using the DC Protein Assay (Bio-Rad). IOVs were prepared and stripped of residual peripheral proteins with KI essentially using the method of Bennett31 (details are provided in the supplemental materials, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Vesicle isolation
To obtain vesicles, erythrocytes were incubated at 42°C under stirring at 180g in a microtube, and the supernatants were collected and centrifuged at 25 000g for 10 minutes at 4°C to eliminate spontaneously formed erythrocyte ghosts. After the addition of phosphatase inhibitors, supernatants were centrifuged for 3 hours at 100 000g on an ultracentrifuge (Beckman) at 4°C to isolate vesicles.
GST-ankyrin binding assay
A fusion protein of GST attached to the COOH-terminus of the 46-kDa D3D4 domain of human erythrocyte ankyrin (GST-D3D4-Ankyrin) was expressed in E coli as described previously.30 The fusion protein was purified by GST-affinity chromatography and dialyzed into ankyrin-binding buffer (5mM phosphate, 1mM EDTA, and 1.5mM sodium azide, pH 8.0).
For binding assays, KI-IOVs were incubated overnight at 4°C with GST-D3D4-Ankyrin. Unbound ankyrin was removed by washing 2 times in binding buffer, and the KI-IOVs were pelleted at 30 000g. Ankyrin binding to KI-IOVs was quantified by either assaying GST activity or measuring the ankyrin concentration using a quantitative dot-blot assay with a polyclonal anti-ankyrin antibody (prepared against the D3D4 domain of ankyrin). Quantitative densitometry of ankyrin was performed using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences) with Odyssey V3.0 software, and expressed as arbitrary fluorescence units.
Triton X-100 extraction of band 3
Erythrocytes were resuspended at 30% hematocrit in PBS-glucose, incubated with increasing concentrations of o-vanadate or diamide, and 0.5% Triton X-100 (Pierce) was added. Membrane proteins were allowed to extract for 15 minutes at 0°C. Samples were centrifuged at 25 000g and the supernatant (Triton X-100 extract) was collected and solubilized in Laemmli buffer (1:1). All SDS-PAGE experiments were performed at least in triplicate.
Immunoblot analysis
Proteins separated by SDS-PAGE were transferred to nitrocellulose filters and probed with anti-phosphotyrosine antibody (Santa Cruz Biotechnology) diluted 1:2000, anti–band 3 antibody diluted 1:50 000, anti-ankyrin antibody diluted 1:5000, anti-spectrin diluted 1:2000, and anti-adducin diluted 1:2000. Secondary antibodies conjugated to infrared fluorescent dyes excitable at 800 nm (IRDye 800CW; LI-COR Biosciences) were then used to visualize the desired antigens using an 800-nm laser scanner (Odyssey; LI-COR Biosciences). To establish the specificity of the anti-phosphotyrosine antibody, proteins were dephosphorylated before gel electrophoresis, as described previously.19 Quantitative densitometry analysis was performed using Odyssey V3.0 software.
Confocal imaging
Washed erythrocytes were diluted to 0.5% hematocrit and labeled with 5 μL/mL of Vybrant DiI (Invitrogen) for 20 minutes at 37°C, followed by washing 3 times with PBS-glucose and 0.1% BSA. Labeled cells were diluted to 0.1% hematocrit and deposited onto precleaned coverslips in a custom-built chamber. O-vanadate (2mM) was added from a 100mM stock solution, and imaging was performed on an Olympus FluoView FV1000 inverted confocal microscope using a 60× oil-immersion objective. Transmission images were obtained similarly, except erythrocytes were not labeled with dye.
For immunofluorescence images, o-vanadate–treated erythrocytes were fixed and permeabilized as described previously.8 Staining was performed using polyclonal anti–band 3 antibody (raised in rabbit against purified human cdb3) and monoclonal anti-phosphotyrosine (py99; Santa Cruz Biotechnology). Band 3 was visualized with Cy2-conjugated anti-rabbit and anti-phosphotyrosine with Cy3-conjugated anti-mouse antibodies (Jackson ImmunoResearch Laboratories). After labeling, resuspended erythrocytes were allowed to attach to coverslips coated with polylysine that were mounted using Aqua-Mount (Lerner Laboratories).
Labeling of band 3 in whole erythrocytes with DIDS-biotin for diffusion measurements
For single-molecule studies, erythrocytes were labeled with 10−11M 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS)–biotin, as described previously.32 These incubation conditions allowed for covalent reaction of the compound with no more than 2 band 3 molecules per cell. DIDS-biotin–labeled cells were incubated with excess streptavidin-conjugated Qdot525 (Invitrogen) and washed 2 times with PBS containing 0.1% BSA. Labeled erythrocytes were allowed to settle onto a precleaned, polylysine-coated coverslip in a custom-built chamber. For o-vanadate treatment, the appropriate concentration was added from a 100mM stock solution to the solution on the coverslip. Video recordings of individual band 3 molecules were collected for 1000 frames at 120 frames/s, and analyzed essentially as described previously.32 Band 3 diffusion was recorded from 0-60 minutes after the addition of o-vanadate, but only data from 30-60 minutes of treatment were ultimately used because phosphorylation was found to be maximal during this time period.
Results
Dose and time dependence of o-vanadate–induced band 3 tyrosine phosphorylation
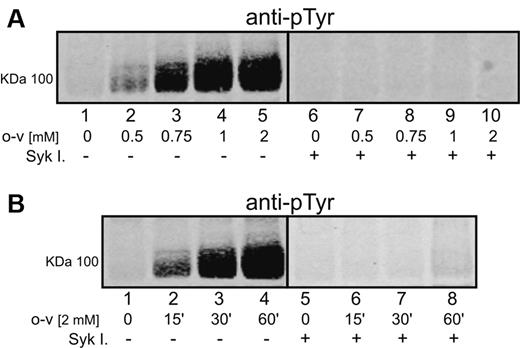
O-vanadate has been well established to induce hyperphosphorylation of band 3 on tyrosine residues, probably by inhibition of the cell's tyrosine phosphatases.29,33 As reported previously,20,34-36 tyrosine phosphorylation of band 3 cannot be detected in untreated erythrocytes (Figure 1A lane 1). However, a gradual increase in band 3 tyrosine phosphorylation could be observed at concentrations between 0.5 and 2mM of o-vanadate (Figure 1A lanes 2-5), with no further enhancement of phosphorylation seen at higher o-vanadate concentrations (data not shown). Band 3 tyrosine phosphorylation was readily apparent after 15 minutes of exposure to o-vanadate, and phosphotyrosine content increased until it reached a maximum at 60 minutes of exposure (Figure 1B lanes 1-4). Longer incubation times (2-4 hours) led to a slow decline in tyrosine phosphorylation and, in agreement with previous studies,33,34 no evidence for significant tyrosine phosphorylation of other major membrane proteins was seen in response to o-vanadate.
Effect of o-vanadate and Syk inhibitors on phosphorylation of band 3 in intact human erythrocytes. Proteins were separated by 8% SDS-PAGE, after which band 3 phosphorylation was analyzed by Western blotting using an anti-phosphotyrosine (anti-pTyr) antibody. Erythrocytes were treated for 1 hour with different concentrations of o-vanadate (o-v; A) and for different incubation times (B) with 2mM o-vanadate in the absence (panel A lanes 1-5; panel B lanes 1-4) or presence (panel A lanes 6-10; panel B lanes 5-8) of Syk inhibitors II and IV (Syk I). Images were acquired using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences).
Effect of o-vanadate and Syk inhibitors on phosphorylation of band 3 in intact human erythrocytes. Proteins were separated by 8% SDS-PAGE, after which band 3 phosphorylation was analyzed by Western blotting using an anti-phosphotyrosine (anti-pTyr) antibody. Erythrocytes were treated for 1 hour with different concentrations of o-vanadate (o-v; A) and for different incubation times (B) with 2mM o-vanadate in the absence (panel A lanes 1-5; panel B lanes 1-4) or presence (panel A lanes 6-10; panel B lanes 5-8) of Syk inhibitors II and IV (Syk I). Images were acquired using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences).
p72 Syk has been reported to be primarily responsible for phosphorylation of tyrosines in the cytoplasmic domain of band 3.29,37 Consistent with this hypothesis, newly available Syk inhibitors were found to inhibit o-vanadate–mediated band 3 tyrosine phosphorylation nearly quantitatively (Figure 1A lanes 6-10 and Figure 1B lanes 5-8). Moreover, characterization of the phosphorylation sites by mass spectrometry20,37 demonstrated the phosphorylation of tyrosines 8, 21, and 359, indicating that all 3 tyrosines in the band 3 cytoplasmic domain were phosphorylated upon o-vanadate treatment. Because mass spectrometry analysis did not reveal any oxidative cross-linking of cysteine-containing peptides, it can be concluded that o-vanadate treatment does not induce oxidative changes in band 3.
Band 3-Ankyrin interactions in KI-IOVs containing tyrosine-phosphorylated band 3
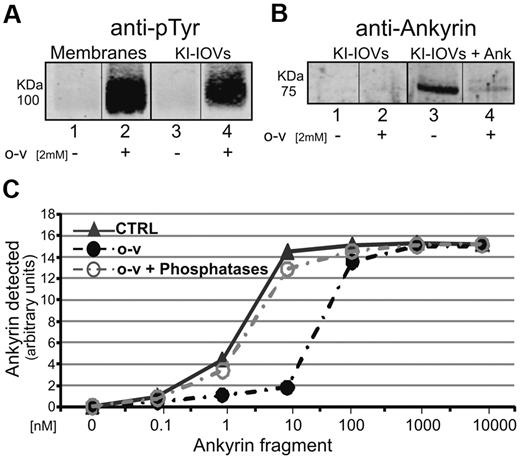
To obtain an initial indication of whether band 3 tyrosine phosphorylation might affect its affinity for ankyrin, we prepared KI-IOVs (IOVs stripped of peripheral proteins with 1M KI) from both control and 2mM o-vanadate–treated erythrocytes and examined their ability to bind ankyrin. As seen in the pull-down assays of Figure 2A, band 3 in KI-IOVs prepared from o-vanadate–treated erythrocytes (isolated in presence of phosphatase inhibitors) retained its highly phosphorylated state (lane 4), whereas band 3 in untreated KI-IOVs from control cells remained unphosphorylated (lane 3). Using the 75-kDa fusion protein containing the D3D4 domain of ankyrin fused to GST (GST-D3D4-Ankyrin, see “GST-ankyrin binding assay”), we observed that phosphorylated KI-IOVs from o-vanadate–treated cells bound negligible amounts of the GST-D3D4-Ankyrin (Figure 2B lane 4), whereas unphosphorylated KI-IOVs from control cells co-pelleted significant amounts of the same ankyrin fusion protein (Figure 2B lane 3). As expected, KI-IOVs that were not incubated with GST-D3D4-Ankyrin did not show any immunostaining band in the 75-kDa region. To confirm that these results were the direct result of band 3-ankyrin interactions, we also immunopelleted band 3 from detergent-solubilized KI-IOVs using anti–band 3 antibodies and observed the same difference in ankyrin association between o-vanadate–treated and untreated samples (data not shown). Finally, to ensure that the observed decrease in ankyrin affinity was not a consequence of some nonspecific effect of o-vanadate on erythrocyte properties, we phosphorylated KI-IOVs directly with the recombinant catalytic subunit of p72 Syk and again demonstrated a loss of ankyrin-binding affinity (data not shown). These data suggest that band 3 tyrosine phosphorylation by p72 Syk greatly reduces its affinity for ankyrin.
Effect of band 3 tyrosine phosphorylation on the ability of erythrocyte membrane KI-IOVs to bind ankyrin. Proteins were separated by 8% SDS-PAGE and analyzed by Western blotting using anti-phosphotyrosine (anti-pTyr) and anti-ankyrin antibodies. (A) Analyzed membrane proteins and KI-IOVs were obtained from control (lanes 1 and 3) and 2mM o-vanadate–treated (lanes 2 and 4) erythrocytes. (B) KI-IOVs obtained from control and o-vanadate–treated erythrocytes before (lanes 1 and 2) and after (lanes 3 and 4) incubation with the D3D4 domain of ankyrin (1nM). Quantification of ankyrin binding to KI-IOVs obtained from untreated (CTRL) and o-vanadate–treated erythrocytes in the absence (o-v) or presence of 6 μL of (400 units) λ phosphatase (o-v + Phosphatases) after incubation with different concentrations of the ankyrin fragment. (C) Quantitative densitometry of ankyrin was performed using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences) with Odyssey V3.0 software and is expressed as arbitrary fluorescence units.
Effect of band 3 tyrosine phosphorylation on the ability of erythrocyte membrane KI-IOVs to bind ankyrin. Proteins were separated by 8% SDS-PAGE and analyzed by Western blotting using anti-phosphotyrosine (anti-pTyr) and anti-ankyrin antibodies. (A) Analyzed membrane proteins and KI-IOVs were obtained from control (lanes 1 and 3) and 2mM o-vanadate–treated (lanes 2 and 4) erythrocytes. (B) KI-IOVs obtained from control and o-vanadate–treated erythrocytes before (lanes 1 and 2) and after (lanes 3 and 4) incubation with the D3D4 domain of ankyrin (1nM). Quantification of ankyrin binding to KI-IOVs obtained from untreated (CTRL) and o-vanadate–treated erythrocytes in the absence (o-v) or presence of 6 μL of (400 units) λ phosphatase (o-v + Phosphatases) after incubation with different concentrations of the ankyrin fragment. (C) Quantitative densitometry of ankyrin was performed using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences) with Odyssey V3.0 software and is expressed as arbitrary fluorescence units.
Quantitation of band 3 ankyrin-binding affinity in KI-IOVs containing tyrosine-phosphorylated band 3
To obtain a more quantitative measure of the impact of band 3 tyrosine phosphorylation on its affinity for ankyrin, increasing concentrations of GST-D3D4-Ankyrin were incubated with KI-IOVs prepared from control and o-vanadate–treated erythrocytes. After centrifuging at 30 000g to collect the KI-IOVs, analysis of bound ankyrin revealed an ankyrin-binding constant of ∼ 1nM for control IOVs and ∼ 25nM for o-vanadate–treated KI-IOVs (Figure 2C). This 25-fold decline in ankyrin affinity in response to tyrosine phosphorylation confirms the qualitative results shown in Figure 2B.
To further establish the role of tyrosine phosphorylation in the above loss of binding affinity, we next dephosphorylated band 3 by incubating the KI-IOVs with a tyrosine phosphatase before the addition of ankyrin. As seen in Figure 2C, phosphatase treatment caused a complete reversal of inhibition of ankyrin binding. Moreover, direct blockade of band 3 phosphorylation in response to o-vanadate by coadministration of the p72 Syk inhibitors used in the experiments shown in Figure 1 was found to completely abrogate the o-vanadate effect (data not shown). These data strongly confirm that tyrosine phosphorylation of band 3 inhibits its binding to ankyrin.
Triton X-100 extractability of band 3 from o-vanadate–treated erythrocytes
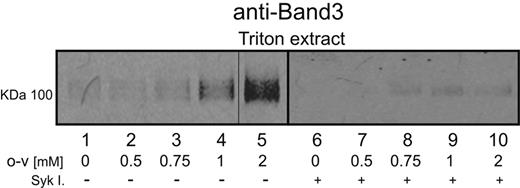
To evaluate whether induction of phosphorylation might influence band 3-ankyrin interactions in situ, the detergent extractability of band 3 from whole erythrocytes was examined in o-vanadate–treated and untreated cells. As seen in the immunoblots of Figure 3 lanes 1-5, a low concentration of Triton X-100 (0.5%) was found to extract little band 3 from untreated erythrocytes. However, as the o-vanadate concentration was increased, the same concentration of Triton X-100 was seen to solubilize increasing quantities of the anion transporter. The largest increase in band 3 extractability was observed after treatment with 2mM o-vanadate, the concentration shown to elicit the highest level of band 3 tyrosine phosphorylation (Figure 1). To further support the causal relationship between band 3 hyperphosphorylation and its extractability, erythrocytes were treated with o-vanadate in the presence of p72 Syk inhibitors, and a marked reduction in o-vanadate–induced band 3 extractability was observed (Figure 3 lanes 6-10).
Effect of band 3 phosphorylation on its extractability from whole erythrocytes. Erythrocytes were treated with different o-vanadate concentrations for 30 minutes at 37°C in the presence (lanes 6-10) or absence (lanes 1-5) of Syk inhibitors. Released band 3 was then extracted by incubation in 0.5% Triton X-100 at 0°C for 15 minutes, and the soluble fraction was separated from the insoluble membrane skeletons by centrifugation. Extracted proteins in the supernatant were separated by 8% SDS-PAGE, and the band 3 content of the supernatant was visualized by Western blotting using an anti–band 3 antibody.
Effect of band 3 phosphorylation on its extractability from whole erythrocytes. Erythrocytes were treated with different o-vanadate concentrations for 30 minutes at 37°C in the presence (lanes 6-10) or absence (lanes 1-5) of Syk inhibitors. Released band 3 was then extracted by incubation in 0.5% Triton X-100 at 0°C for 15 minutes, and the soluble fraction was separated from the insoluble membrane skeletons by centrifugation. Extracted proteins in the supernatant were separated by 8% SDS-PAGE, and the band 3 content of the supernatant was visualized by Western blotting using an anti–band 3 antibody.
Confocal imaging of o-vanadate–treated erythrocytes
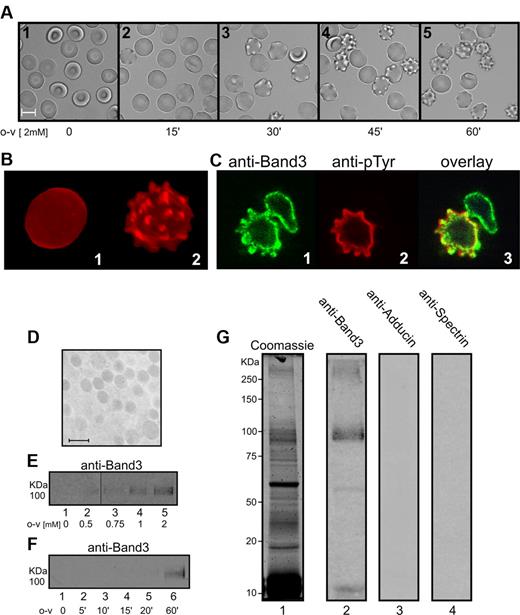
Dissociation of band 3 from ankyrin and the associated spectrin/actin skeleton would be predicted to destabilize the erythrocyte bilayer38 and result in membrane vesiculation. To test this prediction, confocal and bright-field images of o-vanadate–treated cells were collected as a function of time after o-vanadate addition. Within 15 minutes of treatment, mild membrane ruffling was observed (Figure 4A). As time proceeded, spiculation became more pronounced and followed a time course that was roughly correlated with the observed kinetics of band 3 tyrosine phosphorylation (Figure 1B). Untreated control cells observed over the same time period maintained their discoid shape and displayed no gross changes over 60 minutes (data not shown). Labeling of the cells with the fluorescent lipid analog DiI allowed improved visualization of the vesiculation process, revealing that cells treated with o-vanadate accumulated large, rounded protrusions, with some becoming severely spiculated after only 45 minutes of treatment (Figure 4B panel 2). In contrast, cells that were not treated with o-vanadate maintained a smooth, round appearance throughout the duration of the experiment (Figure 4B panel 1). A reduction in deformability of the cells after o-vanadate treatment was also observed (as measured by filtration; supplemental Figure 1) in accordance with a change in surface/volume ratio due to vesiculation. Figure 4C shows images of o-vanadate–treated cells stained with anti–band 3 and anti-phosphotyrosine. In these images, it can also be seen that the membrane deformations extend outward and are correlated with phosphorylation at the membrane, likely of band 3, which remains on the external edge of the cell.
Analysis of the effect of o-vanadate treatment on erythrocyte morphology and vesicle release. (A) Images of 2mM o-vanadate–treated (o-v) erythrocytes at different incubation times. (B) Confocal fluorescence microscopy images (maximum intensity projections) of DiI-labeled untreated erythrocytes (panel 1) and 2mM o-vanadate–treated erythrocytes (panel 2), both at 60 minutes. (C) Confocal microscopy images of erythrocytes treated with 2mM o-vanadate for 1 hour, erythrocytes labeled with anti–band 3 (panel 1) and anti-phosphotyrosine (anti-pTyr; panel 2) antibodies, and an overlay image (panel 3). (D) Electron microscopy image of the vesicles released from o-vanadate–treated erythrocytes. Ultrathin sections were stained with osmium tetroxide, examined, and photographed using a Zeiss EM 900 transmission electron microscope operating at 80 KV. Bar indicates 1 μm. Vesicles released from erythrocytes treated with increasing o-vanadate (o-v) concentrations (E) or with 2mM o-vanadate for different incubation times (F) were collected by ultracentrifugation from the supernatants of cells incubated at 42°C for 45 minutes. The amount of released vesicles was estimated by measuring band 3 using anti–band 3 Western blotting. Vesicle proteins obtained from o-vanadate–treated erythrocytes were separated by 8% SDS-PAGE under reducing conditions. Gels were stained with Coomassie (lane 1) or transferred to nitrocellulose and immunostained with anti–band 3 (lane 2), anti-adducin (lane 3), and anti-spectrin (lane 4) antibodies (G). Images were acquired using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences).
Analysis of the effect of o-vanadate treatment on erythrocyte morphology and vesicle release. (A) Images of 2mM o-vanadate–treated (o-v) erythrocytes at different incubation times. (B) Confocal fluorescence microscopy images (maximum intensity projections) of DiI-labeled untreated erythrocytes (panel 1) and 2mM o-vanadate–treated erythrocytes (panel 2), both at 60 minutes. (C) Confocal microscopy images of erythrocytes treated with 2mM o-vanadate for 1 hour, erythrocytes labeled with anti–band 3 (panel 1) and anti-phosphotyrosine (anti-pTyr; panel 2) antibodies, and an overlay image (panel 3). (D) Electron microscopy image of the vesicles released from o-vanadate–treated erythrocytes. Ultrathin sections were stained with osmium tetroxide, examined, and photographed using a Zeiss EM 900 transmission electron microscope operating at 80 KV. Bar indicates 1 μm. Vesicles released from erythrocytes treated with increasing o-vanadate (o-v) concentrations (E) or with 2mM o-vanadate for different incubation times (F) were collected by ultracentrifugation from the supernatants of cells incubated at 42°C for 45 minutes. The amount of released vesicles was estimated by measuring band 3 using anti–band 3 Western blotting. Vesicle proteins obtained from o-vanadate–treated erythrocytes were separated by 8% SDS-PAGE under reducing conditions. Gels were stained with Coomassie (lane 1) or transferred to nitrocellulose and immunostained with anti–band 3 (lane 2), anti-adducin (lane 3), and anti-spectrin (lane 4) antibodies (G). Images were acquired using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences).
The evaginations that appear on the surface of treated cells rarely spontaneously blebbed off to form free vesicles, but upon exposure to moderate thermal and shear stress, the nascent vesicles were released into the supernatant and could be collected by centrifugation. Electron microscopic images of these collected vesicles (Figure 4D) revealed a relatively uniform spherical size (∼ 1 μm), and analysis of their band 3 content by Western blotting indicated an increase in vesiculation with increasing o-vanadate concentration (Figure 4E) and incubation time (Figure 4F). Preliminary examination of the protein composition of the isolated vesicles by Coomassie staining and Western blotting further revealed that the vesicles contained both normal and aggregated band 3, but were largely depleted of the cytoskeletal proteins spectrin and adducin (Figure 4G). Although mass spectrometry analysis of the SDS-PAGE–separated proteins demonstrated a large amount of hemoglobin, band 3, and catalase in the vesicular fraction (data not shown), a more quantitative proteomic study will be required to fully characterize the vesicle components.
Band 3 diffusion in intact o-vanadate–treated erythrocytes
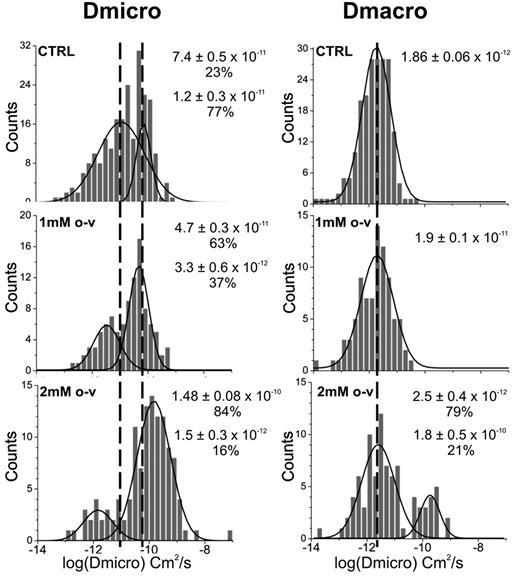
The reduced affinity of band 3 for ankyrin and its enhanced extractability by Triton X-100 upon treatment with o-vanadate suggested that tyrosine phosphorylation of band 3 might result in its detachment from the spectrin/actin skeleton in whole cells. To confirm this hypothesis, we measured the diffusion of band 3 in untreated and o-vanadate–treated erythrocytes using single-particle tracking methods.32 Two diffusion coefficients were measured, one corresponding to band 3 diffusion over short time intervals, termed the microscopic diffusion coefficient (Dmicro), and the other corresponding to its asymptotic diffusion coefficient at long time points, termed the macroscopic diffusion coefficient (DMacro). The first, Dmicro, provides information on the more rapid motions of the protein, which could include simple Brownian motion or oscillation of band 3 while attached to a flexible tether. In contrast, DMacro reflects the ability of band 3 to undergo longer-range excursions, and thereby provides information on the nature and magnitude of diffusional constraints imposed by proximal barriers such as an underlying spectrin/actin skeleton.
As reported previously,32 analysis of individual band 3 trajectories over short time intervals in normal human erythrocytes revealed 2 distinct diffusing populations (Figure 5). The majority of band 3 molecules in this study (∼ 77%) displayed an average Dmicro of 10−11 cm2/s, probably corresponding to those anion transporters that were bound to the spectrin/actin cytoskeleton via either an ankyrin or adducin bridge.14 The other, more mobile population of band 3 (∼ 23%) exhibited an average Dmicro closer to 10−10 cm2/s, perhaps representing unbound/freely diffusing band 3.32 At longer time spans, only a single population of band 3 was detectable and displayed an average DMacro of 10−12 cm2/s. After 30-60 minutes of exposure to 1mM o-vanadate, the proportions of the 2 Dmicro populations, “bound” and “unbound,” shifted to 37% and 63%, respectively, with little change in DMacro (Figure 5). At 2mM o-vanadate, a more significant shift in the distribution of Dmicro values was seen, with the faster diffusing population now comprising 84% of the total band 3. This dose-dependent shift in band 3 from being predominantly confined to being a primarily mobile population in intact erythrocytes suggests a decrease in its interaction with the cytoskeleton. Furthermore, at 2mM o-vanadate, a second, more mobile population was observed over long time periods, with a DMacro of ∼ 10−10 cm2/s. This faster, less restricted long-range diffusion shows that high o-vanadate concentrations can induce a loosening of the cytoskeletal network, permitting a fraction of the band 3 to diffuse without significant hindrance from spectrin “fences.”
Effect of o-vanadate on the microscopic and macroscopic diffusion coefficients of band 3 in intact erythrocytes. Distributions of the logarithms of Dmicro and DMacro obtained from single particle tracking measurements on intact control cells (top row) and o-vanadate–treated cells (1mM, middle row; 2mM, bottom row). As anticipated, the cells used for single-particle diffusion analyses displayed the same morphologic changes as a function of time as the similarly treated cells shown in Figure 4A. Gaussian fits were performed on the histograms to determine the mean values of the distributions and their abundances. The number of Gaussian distributions required to fit the histograms was chosen using the F test with a 95% confidence level.
Effect of o-vanadate on the microscopic and macroscopic diffusion coefficients of band 3 in intact erythrocytes. Distributions of the logarithms of Dmicro and DMacro obtained from single particle tracking measurements on intact control cells (top row) and o-vanadate–treated cells (1mM, middle row; 2mM, bottom row). As anticipated, the cells used for single-particle diffusion analyses displayed the same morphologic changes as a function of time as the similarly treated cells shown in Figure 4A. Gaussian fits were performed on the histograms to determine the mean values of the distributions and their abundances. The number of Gaussian distributions required to fit the histograms was chosen using the F test with a 95% confidence level.
Increased band 3 cross-linking by BS3 after o-vanadate treatment
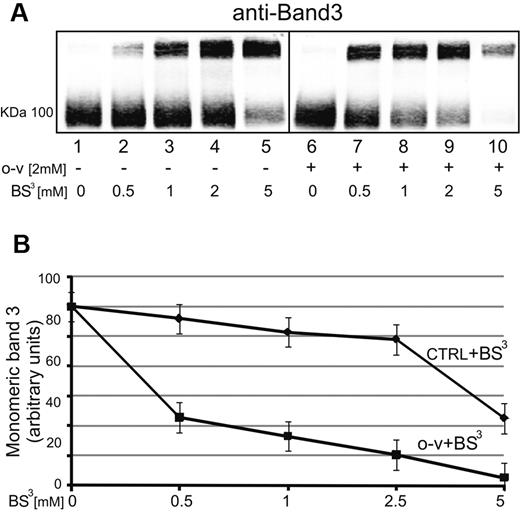
To further establish that band 3 tyrosine phosphorylation leads to enhancement of its lateral mobility, we treated whole erythrocytes with increasing concentrations of BS3, a membrane-impermeable cross-linking agent known to cross-link band 3,39,40 and examined the ability of o-vanadate to enhance formation of high–molecular weight band 3 aggregates. It was more accurate to measure band 3 aggregation as residual monomeric band 3 because at high BS3 concentrations, band 3 aggregates barely entered the gel (Figure 6 lane 10). As seen in Figure 6A-B, treatment with 2mM o-vanadate caused a more intense depletion of monomeric band 3 after BS3 treatment (lanes 6-10). As anticipated, p72 Syk inhibitors completely abrogated this phenomenon (data not shown), confirming the central role of band 3 tyrosine phosphorylation in enhancing its mobility and availability to form clusters.
Effect of o-vanadate on the ability of BS3 to cross-link band 3 into high–molecular weight aggregates. Erythrocytes were treated in the presence (lanes 1-5) or absence (lanes 6-10) of 2mM o-vanadate, and then exposed to increasing concentrations of BS3 (0.5-5mM) before preparation of ghosts and analysis of the molecular weights of band 3 species present by polyacrylamide gel electrophoresis followed by Western blotting (A). The bands in panel A were quantified by densitometry using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences) with Odyssey V3.0 software and plotted against BS3 concentration (B). Lanes 1-5 (without o-vanadate) are plotted in the top curve (CTRL + BS3) and lanes 6-10 (with o-vanadate) are plotted in the bottom curve (o-v + BS3). Band density is expressed as arbitrary fluorescence units and values are means of 3 separate experiments. Bars represent SD from the mean.
Effect of o-vanadate on the ability of BS3 to cross-link band 3 into high–molecular weight aggregates. Erythrocytes were treated in the presence (lanes 1-5) or absence (lanes 6-10) of 2mM o-vanadate, and then exposed to increasing concentrations of BS3 (0.5-5mM) before preparation of ghosts and analysis of the molecular weights of band 3 species present by polyacrylamide gel electrophoresis followed by Western blotting (A). The bands in panel A were quantified by densitometry using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences) with Odyssey V3.0 software and plotted against BS3 concentration (B). Lanes 1-5 (without o-vanadate) are plotted in the top curve (CTRL + BS3) and lanes 6-10 (with o-vanadate) are plotted in the bottom curve (o-v + BS3). Band density is expressed as arbitrary fluorescence units and values are means of 3 separate experiments. Bars represent SD from the mean.
Analysis of the effect of diamide, another inducer of band 3 tyrosine phosphorylation, on ankyrin affinity and band 3 extractability
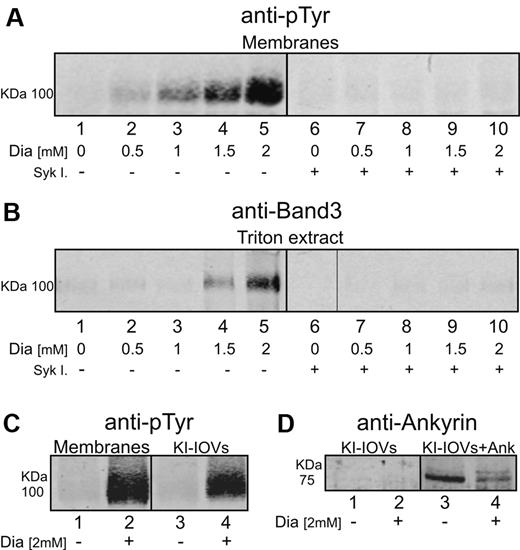
It has been demonstrated recently that diamide-induced oxidation of band 3 can strongly promote its phosphorylation by p72 Syk.20 To determine whether diamide treatment might affect band 3-ankyrin interactions similarly to o-vanadate, the tyrosine phosphorylation and extractability of band 3 were examined in diamide-treated erythrocytes. As shown in Figure 7A, diamide caused the anticipated dose-dependent rise in band 3 tyrosine phosphorylation (lanes 1-5) and, similar to o-vanadate treatment, also promoted an analogous increase in the extractability of band 3 by Triton X-100 (Figure 7B lanes 1-5). Diamide treatment in the presence of p72 Syk inhibitors led to an essentially complete abrogation of both band 3 phosphorylation and extractability without altering diamide's effect on band 3 oxidation (Figure 7A-B lanes 6-10). These data demonstrate that band 3 phosphorylation, and not band 3 oxidation, constitutes the stimulus that promotes its release from the cytoskeleton. In agreement with this finding, hyperphosphorylated KI-IOVs obtained from diamide-treated erythrocytes (Figure 7C lane 4) also showed a decrease in ankyrin binding (Figure 7D lanes 3 and 4).
Effect of diamide treatment on tyrosine phosphorylation and extractability of band 3 from erythrocyte membranes. Erythrocyte membrane proteins were separated by 8% SDS-PAGE and analyzed by Western blotting using anti-phosphotyrosine (anti-pTyr), anti–band 3, and anti-Syk antibodies. Gels were run under reducing conditions. (A) Erythrocytes were treated with different concentration of diamide (Dia) for 45 minutes at 37°C in the absence (lanes 1-5) or presence (lanes 6-10) of Syk inhibitors II and IV (Syk I). (B) 0.5% Triton X-100 (0°C for 15 minutes) was added to erythrocytes treated in the absence (lanes 6-10) or presence of Syk inhibitors (lanes 1-5), spun down, and the supernatant (Triton X-100 extract) was collected for Western blot analysis. (C) Membrane proteins and corresponding KI-IOVs (IOVs) obtained from control cells (lanes 1 and 3) and 2 mM diamide–treated cells (lanes 2 and 4). (D) Western blots of pelleted KI-IOVs obtained from control and diamide-treated erythrocytes after their incubation with (lanes 3 and 4) and without (lanes 1 and 2) ankyrin fragment (1nM). Control KI-IOVs (lanes 1 and 3) were obtained from the same experiment showed in Figure 2B. Images were acquired using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences).
Effect of diamide treatment on tyrosine phosphorylation and extractability of band 3 from erythrocyte membranes. Erythrocyte membrane proteins were separated by 8% SDS-PAGE and analyzed by Western blotting using anti-phosphotyrosine (anti-pTyr), anti–band 3, and anti-Syk antibodies. Gels were run under reducing conditions. (A) Erythrocytes were treated with different concentration of diamide (Dia) for 45 minutes at 37°C in the absence (lanes 1-5) or presence (lanes 6-10) of Syk inhibitors II and IV (Syk I). (B) 0.5% Triton X-100 (0°C for 15 minutes) was added to erythrocytes treated in the absence (lanes 6-10) or presence of Syk inhibitors (lanes 1-5), spun down, and the supernatant (Triton X-100 extract) was collected for Western blot analysis. (C) Membrane proteins and corresponding KI-IOVs (IOVs) obtained from control cells (lanes 1 and 3) and 2 mM diamide–treated cells (lanes 2 and 4). (D) Western blots of pelleted KI-IOVs obtained from control and diamide-treated erythrocytes after their incubation with (lanes 3 and 4) and without (lanes 1 and 2) ankyrin fragment (1nM). Control KI-IOVs (lanes 1 and 3) were obtained from the same experiment showed in Figure 2B. Images were acquired using a laser infrared fluorescence detector (Odyssey; LI-COR Biosciences).
Discussion
Although most early studies dismissed the presence of signaling components in mature erythrocytes as vestiges of previously functional pathways in erythroid precursor cells, more recent studies have suggested that these components can regulate the biology of the mature erythrocyte. In the present study, we demonstrated that tyrosine phosphorylation of band 3 triggers its dissociation from ankyrin and consequent release from the spectrin/actin skeleton. Tyrosine phosphorylation of band 3 in KI-IOVs by 2 distinct protocols was found to result in loss of ankyrin affinity for KI-IOVs in a manner that: (1) was proportional to the level of band 3 tyrosine phosphorylation, (2) could be blocked by p72 Syk inhibitors, and (3) was completely reversed on exposure to phosphatases. Second, lateral diffusion of band 3 in intact erythrocytes was significantly elevated on both microscopic and macroscopic time scales after induction of band 3 tyrosine phosphorylation. Finally, multiple macroscopic membrane changes were triggered by stimulation of band 3 tyrosine phosphorylation. These changes can best be explained by a weakening of bridges between band 3 and the membrane skeleton, and include increased extractability of band 3 from whole cells with Triton X-100, formation of membrane spicules leading to release of band 3-enriched vesicles, and enhancement of the capability of BS3 to cross-link band 3 into high–molecular weight aggregates. Because all of these changes could be prevented by the addition of p72 Syk inhibitors or, in many cases, reversed by exposure to phosphatases, the data provide strong evidence for a weakening of the band 3-ankyrin bridge to the spectrin-actin skeleton by tyrosine phosphorylation of band 3. Therefore, in addition to regulation of the assembly of a glycolytic enzyme complex on band 3,8 tyrosine phosphorylation also controls band 3's interaction with the cytoskeleton.
Although most of the binding studies described herein were focused on the band 3-ankyrin bridge to the membrane skeleton, it should not be ignored that band 3 also provides a bridge to the spectrin-actin junctional complex via its association with adducin. Whereas the effect of band 3 tyrosine phosphorylation on this latter bridge was not examined directly, the band 3 diffusion data suggest that this bridge to adducin is also at least partially disrupted. Therefore, if the slowly diffusing fraction of band 3 (Figure 5) derives from both the ankyrin- and adducin-bound populations of band 3, as was suggested in previous studies,32 then the decrease in size of this population from 77% to 16% of the total band 3 on band 3 phosphorylation cannot be ascribed solely to disruption of the ankyrin bridge. At most, severance of the ankyrin bridge alone would yield a decrease in the slower population to ∼ 30%.14 The emergence of a new population of rapidly diffusing band 3 in macroscopic diffusion analyses of o-vanadate–treated erythrocytes suggests that some alteration in the spectrin skeletal network may also accompany band 3 phosphorylation. We can offer 2 possible explanations for this observation. First, Morrow et al41 and Mohandas et al7 have provided evidence that weakening of the band 3-ankyrin bridge somehow propagates a signal through ankyrin and spectrin that ultimately weakens the spectrin dimer↔tetramer interaction. In this scenario, dissociation of some spectrin tetramers to dimers might enable a fraction of the unattached band 3 to diffuse faster. Second, release of the spectrin network from its attachment to ankyrin roughly midway along the spectrin tetramer might allow the spectrin “fences” to transiently droop or move further away from the membrane. During such fluctuations, unanchored band 3 could migrate more readily from one spectrin corral to another.
The evolution of signaling networks obviously does not occur unless they improve the fitness of the organism. The question therefore arises of how tyrosine phosphorylation–induced dissociation of band 3 from ankyrin might benefit the erythrocyte and thereby the organism. To address this question, we first considered the conditions that lead to tyrosine phosphorylation of band 3 in vivo. As reported by us and by others,10,11,19,35-38,40,42 malarial parasite invasion, shrinkage of the erythrocyte in response to hypertonic stress, and exposure of the cell to oxidative stresses such as those that derive from hemoglobin denaturation/hemichrome formation, thalassemias, sickle cell disease, glucose-6-phosphate dehydrogenase, other antioxidant deficiencies, and natural aging all lead to enhanced band 3 tyrosine phosphorylation.22,23,26 Among these stimuli, oxidative stress appears to be the most proficient, and indeed recent data demonstrate that oxidative cross-linking of band 3 directly induces its association with Syk, which in turn triggers phosphorylation of tyrosines within its cytoplasmic domain.20 Consistent with these findings, only the oxidized fraction of band 3 becomes phosphorylated after imposition of an oxidant stress.20 Because this selectivity would liberate only the oxidized fraction of band 3 from its cytoskeletal anchor, oxidation of band 3 should facilitate collection of this oxidized population into clusters and spicules, leading ultimately to pitting and/or blebbing of the oxidized region from the membrane. Whereas this loss of surface area may not at first be considered beneficial, if regions of the membrane containing oxidized band 3 can be repeatedly removed or “pitted” in this manner, leaving a healthier, more functional erythrocyte to continue its normal function, then the fitness of the entire organism might be improved. Such a cell life–extension mechanism might be especially beneficial to individuals with hemoglobinopathies (eg, sickle cell disease or β-thalassemia), because erythrocytes from these patients might otherwise be cleared too rapidly from the circulation.42,43 Indeed, hemichromes that accumulate in such cells have been reported previously to bind to band 3,10,44 to catalyze band 3 oxidation,43 and to promote its clustering10,42 and the consequent antibody binding, thereby triggering its removal.11 Consistent with this conjecture, we have observed previously that splenectomy of thalassemic patients leads to an enlargement of erythrocyte volume, an increase in cell surface area, and a rise in the content of membrane-bound hemichromes.43
The evolutionary benefit of increased tyrosine phosphorylation to malaria-infected erythrocytes is less obvious. Because the Plasmodium genome does not contain tyrosine kinase,45 one must conclude that the elevated tyrosine phosphorylation of band 3 seen in infected cells derives from erythrocyte tyrosine kinases. Because this phosphorylation arises only early in the infection process (at the ring stage),19 it can be surmised that the accompanying increased band 3 mobility will be limited to this stage of the invasion process. As noted by Ayi,46 these early stages are also characterized by heightened hemichrome formation, suggesting that oxidative stresses could also trigger the phosphorylation process. However, whether this phosphorylation benefits the erythrocyte by perhaps enabling band 3 clustering and its consequent recognition by the immune system12 or if it serves to assist the parasite in erythrocyte membrane remodeling remains to be established.
Finally, analysis of the crystal structure of the cytoplasmic domain of band 3,47 together with information on the binding site of ankyrin on band 3,30,48,49 permits one to inquire why phosphorylation of tyrosines 8 and 21 might inhibit ankyrin binding. Although the mobility of the NH2-terminal 55 residues of band 3 is so high that the residues are not resolved in the diffraction pattern, they are still known to reside on the opposite side of band 3 from the ankyrin-binding site (residues 175-185), suggesting that inhibition of ankyrin binding does not arise from direct steric obstruction. Rather, we speculate that phosphorylation triggers other changes in band 3's conformation or interaction with other proteins, and that these in turn lead to loss of ankyrin affinity. In this respect, it is interesting that ankyrin association inhibits phosphorylation of Y8 and Y21 at the NH2-terminus of band 3,49 strengthening the hypothesis that the N-terminus and ankyrin-binding site on band 3 either overlap or indirectly communicate. The crucial role of the N-terminal residues of band 3 in forming the ankyrin bridge is further evidenced by the observation that antibodies to this sequence block ankyrin binding.49 In addition, an 11–amino acid N-terminal deletion was reported to cause severe membrane instability in the same study in which indications about the inter-independence of Y8 and Y21 phosphorylations were provided.50 It will now be important to examine the sequelae of band 3 tyrosine phosphorylation at the molecular level to characterize the mechanism underlying its effect on ankyrin affinity. Understanding this mechanism should provide insight into the critical membrane alterations in hereditary hematologic disorders and malaria that are characterized by tyrosine hyperphosphorylation of band 3.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health grant GM24417.
National Institutes of Health
Authorship
Contribution: All authors designed the experiments, analyzed the data, and reviewed the manuscript; P.S.L. and F.T. wrote the manuscript; E.F., A.P., K.G., and J.G. performed experiments; and E.F. prepared the images.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Francesco Turrini, Department of Genetics, Biology and Biochemistry, University of Turin, 10126 Turin, Italy; e-mail: francesco.turrini@unito.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal