Abstract
In pathologic settings including retinal ischemia and malignant tumors, robust angiogenesis occurs despite the presence in the microenvironment of antiangiogenic proteins containing thrombospondin structural homology (TSR) domains. We hypothesized that antiangiogenesis mediated by TSR-containing proteins could be blunted by localized down-regulation of their cognate receptor on microvascular endothelial cells (MVECs), CD36. Through screening a panel of endothelial cell agonists, we found that lysophosphatidic acid (LPA) dramatically down-regulated CD36 surface expression on primary MVECs. LPA is a lipid-signaling mediator known to have proangiogenic activity, but the mechanisms are largely unknown. We observed that LPA caused CD36 down-regulation in a dose- and time-dependent manner and was long lasting. Down-regulation occurred at the transcriptional level via a signaling pathway involving specific LPA receptors and protein kinase D. LPA-induced MVEC CD36 repression significantly attenuated in vitro antiangiogenic responses to thrombospondin-1, including blockade of migration, tube formation, and VEGFR-2 signaling in response to fibroblast growth factor-2. In vivo relevance was demonstrated by showing that LPA abrogated thrombospondin-1–mediated inhibition of neovascularization of Matrigel plugs implanted in mice. Our data thus indicate that the proangiogenic mechanism of LPA may in part be via switching off the antiangiogenic switch mediated by TSR proteins and CD36.
Introduction
Angiogenesis, the growth of new blood vessels from existing microvasculature, is essential for organ growth and tissue repair. Under normal conditions, angiogenesis is tightly regulated by a dynamic balance between proangiogenic and antiangiogenic signaling pathways. Loss of balance between these pathways can occur as a consequence of many diseases and can lead to either inadequate or excess angiogenesis. The latter contributes to tumor progression, diabetic retinopathy, macular degeneration, and rheumatoid arthritis.1,2 We have been interested in an endogenous antiangiogenic pathway triggered by proteins containing a conserved domain first identified in the platelet and matrix glycoprotein thrombospondin-1 (TSP-1).3,4 This domain, called the TSP type 1 repeat (TSR), is also found in TSP-2,5 in vasculostatin,6,7 and in other antiangiogenic proteins and has been shown to exert its activity by binding to a specific receptor, CD36, expressed on microvascular endothelial cells (MVECs).8 The antiangiogenic activities of TSP-1 and -2 and vasculostatin are absent or significantly reduced in cd36 knockout mice.4-6
CD36 is a widely expressed cell surface glycoprotein with 2 major classes of ligand in addition to TSR-containing proteins.9,10 On adipocytes, myocytes, specialized neurosensory cells, and gut epithelium, CD36 functions as a transporter and/or sensor of free fatty acids. On phagocytic cells and platelets, CD36 functions in the innate immune response as a scavenger receptor, facilitating binding and internalization of numerous endogenous and exogenous danger signals, including oxidized LDL. In these contexts CD36 has been shown to play a role in chronic inflammation, atherosclerosis, arterial thrombosis, and insulin resistance.11-13
The mechanisms by which CD36 inhibits angiogenesis are based on its ability to transduce signals in MVECs that “turn off” proangiogenic responses and “turn on” antiangiogenic responses in newly formed microvasculature. TSR-CD36 interactions on MVECs inhibit cell migration and tube formation and induce apoptosis by recruiting and activating specific SRC-family and MAPKs, including Fyn, p38, and JNK, directly activating caspases, and inducing expression of endogenous proapoptotic receptors, such as TNFR, Fas, and TRAIL receptors DR4 and DR5, and suppressing AKT activation in response to VEGF.3,4,8,14-16
CD36 expression on monocytes/macrophages and striated muscle cells is highly regulated and has been extensively studied. Monocyte expression is influenced by cytokines such as IL-4 and M-CSF, nuclear hormone receptors such as peroxisome proliferator-activated receptor-γ and liver X receptor, lipids and lipoproteins, and statin and anti-HIV drugs, whereas muscle cell expression is influenced by insulin and energy demands.9,17,18 In contrast, although CD36 is broadly and constitutively expressed in microvascular beds, there is surprisingly little known regarding regulation of its expression on MVECs. Mwaikambo et al19 recently reported that retinal MVEC CD36 expression was up-regulated by hypoxia via the hypoxia-inducible factor-1 transcription factor, suggesting that up-regulation of a natural antiangiogenic pathway may accompany up-regulation of hypoxia-driven proangiogenic pathways, perhaps to provide a “brake” to prevent excess neovascularization.
In many pathologic settings, such as retinal ischemia and malignant tumors, robust angiogenesis occurs despite the abundant presence of TSR-containing proteins in the microenvironment. We thus hypothesized that one mechanism by which TSR-mediated antiangiogenesis could be blunted would be via localized down-regulation of the receptor CD36 on MVECs. In this article we report that the biologically active extracellular lipid-signaling molecule lysophosphatidic acid (LPA) dramatically down-regulated CD36 transcription and expression in primary human dermal MVECs. The down-regulation was long lasting and mediated by a signaling pathway involving specific G protein–coupled LPA receptors and protein kinase D-1 (PKD-1), a Ser/Thr kinase also known as protein kinase Cμ (PKCμ), which induced transcriptional repression of the CD36 gene. LPA treatment of MVECs in vitro abrogated TSP-1–mediated antiangiogenic activities, including fibroblast growth factor-2 (FGF-2)–induced cell migration, branching morphogenesis, and VEGFR signaling. The in vivo relevance of these discoveries was demonstrated by showing that LPA blunted TSP-1 antiangiogenesis in mouse Matrigel assays and that this was associated with loss of neovascular CD36 expression.
LPA is an important regulator of vascular and inflammatory cells. It is produced by activated platelets and leukocytes, and plasma levels are dramatically increased during vascular injury.20 Our data are consistent with studies showing that LPA regulates endothelial cell behavior and angiogenesis21 and that autotaxin, a phospholipase that generates LPA from lysophosphatidylcholine, is angiogenic and essential for vascular development in mice.22 Despite the prominent role of LPA signaling in endothelial cells, the mechanisms by which LPA mediates angiogenesis remain incompletely understood. Our study reveals a novel mechanism by which LPA modulates angiogenesis through interrupting an antiangiogenic pathway and suggests that the LPA–PKD-1–CD36 signaling axis could be a useful target for therapeutic intervention.
Methods
Reagents
1-Palmitoyl LPA and 1-oleoyl LPA and dioctanoylglycerol pyrophosphate (DGPP) were purchased from Avanti Polar Lipids, Ki16425 and antibodies to LPA1 and LPA3 were from Cayman Chemical, TSP-1 was purchased from Calbiochem or isolated from human platelets as described previously,3,4 FGF-2 and Matrigel were from BD Biosciences, bradykinin and the CelLytic cell lysis/extraction reagent were from Sigma-Aldrich, the PKD inhibitor CID 755673 was from Tocris Bioscience, the G-LISA RhoA Activation Assay Biochem Kit and the cell permeable Rho inhibitor C3 Transferase were from Cytoskeleton, SYBR Green was from Applied Biosystems, reverse transcriptase AMV was from Roche Applied Science, the RNeasy Mini Kit was from QIAGEN, and PKD-1 small interfering RNA (siRNA) plasmids were from Addgene. Antibodies to PKD/PKCμ, phospho-PKD (Ser916), phospho-PKD (Ser744/748; which recognizes human PKD-1 phosphorylation site Ser738/742), phospho-Akt (Ser473), Akt, phospho–VEGFR-2 (Tyr1175), VEGFR-2, and vascular endothelial cadherin (VE-cadherin) were from Cell Signaling Technology. Antibody to β-actin and PE- or FITC-conjugated anti-CD36 were from Santa Cruz Biotechnology. Monoclonal antibody to mouse CD36 (clone CRF D-2712) was purchased from Hycult Biotechnology and CD36 antibody (ab78054) was from Abcam. Alexa Fluor 568 phalloidin was from Invitrogen. VECTASHIELD Mounting Medium with 4,6-diamidino-2-phenylindole (DAPI) was purchased from Vector Laboratories.
Endothelial cell culture
Primary human dermal MVECs were purchased from Lonza Walkersville and were maintained in microvascular endothelial cell basal medium-2 (EBM-2) obtained from Lonza Walkersville supplemented with 5% FBS, growth factors, cytokines, supplements, and 200 units/mL penicillin, 100 units/mL streptomycin, and 250 μg/mL amphotericin. Early passage cells (passages 4-8) were grown to 90% confluence, and the medium was replaced with EBM-2 containing 1% FBS overnight before the studies and then replaced again with fresh 1% FBS/EBM-2 for experiments. Mouse cardiac MVECs were isolated as described previously23 and maintained as were the human cells.
Flow cytometry
Endothelial cells exposed to various stimuli or vehicle controls were retrieved from culture dishes by trypsinization, washed with PBS, and then incubated with FITC-conjugated anti-CD36 monoclonal IgG or isotype-matched control IgG. Surface CD36 expression was determined by fluorescence-activated flow cytometry using a FACSCalibur system (BD Biosciences), and the data were analyzed with FlowJo Version 7.2.5 software (Tree Star).
Real-time quantitative RT-PCR
RNA was isolated from MVECs using the RNeasy Mini Kit and then subjected to real-time RT-PCR using an iCycle iQ Multi-Color real-time PCR detection system (Bio-Rad Laboratories). CD36, GAPDH, and cyclophilin A (PPIA) RT2 quantitative PCR Primers were from SABiosciences. GAPDH or PPIA transcripts were amplified in a separate tube to normalize variances in input RNA. Relative Ct values were used to compare changes of mRNA expression.
Immunoprecipitation and immunoblot assays
Immunoprecipitations and immunoblots were performed for CD36 protein expression. For assays of PKD-1, VEGFR-2, and Akt phosphorylation, immunoblots of MVEC lysates were probed with phospho-specific antibodies on duplicate membranes. The membranes were reprobed with non–phospho-specific antibodies as a loading control. Protein concentrations were assayed with a BCA kit (Pierce Chemical) and anti–β-actin was used as an additional loading control for all blots. For some studies, MVEC plasma membranes were isolated before immunoblotting using the Qproteome Plasma Membrane Protein Kit (QIAGEN) according to the manufacturer's instruction.
RNA transcription assay
MVECs cultured in 10-cm dishes were digested with 0.25% trypsin–0.1% EDTA and suspended in 5% FBS/EBM-2. The cells were centrifuged at 500g for 10 minutes, washed once in buffer (10mM Tris-HCl, pH 7.4, 150mM KCl, and 8mM magnesium acetate), and then lysed in the same buffer containing 0.5% Nonidet P-40. The lysates containing intact nuclei were added into 100mM Tris-HCl, 5mM MgCl2, and 600mM sucrose and centrifuged at 500g for 10 minutes. The pellets (nuclei) were subsequently resuspended in 40% glycerol, 50mM Tris-HCl, 5mM MgCl2, and 0.1mM EDTA and immediately stored at −80°C until use. For the nuclear run-on transcription assay, 107 nuclei were used for each reaction on the basis of the method of Zhang et al24 using biotin-labeled RNA and quantitative real-time PCR. In brief, the nuclei were incubated in a reaction buffer (5mM Tris-HCl, pH 8.0, 2.5mM MgCl2, 150mM KCl, and 2.5mM each of ATP, GTP, and CTP) and biotin-16-UTP at 30°C for 45 minutes in a final volume of 60 μL. The reaction was stopped by the addition of 1000 units of RNase-free DNase for 10 minutes at 37°C. The nuclei were subsequently lysed by the addition of buffer containing 10mM Tris-HCl, 1% SDS, and 5mM EDTA. The reaction mixtures were treated with 20 μL of proteinase K (10 mg/mL), and RNA was extracted with TRIzol reagent, ethanol-precipitated, and resuspended in 50 μL RNase-free H2O. The biotinylated RNA was purified by addition of streptavidin particle beads, followed by a 2-hour incubation at 25°C on a rocking platform. Beads were separated by centrifugation at 2000 rpm for 5 minutes and washed once with 2× standard saline citrate–15% formamide for 10 minutes and twice with 2× standard saline citrate for 5 minutes. The biotinylated RNA was used for reverse-transcriptase cDNA synthesis and further quantitative real-time PCR.
siRNA plasmid transfection
Passage 5 cells (5 × 105) were transduced with PKD-1 RNA interference (RNAi) or scrambled control plasmids using the Amaxa Nucleofector system (Lonza Walkersville) specifically optimized for primary human MVECs. A GFP plasmid was used to determine transfection efficiency. After 24 hours, the cells were switched to 1% FBS/EBM-2 overnight and then treated with LPA or control for 24 hours before detection of CD36 and PKD-1 by immunoblot.
Cell migration assay
Human microvascular endothelial cells (HMVECs) plated in 8-μm pore size transwell chambers (Millipore Corporation) were placed in 24-well dishes filled with 1% FBS/EBM-2. After the cells were exposed to LPA (5μM) for 22 hours or vehicle control, FGF-2 (50 ng/mL) and TSP-1 (2nM) in various combinations were added, and 24 hours later the filters were removed and the cells on the upper surface were removed. The filters were then fixed in 4% paraformaldehyde (Electron Microscopy Sciences) for 30-40 minutes at room temperature, mounted with Vectashield mounting medium, and analyzed by fluorescence microscopy after staining with DAPI to count nuclei on the lower surface. Quantification was done using National Institutes of Health (NIH) ImageJ Version 1.40g software.
In vitro tube formation assay
MVECs (2 × 104) were plated on 24-well culture plates coated with growth factor–reduced Matrigel containing LPA (1-5μM), FGF-2 (50 ng/mL), and/or TSP-1 (2nM) in various combinations. After 24 hours the cellular networks were stained with Alexa Fluor 568 phalloidin and visualized with a Leica DM-RXE fluorescence microscope interfaced to a computer with QCapture Pro Version 6.0 software (Media Cybernetics and QImaging). The total tube length and enclosure areas were quantified using NIH ImageJ Verion 1.40g for 6-8 randomly chosen fields per well of double or triple wells for each experimental condition.
In vivo angiogenesis assay
Matrigel plug assays were performed as described previously2,23 by intradermal injection of 8-week-old male C57/BL6 mice with Matrigel premixed with FGF-2, LPA, and TSP-1 in various combinations. Mice were killed 10 days later, and the plugs were removed and fixed in 4% paraformaldehyde overnight at 4°C and then in 10% formaldehyde before embedding in paraffin. The paraffin blocks were cut into 5-μm sections and stained with Masson trichrome. Randomly chosen sections were analyzed microscopically using NIH ImageJ Version 1.40g software to quantify vascularity; 6-8 images were obtained for each specimen. Sections were also analyzed by immunofluorescence microscopy using antibodies to CD36 and CD31 and Alexa-conjugated fluorescent secondary antibodies. Imaging was done with a Leica DM-RXE microscope, interfaced to a computer with QCapture Pro Version 6.0 software or with a Leica confocal microsystem equipped with a 40×/0.75 NA objective and QImaging SRV camera. An additional study using FITC-conjugated LPA (kindly provided by Dr Thomas McIntyre, Cleveland, OH) showed that intact LPA remained detectable in extracts of Matrigel plugs harvested after 1, 3, 7, and 10 days.
All procedures and manipulations of animals were approved by institutional animal care and use committees at Cleveland Clinic in accordance with the US Public Health Service Policy on the Humane Care and Use of Animals and the NIH Guide for the Care and Use of Laboratory Animals (8th edition, revised 2010).
Statistics
Quantitative data are presented as mean ± SD or SEM. Comparisons were done using Student t tests (1-tailed). P < .05 was considered statistically significant.
Results
LPA down-regulates CD36 expression in primary MVECs
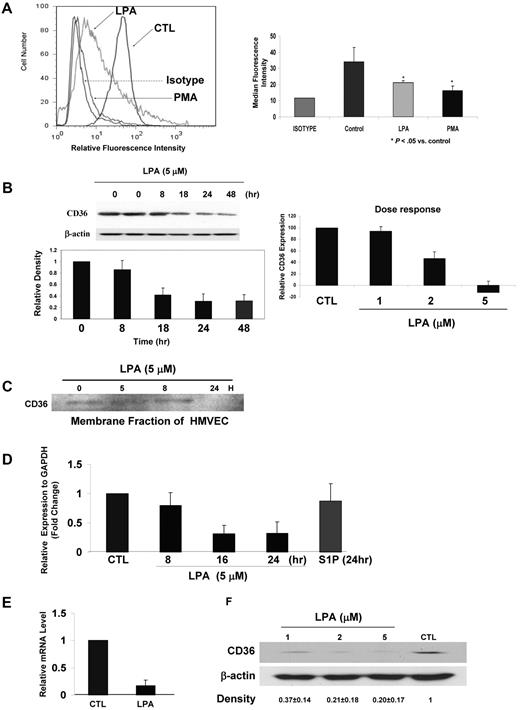
Initial studies aimed to identify pathways regulating CD36 expression on MVECs revealed that exposure of the cells to the PKC activators phorbol 12-myristate 13-acetate (PMA) and/or thymleatoxin led to dramatic down-regulation of surface expression over a 18- to 36-hour period (Figure 1A). We then used fluorescence-activated flow cytometry to screen a battery of endothelial cell agonists known to signal through PKC, including thrombin, histamine, TNF-α, IL-1, bradykinin, and IFN-γ and found that among this group only LPA replicated the PMA effect (Figure 1A). The effect of LPA on CD36 expression was time and dose dependent (Figure 1B), showing an average inhibition of approximately 80% at a concentration of 5μM by 24 hours. The effect was long lasting because even when LPA was withdrawn and replaced with standard complete medium, CD36 expression remained suppressed for at least 24 hours longer (Figure 1B). Western blot analyses of both whole-cell lysates (Figure 1B) and isolated membrane fractions (Figure 1C) confirmed that LPA induced down-regulation of surface CD36 expression and showed that total CD36 levels were correspondingly decreased.
LPA down-regulates CD36 expression in human and mouse MVECs. (A) Representative flow cytometry histogram (left) of human MVECs after exposure in complete medium to LPA (50μM), PMA (100 ng/mL), or vehicle control (CTL) for 36 hours. Cells were analyzed with FITC-conjugated anti-CD36 IgG. An isotype-matched nonimmune IgG was used as a control (brown). On the right is a bar graph showing means ± SD of CD36 median fluorescence intensity for triplicate experiments (P < .05). (B) Time course (left) and dose response (right) of CD36 down-regulation in MVECs exposed to LPA. The time course was done using 5μM LPA, and dose response was assessed at 24 hours. In both cases cells were grown first to 90% confluence and then incubated overnight in “basic” medium containing 1% FBS before addition of LPA. The inset shows a representative blot. The bar graph for the time course shows means ± SEM of band densities (normalized to β-actin control) for 3 independent experiments. The 48-hour bar on the time course indicates relative expression 48 hours after initiation of LPA exposure; in this experiment the LPA-containing medium was removed after 24 hours and replaced with complete medium for an additional 24 hours. The dose response (right panel) was determined by flow cytometry as in panel A. Mean fluorescence intensities relative to the isotype control were obtained for each treatment, and the fluorescence intensity from cells not exposed to LPA was set as 100. (C) Membrane fractions of human MVECs exposed to LPA were obtained by differential centrifugation and then analyzed by Western blot for CD36 expression as in panel B. (D) Quantitative real-time PCR for CD36 mRNA from HMVECs exposed to LPA (5μM) or S1P (1μM) for timed periods from 8 to 24 hours. Data are expressed relative to a control transcript, GAPDH and the mean value for untreated samples were set to 1, and the bar graph shows mean ± SEM of 3 independent experiments. (E) CD36 mRNA transcription (nuclear run-on) assays performed on nuclei isolated from human MVECs treated with LPA (5μM) or vehicle control for 24 hours. Nuclei were incubated with ATP, CTP, GTP, and biotin-16-UTP (2.5mM) at 30°C for 45 minutes, and the biotinylated transcripts were then purified and used for cDNA synthesis and real-time PCR. Data are presented as relative mRNA levels of CD36 compared with GAPDH and are mean ± SEM from n = 3. (F) Mouse cardiac MVEC CD36 protein expression detected by Western blot of whole-cell lysates 24 hours after exposure to LPA (1-5μM). Blots were performed as in panel B using anti-CD36 antibody and then stripped and reprobed with anti–β-actin as a loading control. The gel is representative of 3 separate experiments, and numbers below it show the mean band density ± SD compared with the control lane.
LPA down-regulates CD36 expression in human and mouse MVECs. (A) Representative flow cytometry histogram (left) of human MVECs after exposure in complete medium to LPA (50μM), PMA (100 ng/mL), or vehicle control (CTL) for 36 hours. Cells were analyzed with FITC-conjugated anti-CD36 IgG. An isotype-matched nonimmune IgG was used as a control (brown). On the right is a bar graph showing means ± SD of CD36 median fluorescence intensity for triplicate experiments (P < .05). (B) Time course (left) and dose response (right) of CD36 down-regulation in MVECs exposed to LPA. The time course was done using 5μM LPA, and dose response was assessed at 24 hours. In both cases cells were grown first to 90% confluence and then incubated overnight in “basic” medium containing 1% FBS before addition of LPA. The inset shows a representative blot. The bar graph for the time course shows means ± SEM of band densities (normalized to β-actin control) for 3 independent experiments. The 48-hour bar on the time course indicates relative expression 48 hours after initiation of LPA exposure; in this experiment the LPA-containing medium was removed after 24 hours and replaced with complete medium for an additional 24 hours. The dose response (right panel) was determined by flow cytometry as in panel A. Mean fluorescence intensities relative to the isotype control were obtained for each treatment, and the fluorescence intensity from cells not exposed to LPA was set as 100. (C) Membrane fractions of human MVECs exposed to LPA were obtained by differential centrifugation and then analyzed by Western blot for CD36 expression as in panel B. (D) Quantitative real-time PCR for CD36 mRNA from HMVECs exposed to LPA (5μM) or S1P (1μM) for timed periods from 8 to 24 hours. Data are expressed relative to a control transcript, GAPDH and the mean value for untreated samples were set to 1, and the bar graph shows mean ± SEM of 3 independent experiments. (E) CD36 mRNA transcription (nuclear run-on) assays performed on nuclei isolated from human MVECs treated with LPA (5μM) or vehicle control for 24 hours. Nuclei were incubated with ATP, CTP, GTP, and biotin-16-UTP (2.5mM) at 30°C for 45 minutes, and the biotinylated transcripts were then purified and used for cDNA synthesis and real-time PCR. Data are presented as relative mRNA levels of CD36 compared with GAPDH and are mean ± SEM from n = 3. (F) Mouse cardiac MVEC CD36 protein expression detected by Western blot of whole-cell lysates 24 hours after exposure to LPA (1-5μM). Blots were performed as in panel B using anti-CD36 antibody and then stripped and reprobed with anti–β-actin as a loading control. The gel is representative of 3 separate experiments, and numbers below it show the mean band density ± SD compared with the control lane.
Quantitative real-time PCR assays demonstrated that down-regulation of primary human MVEC surface CD36 expression by LPA was associated with nearly complete loss of CD36 steady-state mRNA (Figure 1D) by ∼ 16 hours. Another biologically active extracellular lipid signaling molecule, sphingosine-1-phosphate (S1P) did not alter CD36 expression, demonstrating specificity. Nuclear run-on transcription assays and mRNA stability assays were then used to determine whether CD36 mRNA regulation by LPA occurs at the transcriptional level. Figure 1E shows that the relative levels of CD36 nuclear mRNA were significantly decreased in cells exposed to LPA for 24 hours, whereas RNA stability assays using actinomycin D demonstrated no effect (data not shown), indicating transcriptional repression of CD36 by LPA.
To determine whether mouse models could be used to study this system, we assessed CD36 expression on primary MVECs isolated from mouse tissues and found that it was also down-regulated by LPA. Figure 1F is a Western blot of mouse cardiac MVECs showing loss of detectable CD36 expression after exposure to 1, 2, and 5μM LPA for 24 hours.
CD36 transcriptional repression by LPA is mediated by specific G protein–coupled receptors
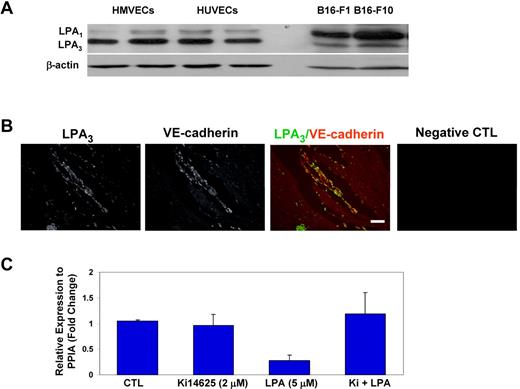
LPA signaling is mediated by members of a family of plasma membrane G protein–coupled receptors25 with various degrees of specificity for phospholipids such as LPA, platelet-activating factor, oxidized phospholipids, lysophosphatidylcholine, and S1P. As a first step in dissecting the MVEC signaling pathway involved in regulating CD36, we used Western blots with antibodies specific for LPA1 and/or LPA3 to demonstrate that HMVECs in culture expressed LPA3 and to a lesser extent LPA1 (Figure 2A). In contrast, 2 different murine B16 melanoma cell lines expressed mainly LPA1. Indirect immunofluorescence microscopy of Matrigel plugs removed from mice 10 days after implantation showed abundant expression of LPA3 (Figure 2B) in the neovasculature. LPA3 immunofluorescence colocalized with the endothelial cell marker, VE-cadherin, demonstrating that migrating endothelial cells in vivo express LPA3. We then pretreated HMVECs in culture with a pharmacologic antagonist, Ki14625, which blocks LPA1 and LPA3 before stimulating the cells with LPA (5μM) for 24 hours. The antagonist attenuated LPA-induced down-regulation of CD36 expression by ∼ 80% (Figure 2C), strongly suggesting that LPA3 and/or LPA1 mediate CD36 repression. Ki14625 itself showed little impact on CD36 expression.
LPA receptors LPA1 and LPA3 regulate CD36 MVEC expression. (A) Lysates prepared from confluent cultures of human umbilical vein endothelial cells (HUVECs), HMVECs and 2 strains of murine B16 melanoma cells were analyzed by Western blot using an antibody that recognizes LPA1 (top band) and LPA3 (bottom band). Blots were stripped and reprobed with anti–β-actin as a loading control. (B) Matrigel containing FGF-2 was injected subcutaneously into mice, and after 10 days the plugs were removed, sectioned, and analyzed by indirect immunofluorescence microscopy with a Leica DM-RXE microscope equipped with a 10×/0.30 NA objective using antibodies to LPA3 (green) or VE-cadherin (red). A representative merged image is shown in the third panel. The far right image shows a control (CTL) with secondary antibody alone. Scale bar = 100 μm. (C) HMVECs were pretreated with the LPA1,3 antagonist Ki14625 (Ki; 2μM) or vehicle control followed by LPA (5μM) for 24 hours. Quantitative real-time PCR of CD36 mRNA was then done as in Figure 1D. Data are expressed relative to a control transcript, PPIA. The mean value for untreated samples was set to 1, and the bar graph shows mean ± SEM of 3 independent experiments.
LPA receptors LPA1 and LPA3 regulate CD36 MVEC expression. (A) Lysates prepared from confluent cultures of human umbilical vein endothelial cells (HUVECs), HMVECs and 2 strains of murine B16 melanoma cells were analyzed by Western blot using an antibody that recognizes LPA1 (top band) and LPA3 (bottom band). Blots were stripped and reprobed with anti–β-actin as a loading control. (B) Matrigel containing FGF-2 was injected subcutaneously into mice, and after 10 days the plugs were removed, sectioned, and analyzed by indirect immunofluorescence microscopy with a Leica DM-RXE microscope equipped with a 10×/0.30 NA objective using antibodies to LPA3 (green) or VE-cadherin (red). A representative merged image is shown in the third panel. The far right image shows a control (CTL) with secondary antibody alone. Scale bar = 100 μm. (C) HMVECs were pretreated with the LPA1,3 antagonist Ki14625 (Ki; 2μM) or vehicle control followed by LPA (5μM) for 24 hours. Quantitative real-time PCR of CD36 mRNA was then done as in Figure 1D. Data are expressed relative to a control transcript, PPIA. The mean value for untreated samples was set to 1, and the bar graph shows mean ± SEM of 3 independent experiments.
CD36 transcriptional repression by LPA receptors is mediated by PKD-1
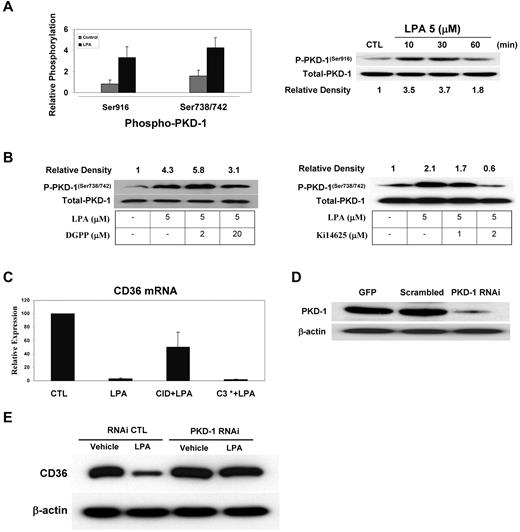
To define relevant signaling cascades downstream of LPA receptors, we examined PKD-1, which has recently been shown to mediate LPA signals in fibroblast cell lines and to regulate angiogenesis.26,27 Using a Western blot assay with antibodies to specific phosphorylated forms of PKD-1, we found that LPA induced a 3- to 5-fold increase in phosphorylation of both Ser916 and Ser738/742 in MVECs (Figure 3A). The Ser916 autophosphorylation site was detected as early as 10 minutes after LPA exposure, whereas phosphorylation of the activation loop sites Ser738 and Ser742 was seen after 30 minutes. When the cells were pretreated with specific LPA receptor antagonists, DGPP against LPA3 or Ki14625 against both LPA1 and LPA3, we found that Ki14625 reduced LPA-induced PKD-1 active site phosphorylation to baseline levels, whereas DGPP partially diminished LPA-induced phosphorylation (Figure 3C).
PKD-1 mediates LPA-induced down-regulation of CD36. (A) HMVECs were exposed to LPA (5μM) or vehicle control for 30 minutes, and then cell lysates were prepared and analyzed by Western blot using antibodies specific for PKD-1 phosphorylated (P-PKD-1) at Ser916 or Ser738/742. Blots were stripped and reprobed with an antibody to total PKD-1 as a loading control. The bar graph shows relative phosphorylation levels (mean ± SEM) from 3 independent experiments. The blot is a representative image of a time course of Ser916 phosphorylation. Relative band densities are shown below the blot. (B) MVECs were treated with the LPA3 antagonist DGPP (2-20μM) or the LPA1,3 antagonist Ki14625 (1-2μM) for 30 minutes followed by LPA (5μM) for 30 minutes. Western blots for phospho–PKD-1 (Ser738/742) were performed as in panel A. Relative band densities are shown above the blot. (C) MVECs were pretreated with a selective PKD inhibitor, CID 755673 (CID; 25μM), or C3 transferase (C3*; 5 μg/mL) for 30 minutes followed by LPA (5μM) for 24 hours. Quantitative real-time PCR for CD36 mRNA was then performed as in Figure 1, and relative CD36 expression was normalized with PPIA. (D) Lysates from MVECs transfected with pmaxGFP (GFP), scrambled RNAi plasmids (Scramble), or pSUPER PKD-1 RNAi were analyzed by Western blot using an antibody to PKD-1. Blots were stripped and reprobed with anti–β-actin as the loading control. (E) MVECs transfected with RNAi plasmids as in panel D were treated with LPA (5μM) for 24 hours and analyzed by Western blot for CD36 expression. Blots were stripped and reprobed with anti–β-actin as the loading control (CTL).
PKD-1 mediates LPA-induced down-regulation of CD36. (A) HMVECs were exposed to LPA (5μM) or vehicle control for 30 minutes, and then cell lysates were prepared and analyzed by Western blot using antibodies specific for PKD-1 phosphorylated (P-PKD-1) at Ser916 or Ser738/742. Blots were stripped and reprobed with an antibody to total PKD-1 as a loading control. The bar graph shows relative phosphorylation levels (mean ± SEM) from 3 independent experiments. The blot is a representative image of a time course of Ser916 phosphorylation. Relative band densities are shown below the blot. (B) MVECs were treated with the LPA3 antagonist DGPP (2-20μM) or the LPA1,3 antagonist Ki14625 (1-2μM) for 30 minutes followed by LPA (5μM) for 30 minutes. Western blots for phospho–PKD-1 (Ser738/742) were performed as in panel A. Relative band densities are shown above the blot. (C) MVECs were pretreated with a selective PKD inhibitor, CID 755673 (CID; 25μM), or C3 transferase (C3*; 5 μg/mL) for 30 minutes followed by LPA (5μM) for 24 hours. Quantitative real-time PCR for CD36 mRNA was then performed as in Figure 1, and relative CD36 expression was normalized with PPIA. (D) Lysates from MVECs transfected with pmaxGFP (GFP), scrambled RNAi plasmids (Scramble), or pSUPER PKD-1 RNAi were analyzed by Western blot using an antibody to PKD-1. Blots were stripped and reprobed with anti–β-actin as the loading control. (E) MVECs transfected with RNAi plasmids as in panel D were treated with LPA (5μM) for 24 hours and analyzed by Western blot for CD36 expression. Blots were stripped and reprobed with anti–β-actin as the loading control (CTL).
Of importance, CID 755673, a selective PKD pharmacologic inhibitor,28 significantly attenuated the effects of LPA on CD36 mRNA (Figure 3C). The inhibitor had no effect by itself. To confirm these results, endogenous PKD-1 expression was knocked down in HMVECs with a specific siRNA plasmid. Western blots showed that the siRNA plasmids reduced PKD-1 expression by > 80% compared with a scrambled control plasmid (Figure 3D). Figure 3E shows that cells transfected with the PKD-1 siRNA plasmid maintained CD36 protein expression after a 24-hour exposure to LPA, whereas expression was decreased by > 70% by LPA in cells transfected with the control constructs.
LPA abrogates TSP-1–mediated antiangiogenic activities of MVECs in vitro
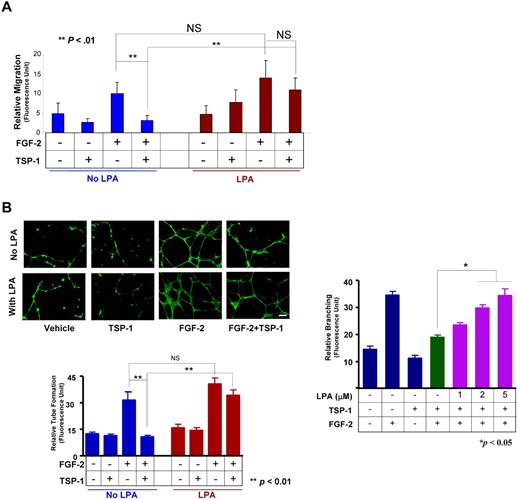
To show the functional consequences of LPA-mediated CD36 down-regulation, we performed in vitro Boyden chamber cell migration assays in MVECs pretreated with LPA (5μM) for 22-24 hours. Both LPA-treated and untreated cells showed robust migration in response to FGF-2, with no statistically significant differences between the groups (Figure 4A). However, whereas coexposure of the cells to TSP-1 suppressed FGF-2–induced migration in control cells (P < .05), no suppression was seen in cells preexposed to LPA. TSP-1 alone or LPA alone produced no statistically significant effect on migration.
LPA abrogates TSP-1–mediated inhibition of migration and tubelike structure formation in vitro. (A) MVECs treated with LPA (5μM) or the vehicle control for 22 hours were subjected to migration assays using a modified Boyden chamber assay in which cells were exposed to FGF-2 (50 ng/mL), TSP-1 (2nM), or their combination. Migration was assessed microscopically after 24 hours by staining nuclei with DAPI (NS, not significant; **P < .01). (B) MVECs were exposed to 5μM (left) or 1-5μM of LPA (right) and then cultured for 24 hours in Matrigel containing FGF-2 (50 ng/mL), TSP-1 (2nM), or their combination. Cells were imaged with a Leica DM-RXE fluorescence microscope equipped with a 20×/0.50 NA objective interfaced to a computer with QCapture Version 6.0 software and the extent of the tubelike (cordlike) structure was quantified microscopically using ImageJ Version 1.40g software. Representative images are shown in the top left panel (scale bar = 50 μm); bar graphs show mean ± SEM (**P < .01; *P < .05).
LPA abrogates TSP-1–mediated inhibition of migration and tubelike structure formation in vitro. (A) MVECs treated with LPA (5μM) or the vehicle control for 22 hours were subjected to migration assays using a modified Boyden chamber assay in which cells were exposed to FGF-2 (50 ng/mL), TSP-1 (2nM), or their combination. Migration was assessed microscopically after 24 hours by staining nuclei with DAPI (NS, not significant; **P < .01). (B) MVECs were exposed to 5μM (left) or 1-5μM of LPA (right) and then cultured for 24 hours in Matrigel containing FGF-2 (50 ng/mL), TSP-1 (2nM), or their combination. Cells were imaged with a Leica DM-RXE fluorescence microscope equipped with a 20×/0.50 NA objective interfaced to a computer with QCapture Version 6.0 software and the extent of the tubelike (cordlike) structure was quantified microscopically using ImageJ Version 1.40g software. Representative images are shown in the top left panel (scale bar = 50 μm); bar graphs show mean ± SEM (**P < .01; *P < .05).
Similar results were seen with in vitro Matrigel tube formation assays (Figure 4B). Both LPA-treated and untreated cells showed vigorous network formation in response to FGF-2, with no statistically significant differences between the groups. Exposure of the cells to TSP-1 suppressed FGF-2–induced tubelike structure formation to baseline levels in control cells (P < .05), whereas no suppression was seen in the cells preexposed to LPA. The LPA effect was dose-dependent, with full restoration of tubelike structure formation seen at 5μM. TSP-1 alone or LPA alone produced no statistically significant effect.
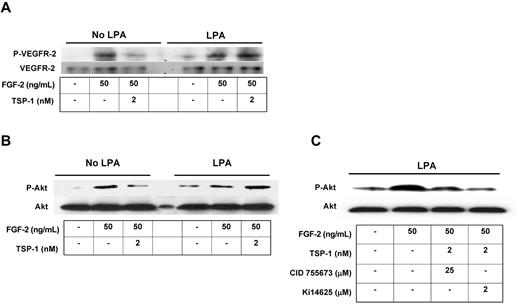
It has been shown previously that FGF-2 induces VEGFR-2 activation and subsequent downstream phosphorylation of Akt-1 in MVECs30 and that this can be attenuated by TSP-1.16,31 To show that the effects of TSP-1 on FGF-2–mediated intracellular signaling can be blocked by LPA, we assessed VEGFR-2 and Akt phosphorylation by Western blot. Both LPA-treated and untreated cells showed increased phosphorylation of VEGFR-2 (Figure 5A) and Akt Ser473 (Figure 5B) in response to FGF-2. Treatment of the cells with TSP-1 suppressed FGF-2–induced phosphorylation of both VEGFR-2 and Akt in control cells; however, no suppression was seen in cells preexposed to LPA. Furthermore, when cells were pretreated with a selective PKD inhibitor or LPA1,3 receptor antagonist before 24 hours of LPA exposure, TSP-1–mediated inhibition of FGF-2–induced Akt phosphorylation was maintained (Figure 5C). These results indicate that CD36 signaling regulates crosstalk between FGF-2 and VEGF and that LPA interrupts this regulation via PKD-1 and LPA G protein–coupled receptor signaling.
LPA abrogates TSP-1–mediated inhibition of angiogenic signaling via LPA1,3 and PKD-1. MVECs were exposed to LPA (5μM) for 24 hours and then were treated with FGF-2 (50 ng/mL) with or without TSP-1 (2nM) for 30 minutes. Cell lysates were subjected to Western blot using antibodies to phospho-VEGFR-2Tyr1175 (P-VEGFR; A) or phospho-Akt (P-Akt; B). (C) Cells were pretreated with CID 755673 (25μM) or Ki14625 (2μM) for 30 minutes followed by LPA for 24 hours and then analyzed as in panel B. Blots were stripped and reprobed with antibodies to total VEGFR-2 or Akt as loading controls. Blots are representative of 2 independent experiments.
LPA abrogates TSP-1–mediated inhibition of angiogenic signaling via LPA1,3 and PKD-1. MVECs were exposed to LPA (5μM) for 24 hours and then were treated with FGF-2 (50 ng/mL) with or without TSP-1 (2nM) for 30 minutes. Cell lysates were subjected to Western blot using antibodies to phospho-VEGFR-2Tyr1175 (P-VEGFR; A) or phospho-Akt (P-Akt; B). (C) Cells were pretreated with CID 755673 (25μM) or Ki14625 (2μM) for 30 minutes followed by LPA for 24 hours and then analyzed as in panel B. Blots were stripped and reprobed with antibodies to total VEGFR-2 or Akt as loading controls. Blots are representative of 2 independent experiments.
LPA abrogates TSP-1–mediated antiangiogenic responses in vivo
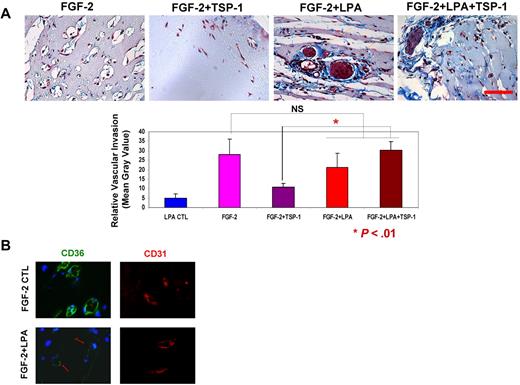
To determine the in vivo relevance of LPA-mediated CD36 down-regulation, we used Matrigel plug angiogenesis assays in which combinations of FGF-2, LPA, and TSP-1 were added to Matrigel solutions implanted subcutaneously into mice. After 10 days, the plugs were removed, embedded, sectioned, and stained to assess the degree of neovascularization and level of CD36 expression. As reported previously by our group and others,3,4 TSP-1 alone had no effect, but it inhibited FGF-2–induced vascular invasion and network formation by ∼ 3-fold (Figure 6A). In the presence of LPA, FGF-2–mediated invasion was not significantly changed, but TSP-1–mediated inhibition was lost. Indirect immunofluorescence microscopy showed that there was abundant expression of CD36 in the neovasculature in the FGF-2–containing Matrigel plugs, whereas CD36 expression was markedly attenuated in the plugs containing both FGF-2 and LPA (Figure 6B). These data suggest that LPA-induced long-lasting down-regulation of CD36 in vivo and effectively “turned off” the TSP-1–mediated antiangiogenic switch.
LPA abrogates TSP-1–mediated antiangiogenesis in vivo. Matrigel solutions containing FGF-2 (100 ng/mL) alone or in combination with TSP-1 (400 ng/mL) and/or LPA (5μM) were implanted subcutaneously into C57/BL6 mice. After 10 days, the plugs were removed, sectioned, and stained with Masson trichrome to measure neovascularization (A) or antibodies to CD36 (green) and CD31 (red) to assess relative expression levels (B). Vascular invasion was quantified from digital images using ImageJ Version 1.40g software. Representative images are shown in panel A top (scale bar = 50 μm). Bar graph shows mean ± SEM (NS, nonsignificant; *P < .01). Arrows in panel B indicate new blood vessels with weak CD36 expression.
LPA abrogates TSP-1–mediated antiangiogenesis in vivo. Matrigel solutions containing FGF-2 (100 ng/mL) alone or in combination with TSP-1 (400 ng/mL) and/or LPA (5μM) were implanted subcutaneously into C57/BL6 mice. After 10 days, the plugs were removed, sectioned, and stained with Masson trichrome to measure neovascularization (A) or antibodies to CD36 (green) and CD31 (red) to assess relative expression levels (B). Vascular invasion was quantified from digital images using ImageJ Version 1.40g software. Representative images are shown in panel A top (scale bar = 50 μm). Bar graph shows mean ± SEM (NS, nonsignificant; *P < .01). Arrows in panel B indicate new blood vessels with weak CD36 expression.
Discussion
CD36 is expressed broadly and constitutively in microvascular beds, but despite its importance in vascular biology, there is surprisingly little known regarding regulation of its expression. We hypothesized the existence of mechanisms to blunt the antiangiogenic activity of TSR-1 proteins at the receptor level via down-regulation of CD36 expression. Others have reported immunohistochemical32 and mRNA expression profile33 data showing that CD36 expression in tumor microvasculature can vary and that expression levels correlate with degree of angiogenesis and prognosis in human cancers, but no mechanisms have been described. To address this issue we used pharmacologic probes to identify potential pathways by which CD36 expression could be down-regulated in early passage cultured human dermal MVECs and found that activation of PKC with the broad-spectrum activator PMA led to substantial loss of surface CD36 protein expression. We then identified LPA as a “physiologic” endothelial cell agonist that could also down-regulate CD36 in both human and murine MVECs. It is interesting that mouse cells were modestly more sensitive to LPA than human cells. Down-regulation was at the transcriptional level, as assessed by nuclear run-on experiments and RT-PCR and led to loss of cell surface protein expression over an 18- to 24-hour period that was long lasting. The potential importance of these observations is highlighted by our studies showing that exposure of MVECs to LPA in vitro or in vivo resulted in loss of antiangiogenic responses to TSP-1; that is, in these settings MVECs maintained their capacity to migrate, invade, undergo tubelike structure formation, and activate VEGFR-2 signals such as VEGFR-2 and Akt phosphorylation in response to FGF-2 even in the presence of TSP-1.
LPA (1-acyl/alkyl-sn-glycerol-3-phosphate) is a bioactive phospholipid derived primarily by the extracellular action of phospholipase D (also known as autotaxin)20 from precursor phospholipids (lysophosphatidylcholine) that are produced in cell membranes34 or lipoproteins by the action of phospholipase A1/2 and/or lecithin-cholesterol acyltransferase.34,35 LPA can also be produced by deacylation of phosphatidic acid by phospholipase A1/2, acylation of glycerol 3-phosphate by glycerophosphate acetyltransferase, or phosphorylation of monoacylglycerol by acylglycerol kinase.34 LPA is found in the plasma in modest concentrations (∼ 100-500nM) but larger amounts (> 2μM) are found in plasma and tissues in the setting of an inflammatory response, thrombosis, atherosclerosis, and in some cancers (eg, ovarian and breast).35-37 Autotaxin released from platelets is probably the primary source of LPA-generating activity in these settings, and autotoxin expression has been associated with increased angiogenesis. Interestingly, From the perspective of CD36 biology, oxidized LDL is a particularly rich source of both LPA precursors and LPA.
LPA signaling has been implicated in a variety of biologic processes including vascular development, neurite remodeling, inflammation, tumor progression, wound healing, and ischemia/reperfusion injury.20 Studies have demonstrated proangiogenic activity of LPA in cancer, atherosclerosis, and chronic inflammation,36-39 and LPA has been reported to directly stimulate proliferation and migration of human umbilical and adult bovine aortic and pulmonary artery endothelial cells in vitro39,40 and up-regulate hypoxia-inducible factor-1α and VEGF expression in tumors and thus indirectly promote tumor angiogenesis41 and to promote vascular maturation in vivo in a chicken chorioallantoic membrane assay.21 In our studies of microvascular endothelial cells we did not observe a significant effect of LPA on migration. However, our data suggest that in addition to promoting angiogenic activity in endothelial and tumor cells, LPA may also promote angiogenesis by down-regulating an important endogenous antiangiogenic pathway.
LPA induces biologic responses via high-affinity interactions with a group of G protein–coupled receptors, originally termed EDG-2, -4, and -7, but now known as LPA1, LPA2, and LPA3.20 Another group of receptors termed LPA4-6 related to the P2Y purinergic family have also been identified as possible LPA receptors.35,42 The precise roles of specific LPA receptor subtypes have not been fully characterized in the vascular system. Here we showed that human MVECs in culture expressed abundant levels of LPA3 with less LPA1, in contrast to melanoma cells, which express mainly LPA1. We found that an LPA3 antagonist, DGPP, inhibited LPA-elicited PKD-1 activation and that Ki14625, an antagonist of both LPA1 and LPA3, not only significantly diminished LPA-elicited PKD-1 phosphorylation but also abrogated LPA-mediated down-regulation of CD36. LPA receptors differ in their affinity for specific molecular species of LPA; for example, unsaturated LPA has specificity toward LPA3.43 Naturally occurring unsaturated LPA has been shown to accumulate in human atherosclerotic plaques.37 It is thus reasonable to speculate that down-regulation of MVEC CD36 by plaque LPA could contribute to neointimal angiogenesis, which is known to promote plaque progression.
Our studies identified the calcium/calmodulin-dependent Ser/Thr kinase PKD-1 as a critical signaling component downstream of LPA receptors in mediating MVEC CD36 transcriptional repression. We showed that PKD pharmacologic inhibition or selective siRNA knockdown of PKD-1 blocked LPA-mediated CD36 down-regulation and restored TSP-1–induced inhibition of angiogenic signals. These studies are consistent with reports that LPA can induce PKD activation in mouse Swiss 3T3, Rat-1, and IEC6 cells. In these cells, PKD activation was shown to be PKC dependent,44 which explains our finding that direct PKC activation by PMA also down-regulated MVEC CD36. Although there are 3 known isoforms of PKD and both PKD-1 and -2 have been implicated in endothelial cell angiogenic responses, our siRNA studies suggest that PKD-1 is the dominant isoform in the LPA/CD36 pathway. Unfortunately, data on genetically engineered mouse models are limited because PKD-1–null mice are embryonic lethal. In a recent study, Cantrell and colleagues reported data from novel models in which catalytically inactive PKD-1 and -2 mutant alleles were knocked-in to the appropriate loci or in which PKD-2 was deleted by a gene trap strategy.45 This work confirmed the role of PKD-1 in embryogenesis and showed that PKD-2 is not necessary for normal development but is the predominant isoform in lymphocytes. No studies of angiogenesis in these mice have been done.
PKD-1 transduces signals to multiple pathways, including vesicular transport, transcriptional activation, and chromatin remodeling, and it has been implicated in several important cardiovascular processes including endothelial cell migration, proliferation, and apoptosis and cardiac and smooth muscle cell hypertrophy.46 That PKD-1 can mediate endothelial cell proangiogenic responses to VEGF47,48 as well as abrogate CD36-mediated antiangiogenic responses demonstrates that targeting PKD-1 could be a powerful way to inhibit angiogenesis.
LPA signaling through Rho GTPases has been implicated in down-regulation of monocyte/macrophage CD36 by LPA.29 Although we found that LPA stimulated RhoA activity in HMVECs (not shown), a cell-permeable inhibitor that selectively inactivates RhoA, RhoB, and RhoC GTPase activity had no effect on LPA-mediated CD36 down-regulation (Figure 3C), suggesting difference in endothelial and macrophages regulatory pathways.
In summary, our studies have defined a novel pathway by which production of LPA in angiogenic microenvironments activates a specific signaling pathway in MVECs, leading to loss of surface expression of the receptor CD36. Such receptor loss leads to loss of responsiveness to endogenous or exogenous TSR-containing antiangiogenic proteins, thereby promoting angiogenesis. Specific targeting of the LPA–PKD-1–CD36 signaling axis could thus have potential value in treating human diseases associated with aberrant angiogenesis or in preventing resistance to TSR-mediated antiangiogenic therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We appreciate the technical help from Drs Angela Ting, Jinbo Liu, and Thomas McIntyre.
This work was supported in part by the National Institutes of Health (grant HL085718 to R.L.S.).
National Institutes of Health
Authorship
Contribution: B.R. designed the studies, performed experiments, wrote the paper, and analyzed data; J.H. and S.S. performed experiments and analyzed data; and R.L.S. designed the studies, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Roy L. Silverstein, MD, Chair, Department of Cell Biology, Lerner Research Institute, NC10, Cleveland Clinic Foundation, 9500 Euclid Ave, Cleveland, OH 44195; e-mail: silverr2@ccf.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal