Abstract
Hemophagocytic lymphohistiocytosis (HLH) is a rare inflammatory disorder with a poor prognosis for affected individuals. To find a means of suppressing the clinical phenotype, we investigated the cellular and molecular mechanisms leading to HLH in Unc13djinx/jinx mice, in which cytolytic function of NK and CD8+ T cells is impaired. Unc13djinx/jinx mutants infected with lymphochoriomeningitis virus (LCMV) present typical clinical features of HLH, including splenomegaly, elevated serum IFNγ, and anemia. Proteins mediating cell-cell contact, cytokine signaling or Toll-like receptor (TLR) signaling were analyzed. We show that neither the integrin CD18, which is involved in adhesion between antigen-presenting cells and effector T cells, nor tumor necrosis factor (TNF) made nonredundant contributions to the disease phenotype. Disruption of IFNγ signaling reduced immune cell activation in Unc13djinx/jinx mice, but also resulted in uncontrolled viral proliferation and exaggerated release of inflammatory cytokines. Abrogating the function of myeloid differentiation primary response gene 88 (MyD88) in Unc13djinx/jinx mice suppressed immune cell activation and controlled cytokine production in an IL-1 receptor 1 (IL-1R1)–independent way. Our findings implicate MyD88 as the key initiator of myeloid and lymphoid proliferation in HLH, and suggest that blockade of this signaling molecule may reduce immunopathology in patients.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare disorder of the immune system. The familial form (FHL) arises in children and is caused by mutations that impair the cytotoxic activity of NK cells and CD8+ T cells. The second form, sporadic HLH, may arise in individuals with pathogen infections or with autoimmune diseases or malignancies.1 Both HLH variants are believed to be initiated by viral infections.2 These pathogenic triggers have remained elusive, although Epstein-Barr virus has been proposed as one cause.3,4
After symptomatic onset of HLH, survival declines rapidly despite chemotherapy with cytotoxic and immunosuppressive drugs.5 To date, hematopoietic stem cell transplantation is the best long-term therapy for familial HLH.
We previously described an N-ethyl-N-nitrosourea (ENU)–germ line mutant called jinx in which a mutation in Unc13d prevents the release of cytolytic granules by NK cells and CD8+ T cells.6 We demonstrated that clinical features of the HLH-like syndrome observed in humans are recapitulated in Unc13djinx/jinx mice infected with lymphochoriomeningitis virus (LCMV). Unc13djinx/jinx remains the only animal model of human type 3 FHL, which is caused by mutations in MUNC13-4, the human ortholog of Unc13d.
On infection of Unc13djinx/jinx mutants, a positive feedback loop is initiated between CD8+ T cells and antigen-presenting cells (APCs) such as macrophages, leading to their overactivation and HLH-like symptoms.6 We hypothesized that interrupting this positive feedback loop could ameliorate the onset and/or extent of HLH-like disease and potentially reveal novel therapeutic targets for human HLH. We therefore examined the impact of disrupting cell adhesion molecules, effector cytokines, and innate immune sensors on the progression of HLH-like disease in Unc13djinx/jinx mutants, and identified myeloid differentiation primary response gene 88 (MyD88) as a key protein required for the development of the syndrome.
Methods
Mice
All mouse studies were performed in accordance with institutional regulations governing animal care and use. C57BL/6J mice were bred locally at The Scripps Research Institute. The following mutants have been previously reported and are described at http://mutagenetix.scripps.edu: Unc13djinx/jinx6 (MGI: 3628822; MMRRC: 016137-UCD), Myd88poc/poc7 (MGI: 3641255; MMRRC:010475-UCD), and TnfPanR1/PanR18 (MGI:3616888; MMRRC:010462-UCD). Itgb2Joker/Joker mice (MGI:3808883; MMRRC:016138-UCD) carry an A to T transversion in the acceptor splice site of intron 6 (TCCCAG→TCCCTG) in the Itgb2 gene, resulting in a complete deletion of exon 7. The mutated CD18 protein cannot associate with CD11a, CD11b, or CD11c.9 Interferon γ receptor (IFNγR)–deficient mice were kindly provided by Dr Charles D. Surh (The Scripps Research Institute) and B6.129S7-Il1r1tm1Imx/J, mice were obtained from The Jackson Laboratory. Compound mutants were generated by intercrossing and genotyping the respective F1 progeny.
Complete blood counts and serum cytokine detection
Blood samples were taken from the retro-orbital plexus of mice and analyzed using a Hemavet 950 veterinary hematology system (Fisher Scientific). Alternatively, IFNγ or TNF were assayed in the serum by ELISA (eBioscience).
Viruses, focus-forming unit assay
The Armstrong strain of LCMV was injected intravenously at a dose of 2 × 105 plaque-forming units per mouse. Viral titers were determined after organ homogenization by standard plaque assays on VERO cells.
Antibodies and intracellular cytokine staining
The following antibodies were used for flow cytometry, to stain splenocytes: CD11b (M1/70), CD3ϵ (145-2C11), CD8α (53-6.7), CD80 (B7-1), CD86 (GL1), F4/80 (BM8), IFNγ (XMG1.2), TNF (MP6-XT22). Specific CD8+ T-cell responses were determined 12 days after infection, from splenocytes, by intracellular IFNγ and TNF staining after a 5-hour stimulation with 10−7 M LCMV GP33 (KAVYNFATM) peptide as described before.6 Specific cytokine secretion after stimulation with LCMV-derived peptide was measured as (% cytokine+ CD8+ T cells after peptide stimulation) − (% cytokine+ CD8+ T cells after stimulation without peptide). For spontaneous cytokine production, % cytokine+ CD8+ T cells after stimulation without peptide are indicated.
Histology
For histologic analysis, tissues were fixed in 10% buffered formalin. Paraffin-embedded sections were stained with H&E.
Statistics
The statistical significance of differences was determined by unpaired Student 2-tailed t test. Differences with a P < .05 were considered statistically significant. For all figures: ns indicates not significant; *P < .05; **P ≤ .01; ***P ≤ .001.
Results
Disruption of CD18 does not affect HLH-like syndrome
Lymphocyte-function associated antigen (LFA-1) is a heterodimeric integrin that is involved in lymphocyte migration and intercellular adhesion. Because LFA-1 is found at the immunologic synapse between LCMV-infected target and CD8+ T cells,10 we hypothesized that impairing its function would restrain uncontrolled CD8+ T-cell proliferation in LCMV-infected Unc13djinx/jinx mice. To test this, we bred Unc13djinx/jinx mutants with Joker mice, in which a point mutation in the Itgb2 gene precludes surface expression of CD18, the β chain of the LFA-1 complex.9
Despite a decreased response on day 7 after LCMV infection, when T-cell expansion peaks (supplemental Figure 1A, available on Blood Web site; see the Supplemental Materials link at the top of the online article), the LCMV GP33-specific T-cell response in Unc13djinx/jinx;Itgb2Joker/Joker mice was as severe as in Unc13djinx/jinx mice on day 12, when T cells usually contract (supplemental Figure 1B-C). The Joker mutation did not alter the spontaneous production of IFNγ in jinx CD8+ T cells 7 days after infection (supplemental Figure 1D). A similar differential behavior among CD8+ T-cell populations has been previously reported in LCMV-infected, CD18-deficient mice around the peak of the T-cell response.11 On day 12 after infection, Unc13djinx/jinx;Itgb2Joker/Joker CD8+ T cells accumulated, produced IFNγ spontaneously and displayed an activated phenotype (supplemental Figure 1C-E). Furthermore, both Unc13djinx/jinx;Itgb2Joker/Joker and Unc13djinx/jinx mice displayed hematologic symptoms of HLH-like disease on day 12, namely low hematocrit, leukocytosis and neutrophilia (supplemental Figure 1F-H, respectively). Therefore, inhibiting integrin-dependent interaction is not sufficient to dampen the later progression of HLH-like syndrome in Unc13djinx/jinx mice.
The development of HLH-like syndrome is TNF-independent but IFNγ-dependent
HLH-affected individuals exhibit elevated concentrations of circulating cytokines including TNF and IFNγ, which are indicative of a poor clinical outcome in young patients.12,13 We evaluated the role of these cytokines in the development of experimental HLH. To this end, Unc13djinx/jinx mutants were bred to TnfPanR1/PanR1 mice, in which TNF bioactivity is completely abrogated,8 and to IFNγ receptor (IFNγR)–deficient mice. On day 12 after LCMV infection, Unc13djinx/jinx and Unc13djinx/jinx;TnfPanR1/PanR1 mice had enlarged spleens (supplemental Figure 2A), with elevated proportions of CD8+ T cells and macrophages (supplemental Figure 2B-C). By contrast, Unc13djinx/jinx;Ifngr−/− mice spleens did not develop splenomegaly and the proportions of splenic CD8+ T cells and macrophages were comparable with those observed in wild-type mice (supplemental Figure 2B-C). However, circulating levels of IFNγ and TNF in Unc13djinx/jinx;Ifngr−/− mice were approximately 40-fold and 5-fold higher than in Unc13djinx/jinx controls on day 12 after LCMV infection (supplemental Figure 2D and E, respectively). A substantial elevation in the concentration of serum IFNγ was already observable in Unc13djinx/jinx;Ifngr−/− mice relative to Unc13djinx/jinx mice on day 7 after LCMV infection (supplemental Figure 2F).
These data demonstrate that TNF signaling has no major role in the development of HLH-like syndrome in LCMV-infected Unc13djinx/jinx mice, whereas IFNγ/IFNγR signaling is required for uncontrolled APC and CD8+ T-cell proliferation. Unc13djinx/jinx;Ifngr−/− mice became hunched and weak by day 12 after infection, with 100 000-fold higher viral loads in their kidneys than Unc13djinx/jinx mice (supplemental Figure 2G); LCMV infection led to a fatal outcome in some of these mice (data not shown). Unresolved viral proliferation in the compound mutants is consistent with previous studies showing that IFNγ-deficient mice are susceptible to LCMV14 and that elevated viral loads in Ifngr−/− mice are further aggravated by the absence of perforin.15,16 In addition, administration of an IFNγ-blocking antibody to perforin-deficient mice during the peak CD8+ T-cell response to LCMV resulted in remission of HLH-like symptoms.17,18 Despite the use of different LCMV strains, these complementary observations suggest that the production of IFNγ at early times during viral infection may be critical to generate an initial antiviral state by priming immune responses in HLH prone individuals, before high quantities of IFNγ secreted by activated CD8+ T cells trigger the development of immunopathology. In our model of IFNγR-deficiency, this early antiviral state may be prevented, resulting in increased viral susceptibility. Early blockade of IFNγ/IFNγR signaling may therefore be deleterious in HLH-affected individuals. Alternatively, and as previously reported in another model of virus infection, deficiency of IFNγR may result in a different effect than deficiency of its ligand, IFNγ.19
Disrupting MyD88 signaling abolishes the development of HLH-like syndrome
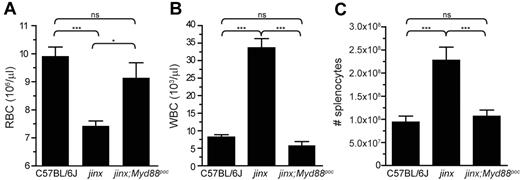
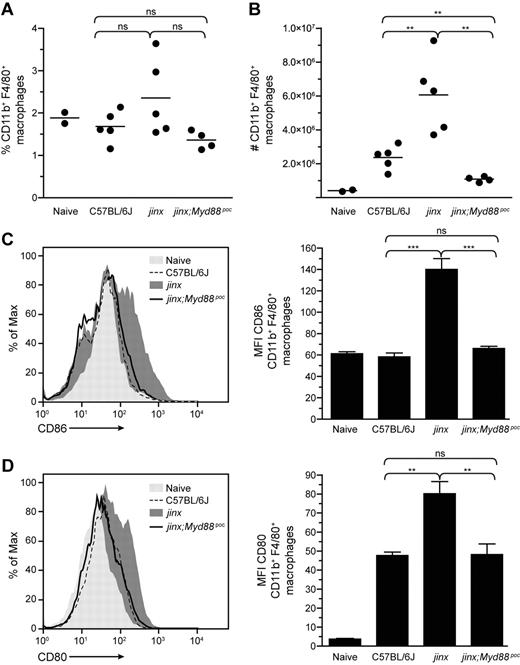
TLRs are sensors of pathogens, and most TLRs recruit the adaptor MyD88 on stimulation. During LCMV infection, MyD88 expressed by APCs is implicated in proinflammatory cytokine production20 and CD8+ T-cell expansion,21,22 2 hallmarks of HLH. To examine the effects of a disruption of MyD88 signaling in the development of HLH-like disease in Unc13djinx/jinx mice, we introduced the pococurante (poc) allele of Myd887 onto the Unc13djinx/jinx background. LCMV-infected Unc13djinx/jinx;Myd88poc/poc mice and wild-type controls displayed comparable densities of red blood cells (RBCs) and white blood cells (WBCs) on day 12 (Figure 1A-B). LCMV infection did not affect hematocrit, hemoglobin, circulating lymphocytes, neutrophils, and monocytes in Unc13djinx/jinx;Myd88poc/poc mice (supplemental Figure 3), and the spleen cellularity of these double mutants was half that observed in Unc13djinx/jinx mice (Figure 1C). Moreover, whereas the proportion of splenic macrophages in Unc13djinx/jinx; Myd88poc/poc mice was comparable with the one in Unc13djinx/jinx mice after infection (Figure 2A), the absolute number of these cells was 6-fold lower than in Unc13djinx/jinx mice (Figure 2B). Unc13djinx/jinx;Myd88poc/poc macrophages did not up-regulate CD86 (Figure 2C) and CD80 (Figure 2D) on their surface as opposed to Unc13djinx/jinx macrophages. Because MyD88 is not involved in the up-regulation of CD86 and CD80 on APCs20 or in antigen presentation in LCMV-infected wild-type mice,22 we hypothesize that the down-modulation of these molecules at the surface of Unc13djinx/jinx;Myd88poc/poc macrophages is cell-extrinsic and possibly attributed to an indirect effect mediated by other cells.
Lack of anemia and leukocyte hyperproliferation in wild-type and Unc13djinx/jinx;Myd88poc/poc mice after infection. Blood samples and spleens were collected from control and double mutant mice at day 12 after LCMV infection. Circulating red blood cells (A) and white blood cells (B) and total splenocytes (C) were enumerated. jinx, Unc13djinx/jinx mice; jinx;Myd88poc, Unc13djinx/jinx;Myd88poc/poc mice. Representative data of 3 independent experiments are shown; n = 5 per group. Error bars show SEM.
Lack of anemia and leukocyte hyperproliferation in wild-type and Unc13djinx/jinx;Myd88poc/poc mice after infection. Blood samples and spleens were collected from control and double mutant mice at day 12 after LCMV infection. Circulating red blood cells (A) and white blood cells (B) and total splenocytes (C) were enumerated. jinx, Unc13djinx/jinx mice; jinx;Myd88poc, Unc13djinx/jinx;Myd88poc/poc mice. Representative data of 3 independent experiments are shown; n = 5 per group. Error bars show SEM.
Absence of proliferation and expression of costimulatory molecules by Unc13djinx/jinx;Myd88poc/poc macrophages after infection. Twelve days after LCMV infection, splenocytes from mice were stained for CD11b and F4/80 expression to evaluate the percentage of activated macrophages (A) and their absolute numbers (B). Each dot represents an individual mouse. Histograms in panels C and D show the surface expression of CD86 and CD80 on CD11b+F4/80+ macrophages, respectively. The respective MFI are reported on associated graphs. jinx, Unc13djinx/jinx mice; jinx;Myd88poc, Unc13djinx/jinx;Myd88poc/poc mice. Representative data of 3 independent experiments are shown for panels A and B and 2 independent experiments for panels C and D; n ≥ 4 per group of infected mice. Error bars show SEM.
Absence of proliferation and expression of costimulatory molecules by Unc13djinx/jinx;Myd88poc/poc macrophages after infection. Twelve days after LCMV infection, splenocytes from mice were stained for CD11b and F4/80 expression to evaluate the percentage of activated macrophages (A) and their absolute numbers (B). Each dot represents an individual mouse. Histograms in panels C and D show the surface expression of CD86 and CD80 on CD11b+F4/80+ macrophages, respectively. The respective MFI are reported on associated graphs. jinx, Unc13djinx/jinx mice; jinx;Myd88poc, Unc13djinx/jinx;Myd88poc/poc mice. Representative data of 3 independent experiments are shown for panels A and B and 2 independent experiments for panels C and D; n ≥ 4 per group of infected mice. Error bars show SEM.
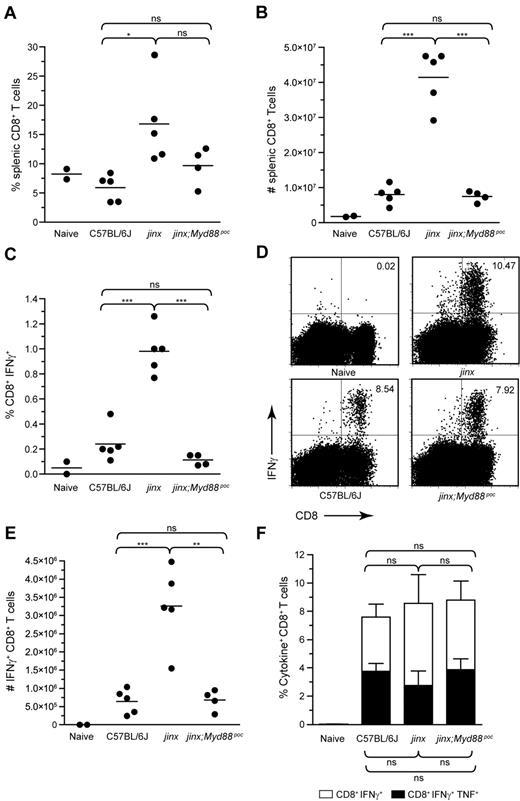
As the ablation of MyD88 signaling in the Unc13djinx/jinx background affects the expression of costimulatory molecules on APCs, T-cell activation in Unc13djinx/jinx;Myd88poc/poc mice may be dampened as well. The proportions and the absolute numbers of splenic CD8+ T cells in Unc13djinx/jinx;Myd88poc/poc mice and wild-type mice were similar (Figure 3A-B). The percentage of CD8+ T cells that spontaneously produced IFNγ in wild-type and Unc13djinx/jinx;Myd88poc/poc mice were comparable and approximately one-tenth the percentage observed in Unc13djinx/jinx mice on day 12 after infection (Figure 3C). In vitro stimulation of splenocytes with the LCMV-derived peptide GP33 showed that the total percentage of IFNγ-producing CD8+ T cells (Figure 3D) and their absolute numbers (Figure 3E) were high in Unc13djinx/jinx mice, but significantly lower and equivalent to wild-type in Unc13djinx/jinx;Myd88poc/poc mice. The percentage of LCMV GP33-specific cytokine-producing CD8+ T cells was comparable in all strains (Figure 3F).
Absence of hyperproliferation and overactivation of Unc13djinx/jinx;Myd88poc/poc CD8+ T cells on day 12 after LCMV infection. Splenocytes from mice were stained to evaluate the percentage of CD8+ T cells (A) and their absolute numbers (B). Each dot represents an individual mouse. (C) Graph reporting the percentages of CD8+ T cells that spontaneously secrete IFNγ after 5-hour incubation in media without peptide stimulation. (D-E) IFNγ production by CD8+ T cells after stimulation with LCMV GP33. The percentage of IFNγ+ cells among the CD8+ T-cell population is indicated in representative dot plots (D). Absolute counts of LCMV GP33-specific IFNγ+CD8+ T cells are shown in panel E. (F) Histogram reporting the percentages of LCMV GP33-specific CD8+ T cells that secrete either IFNγ alone (white rectangles) or IFNγ and TNF (black rectangles). Representative data of 3 independent experiments are shown for (A-F); n ≥ 4 per group of infected mice. Error bars show SEM.
Absence of hyperproliferation and overactivation of Unc13djinx/jinx;Myd88poc/poc CD8+ T cells on day 12 after LCMV infection. Splenocytes from mice were stained to evaluate the percentage of CD8+ T cells (A) and their absolute numbers (B). Each dot represents an individual mouse. (C) Graph reporting the percentages of CD8+ T cells that spontaneously secrete IFNγ after 5-hour incubation in media without peptide stimulation. (D-E) IFNγ production by CD8+ T cells after stimulation with LCMV GP33. The percentage of IFNγ+ cells among the CD8+ T-cell population is indicated in representative dot plots (D). Absolute counts of LCMV GP33-specific IFNγ+CD8+ T cells are shown in panel E. (F) Histogram reporting the percentages of LCMV GP33-specific CD8+ T cells that secrete either IFNγ alone (white rectangles) or IFNγ and TNF (black rectangles). Representative data of 3 independent experiments are shown for (A-F); n ≥ 4 per group of infected mice. Error bars show SEM.
Taken together, CD8+ T-cell responses in Unc13djinx/jinx;Myd88poc/poc mice were lower than in Unc13djinx/jinx mutants, consistent with the reduced activation state of their macrophages. Therefore, inhibition of MyD88 signaling in the Unc13djinx/jinx background not only prevented overproliferation of immune cells, but also dampened antigen-presenting cell maturation and overactivation of CD8+ T cells.
Absence of MyD88 signaling in Unc13djinx/jinx;Myd88poc/poc mice prevents severe systemic inflammation and viral proliferation
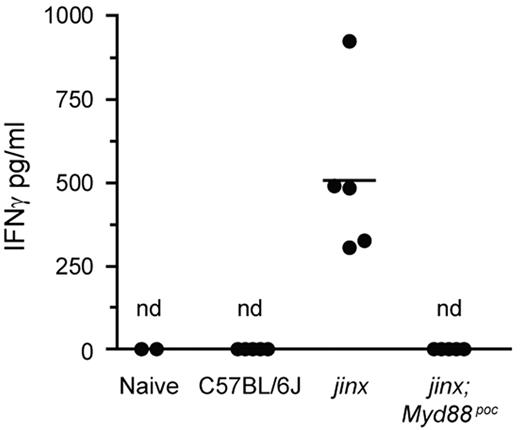
Although they exhibited similar spleen size, complete blood counts, macrophage and CD8+ T-cell numbers as wild-type mice, Unc13djinx/jinx;Ifngr−/− mice produced overwhelming amounts of systemic IFNγ and TNF (supplemental Figure 2D-E). Serum IFNγ concentration was higher in Unc13djinx/jinx;Myd88poc/poc double mutants than in wild-type controls on day 7 at the peak of the CD8+ T-cell response (supplemental Figure 2F). However, 12 days after infection with LCMV, IFNγ was undetectable in Unc13djinx/jinx;Myd88poc/poc mice (Figure 4) which additionally displayed only a moderate quantity of serum TNF (supplemental Figure 2E). These observations show that compared with deletion of the Ifngr gene, the Myd88poc mutation mitigates systemic inflammation in a remarkably effective manner. Although IFNγ may play a major role in the activation of APCs by primed CD8+ T cells, it has nonetheless nonredundant functions in controlling viral proliferation, and is therefore necessary for host survival.14 LCMV tends to persist in kidneys,23 and whereas Unc13djinx/jinx;Ifngr−/− compound mutants failed to clear the virus from the kidney on day 12 after inoculation, no infectious particles were detected in Unc13djinx/jinx;Myd88poc/poc mice (supplemental Figure 2G). Noteworthy, LCMV was controlled in blood and spleens of Unc13djinx/jinx;Myd88poc/poc and Unc13djinx/jinx;Ifngr−/− mice on day 12 (data not shown).
Systemic IFNγ 12 days after LCMV infection. Serum IFNγ was measured by ELISA. Each dot represents an individual mouse. Nd indicates not detected; jinx, Unc13djinx/jinx mice; jinx;Myd88poc, Unc13djinx/jinx;Myd88poc/poc mice.
Systemic IFNγ 12 days after LCMV infection. Serum IFNγ was measured by ELISA. Each dot represents an individual mouse. Nd indicates not detected; jinx, Unc13djinx/jinx mice; jinx;Myd88poc, Unc13djinx/jinx;Myd88poc/poc mice.
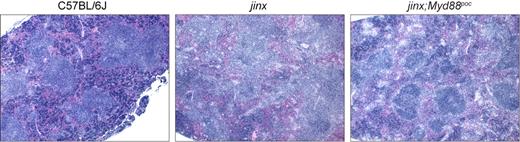
Finally, we performed histologic analysis to further investigate the dampening effects of MyD88 disruption on HLH-like symptoms in Unc13djinx/jinx mice. Sections from spleens revealed absent or disrupted B-cell follicles with infiltrations of activated lymphocytes and macrophages in LCMV-infected Unc13djinx/jinx mice. Unc13djinx/jinx;Myd88poc/poc compound mutants, however, displayed distinct germinal centers, although their overall splenic architecture was to some extent less organized than in wild-type controls (Figure 5).
Histologic appearance of the spleen in LCMV-infected mutant and wild-type mice. Hematoxylin and eosin staining of sections of spleen from representative mutant and wild-type mice 12 days after LCMV infection. Images were acquired using an Olympus AX70 microscope equipped with a 4× UPlanFl objective with a numeric aperture of 0.13, a SPOT-RT slider camera and SPOT software Version 4.6. 40× magnification is displayed. jinx indicates Unc13djinx/jinx mice; jinx;Myd88poc, Unc13djinx/jinx;Myd88poc/poc mice.
Histologic appearance of the spleen in LCMV-infected mutant and wild-type mice. Hematoxylin and eosin staining of sections of spleen from representative mutant and wild-type mice 12 days after LCMV infection. Images were acquired using an Olympus AX70 microscope equipped with a 4× UPlanFl objective with a numeric aperture of 0.13, a SPOT-RT slider camera and SPOT software Version 4.6. 40× magnification is displayed. jinx indicates Unc13djinx/jinx mice; jinx;Myd88poc, Unc13djinx/jinx;Myd88poc/poc mice.
Discussion
We have shown that mutational inactivation of MyD88 prevents HLH-like disease in Unc13djinx/jinx mice by abrogating the positive feedback loop between CD8+ T cells and APCs while permitting effective control of infection. Replicating virus was absent in Unc13djinx/jinx;Myd88poc/poc mutants, in contrast with a large infectious reservoir in Unc13djinx/jinx;Ifngr−/− kidneys (supplemental Figure 2G). This has important clinical implications toward a strategy to eliminate HLH immunopathology while maintaining antiviral immunity. Several mechanisms and effector cell types may account for the balance between immunopathology and protection observed in Unc13djinx/jinx;Myd88poc/poc mice.
Besides TLR signaling, MyD88 mediates signaling from the IL-1 receptor family, which includes the IL-18 receptor expressed by CD8+ T cells. In a LCMV model of neuropathogenesis, disruption of the IL-18 or IL-1 receptors did not affect CD4+ T-cell response whereas disruption of MyD88 did.24 IL-18 synergizes with IL-12 in maintaining IFNγ secretion by LCMV-specific CD8+ T cells.25,26 Furthermore, administration of an IL-18–blocking antibody to LCMV-infected perforin-deficient mice, which also exhibit HLH-like clinical features, did not improve the survival of treated animals.17 Therefore abrogation of IL-18 signaling in CD8+ T cells by compound homozygosity for the Myd88poc mutation is unlikely to explain rescue of HLH-like disease in Unc13djinx/jinx;Myd88poc/poc mice.
High levels of IL-1 are found in the serum of HLH-affected patients,13 and the administration of IL-1 receptor antagonists seems beneficial for patients affected with the reactive form of HLH.27 To determine the degree of involvement of IL-1 in the development of the HLH-like syndrome in response to LCMV infection, Unc13djinx/jinx mice were bred to IL-1R1–deficient mice. Disrupting IL-1R1 signaling neither decreased the maturation state of Unc13djinx/jinx macrophages (supplemental Figure 4A) nor restrained activation of Unc13djinx/jinx CD8+ T cells in response to LCMV (supplemental Figure 4B-C), suggesting that the rescue of LCMV-infected Unc13djinx/jinx mice by concomitant MyD88-deficiency does not result from impairment of IL-1 receptor signaling.
Our data exclude a role for TNF and IL-1 for the progression of HLH-like disease in Unc13djinx/jinx mutants (supplemental Figures 2 and 4, respectively). Yet IL-6–producing macrophages have been identified in liver biopsies and blood of patients suffering from hemophagocytic syndrome28,29 and significant amount of IL-6 was found in sera of mice displaying symptoms of HLH.17 On LCMV infection, MyD88-deficiency results in reduced production of IL-6,20,22 which has been suggested to support T-cell proliferation.30 However, Unc13djinx/jinx;Myd88poc/poc double mutants displayed serum IL-6 concentrations that were not significantly different from control C57BL/6J or Unc13djinx/jinx mice on day 7 after LCMV infection (supplemental Figure 4D). These data suggest a minor role of this cytokine for the outcome of the T-cell response in our model.
The observation that mice with deficiencies in both TLR7 and TLR9 mount normal adaptive immune responses to LCMV supports a TLR-independent role of MyD88 for containing the infection.20 Alternatively, sensors independent of MyD88 may be sufficient to induce normal adaptive responses to LCMV in MyD88-deficient mice. Recently, the endoplasmic RNA helicases RIG-I and MDA-5 were shown to bind LCMV-derived RNA and trigger type I IFN production.31 Additional cell-intrinsic roles have also been ascribed to MyD88 as for instance its ability to regulate expression of effector immune responses in IFNγ-activated macrophages.32 MyD88 has been shown to interact with IFNγR thereby transducing IFNγ-mediated signals that induce a pathway for the control of expression and stability of many short-lived mRNAs, including those encoding TNF and IFNγ-inducible protein 10 (IP-10).33
During LCMV infection, the priming of the immune response largely depends on LCMV-infected APCs.34,35 It has been suggested that impaired type I IFN production by plasmacytoid dendritic cells is an underlying cause of reduced priming of endogenous T cells in Myd88−/− mice.20 However, MyD88-deficient APCs were shown to be as efficient as wild-type APCs in priming infused, MyD88-competent T cells with a transgenic T-cell receptor specific for the immunodominant epitope of LCMV.22 Furthermore, serum IFNβ remained undetectable in LCMV-infected mice on day 7, although substantial concentrations of IFNγ were measured (data not shown and supplemental Figure 2F), thereby further emphasizing the importance of IFNγ for the modulation of the T-cell response in Unc13djinx/jinx mice.
Thus, a need for MyD88 in costimulation and antigen presentation leading to T-cell activation is unlikely to be a critical driver of HLH-like disease per se in LCMV-infected Unc13djinx/jinx mice, at least during the acute stage of the infection. Yet, alteration of T-cell responses observed in the MyD88-deficient state might potentially result from a requirement for MyD88 within APCs.
MyD88 is expressed by T cells and required for their function. Therefore, Unc13djinx/jinx;Myd88poc/poc CD8+ T cells might have an intrinsic defect imparted by the Myd88 mutation. In mice infected with LCMV, MyD88 signaling is necessary for sustained expansion of effector CD8+ T cells,21,22 especially during systemic infection with the faster-replicating Traub strain of LCMV.36 However MyD88 is not involved in early activation and proliferation of antigen-activated CD8+ T cells nor in the generation and maintenance of functional memory CD8+ T cells.37 Considering the importance of IFNγ-producing CD8+ T cells for the development of LCMV-induced HLH disease,17 it is plausible that disruption of MyD88 rescues the disease in Unc13djinx/jinx;Myd88poc/poc mutants because fewer CD8+ T cells secreting less IFNγ are induced. This may, in turn, result in fewer APCs being activated, thereby restricting the stimulation of CD8+ T cells. In addition to such a quantitative effect on APC activation, the transcription of many IFNγ-dependent genes in macrophages also relies on MyD88,32 which may further affect those macrophages that become activated in Unc13djinx/jinx;Myd88poc/poc mice.
In summary, our data demonstrate no role for CD18, TNF, and IL-1R1 in the progression of HLH in our model. Furthermore, we identified MyD88 as a candidate drug target superior to IFNγ, given the unrestrained viral load resulting from a disruption of IFNγ signaling.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Christine Domingo, Sara Kalina, Diantha LaVine, Terrence Robinson, and Charles Ross for technical support. Dr Nissi Varki, University of California, San Diego helped with the evaluation of the histology. We are indebted to Carrie N. Arnold, Amanda L. Blasius, Eva Marie Y. Moresco, Sophie Ugolini, and Eric Vivier for critical review of the manuscript.
This work was supported by National Institutes of Health (NIH) contract HHSN2722007000 38C (to B.B.) and NIH grants 5P01AI070167 (to B.B.) and AI09484 (to M.B.O.), by fellowships from EMBO and the Swiss National Science Foundation (to P.K.), and by NIH training grant AI007244 (to D.P.).
National Institutes of Health
Authorship
Contribution: P.K. and K.C. conducted experiments and performed data analyses; D.P. conducted experiments, performed data analyses, and provided critical discussions; M.B.O. provided critical discussions; and P.K., K.C., and B.B. designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruce Beutler, MD, Professor and Chair, Department of Genetics, The Scripps Research Institute, 10550 N Torrey Pines Rd, SP-293, La Jolla, CA 92037; e-mail: bruce@scripps.edu.
References
Author notes
P.K. and K.C. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal