Abstract
Exit from quiescence and reentry into cell cycle is essential for HSC self-renewal and regeneration. Skp2 is the F-box unit of the SCF E3-ligase that targets the CDK inhibitors (CKIs) p21Cip1, p27Kip1, p57Kip2, and p130 for degradation. These CKIs inhibit the G1 to S-phase transition of the cell cycle, and their deletion results in increased cell proliferation and decreased stem cell self-renewal. Skp2 deletion leads to CKIs stabilization inducing cell-cycle delay or arrest, and conversely, increased Skp2 expression is often found in cancers. Here, we show that SKP2 expression is increased in HSC and progenitors in response to hematopoietic stress from myelosuppression or after transplantation. At steady state, SKP2 deletion decreased the mitotic activity of HSC and progenitors resulting in enhanced HSC quiescence, increased HSC pool size, and maintenance. However, the inability to rapidly enter cell cycle greatly impaired the short-term repopulating potential of SKP2 null HSC and their ability to regenerate after myeloablative stress. Mechanistically, deletion of SKP2 in HSC and progenitors stabilized CKIs in vivo, particularly p27Kip1, p57Kip2, and p130. Our results demonstrate a previously unrecognized role for SKP2 in regulating HSC and progenitor expansion and hematopoietic regeneration after stress.
Introduction
The molecular mechanisms regulating adult hematopoietic stem cell fate decisions during the BM response to stress are not yet well defined. Several molecules have been implicated in the regulation of HSC quiescence and cell-cycle entry in particular cell-cycle regulators such as the cyclin CDK inhibitors (CKIs).1 CKIs regulate directly the activity of cyclin/CDK complexes, specifically the CIP/KIP family members (p21Cip1, p27Kip1, p57Kip2), which preferentially inhibit CDK2 activity at the G1-S-phase transition.2,3 Overexpression of each of these CKIs has been shown to induce growth arrest, whereas diminished expression is linked to increased proliferation in progenitors or loss of self-renewal in HSC.4-6 Their regulation occurs predominantly at the posttranslational level and involves proteasome-dependent degradation mediated by SCF complexes.3,7
The authors of recent reports have indicated that the regulation of proteasomal degradation and protein stability by SCF complexes may contribute to the regulation of HSC self-renewal and differentiation.8,9 The Skp1-Cullin-F-box (SCF) complex is a multi-unit E3 ubiquitin ligase in which the F-box family of proteins confers substrate specificity.10 In the SCFSkp2 complex, the F-box SKP2 protein is necessary for ubiquitination and degradation of p27Kip,11 p21Cip1,4 and p57Kip212 and Rb family member p130.13
SKP2 overexpression causes quiescent cells to enter the cell cycle14 and is frequently associated with human cancers.15,16 Conversely, its down-regulation is critical for cell-cycle arrest,17 and its deletion restricts oncogenicity and induces senescence.18 SCFSKP2 is undoubtedly the major ubiquitin ligase regulating the abundance of cell-cycle regulatory proteins at the G1-S transition. The fact that SKP2 controls the destruction of several CKIs counteracting S-phase entry underscores its central role as a cell-cycle regulator and the importance of its control.
Transcriptional regulation appears to be the primary mechanism of Skp2 induction in response to extracellular signals.19,20 Skp2 transcripts are reduced by treatment with antimitogenic signals,20,21 resulting in p27Kip1 accumulation and G1 cell-cycle arrest. Conversely, growth factor signaling mediated by the PI3 kinase/Akt21 and the Ras/MAP kinase20 pathways correlates with increased levels of SKP2, resulting in p27Kip1 degradation and cell-cycle progression. We have previously found that the Notch pathway, which is involved in stem cell regulation, induces the transcriptional activation of Skp2, promoting down-regulation of p21Cip1 and p27Kip1 and inducing accelerated cell-cycle progression in hematopoietic cells.22 Furthermore, TGF-β, a critical mediator of HSC quiescence,23 has been shown to promote SKP2 degradation and stabilization of p27Kip1.24 Although SKP2 has been extensively studied in several cellular systems, its role in hematopoiesis, and in particular in HSC and progenitors, has not yet been investigated.
Here, we show that SKP2 inactivation delays entry of HSC and progenitors into the cell cycle, resulting in enhanced HSC quiescence, increased pool size, and increased HSC maintenance. However, the inability to rapidly and efficiently enter the cell cycle greatly impairs the ability of HSC and progenitors to regenerate hematopoiesis after myeloablative stress and to sustain short-term engraftment after transplantation.
Methods
Mice
Mixed-background Skp2−/− (C57BL/6J-129)25 or Skp2−/− mice backcrossed with C57BL/6J (The Jackson Laboratory) for 6 generations, were used. B6.SJL-PtrcaPep3b/BoyJ mice (BoyJ; CD45.1; In Vivo Therapeutics Core, Wells Center, Indiana University) were used for competitive repopulation assay (CRA). All animal studies were reviewed and approved by the Indiana University Laboratory Animal Resource Center Committee on Animal Research.
Cell cultures and proliferation assay
BM cells from Skp2+/+ and Skp2−/− mice were harvested from femurs as described.26 The lineage cell depletion kit (Miltenyi Biotec) was used, and Lin− cells were seeded at 5 × 105 cells/mL in IMDM10 media (Gibco, Invitrogen) supplemented with SCF (50 ng/mL) and IL-3 (50 ng/mL; Miltenyi Biotec) in 96-well plates and cultured at 37°C, 5% CO2.
Transplantation, homing assays, and 5-fluoracil treatment
Because of their mixed genetic background, BM transplantations in C57BL/6J-129 Skp2−/− mice were performed with the male-female model. A total of 2 × 106 BM donor cells from males littermates (8-10 weeks of age; Skp2−/−; Skp2+/+) were injected into lethally irradiated (11 Gy) syngeneic Skp2+/+ females. In secondary transplants, 2 × 106 BM cells from the primary recipients of Skp2−/− or Skp2+/+ cells were harvested at week 12 after transplantation and injected in secondary irradiated Skp2+/+ female mice. Engraftment was evaluated at 4-week intervals by collecting the peripheral blood (PB) for analysis of white blood cells (WBCs), lineage markers, and Y chromosome content. After backcrossing with C57BL/6J-CD45.2 mice, competitive repopulation assay (CRA) was performed. Lin− cells were purified (Miltenyi Biotec) from Skp2+/+ or Skp2−/− CD45.2 mice; 7.5 × 104, 5 × 104, or 2.5 × 104 cells were admixed with 105 competitive BM cells from BoyJ;CD45.1 and injected into lethally irradiated BoyJ;CD45.1. Engraftment was evaluated at 4-week intervals by collecting PB for analysis of chimerism (CD45.2/CD45.1) and lineage markers. In secondary transplantations, primary recipients were killed at week 12 after undergoing transplantation. Sorted CD45.2 Lin− donor cells (7.5 × 104) were admixed with 105 of competitive cells and injected into lethally irradiated BoyJ;CD45.1.
For homing and early phase engraftment analysis, 1 × 106 Lin− cells from Skp2+/+ or Skp2−/− CD45.2 animals were injected into lethally irradiated BoyJ;CD45.1. BM cells were harvested from cohorts of animals killed at 48, 72, and 96 hours after injection, and CD45.2 cells werer sorted for analysis. 5-Fluorouracil (5-FU; Abraxis BioScience; Celgene) was administered intraperitoneally at 150 mg/kg.
Flow cytometric analysis and cell sorting
Antibody staining, FACS analysis, and sorting were performed as previously described.26,27 BM lineage antibody cocktail and antibodies were used as described in Rodriguez et al.26 All antibodies were purchased from BD Biosciences or eBioscience. Intracytoplasmic staining was performed on fixed samples previously labeled with surface markers to identify specific subsets. After fixation and permeabilization (BD Biosciences kit), samples were incubated with antibodies (Santa Cruz Biotechnology) directed to p27 (C-19), p130 (C-20), p57 (H-91), CyclinE (M-20), or c-Myc (N-262) and followed by secondary antibody (rabbit anti–mouse). Cells were sorted or collected by the use of LSRII(407), FACSCalibur, FACSVantage, or FACSAria (BD Biosciences). Data were analyzed with FlowJo software. For analysis of rare populations, 1 × 106 to 5 × 106 events were collected.
Cell-cycle analysis and apoptosis
Mice were injected with BrdU (1.5 mg/20 g intraperitoneally) 24 hours before sacrifice. BM cells were stained to identify the indicated subsets. Unfractionated, labeled BM cells, or sorted populations were fixed and stained with an anti-BrdU antibody and 7-aminoactinomycin D (7-AAD) following the manufacturer's instructions (BrdU-APC flow kit; BD Biosciences). Apoptosis was measured by the use of annexin V and propidium iodide (PI) staining (BD Biosciences).
Immunoblot analysis
Western blots were performed as described.28 The following antibodies were used: p21 (F-5), p27 (C-19), p130 (C-20), p57 (H-91), or CyclinE (M-20) (Santa Cruz Biotechnology). β-Actin (Santa Cruz Biotechnology) was used to normalize bands in immunoblots, and ImageJ software was used for quantification.
Real-time quantitative RT-PCR analysis
RNA isolation, cDNA, and quantitative RT-PCR were performed as described.26 For each gene analyzed, a calibration curve was performed, and all the oligonucleotides (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) were tested to ensure specificity and sensitivity. In each sample, each transcript was normalized with GAPDH. Reaction efficiency and ratios between target and GAPDH were calculated following the method described previously.29 Relative expression was calculated by arbitrarily choosing a gene (ie, Skp2) and setting its value as 1 and calculating respective fold change of the other genes. Y chromosome detection was performed as previously described.30
Statistical analysis
Statistical significance was assayed by the Student t test; significance is demonstrated by *P < .05 and **P < .005 in the figures.
Results
Dynamic regulation of Skp2 expression during hematopoietic cell differentiation and cell-cycle entry
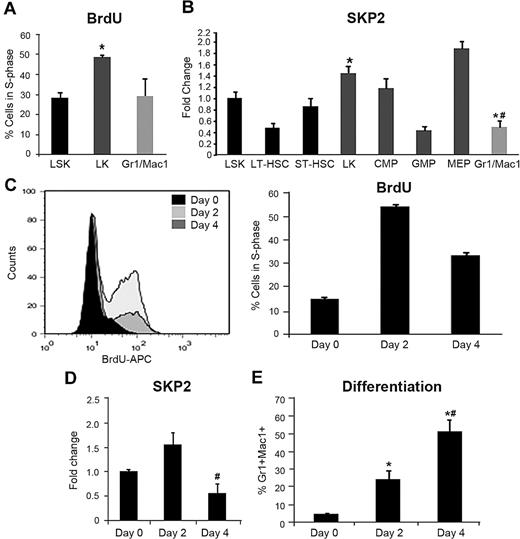
To determine the transcriptional expression of Skp2 during differentiation and cell-cycle entry, we examined mRNA in different hematopoietic compartments. Compared with BM, Skp2 expression was greater in the thymus (an organ characterized by high proliferative activity) but considerably lower in the spleen and liver, organs characterized by low cellular turnover (supplemental Figure 1A). To refine the analysis of cells in the BM, we purified and analyzed distinct hematopoietic subsets, including LSK (negative for lineage markers, Lin− and positive for Sca1 and c-Kit, a subset greatly enriched for HSCs), LK (Lin− c-Kit+ Sca1− that originate directly from the LSK subset and are enriched in highly proliferating erythromyeloid progenitors), and Gr1+Mac1+ (myeloid cells generated by the LK subset that include both immature cells still cycling and terminally differentiated, noncycling cells). The LSK subset was further subdivided in long-term HSCs (LT-HSCs) and short-term HSCs (ST-HSCs); LK cells were further distinguished in common myeloid progenitors (CMP), granulocytic-monocytic progenitors (GMP), and megakaryocitic-erythroid progenitors (MEP). Skp2 expression was greater in the subsets characterized by a greater proliferative rate (LK; MEP and CMP) and lower in the more quiescent LT-HSC and in the differentiating Gr1+Mac1+ fraction (Figure 1A-B). To complement this analysis, we determined the mRNA levels of major SKP2 targets, including p21Cip1, p27Kip1, p57Kip2, p130, and cyclin E. CKI expression was observed in all subsets, with significant levels of p57Kip2 and p130 in LSK cells (supplemental Figure 1B).
Skp2 expression reflects the cell-cycle status of hematopoietic cells during differentiation. (A) BM was harvested from C57BL/6J mice 24 hours after injection with BrdU and labeled in conjunction with the surface markers necessary to identify the indicated cell subsets. BrdU incorporation and DNA content (7-AAD) were measured in gated LSK, LK and Gr1+Mac1+ populations. Bars represent average of cells positive for BrdU. n = 4-6 of 3 independent experiments. *P < .05 vs LSK. (B) Fold change in Skp2 mRNA expression by quantitative RT-PCR in sorted LSK, LT-HSC, ST-HSC, LK, CMP, GMP, MEP, and Gr1+Mac1+ populations. n = 2-4 samples (each sample a pool of 5-6 mice) in 2 independent experiments. *P < .05 vs LSK #P < .05 vs LK. (C) Purified Lin− cells were cultured in the presence of SCF and IL-3 for 0, 2, or 4 days and analyzed for S-phase by BrdU incorporation (3-hour pulse) and DNA content (7-AAD); values in the bar graph represent average of percentage of cells in S-phase in 2 experiments. (D) Relative expression of Skp2 by q-RT-PCR in Lin− cells harvested at day 0, 2, and 4 of culture. (E) Kinetics of myeloid differentiation measured as expression of Gr1 and Mac1 by immunophenotypic analysis. Values are average of cells expressing Gr1+Mac1+ in the total population. In panels D and E, n = 4-6 samples in 3 independent experiments. Data are expressed as mean ± SEM *P < .05 vs day 0. #P < .05 vs day 2.
Skp2 expression reflects the cell-cycle status of hematopoietic cells during differentiation. (A) BM was harvested from C57BL/6J mice 24 hours after injection with BrdU and labeled in conjunction with the surface markers necessary to identify the indicated cell subsets. BrdU incorporation and DNA content (7-AAD) were measured in gated LSK, LK and Gr1+Mac1+ populations. Bars represent average of cells positive for BrdU. n = 4-6 of 3 independent experiments. *P < .05 vs LSK. (B) Fold change in Skp2 mRNA expression by quantitative RT-PCR in sorted LSK, LT-HSC, ST-HSC, LK, CMP, GMP, MEP, and Gr1+Mac1+ populations. n = 2-4 samples (each sample a pool of 5-6 mice) in 2 independent experiments. *P < .05 vs LSK #P < .05 vs LK. (C) Purified Lin− cells were cultured in the presence of SCF and IL-3 for 0, 2, or 4 days and analyzed for S-phase by BrdU incorporation (3-hour pulse) and DNA content (7-AAD); values in the bar graph represent average of percentage of cells in S-phase in 2 experiments. (D) Relative expression of Skp2 by q-RT-PCR in Lin− cells harvested at day 0, 2, and 4 of culture. (E) Kinetics of myeloid differentiation measured as expression of Gr1 and Mac1 by immunophenotypic analysis. Values are average of cells expressing Gr1+Mac1+ in the total population. In panels D and E, n = 4-6 samples in 3 independent experiments. Data are expressed as mean ± SEM *P < .05 vs day 0. #P < .05 vs day 2.
To determine the relationship between Skp2 expression and cell-cycle entry and differentiation, we purified Lin− cells and cultured them for 4 days (Figure 1C-E). After cytokine stimulation, the number of cells in active S-phase increased from 14% at day 0 to 54% at day 2. Skp2 expression peaked on day 2 after cytokine stimulation, correlating with entry of these cells into S-phase, and decreased on day 4, correlating with overall decline in cell cycle (Figure 1C-D) and increased terminal differentiation (Figure 1E). Taken together, these results show that Skp2 expression is up-regulated as primitive cells enter cell cycle and is down-regulated during terminal differentiation, establishing a strong correlation between Skp2 expression and the proliferative characteristics of various hematopoietic subsets.
Loss of SKP2 results in increased HSC pool size and perturbs cell-cycle entry in different hematopoietic subsets
To determine the requirement for SKP2 in hematopoiesis, we analyzed the BM compartment of mice in which SKP2 was deleted by gene targeting.25 Skp2-null mice are viable, smaller than control littermates, but have a normal life span and no obvious defects in hematopoiesis.25 In each experiment, we confirmed the deletion of Skp2 by PCR (supplemental Figure 2A). Analysis of the PB showed no alteration in hematocrit or in WBC counts (supplemental Figure 2B). BM cellularity was decreased in Skp2−/− mice compared with Skp2+/+ (∼ 30% lower; supplemental Figure 2C). Immunophenotypic analysis of mature subsets in the BM did not show significant differences in erythroid progenitors, T cells, B cells, and neutrophils (supplemental Figure 3). However, an examination of the BM stem/progenitor cell compartment revealed significant differences between Skp2−/− and Skp2+/+ mice.
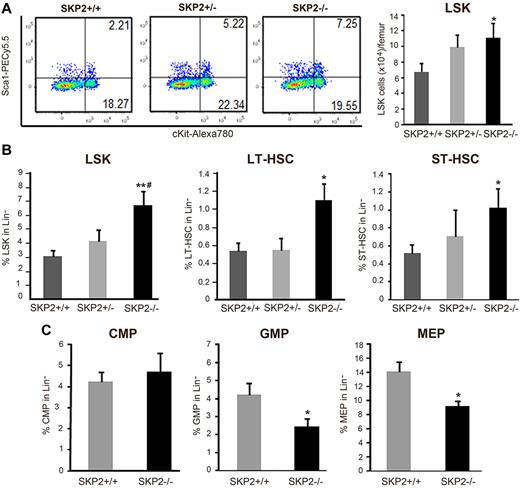
A detailed analysis demonstrated that the frequency and absolute number of LSK cells was significantly increased in Skp2−/− mice compared with their Skp2+/+ littermates (2- to 3-fold; P = .0007 and P = .04; Figure 2A-B). Further distinction of the LSK subset in IL-7R−CD34−FLT3− (LT-HSC) and LSK IL7R−CD34+FLT3+ (ST-HSC) indicated that both LT-HSCs and ST-HSCs were greatly augmented in Skp2−/− BM (Figure 2B; supplemental Figure 2D). Similar results were found when the signaling lymphocytic activation molecule (SLAM) code was used to identify LT-HSCs31 (data not shown). Skp2+/− heterozygous mice showed values intermediate between control and null animals as well as values close to the Skp2+/+ (Figure 2A-B; supplemental Figure 2D). Further analysis of distinct Lin− subsets as described26,27 demonstrated that Skp2-null mice have equivalent percentages of CMPs (Lin−IL7R−Sca−Kit+CD34+FcγRII/IIIlow) but a significant reduction in the frequency and in the absolute numbers of GMPs (Lin−IL7R−Sca−Kit+CD34+FcγRII/IIIhi) and MEPs (Lin−IL7R−Sca−Kit+CD34−FcγRII/III−), subsets constituting the majority of actively proliferating LK fraction (Figure 2C; supplement Figure 2E).
Loss of SKP2 increases the size of the LSK pool and decreases the number of hematopoietic progenitors. (A) Dot blots indicate the LSK population by showing Sca1 and c-Kit expression on Lin− cells in a representative experiment. Numbers indicate the percentage of cells in each quadrant. Bar graph on the right summarizes 8 independent experiments (n = 15-18). Values are average of absolute number of LSK cells per femur. (B) Bar graphs represent average percentage of each population in the gated Lin− population; n = 15-18. LSK cells are shown on the left and were further subdivided into LT-HSC (LSK IL7R−CD34−Flt3−; middle) and ST-HSC (LSK IL7R−CD34+Flt3+; right); n = 6-10. (C) LK cells (Lin− Sca1− c-Kit+ IL7R−) were analyzed for CD34 and FcγRII/III expression to determine CMP (CD34+ FcγRII/IIIlo), GMP (CD34+ FcγRII/IIIhi), and MEP (CD34− FcγRII/IIIlo) progenitors. Values indicate average percentages in the Lin− population; n = 11-15 mice. Data expressed as mean ± SEM *P < .05 vs Skp2+/+. **P < .005 vs Skp2+/+. #P < .05 vs Skp2+/−.
Loss of SKP2 increases the size of the LSK pool and decreases the number of hematopoietic progenitors. (A) Dot blots indicate the LSK population by showing Sca1 and c-Kit expression on Lin− cells in a representative experiment. Numbers indicate the percentage of cells in each quadrant. Bar graph on the right summarizes 8 independent experiments (n = 15-18). Values are average of absolute number of LSK cells per femur. (B) Bar graphs represent average percentage of each population in the gated Lin− population; n = 15-18. LSK cells are shown on the left and were further subdivided into LT-HSC (LSK IL7R−CD34−Flt3−; middle) and ST-HSC (LSK IL7R−CD34+Flt3+; right); n = 6-10. (C) LK cells (Lin− Sca1− c-Kit+ IL7R−) were analyzed for CD34 and FcγRII/III expression to determine CMP (CD34+ FcγRII/IIIlo), GMP (CD34+ FcγRII/IIIhi), and MEP (CD34− FcγRII/IIIlo) progenitors. Values indicate average percentages in the Lin− population; n = 11-15 mice. Data expressed as mean ± SEM *P < .05 vs Skp2+/+. **P < .005 vs Skp2+/+. #P < .05 vs Skp2+/−.
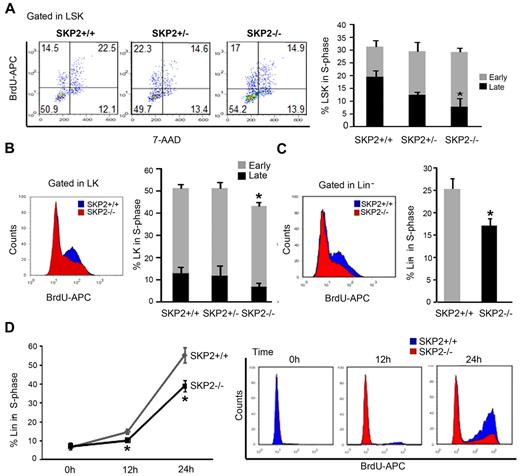
To determine the impact of SKP2 loss on the cell cycle, we performed in vivo BrdU incorporation. The loss of SKP2 did not significantly impact proliferation of whole BM. However, analysis of stem and progenitor cells revealed that SKP2 deficiency differentially affected specific subsets (Figure 3A-C). At steady state, the overall percentage of cells in S-phase was not significantly altered in the Skp2−/− LSK subset (Skp2−/−, 28% vs Skp2+/+, 31%); yet, the percentage of cells in late S-phase of the cell cycle was significantly decreased in Skp2−/− LSK cells (7.9% vs 19.6%; P = .02), indicating diminished cell-cycle entry (Figure 3A). Indeed, overall cell cycle was significantly decreased in LSK Skp2−/− cells in nonhomeostatic conditions (Figure 5C). Actively proliferating Skp2−/− LK cells demonstrated a significant decrease in the percentage of S-phase cells (P = .04), particularly in late S-phase (Figure 3B). In the Lin− cell fraction, a significantly lower percentage of cells in the late S-phase of cell cycle was observed in the Skp2−/− Lin− cells (17% vs 25%; Figure 3C; P = .03).
Loss of SKP2 differentially affects cell-cycle kinetics in distinct hematopoietic subsets. In vivo BrdU incorporation and DNA content were analyzed in immunophenotypically defined hematopoietic subsets from Skp2+/+, Skp2+/−, and Skp2−/− mice: (A) LSK. Left, representative dot blot of BrdU analysis gated on LSK cells. Numbers indicate the percentage of LSK cells in each quadrant; left top quadrant indicates early S-phase; right top quadrant indicates late S-phase. Bar graph on the right summarizes average percentage of LSK cells positive for BrdU and shows distribution between early S-phase (top light columns in the bar) and late S-phase (bottom dark columns in the bar); (B) LK. On the left, histogram shows BrdU incorporation in LK cells in a representative experiment; overlaid are intensity of BrdU fluorescence in LK Skp2−/− cells (red) and of BrdU fluorescence LK Skp2+/+ cells (blue). Bar graph on the right summarizes the average percentage of LK cells positive for BrdU as in panel A. (C) Lin− cells. On the left, histogram shows BrdU incorporation in Lin− cells in a representative experiment. Lin− cells were purified and subjected to a BrdU pulse for 3 hours. Bar graph on the right summarizes average percentage of Lin− cells positive for BrdU. (A-C) n = 6 in 4 independent experiments. (D) Lin− purified cells were starved overnight with 2% FBS media supplemented with SCF and then stimulated with SCF and IL-3 for 0, 12, and 24 hours. Line graph summarizes average percentage of cells positive for BrdU. On the right, dot blot shows BrdU incorporation in a representative experiment. n = 5 in 2 independent experiments. All values are expressed as mean ± SEM *P < .05 vs Skp2+/+.
Loss of SKP2 differentially affects cell-cycle kinetics in distinct hematopoietic subsets. In vivo BrdU incorporation and DNA content were analyzed in immunophenotypically defined hematopoietic subsets from Skp2+/+, Skp2+/−, and Skp2−/− mice: (A) LSK. Left, representative dot blot of BrdU analysis gated on LSK cells. Numbers indicate the percentage of LSK cells in each quadrant; left top quadrant indicates early S-phase; right top quadrant indicates late S-phase. Bar graph on the right summarizes average percentage of LSK cells positive for BrdU and shows distribution between early S-phase (top light columns in the bar) and late S-phase (bottom dark columns in the bar); (B) LK. On the left, histogram shows BrdU incorporation in LK cells in a representative experiment; overlaid are intensity of BrdU fluorescence in LK Skp2−/− cells (red) and of BrdU fluorescence LK Skp2+/+ cells (blue). Bar graph on the right summarizes the average percentage of LK cells positive for BrdU as in panel A. (C) Lin− cells. On the left, histogram shows BrdU incorporation in Lin− cells in a representative experiment. Lin− cells were purified and subjected to a BrdU pulse for 3 hours. Bar graph on the right summarizes average percentage of Lin− cells positive for BrdU. (A-C) n = 6 in 4 independent experiments. (D) Lin− purified cells were starved overnight with 2% FBS media supplemented with SCF and then stimulated with SCF and IL-3 for 0, 12, and 24 hours. Line graph summarizes average percentage of cells positive for BrdU. On the right, dot blot shows BrdU incorporation in a representative experiment. n = 5 in 2 independent experiments. All values are expressed as mean ± SEM *P < .05 vs Skp2+/+.
To further validate the impact of SKP2 on cell-cycle entry, we synchronized Skp2−/− and Skp2+/+ Lin− cells in G0/G1 and stimulated them to proliferate for short-term in vitro. Skp2−/− Lin− cells showed a significant delay in entering the cell cycle, as shown by the lower number of cells incorporating BrdU at 24 hours (39% vs 55%; Figure 3D; P = .01). Evaluation by annexin V and PI in vivo revealed negligible differences between Skp2−/− and Skp2+/+ BM cells (supplemental Figure 1C).
Taken together, these data demonstrate that, in homeostatic conditions, the loss of SKP2 results in altered cell cycle in specific subsets. Specifically, SKP2 deficiency resulted in decreased numbers of highly cycling erythromyeloid progenitors and in a significant increase in the pool size of the more quiescent LT-HSC and ST-HSC subsets.
SKP2 expression is critical for efficient regeneration of the hematopoietic compartment after 5-FU and BM transplantation stress
Our results indicate that SKP2 is a positive regulator of cell-cycle progression among HSC and progenitor cells and that its deletion imparts a “brake,” enhancing HSC quiescence and restricting progenitor expansion. Thus, we hypothesized that SKP2 may play a critical role during hematopoietic stress. To gain further insight into the role of SKP2 in stress hematopoiesis, we evaluated the expression profile of SKP2 after 5-FU treatment. The chemotherapeutic agent 5-FU is cytotoxic to proliferating cells and stimulates surviving stem cells out of quiescence into cell cycle. As SKP2 promotes cell-cycle entry and its availability is largely regulated at the transcriptional level, we predicted that its levels may increase in response to myeloablation.
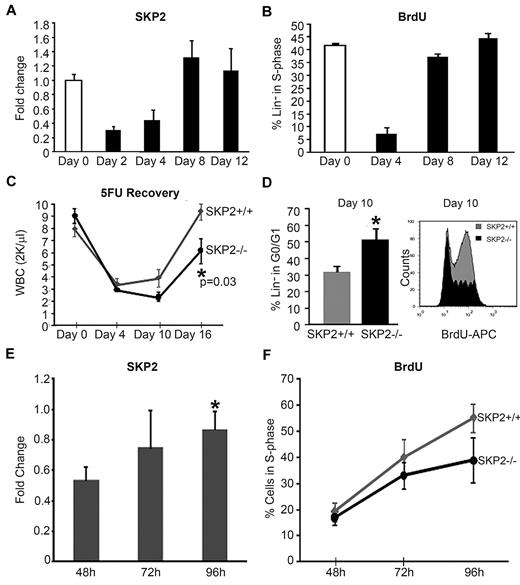
Indeed, we found that Skp2 expression in BM Lin− cells increased progressively after 5-FU treatment, with the greatest levels observed on days 8 and 12, correlating with the number of cells in S-phase and preceding the visible recovery of WBCs in the PB (Figure 4A-C). Next, we determined the requirement for SKP2 in the regeneration of the hematopoietic compartment after 5-FU treatment. Cohorts of Skp2−/−, Skp2+/−, and Skp2+/+ littermates were treated with 5-FU, and the kinetics of hematopoietic recovery were determined. As shown in Figure 4C, at day 4 after treatment, the decrease in WBCs was similar in both Skp2−/− and Skp2+/+ mice, but Skp2−/− mice showed a significant delay in recovery, as indicated by the lower WBC counts on day 10 and 16 after treatment (∼ 50% lower; P = .03). Interestingly, Skp2+/− heterozygous mice showed variable response to 5-FU treatment: 65% of treated mice showed a delayed recovery similar to Skp2−/− mice, whereas 35% behaved like Skp2+/+ littermates and showed full recovery (supplemental Figure 4A). Cell-cycle analysis of BM Lin− cells showed that at day 4 after 5-FU treatment cells from Skp2−/− and Skp2+/+ were equally synchronized in G0/G1, whereas at day 10 a greater percentage of Skp2−/− Lin− cells were still in G0/G1 phase (51% vs 31%), demonstrating a significant delay in entering the cell-cycle S-phase (Figure 4D), which correlated with the delay in WBC recovery at day 10 and 16.
Skp2 expression is critical for regeneration after stress. (A) Analysis of Skp2 expression after 5-FU treatment in Skp2+/+ mice. Q-RT-PCR for Skp2 was performed in Lin−-sorted cells at days 0 (white bar), 2, 4, 8, and 12 (black bars) after 5-FU injections. n = 6-10 in 4 independent experiments. (B) In vivo BrdU incorporation and DNA content were analyzed in Skp2+/+ Lin−-sorted cells at the indicated time points after 5-FU injections. Bar graph shows the percentage of cells positive for BrdU. n = 3 in 2 independent experiments. (C) WBC counts in Skp2+/+ and Skp2−/− mice after 5-FU treatment at the indicated time points. n = 6 in 2 independent experiments. (D) Left, bar graph values indicated the percentage of Lin− cells in G0/G1 phase at day 10 after 5-FU injection (n = 3 in a representative experiment). Right, histogram shows BrdU incorporation; overlaid are intensity of BrdU fluorescence in Lin−Skp2−/− cells (light gray) and of BrdU fluorescence Lin−Skp2+/+ cells (dark gray). *P < .05 vs Skp2+/+. (E-F) CD45.2 Lin−-purified donor cells (1 × 106) were transplanted into lethally irradiated CD45.1 mice. CD45.2+ cells were sorted and analyzed at 48, 72, and 96 hours after transplantation. (E) Fold change in Skp2 expression by quantitative RT-PCR in CD45.2 cells sorted at the indicate time points. n = 4 in 2 independent experiments. *P < .05 vs 48 hours. (F) Kinetics of BrdU incorporation in Skp2+/+ (gray line) and Skp2−/− (black line) mice; values in the line graph represent average percentage of cells positive for BrdU. n = 2-4. Panels A-C expressed as mean ± SEM; D-F expressed as mean ± SD.
Skp2 expression is critical for regeneration after stress. (A) Analysis of Skp2 expression after 5-FU treatment in Skp2+/+ mice. Q-RT-PCR for Skp2 was performed in Lin−-sorted cells at days 0 (white bar), 2, 4, 8, and 12 (black bars) after 5-FU injections. n = 6-10 in 4 independent experiments. (B) In vivo BrdU incorporation and DNA content were analyzed in Skp2+/+ Lin−-sorted cells at the indicated time points after 5-FU injections. Bar graph shows the percentage of cells positive for BrdU. n = 3 in 2 independent experiments. (C) WBC counts in Skp2+/+ and Skp2−/− mice after 5-FU treatment at the indicated time points. n = 6 in 2 independent experiments. (D) Left, bar graph values indicated the percentage of Lin− cells in G0/G1 phase at day 10 after 5-FU injection (n = 3 in a representative experiment). Right, histogram shows BrdU incorporation; overlaid are intensity of BrdU fluorescence in Lin−Skp2−/− cells (light gray) and of BrdU fluorescence Lin−Skp2+/+ cells (dark gray). *P < .05 vs Skp2+/+. (E-F) CD45.2 Lin−-purified donor cells (1 × 106) were transplanted into lethally irradiated CD45.1 mice. CD45.2+ cells were sorted and analyzed at 48, 72, and 96 hours after transplantation. (E) Fold change in Skp2 expression by quantitative RT-PCR in CD45.2 cells sorted at the indicate time points. n = 4 in 2 independent experiments. *P < .05 vs 48 hours. (F) Kinetics of BrdU incorporation in Skp2+/+ (gray line) and Skp2−/− (black line) mice; values in the line graph represent average percentage of cells positive for BrdU. n = 2-4. Panels A-C expressed as mean ± SEM; D-F expressed as mean ± SD.
BM transplantation is another condition in which donor stem/progenitor cells are synchronously induced to enter the cell cycle to rapidly reconstitute the host hematopoietic system. Thus, we predicted that Skp2 levels may increase in hematopoietic stem/progenitors after transplantation. We determined the dynamics of Skp2 expression during the early phase of engraftment. Skp2 levels, which were at limit of detection at 24 hours (data not shown), increased progressively at 72 and 96 hours after transplantation (Figure 4E), correlating with increased cell cycle. Donor cells showed low BrdU incorporation at 48 hours (16%) and active cell proliferation at 72 and 96 hours after transplantation, with a large fraction of cells in the S-phase of the cell cycle (50%-60%; Figure 4F). Thus, after homing to the BM, donor stem/progenitor cells up-regulate Skp2 expression and enter into cell cycle by day 3 after transplant. Next, we determined whether SKP2 is critical for this process. Lin− cells from C57BL/6 CD45.2 Skp2−/− or Skp2+/+ mice were transplanted into lethally irradiated CD45.1 mice and were recovered at sequential time points. In contrast to Skp2+/+, Skp2−/− CD45.2 cells showed a distinct lower percentage of BrdU incorporation at 96 hours (38% vs 55%; Figure 4F). In agreement with the reduced cell cycle, mice transplanted with CD45.2 Skp2−/− cells showed reduced total BM donor cell number 4 weeks after transplantation, accounting for 60% growth relative to Skp2+/+ donor cells (data not shown). Taken together, these findings indicate that SKP2 is a critical mediator of rapid cell-cycle entry in stem/progenitor cells and is required for efficient hematopoietic regeneration in response to myeloablation and after transplantation.
Loss of SKP2 results in defective short-term engraftment and enhances HSC quiescence.
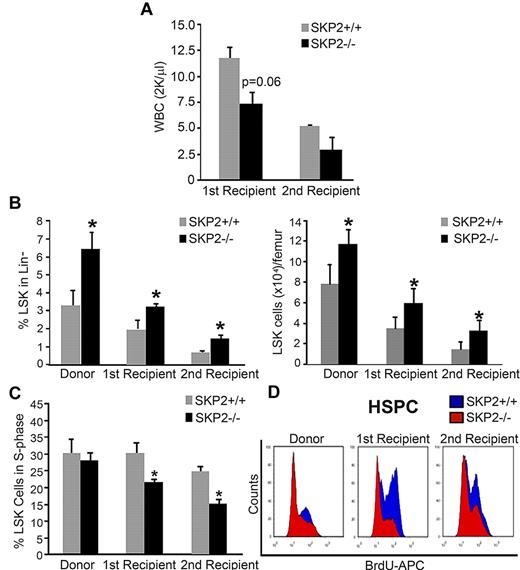
Next, we determined the ability of Skp2−/− cells to support short- and long-term engraftment in sequential BM transplants and CRAs. Total BM cells (2 × 106) from male Skp2−/− or Skp2+/+ littermates were transplanted into lethally irradiated syngeneic Skp2+/+ female mice. PCR for Y chromosome detection was used at the time of assay to monitor chimerism, which was > 95% (supplement Figure 4B). At week 10 after transplantation, recipients of Skp2+/+ donor cells were fully reconstituted with 100% recovery in WBC counts. In contrast, recipients of Skp2−/− donor cells showed an average WBC recovery of 61%. At 12 weeks after transplantation, primary recipients were killed, and secondary transplantations were performed. WBC evaluation in secondary recipients showed a slower recovery in mice receiving Skp2−/− cells in comparison with mice receiving Skp2+/+ cells (Figure 5A). Analysis of the BM of both primary and secondary recipients at week 12, indicated that mice undergoing transplantation with Skp2−/− donor cells recapitulated the HSC phenotype of the original donor, maintaining greater frequency and increased absolute number of LSK cells (2-fold increase; Figure 5B). Of importance, the quiescent state of Skp2−/− LSK cells increased significantly during the serial transplantations: the percentage of LSK cells in the S-phase of the cell cycle was significantly decreased in Skp2−/− compared with Skp2+/+ mice (15% vs 25%; P = .02 in secondary recipients; Figure 5C). Similar results were observed in the LK subset. Figure 5D shows in vivo cell-cycle analysis of stem and progenitor cells.
Loss of SKP2 results in defective short-term engraftment after BM transplantation. BM cells (2 × 106) from male Skp2+/+ (gray bars) or Skp2−/− (black bars) mice were transplanted into lethally irradiated syngeneic Skp2+/+ female nice. (A) WBC recovery at week 10 after transplantation in primary recipients and at week 5 after transplantation in secondary recipients. (B) Analysis of the LSK frequencies (left bar graph) and absolute LSK cell numbers (right bar graph) in the BM of Skp2−/− and Skp2+/+ donors and of first and secondary recipients at week 12 after transplantation. (C) Analysis of BrdU incorporation in LSK cells from Skp2−/− and Skp2+/+ donors and from first and secondary recipients at week 12 after transplantation. (D) Histograms show a representative experiment of BrdU incorporation in LSK and LK cells in Skp2−/− and Skp2+/+ donors and in first and secondary recipients at week 12 after transplantation. Overlaid are intensity of BrdU fluorescence in Skp2−/− cells (red) and Skp2+/+ cells (blue). For all graphs n = 4-8 in 2 independent experiments. Data are expressed as mean ± SEM *P < .05 vs Skp2+/+.
Loss of SKP2 results in defective short-term engraftment after BM transplantation. BM cells (2 × 106) from male Skp2+/+ (gray bars) or Skp2−/− (black bars) mice were transplanted into lethally irradiated syngeneic Skp2+/+ female nice. (A) WBC recovery at week 10 after transplantation in primary recipients and at week 5 after transplantation in secondary recipients. (B) Analysis of the LSK frequencies (left bar graph) and absolute LSK cell numbers (right bar graph) in the BM of Skp2−/− and Skp2+/+ donors and of first and secondary recipients at week 12 after transplantation. (C) Analysis of BrdU incorporation in LSK cells from Skp2−/− and Skp2+/+ donors and from first and secondary recipients at week 12 after transplantation. (D) Histograms show a representative experiment of BrdU incorporation in LSK and LK cells in Skp2−/− and Skp2+/+ donors and in first and secondary recipients at week 12 after transplantation. Overlaid are intensity of BrdU fluorescence in Skp2−/− cells (red) and Skp2+/+ cells (blue). For all graphs n = 4-8 in 2 independent experiments. Data are expressed as mean ± SEM *P < .05 vs Skp2+/+.
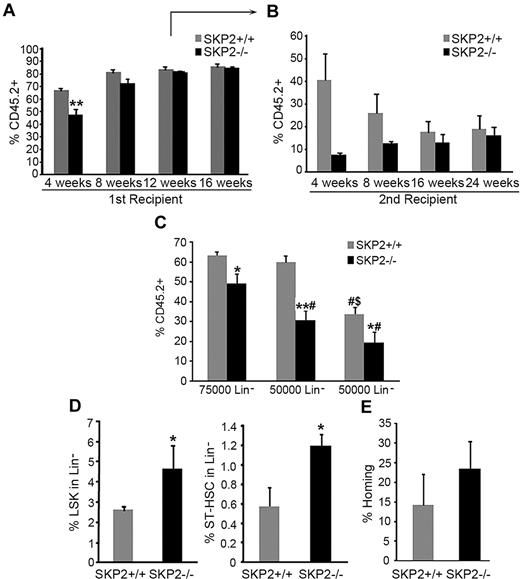
To better follow the kinetics of engraftment of SKP2-deficient cells, we performed CRA. Equivalent numbers of CD45.2 Lin− cells purified from BM of Skp2−/− or Skp2+/+ mice were transplanted into lethally irradiated CD45.1 recipients together with 105 CD45.1 competitor cells. PB chimerism (CD45.2 vs CD45.1) was evaluated at 4-week intervals after transplantation. The transplant of Skp2−/− cells (75 × 104) resulted in significantly lower percent engraftment at 4 weeks (47% vs 69%; P = .004); at week 8, 40% of mice that received Skp2−/− cells showed engraftment levels < 40%, whereas 60% showed similar engraftment as mice receiving Skp2+/+ cells (Figure 6A; figure shows average engraftment of all mice). At weeks 12 and 16, there was no difference between the 2 groups.
Loss of SKP2 results in defective short-term engraftment in competitive repopulation assays. CRA. BM CD45.2 Lin− cells purified from Skp2+/+ (gray bars) or Skp2−/− (black bars) mice were transplanted into lethally irradiated CD45.1 recipients together with 105 competitor cells (CD45.1). Analysis of CD45.2+ cells in PB was performed at 4, 8, 12, 16, and 24 weeks after transplantation. (A) Transplantation was performed with 7.5 × 104 donor cells. Bar graph represents average expression of CD45.2 donor cells in the PB. Each group n = 12-18 mice in 3 independent experiments. (B) Lin−CD45.2+ cells were sorted at week 12 after transplantation from primary recipients and 7.5 × 104 donor cells transplanted in secondary recipients. Bar graph represents average expression of CD45.2 donor cells in the PB. n = 3 from a representative experiment. (C) Lin− CD45.2+ cells from Skp2−/− and Skp2+/+ mice were sorted and transplanted in recipients at decreasing doses: 7.5 × 104, 5 × 104, or 2.5 × 104. Bar graph represents average expression of CD45.2 donor cells in the PB. n = 4-6. #P < .05 vs 7.5 × 104 cells. $P < .05 vs 5 × 104 cells. (D) Analysis of the LSK (left) and ST-HSC (right) content in BM of recipients at week 12 after transplantation. n = 3 from a representative experiment. (E) Homing at 48 hours. Bar graph shows percentage of CD45.2+ donor cells recovered at 48 hours after transplantation of Lin− cells into CD45.1 recipients; n = 4 in 2 independent experiments. Data expressed as mean ± SEM *P < .05 vs Skp2+/+. **P < .005 vs Skp2+/+.
Loss of SKP2 results in defective short-term engraftment in competitive repopulation assays. CRA. BM CD45.2 Lin− cells purified from Skp2+/+ (gray bars) or Skp2−/− (black bars) mice were transplanted into lethally irradiated CD45.1 recipients together with 105 competitor cells (CD45.1). Analysis of CD45.2+ cells in PB was performed at 4, 8, 12, 16, and 24 weeks after transplantation. (A) Transplantation was performed with 7.5 × 104 donor cells. Bar graph represents average expression of CD45.2 donor cells in the PB. Each group n = 12-18 mice in 3 independent experiments. (B) Lin−CD45.2+ cells were sorted at week 12 after transplantation from primary recipients and 7.5 × 104 donor cells transplanted in secondary recipients. Bar graph represents average expression of CD45.2 donor cells in the PB. n = 3 from a representative experiment. (C) Lin− CD45.2+ cells from Skp2−/− and Skp2+/+ mice were sorted and transplanted in recipients at decreasing doses: 7.5 × 104, 5 × 104, or 2.5 × 104. Bar graph represents average expression of CD45.2 donor cells in the PB. n = 4-6. #P < .05 vs 7.5 × 104 cells. $P < .05 vs 5 × 104 cells. (D) Analysis of the LSK (left) and ST-HSC (right) content in BM of recipients at week 12 after transplantation. n = 3 from a representative experiment. (E) Homing at 48 hours. Bar graph shows percentage of CD45.2+ donor cells recovered at 48 hours after transplantation of Lin− cells into CD45.1 recipients; n = 4 in 2 independent experiments. Data expressed as mean ± SEM *P < .05 vs Skp2+/+. **P < .005 vs Skp2+/+.
Multilineage analysis of PB showed similar reconstitution of all lineages at all time points (data not shown). Analysis of BM in a cohort of mice at week 12 demonstrated maintenance of a greater frequency and absolute number of LSK in Skp2−/− BM, both LT-HSCs and ST-HSCs (Figure 6D; supplemental Figure 4C). Despite the greater frequency of ST-HSCs, short-term engraftment of Skp2−/− Lin− donor cells in secondary recipients was significantly lower compared with Skp2+/+ Lin− donor cells (weeks 4 and 8; Figure 6B). However, differences in engraftment between Skp2+/+ and Skp2−/− cells decreased with time as Skp2−/− engraftment levels progressively increased to control levels and in correlation with restoration of homeostasis (Figure 6A-B).
To further assess the impact of SKP2 deletion on short-term engraftment, we performed transplantations with limiting numbers of donor cells. The inefficiency of Skp2−/− cells to sustain short-term engraftment was more apparent when lower cell doses were used for transplantation (Figure 6C). These effects were not due to differences in homing because there was no significant difference in the homing potential of Skp2−/− and Skp2+/+ cells (Figure 6E). Taken together, these results show that by decreasing cell-cycle entry, loss of SKP2 impairs stem/progenitors ability to recover at short-term after acute stress (when rapid cell-cycle entry is required) but may result in a relative advantage to engraft at long term because of the increased quiescence.
Absence of SKP2 stabilizes CKIs levels
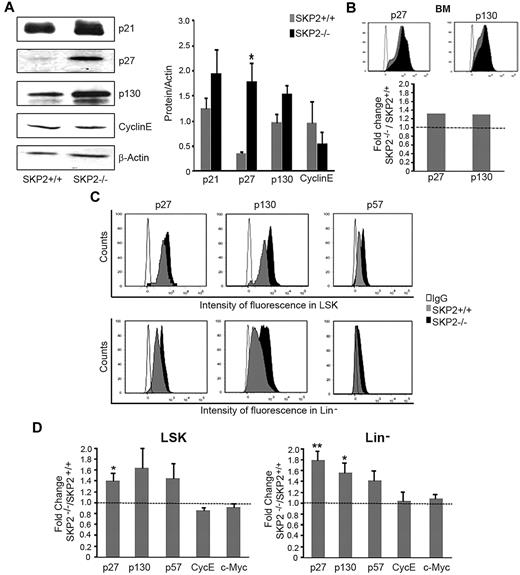
The reduction in the cell cycle observed in stem and progenitor cells in the absence of SKP2 suggested the involvement of cell-cycle inhibitors. SKP2 targets proteasome-mediated degradation of several CKIs.10 Thus, we analyzed the levels of well-characterized SKP2 targets, ie, p21Cip1, p27Kip1, p57Kip2, and p130, which are all CKIs inhibiting the G1-S transition. We also determined the levels of cyclin E and c-Myc, which, conversely, are positive cell-cycle regulators reported to be targets of SKP2.32 We detected a significant increase in the levels of p27Kip1 and substantially greater levels of p21Cip1 and p130 in BM cells by Western blot analysis and by intracytoplasmic staining (Figure 7A-B). We did not find increase in cyclin E (Figure 7A) or differences in overall expression of p57Kip2 and c-Myc in total BM (supplemental Figure 4D and data not shown).
Loss of SKP2 stabilizes target proteins. (A) Detection by Western blot of p21Cip1, p27Kip1, p130, cyclin E, and β-actin protein levels in BM extracts from Skp2−/− and Skp2+/+ mice in a representative experiment. Middle, quantification of the bands normalized by β-actin. n = 3-8 from 2 independent experiments. (B) Histograms in the top panel shows intensity of fluorescence for p27Kip1 and p130 in the same BM samples processed for Western blot in panel A. Bar graph at the bottom shows average fold increase (in MIF) of p27Kip1 and p130 in BM cells in Skp2−/− vs Skp2+/+ mice. Of note, MIF is measured in a logarithmic scale; thus, small differences in fluorescence are equivalent to greater differences in the number of molecules per cell, as seen by comparison of intracytoplasmic labeling in panel B with the levels of protein in the immunoblots in panel A. (C) Expression levels for p27Kip1, p130, and p57Kip2 were determined in specific subsets by immunophenotypical staining with antibody directed to surface markers followed by intracytoplasmic staining. Histograms show intensity of fluorescence of p27Kip1, p130, and p57Kip2 in LSK and Lin− populations in Skp2+/+ and Skp2−/− mice in a representative experiment. Histogram overlaid are intensity of fluorescence in Skp2−/− cells (black) and Skp2+/+ cells (gray). IgG control is shown in white. (D) Bar graphs show average fold increase in MIF of p27Kip1, p130, and p57Kip2, cyclin E, and c-Myc in LSK and Lin− populations in SKP2−/− mice. n = 3-9 in 2-4 independent experiments. Data are expressed as mean ± SEM *P < .05 vs Skp2+/+ **P < .005 vs Skp2+/+.
Loss of SKP2 stabilizes target proteins. (A) Detection by Western blot of p21Cip1, p27Kip1, p130, cyclin E, and β-actin protein levels in BM extracts from Skp2−/− and Skp2+/+ mice in a representative experiment. Middle, quantification of the bands normalized by β-actin. n = 3-8 from 2 independent experiments. (B) Histograms in the top panel shows intensity of fluorescence for p27Kip1 and p130 in the same BM samples processed for Western blot in panel A. Bar graph at the bottom shows average fold increase (in MIF) of p27Kip1 and p130 in BM cells in Skp2−/− vs Skp2+/+ mice. Of note, MIF is measured in a logarithmic scale; thus, small differences in fluorescence are equivalent to greater differences in the number of molecules per cell, as seen by comparison of intracytoplasmic labeling in panel B with the levels of protein in the immunoblots in panel A. (C) Expression levels for p27Kip1, p130, and p57Kip2 were determined in specific subsets by immunophenotypical staining with antibody directed to surface markers followed by intracytoplasmic staining. Histograms show intensity of fluorescence of p27Kip1, p130, and p57Kip2 in LSK and Lin− populations in Skp2+/+ and Skp2−/− mice in a representative experiment. Histogram overlaid are intensity of fluorescence in Skp2−/− cells (black) and Skp2+/+ cells (gray). IgG control is shown in white. (D) Bar graphs show average fold increase in MIF of p27Kip1, p130, and p57Kip2, cyclin E, and c-Myc in LSK and Lin− populations in SKP2−/− mice. n = 3-9 in 2-4 independent experiments. Data are expressed as mean ± SEM *P < .05 vs Skp2+/+ **P < .005 vs Skp2+/+.
Next, we determined the protein levels of these molecules in the different hematopoietic subsets by using intracytoplasmic staining in conjunction with stem/progenitor cell markers. We observed a significant increase of p27Kip1 and a marked increase of p57Kip2 and p130 in Skp2−/− LSK and Lin− subsets compared with Skp2+/+; (1.5- to 2.0-fold change in mean intensity of fluorescence, [MIF]; Figure 7C-D). Levels of p27Kip1, p57Kip2, and p130 also were significantly greater in LK and in Gr1+Mac1+ subsets (supplemental Figure 4E). Cyclin E and c-Myc levels were unchanged (Figure 7D; supplemental Figure 4E).
Taken together, these results demonstrate that deletion of SKP2 in stem/progenitor cells results in the stabilization of known CKIs, in particular p27Kip1, p57Kip2, and p130, providing a mechanism for the decreased cell cycle in these cells. Elevated levels of these CKIs delay or block cell-cycle entry by preferentially inhibiting CDK2 activity in the late phase of G1-S transition.3 Indeed, this effect is noticeable in the LSK cells at steady state, in which lower percentages of cells were observed in late S-phase in Skp2−/− BM (Figure 3A).
Discussion
In this report, we demonstrated that SKP2, the F-box protein of the SCFSKP2 E3 ubiquitin ligase complex, is a regulator of HSC pool size and quiescence and plays a key role in enabling HSC and progenitors to regenerate after myeloablation and BM transplantation. We observed that Skp2 expression is dynamically regulated during proliferation and differentiation of hematopoietic cells. By using a mouse model of SKP2 targeted deletion,25 we found that SKP2 loss resulted in a significantly greater frequency of HSC at steady state, which was maintained in primary and secondary transplant recipients. Cell-cycle analysis by in vivo BrdU incorporation indicated an attenuated cell-cycle activity in Skp2−/− LSK cells at steady state, as revealed by a significantly lower fraction of Skp2−/− LSK cells in the late S-phase of the cell cycle, and demonstrated a significant decrease in cell cycling of Skp2−/− LSK cells after BM transplantation. The subset of greater proliferating erythromyeloid progenitors exhibited a significant decrease in cells in S-phase in Skp2−/− mice, which resulted in a notable reduction of MEPs and GMPs. The more mature subsets (Lin+c-Kit+ and Gr1+Mac1+ cells) showed also decreased cell-cycle activity, which correlated with accelerated myeloid cell differentiation in vitro (data not shown). Thus, at steady state, SKP2 deletion affected primitive subsets pool size and proliferative rates without disrupting overall hematopoiesis and representation of mature lineages; in fact, Skp2−/− mice did not demonstrate any noticeable blood alterations. It is possible, that the accelerated differentiation occurring in the more mature subsets in the absence of SKP2 may compensate for the lower number of progenitors contributing to homeostatic maintenance.
A critical requirement for SKP2 was highlighted under conditions of stress. We observed that Skp2 expression in stem/progenitor cells was up-regulated when rapid entry in cell cycle and cell expansion was needed in response to myeloablative stress or transplantation. As anticipated, SKP2-null stem/progenitor cells showed a significant delay in entering S-phase after treatment with 5-FU, resulting in a substantially less efficient hematopoietic regeneration. In agreement with these results, competitive repopulation assays demonstrated that Skp2−/− stem and progenitor cells were greatly impaired in reconstituting hematopoiesis at short term, a process that requires rapid cell expansion. Defective short-term engraftment was also validated in noncompetitive transplants when mixed-background mice were used. In both models—competitive and noncompetitive—short-term engraftment was further decreased in secondary transplants, and it was cell dose–dependent. Importantly, we ruled out that defective engraftment was because of decreased homing ability of Skp2−/− HSC and progenitors. Interestingly, we observed a trend for a greater homing efficiency of Skp2−/− cells compared with control mice, which is in agreement with previous results correlating the quiescent status of HSC and their homing ability.33,34
As expected, because of increased HSC quiescence, long-term reconstitution was not affected or slightly improved in mice transplanted with Skp2−/− cells. Despite the decreased ability to sustain short-term engraftment, Skp2−/− BM maintained greater frequencies of ST-HSCs and LT-HSCs, which also exhibited a more quiescent status for up to 9 months. At later time points after transplantation, chimerism in mice receiving Skp2−/− cells progressively increased to reach control percentages, suggesting that LT-HSC and their self-renewal are preserved in the absence of SKP2. Taken together, these results indicate that SKP2 deficiency results in a delayed cell-cycle entry that under homeostatic conditions favors HSC quiescence and decreased progenitor proliferation, whereas it impairs the ability of HSC and progenitors to rapidly divide and regenerate following stress.
Studies aimed at understanding the role of CKIs in hematopoiesis have shown a critical and distinct role of p21Cip1, p27Kip1, and p107/p130 in the regulation of the stem cell and progenitor cell pool, respectively.6,35,36 By using Western blot analysis and intracytoplasmic staining, we detected increased stabilization of p21Cip1, p27Kip1, p57Kip2, and p130 in the whole Skp2−/− BM cells and in subfractions of marrow cells, including LSK, LK, Lin−, and myeloid cells. In our study, the combinatorial elevation of these CKIs induced by SKP2 inactivation mirrored the opposite phenotype induced by the deletion of these individual CKIs. Deletion of p21Cip1 was shown to decrease HSC self-renewal in serial transplantation,36 and deletion of p27Kip1 did not affect HSC but led to rapid expansion of progenitor cells, indicating a strong function in controlling their cell-cycle entry.35
Recently, in a study conducted in a triple knockout for Rb, p107, and p130 the authors argued for distinct functions of p107 and p130 in the regulation of HSC homeostasis.6 In contrast to Rb-deficient mice, mice deficient in all 3 Rb family members showed increased HSC proliferation associated with decreased HSCs in transplanted recipient mice and decreased homing. Furthermore, HSCs deficient in all 3 Rb family members outcompeted controls in short-term repopulation assays but were incapable of long-term reconstitution. Thus, accumulation of CKIs in Skp2−/− hematopoietic progenitors results in the opposite effects observed when loss of these CKIs occurs. On the basis of these results, and considering the elevated levels of p130 that we observed in Skp2−/− primitive subsets, p130 may be an attractive candidate to play a major role in mediating the SKP2 induced phenotype. However, further studies are necessary to elucidate the role of specific factors regulated by SKP2. Finally, the role of p57Kip2 in other cellular models and its high expression in HSC37 suggest that it may have a relevant role in HSC homeostasis. Our observation that p57Kip2 is accumulated in the absence of SKP2 in HSC suggest its contribution to regulation of quiescence. However, the function of p57Kip2 in HSC needs to be further assessed.
Few other studies describe the involvement of the E3 ubiquitin ligase in the regulation of hematopoietic cells.8,9,38 Recently, it has been shown that the deletion of Fbw7, the F-box of the SCF complex that targets c-Myc, Notch, and cyclin E for degradation, drives HSCs into active phases of cell cycle, resulting in decreased LSK numbers and stem cell exhaustion.9 Conversely, increased expression of Fbw7 induces HSC quiescence in vitro.39 Although, both SKP2 and Fbw7 are F-box proteins for the same SCF E3 ubiquitin ligase complex and share common targets,32 their individual deletion leads to opposite HSC phenotypes. Furthermore, loss of Fwb7 is embryonically lethal and in mice in whom Fwb7 is conditionally deleted, there is a strong impact on hematopoiesis at steady state. Surprisingly, despite the ability of SKP2 to control the degradation of a large number of substrates, its loss does not affect development, and mice lacking expression have a normal life span and hematopoiesis. It is possible that other compensatory mechanisms have evolved during development that mitigate the effects of SKP2 loss in the adult life at steady state but not during stress. Indeed, many of the substrates of SKP2 are also targeted for degradation by other ubiquitin ligases.
To investigate possible compensatory effects of other F-box proteins, we examined the levels of Fbw7 and β-TrCP32 in SKP2-null cells and did not find a substantial difference from control cells. It is also important to consider that SKP2 not only stabilizes several CKIs, which are negative regulators of the cell cycle, but also c-Myc and cyclin E,25,40 positive cell-cycle regulators. We did not find a noticeable accumulation of c-Myc or cyclin E in hematopoietic cells in the absence of SKP2. It is possible that in the absence of SKP2, the balance between elevated levels of CKIs and cyclin E may contribute to restricting or allowing cell-cycle entry in specific subsets. Although in BM cells of Skp2−/− mice the inhibitory functions of CKIs prevail over the positive effectors of the cell cycle, the modulation of the fine stoichiometry between CKIs and cyclin E may account for the variability in phenotype observed in Skp2+/− heterozygous mice and in the primary transplants at week 8 (Figure 2A-B; Figure 3A-D; supplemental Figure 2 and Figure 6A).
Finally, it is important to mention that SKP2 is the downstream target of signaling pathways regulating stem cell expansion and quiescence, like Notch and TGFβ signaling, respectively.22,24 We have previously demonstrated that Notch signaling promotes transcriptional activation of Skp2 by binding of the CBF-1(RBP-J)/Notch complex to the Skp2 promoter.22 Although a critical role for Notch signaling in HSC self-renewal has been ruled out by studies in Notch loss-of-function models, there is ample evidence to suggest that increased activation of Notch signaling could contribute to hematopoiesis during stress responses.22,41-43 As such, the Notch/SKP2/CKIs pathway links microenvironment cues with the cell-cycle control of HSC and progenitor cells. It is possible that the Notch/SKP2/CKIs axis is important to respond to hematopoietic stress under which BM stem/progenitor cells have to respond to physiologic stimuli with expansion and regeneration of all their components. Thus, the identification of SKP2 as a physiologic regulator of cell-cycle progression of HSC and progenitors during stress has significant implications in the biology of stem cells. These findings increase our understanding of the mechanisms involved in BM recovery after chemotherapy and radiation, BM transplantation, and inflammation and may provide new insights for therapeutic applications.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Mark Kaplan and Mervin Yoder for critical discussions of the manuscript. We thank the IU Division of Neonatal-Perinatal Medicine for their support.
This work was supported by the National Institutes of Health (grant NIH-R01-HL068256-05A2 to N.C.).
National Institutes of Health
Authorship
Contribution: S.R. designed and performed experiments and wrote the manuscript; L.W., C.M., and C.L.C. performed experiments; E.F.S. designed the experiments and provided useful discussions; K.N. provided the animal model; and N.C. designed the study and the experiments, analyzed data, supervised the study, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Nadia Carlesso, MD, PhD, Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, 1044 W Walnut, Bldg R4-Room 166, Indianapolis, IN 46202; e-mail: ncarless@iupui.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal