To the editor:
The placental syncytiotrophoblast layer is a barrier that protects the fetus from infection and cancer metastasis. Even if mother-derived cells enter the fetus, they are usually rejected and cannot be engrafted. Materno-fetal transmission of leukemia/lymphoma is therefore extremely rare.1-4 Recently, Isoda et al demonstrated that maternal leukemic cells were not rejected by an infant when they lost noninherited maternal HLA antigens.5 On the basis of the following study, we propose a novel mechanism accounting for the vertical transmission of cancer.
A male infant born at 30 weeks' gestation weighing 1659 g maintained good health until 7 months of age. At age 8 months, his scrotum appeared enlarged, and he was referred to our hospital. MRI examination revealed a testicular tumor and the infant underwent left orchiectomy (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Immunohistochemical analyses showed that tumor cells expressed LCA, TdT, CD3, and CD56, but did not express CD10, CD20, CD79a, CD34, or ALK (supplemental Figure 2A). Because the tumor cells were positive for Epstein-Barr virus–encoded small RNA (EBER), the diagnosis was EBV-associated NK/T-cell lymphoma, which is a rare histology in children. Multiagent chemotherapy was initiated, then he received cord blood transplantation.
His mother was a 32-year-old Japanese woman whose pregnancy was unremarkable until the 7th month of gestation. Two weeks before delivery, she was transported to our hospital because of thrombocytopenia (68 000/μL) and liver dysfunction (glutamic-oxaloacetic transaminase 513 U/mL, total bilirubin 6.72 mg/d, LDH 5681 U/mL). HELLP syndrome was diagnosed and an emergency cesarean section was performed; however, her condition deteriorated.
A bone marrow smear showed hypoplasia with large granular lymphocytes expressing cytoplasmic CD3 and CD56 (supplemental Figure 2B). Because a high EBV DNA (2.8 × 106 copies/μg DNA of peripheral CD8+ lymphocytes) was detected, she was diagnosed with EBV-related NK/T-cell leukemia, which is prevalent in East Asia and is often fatal.6 Although she was treated with high-dose steroid, etoposide, and cyclosporine, she died of hepatic failure and disseminated intravascular coagulation on postpartum day 25.
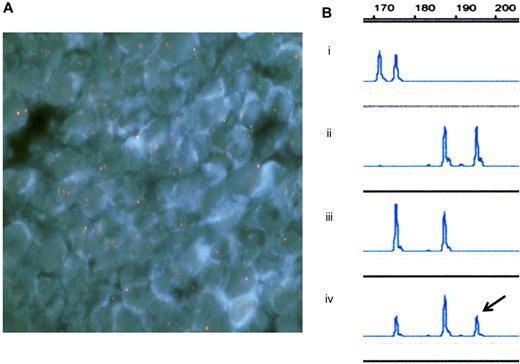
Because of the similarities in disease phenotype between the mother and the infant, we attempted to identify the origin of the infant's lymphoma using 2 independent methods: FISH and microsatellite analysis. Procedures were carried out according to the Declaration of Helsinki and with the informed consent of the family. As a result, the infant's tumor cells were found to be of maternal origin (Figure 1A-B).
Fluorescence in situ hybridization (FISH) analysis of the infant's tumor tissue (a resected sample) and microsatellite analysis. (A) FISH analysis with probes for X (red) and Y (green) chromosomes showed the chimeric pattern; XX in tumor tissue and XY in normal tissue. Image acquisition information: Olympus BX-51 microscope; UPlanApo 100×/1.35 oil objective; DAPI nuclear stain; Nikon DXM1200 camera. (B) Microsatellite analysis on chromosome 3 was carried out using DNA samples extracted from (1) the father's peripheral blood, (2) the mother's bone marrow film, (3) the infant's peripheral blood, and (4) the infant's tumor tissue (a resected sample). The resected tumor tissue had 3 peaks and the right one (arrow) was the noninherited maternal DNA marker. Analysis of the peak height indicated that 55% of the resected tissue was of maternal origin.
Fluorescence in situ hybridization (FISH) analysis of the infant's tumor tissue (a resected sample) and microsatellite analysis. (A) FISH analysis with probes for X (red) and Y (green) chromosomes showed the chimeric pattern; XX in tumor tissue and XY in normal tissue. Image acquisition information: Olympus BX-51 microscope; UPlanApo 100×/1.35 oil objective; DAPI nuclear stain; Nikon DXM1200 camera. (B) Microsatellite analysis on chromosome 3 was carried out using DNA samples extracted from (1) the father's peripheral blood, (2) the mother's bone marrow film, (3) the infant's peripheral blood, and (4) the infant's tumor tissue (a resected sample). The resected tumor tissue had 3 peaks and the right one (arrow) was the noninherited maternal DNA marker. Analysis of the peak height indicated that 55% of the resected tissue was of maternal origin.
Although HLA loss in the tumor tissue is one of the escape mechanisms for evading the immune surveillance system, tumor cells losing HLA-C antigens are recognized and removed by NK cells. Recently, Villalobos et al demonstrated that the uniparental disomy of the HLA-C locus as well as the loss of mismatched HLA-A and -B alleles were associated with leukemic relapse after HLA-haploidentical transplantation.7 We could not precisely determine whether the tumor tissue lost noninherited maternal HLA alleles in the present study (supplemental Table 1). However, it was crucial that the maternal leukemic cells transmigrated into the paratesticular area, a sanctuary from the immuno-surveillance system, where they were engrafted and proliferated.
Authorship
Contribution: H.Y. designed the research and wrote the paper; H.O. and M.I. performed the research; and S.K., M.K., H.S., M.C., and H.M. analyzed the data and supervised the research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Hiroshi Yagasaki, Nihon University, Oyaguchi-kamicho 30-1, Itabashi-ku, Tokyo, Japan 173-8610; e-mail: yagasaki@med.nihon-u.ac.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal