Abstract
Dendritic cell immunoreceptor (DCIR) is a C-type lectin receptor expressed at high levels on dendritic cells (DCs). This surface molecule acts as an attachment factor for HIV-1 on DCs and contributes to trans- and cis-infection pathways. Moreover, DICR is induced by HIV-1 in CD4+ T cells and promotes virus replication in this cell type. Nothing is known hitherto about the DCIR-dependent signaling, which is induced following HIV-1 ligation. First, specific pharmacologic inhibitors were tested on HIV-1 binding/entry and, second, specific antisense oligonucleotides targeted, more specifically kinases and phosphatases, were used. Our results show that SHP-1, SHP-2, Syk, and Src kinases (ie, Src, Fyn, and Hck) as well as PKC-α and MAP kinases (ie, Erk1/2 and p38) are all involved in the DCIR-mediated signal transduction pathway triggered by HIV-1. By mutagenesis and through the use of intracellular phosphorylated peptides, we show as well a pivotal role for the tyrosine and threonine residues of the DCIR immunoreceptor tyrosine-based inhibitory motif (ITIM). Our data suggest for the first time an involvement of ITIM domain in HIV-1–mediated signaling events and a relationship between phosphorylation events and DCIR function with respect to HIV-1 biology.
Introduction
It is known that cell-free virions do not efficiently cross genital epithelial cells. Instead HIV-1 uses primarily dendritic cells (DCs) to penetrate the mucosal epithelium.1,2 The virus is then transferred and disseminated from this entry site to T-cell zones in secondary lymphoid organs, where it can productively infect residing CD4+ T cells. The infection process causes a marked depletion of CD4+ T cells,3 progressive impairment of the immune system, as well as chronic hyperactivation of both CD4+ and CD8+ T cells.4 The initial attachment step of HIV-1 to DCs may occur through several complex interactions between the virus and the target cell surface (reviewed in Clapham and McKnight5 and in Ugolini et al6 ) such as the association between the oligosaccharides found on the external envelope glycoprotein gp120 and the mannose receptor (CD206), langerin (CD207), or DC-SIGN.7,8 This will result in virus capture and transmission to CD4+ T cells in an effective trans mode9 (ie, transfer of virions bound onto DCs and/or localized in endosomes). Another lectin receptor, that is, the dendritic cell immunoreceptor (DCIR), can behave as an HIV-1 attachment factor for HIV-1 to participate actively in trans infection of CD4+ T cells.10 DCIR also contributes to cis-infection events, that is, infection of surrounding CD4+ T cells by virions produced by DCs productively infected with HIV-1.10
DCIR is a member of a recently described family of C-type lectin receptors (CLRs), which includes DCAR, dectin-2, BDCA-2, MCL, and MINCLE. These receptors carry a single carbohydrate recognition domain (CRD) at the COOH terminal and generally lack consensus signaling motifs in their cytoplasmic region.11 Several members of the family, however, possess a positively charged residue in their transmembrane region, via which they associate with immunoreceptor tyrosine-based activation motif (ITAM)–containing adaptor molecules, such as the Fc receptor γ chains. Importantly, DCIR is the only family member harboring an immunoreceptor tyrosine-based inhibitory motif (ITIM), which is involved in modulation of cellular responses.12 DCIR is expressed on the surface of most antigen-presenting cells (ie DCs, monocytes, macrophages, and B cells), as well as on granulocytes, and it is differentially expressed depending on the DC maturation status.13 Lipopolysaccharide, IL-4, and TNF-α down-regulate DCIR expression on neutrophils.14
The ITIM domain of DCIR contains the consensus sequence S/I/V/LxYxxI/V/L, including a tyrosine residue at position 7 (ie, ITYAEV).13 Generally, when ITIM-containing receptors are engaged, they become tyrosine-phosphorylated and then transmit signals by binding and activating Src homology-2 domain (SH2)–containing tyrosine phosphatases (eg, SHP-1 and SHP-2) and/or the SH2-containing tyrosine inositol phosphate (SHIP).15 In the case of DCIR, one study has demonstrated that, when phosphorylated, it recruits both SHP-1 and SHP-2.16 Nevertheless, there is still no evidence of the possible recruitment of phosphatases and/or tyrosine kinases (TKs) after engagement of DCIR by HIV-1.
Although a previous work demonstrated that DCIR is involved in the interaction between HIV-1 and DCs,10 nothing is known on the precise contribution of DCIR-mediated intracellular signal transducers in virus capture, infection, and eventual transfer. The possible interaction between its ITIM with non-receptor TKs (NRTKs) and protein tyrosine phosphatases (PTPs) in this process also deserves some attention. The aim of this study was thus to characterize the mechanisms of DCIR signaling not only in a cell line stably expressing DCIR but also in primary human cells. Our results show that SHP-1, SHP-2, Syk, Src, Hck, Fyn, PKC-α, Erk1/2, and p38 are involved in HIV-1–mediated DCIR signaling and that the tyrosine and threonine residues located in its ITIM play a crucial role in DCIR-directed effector functions.
Methods
Ethics statement
Peripheral blood mononuclear cells (PBMCs) were isolated from blood samples originating from healthy donors in accordance with the guidelines of the Bioethics Committee from the CHUL. The institutional review board from the CHUL approved the study. All subjects signed an ethics board–approved informed consent form in accordance with the Declaration of Helsinki.
Reagents
IL-4 was purchased from R&D Systems, and GM-CSF was obtained from Genscript. SHP-1 inhibitor SSG was provided by Glaxo-Wellcome Ltd. Syk inhibitor piceatannol, Src inhibitor PP2, protein kinase C (PKC) inhibitor Gö6976, PKA inhibitor H89, p38 inhibitor SB203580, MEK1 inhibitor PD98059, BTK inhibitor LFM-A13, and PI3K inhibitor wortmannin were all purchased from Calbiochem. Phycoerythrin (PE)–labeled anti-DCIR monoclonal antibody (Ab) (clone 216110) and the corresponding control isotype Ab were purchased from R&D Systems. The Ab specific for Syk was purchased from Upstate Biotechnology, and SHP-1 (C-19), SHP-2 (C-18), Fgr (N-47), Lyn (44), Lck (3A5), Hck (N-30), PKC-δ (C-20), and actin (I-19) Abs were obtained from Santa Cruz Biotechnology. Abs recognizing p38, Erk1/2 (137F5), and Src were purchased from Cell Signaling Technology. A Fyn Ab was a kind gift from Paul H. Naccache (CHUL). The PKC-α Ab was purchased from BD Biosciences and the PKC-β Ab was obtained from Signalway Antibody.
Cells and flow cytometric analysis
The Raji-CD4 cell line is a B-cell line susceptible to HIV-1 infection by stable transfection with a cDNA encoding human CD4.17 Raji-CD4 cells stably expressing DCIR were obtained following retroviral transduction. In brief, wild-type cDNA encoding for human DCIR was subcloned in the bicistronic retroviral vector MSCV-IRES-eGFP. Viral particles pseudotyped with the vesicular stomatitis virus G protein were generated in 293T cells using a standard calcium-phosphate transfection protocol. Virus stocks were concentrated by ultracentrifugation and titered on HeLa cells. Transduction of Raji-CD4 cells was carried out by mixing cells with serially diluted virus supernatants in culture medium supplemented with 3% FBS. After 24 hours of culture with viral supernatants, culture medium supplemented with 10% FBS was added. After 24 additional hours, cells were washed extensively and those expressing high levels of DCIR were sorted by flow cytometry based on eGFP expression. Primary human DCs were generated from purified human monocytes (ie, CD14+ cells) using a previously described procedure.10 Briefly, CD14+ cells were cultured in 6-well plates at a density of 106 cells/mL in 3 mL of RPMI-1640 medium supplemented with 10% FBS. To generate immature monocyte-derived dendritic cells (IM-MDDCs), purified monocytes were cultured in complete culture medium that was supplemented every other day with GM-CSF (1000 U/mL) and IL-4 (200 U/mL) for 7 days. Experiments were performed with cell preparations that contained a minimal amount of contaminants (ie, IM-MDDCs with a purity > 95% based on expression of specific surface markers). Cell-surface expression of DCIR was monitored by flow cytometry (Epics ELITE ESP, Coulter Electronics) as described previously.10
Production of viral stocks for binding/entry and infection assays
The HIV-1 infectious molecular clones used in this study include NL4-3 (X4-tropic), which was used in experiments with parental Raji-CD4 cells (DCIR-negative) and Raji-CD4-DCIR stable transfectants, and NL4-3balenv (R5-tropic), which was used in studies with IM-MDDCs. Virions were produced on transient transfection of human embryonic kidney 293T cells. The virus-containing supernatants were filtered through a 0.22-μm cellulose acetate syringe filter, ultracentrifuged, and normalized for virion content using an in-house sensitive double-antibody sandwich ELISA specific for the viral p24 protein.18 Preparations of NL4-3 were produced also by acute infection of Raji-CD4 cells.
Virus binding/entry and infection assays
Where indicated, parental Raji-CD4 cells or Raji-CD4-DCIR stable transfectants (1 × 106) were either pretreated with pharmacologic inhibitors for 10 minutes or transfected with sense or antisense oligonucleotides. Cells were then pulsed with NL4-3 (100 ng of p24) for 60 minutes at 37°C. Next, the virus-cell mixture was washed 3 times with PBS to remove unbound virus and resuspended in PBS containing 1% BSA. To assess virus binding/entry, the p24 content was determined by our in-house ELISA test. For the virus infection assay depicted in Figures 1 and 5, Raji-CD4 and Raji-CD4-DCIR (1 × 106 cells) were exposed to NL4-3 (100 ng of p24) for 2 hours at 37°C. After 3 washes with PBS to remove excess virus, cells were maintained in culture for up to 9 days. Cell-free culture supernatants were collected at the indicated time points and assayed for the p24 content. For the infection assay performed before measuring virus-specific Tat mRNA, Raji-CD4 and Raji-CD4-DCIR cells were either treated with pharmacologic inhibitors or transfected with oligonucleotides. Cells were then pulsed with NL4-3 during 24 hours. Next, RNA was extracted and the expression level of Tat mRNA was measured by a quantitative real-time PCR. For assessing the effect of ITIM peptides on virus binding/entry in IM-MDDCs, cells (3 × 105 cells in a final volume of 300 μL) were first transfected with one of the ITIM peptides tested and next exposed to NL4-3balenv (30 ng of p24) for 60 minutes at 37°C. After 3 washes with PBS, cells were resuspended in PBS containing 1% BSA. The p24 content was determined by ELISA. As for the effect of ITIM peptides on virus replication in IM-MDDCs, cells (3 × 105) were initially exposed to NL4-3balenv (30 ng) for 2 hours at 37°C. After 3 washes with PBS, cells were maintained in complete RPMI-1640 culture medium supplemented with GM-CSF and IL-4 in 96-well plates in a final volume of 200 μL. Every 3 days and for a period lasting 9 days, half of the medium was removed and kept frozen at −20°C until assayed. Virus production was estimated by the p24 content.
Gene silencing with sense and antisense oligonucleotides
Raji-CD4 and Raji-CD4-DCIR cells (1 × 106 cells) were washed with OptiMEM (Invitrogen Life Technologies) without serum and antibiotics. The phosphorothioate oligodeoxynucleotides (listed in Table 1; Invitrogen Life Technologies) were transfected at a final concentration of 2.5, 5, 10, 50, and 100μM/well using Oligofectamine according to the manufacturer's instructions (Invitrogen Life Technologies). The overall efficiency of gene silencing was monitored by Western blot analysis. Only data from studies using phosphorothioate oligodeoxynucleotides at a final concentration of 10μM/well are displayed in the article.
Sequences of sense and antisense oligonucleotides
| Oligonucleotides . | Antisense/sense . | Sequence (5′-3′) . |
|---|---|---|
| Syk | Antisense | CATGCTTCAGGGGCCGG |
| Sense | CCGGCCCCTGAAGCATG | |
| SHP-1 | Antisense | CTTGAGCAGGGTCTCTGCATCC |
| Sense | GGATGCAGAGACCCTGCTCAAG | |
| SHP-2 | Antisense | CTCCGCGATGTCATGTTCCT |
| Sense | AGGAACATGACATCGCGGAG | |
| PKC-α | Antisense | CCAGTCACTCGCACCATCGC |
| Sense | CAGCCATGGTTCCCCCCAAC | |
| PKC-β | Antisense | GCGCGCGTTCATCCGACT |
| Sense | TCAGCCATCTTGCGCGCG | |
| PKC-γ | Antisense | AGCACCAACAATCAACGG |
| Sense | GGCCCCACCAGTCTACTG | |
| PKC-δ | Antisense | TTTTCCGAGGTAGTACCGTG |
| Sense | GTGCCATGATGGAGCCTTTT | |
| PKC-ϵ | Antisense | ATTGAACACTACCATGGT |
| Sense | TGGTACCATCACAAGTTA | |
| Erk | Antisense | AGCCGCCGCCGCCGCCGCCA |
| Sense | ATGGCGGCGGCGGCGGCGGCA | |
| Fgr | Antisense | ACACACAGCCCATTCCAGGT |
| Sense | ACCTGGAATGGGCTGTGTGT | |
| Fyn | Antisense | GATAAAGAAGCAGCGAA |
| Sense | TTCGCTGCTTCTTTATC | |
| Lyn | Antisense | CCATATTTCCCGCTCGCGTG |
| Sense | CACGCGAGCGGGAAATATGG | |
| Hck | Antisense | TTCTCGACCCCATCCTGGC |
| Sense | GCCAGGATGGGGTCGAGAA | |
| p38 | Antisense | GTCTTGTTCAGCTCCTGC |
| Sense | GCAGGAGCTGAACAAGAC | |
| Src | Antisense | ATAGAGGGCCACAAAGGT |
| Sense | ACCTTTGTGGCCCTCTAT |
| Oligonucleotides . | Antisense/sense . | Sequence (5′-3′) . |
|---|---|---|
| Syk | Antisense | CATGCTTCAGGGGCCGG |
| Sense | CCGGCCCCTGAAGCATG | |
| SHP-1 | Antisense | CTTGAGCAGGGTCTCTGCATCC |
| Sense | GGATGCAGAGACCCTGCTCAAG | |
| SHP-2 | Antisense | CTCCGCGATGTCATGTTCCT |
| Sense | AGGAACATGACATCGCGGAG | |
| PKC-α | Antisense | CCAGTCACTCGCACCATCGC |
| Sense | CAGCCATGGTTCCCCCCAAC | |
| PKC-β | Antisense | GCGCGCGTTCATCCGACT |
| Sense | TCAGCCATCTTGCGCGCG | |
| PKC-γ | Antisense | AGCACCAACAATCAACGG |
| Sense | GGCCCCACCAGTCTACTG | |
| PKC-δ | Antisense | TTTTCCGAGGTAGTACCGTG |
| Sense | GTGCCATGATGGAGCCTTTT | |
| PKC-ϵ | Antisense | ATTGAACACTACCATGGT |
| Sense | TGGTACCATCACAAGTTA | |
| Erk | Antisense | AGCCGCCGCCGCCGCCGCCA |
| Sense | ATGGCGGCGGCGGCGGCGGCA | |
| Fgr | Antisense | ACACACAGCCCATTCCAGGT |
| Sense | ACCTGGAATGGGCTGTGTGT | |
| Fyn | Antisense | GATAAAGAAGCAGCGAA |
| Sense | TTCGCTGCTTCTTTATC | |
| Lyn | Antisense | CCATATTTCCCGCTCGCGTG |
| Sense | CACGCGAGCGGGAAATATGG | |
| Hck | Antisense | TTCTCGACCCCATCCTGGC |
| Sense | GCCAGGATGGGGTCGAGAA | |
| p38 | Antisense | GTCTTGTTCAGCTCCTGC |
| Sense | GCAGGAGCTGAACAAGAC | |
| Src | Antisense | ATAGAGGGCCACAAAGGT |
| Sense | ACCTTTGTGGCCCTCTAT |
Transfection of IM-MDDCs with ITIM peptides
The following short competitive peptides were used in our study: nonphosphorylated ITIM (TAMRA-EITYAEVRFKNEFKS-OH), ITIM phosphorylated on tyrosine (TAMRA-EITY(PO3H2)AEVRFKNES-OH) or threonine residue (TAMRA-EIT(PO3H2)YAEVRFKNEFKS-OH), and a control peptide (TAMRA-KENFKRFVAYETIES-OH). The listed peptides were introduced into IM-MDDCs (1 × 106 cells) with the Pro-Ject transfection system (Pierce Biotechnology). Complexes were formed by incubating 100 μg of ITIM peptides with 10 μL of the Pro-Ject reagent in a total volume of 100 μL of PBS. As all peptides were labeled with the fluorescent dye TAMRA, the transfection efficiency was controlled by flow cytometry.
Electrophoresis and Western blotting
Raji-CD4 and Raji-CD4-DCIR cells (1 × 106 cells) were either left untreated or treated with sense and antisense oligonucleotides. The equivalent of 2 × 104 cells was transferred into 2× sample buffer. Samples were boiled for 10 minutes and kept at −20°C until subjected to a Western blot analysis. In brief, samples were first loaded onto SDS-PAGE 10% and proteins were next transferred to Immobilon PVDF membranes (Millipore). Immunoblotting was performed with appropriate Abs depending on the transfected oligonucleotide. To measure the amount of protein loaded in the gel, the membrane was stripped again and immunoblotted with an antiactin Ab (dilution 1:5000). Proteins were detected with an enhanced chemiluminescence reagent (Pierce) followed by exposure to Kodak films.
Quantitative real-time PCR
Expression levels of viral TAT splice transcripts were determined using a Rotor-Gene system (Corbett Life Science). Briefly, total RNA (1 × 106 cells) was isolated using the Illustra RNAspin Mini Isolation Kit (GE Healthcare Life Sciences). After elution, the amount and quality of RNA was assessed by measuring the absorbance at 260 and 280 nm. RNA was reverse-transcribed using Superscript III Reverse Transcriptase (Invitrogen). We then proceeded to a quantification of viral transcripts by using the TaqMan Universal PCR MaterMix system from Applied Biosystems with primers designed for TAT splice (TAT splice-F [GAAGCATCCAGGAAGTCAGC], TAT splice-R [CTATTCCTTCGGGCCTGTC], 18S-F [TAGAGGGACAAGTGGCGTTC], and 18S-R [CGCTGAGCCAGTCAGTGT]). Normalization on 18S mRNA levels was performed to obtain final expression values. A standard curve was drawn for each gene of interest using serial dilutions of pooled RNA from all samples. The sequences for the probes are: for TAT splice 5′ d FAM-TATCAAAGCAACCCACCCACCTCC-BHq-1 3′, and for 18S 5′ d FAM-AACAGGTCTGTGATGCCCTT-BHQ-1 3′. Two microliters of cDNA were used in each reaction. Primers were used at 5μM in the reaction and probes at 2μM.
Statistical analysis
Statistical analyses were carried out according to the methods outlined in Zar19 and Sokal and Rohlf.20 Means were compared using the Student t test, or a single-factor ANOVA followed by Dunnett multiple comparisons when more than 2 means were considered. P values of < .05 were deemed statistically significant. Calculations were performed with the GraphPad Prism software Version 3.03.
Results
Enhancement of HIV-1 binding/entry and virus infection in cells stably expressing DCIR
To monitor the impact of DCIR signaling in the context of acute HIV-1 infection, and also to limit the contribution of other lectins to this phenomenon, we used a cell line stably expressing DCIR on its surface. We selected Raji-CD4 cells as a previous work has used them as an experimental model system to define the contribution of DC-SIGN in HIV-1 capture and transfer processes.21 Importantly, Raji-CD4 do not express others CLRs such as DC-SIGN and mannose receptor that can also serve as attachment factors for HIV-1 (A.A.L., unpublished observations). Briefly, Raji-CD4 cells were transduced with a retroviral vector expressing the human c-type lectin DCIR. Flow cytometric analysis shows that a significant proportion of such transduced Raji-CD4-DCIR cells are positive for DCIR after several cell passages (Figure 1A). Furthermore, we verified that expression of CD4, the HIV-1 primary receptor leading to virus fusion and cis infection processes, was comparable for both Raji-CD4 and Raji-CD4-DCIR cell lines (data not shown). Importantly, virus attachment/entry and productive infection are all augmented in Raji-CD4-DCIR stable transfectants compared with parental Raji-CD4 cells (Figure 1B-C).
HIV-1 binding/entry and replication are increased in DCIR-expressing stable transfectants. (A) Raji-CD4 cells were infected with either a retroviral control vector (left) or a retroviral vector encoding for human DCIR (right). Forty-eight hours after transduction, cells expressing high levels of DCIR were isolated by flow cytometry based on eGFP expression. Surface expression of DCIR was monitored by flow cytometry using a combination of PE-labeled anti-DCIR antibody (dotted lines) and a control isotype-matched Ab (continuous lines). Data shown correspond to a single experiment representative of 3 independent experiments. (B) Raji-CD4 and Raji-CD4-DCIR cells were exposed to NL4-3 for 60 minutes. After 3 washes with PBS to remove nonadsorbed virus, cell-associated virus (attached and internalized) was quantified by measuring the p24 content. Data shown correspond to the means ± SD of triplicate samples from 3 independent experiments. (C) Raji-CD4 and Raji-CD4-DCIR were exposed to NL4-3 for 2 hours. After 3 washes with PBS to remove excess virus, cells were maintained in culture for up to 9 days. Cell-free culture supernatants were collected at the indicated time points and assayed for the p24 content. Data shown correspond to the means ± SD of triplicate samples from 3 independent experiments. The statistical significance of differences between Raji-CD4 and Raji-CD4-DCIR is denoted by asterisks: *P < .05. D.P.I. indicates days postinfection.
HIV-1 binding/entry and replication are increased in DCIR-expressing stable transfectants. (A) Raji-CD4 cells were infected with either a retroviral control vector (left) or a retroviral vector encoding for human DCIR (right). Forty-eight hours after transduction, cells expressing high levels of DCIR were isolated by flow cytometry based on eGFP expression. Surface expression of DCIR was monitored by flow cytometry using a combination of PE-labeled anti-DCIR antibody (dotted lines) and a control isotype-matched Ab (continuous lines). Data shown correspond to a single experiment representative of 3 independent experiments. (B) Raji-CD4 and Raji-CD4-DCIR cells were exposed to NL4-3 for 60 minutes. After 3 washes with PBS to remove nonadsorbed virus, cell-associated virus (attached and internalized) was quantified by measuring the p24 content. Data shown correspond to the means ± SD of triplicate samples from 3 independent experiments. (C) Raji-CD4 and Raji-CD4-DCIR were exposed to NL4-3 for 2 hours. After 3 washes with PBS to remove excess virus, cells were maintained in culture for up to 9 days. Cell-free culture supernatants were collected at the indicated time points and assayed for the p24 content. Data shown correspond to the means ± SD of triplicate samples from 3 independent experiments. The statistical significance of differences between Raji-CD4 and Raji-CD4-DCIR is denoted by asterisks: *P < .05. D.P.I. indicates days postinfection.
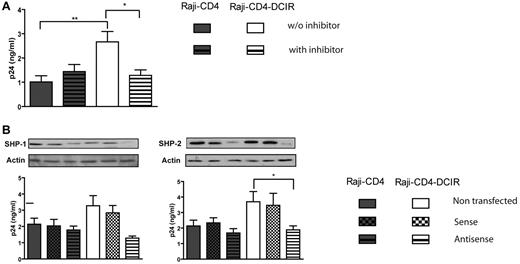
SHP-1 and SHP-2 are involved in DCIR signaling induced by HIV-1
To evaluate the involvement of PTPs in DCIR signaling after HIV-1 binding, we used sodium stibogluconate (SSG), a potent in vitro inhibitor of PTPases such as SHP-1, SHP-2, and PTP-1B.22 A treatment of Raji-CD4-DCIR cells with SSG for 10 minutes before pulsing with NL4-3 leads to a decrease in HIV-1 binding/entry, whereas it has no similar effect in parental Raji-CD4 cells (Figure 2A). To determine more specifically which PTPase(s) is implicated in the signal transduced by DCIR after HIV-1 binding, we used specific antisense oligonucleotides directed against SHP-1 and SHP-2 DNA. We found that both PTPs are involved in the HIV-1–mediated signal transduction events through DCIR (Figure 2B). It must be noted that Western blot (see small inserts in Figures 2,Figure 3,Figure 4–5) and a loading control marker consisting of actin were performed systematically in all antisense oligonucleotide assays presented in this work.
DCIR-mediated enhancing effect on HIV-1 binding/entry involves SHP-1 and SHP-2. (A) Raji-CD4 and Raji-CD4-DCIR cells were either left untreated or treated with SSG (100 μg/mL) for 10 minutes at 37°C. Thereafter, cells were pulsed with NL4-3 for 60 minutes. After 3 washes with PBS to remove nonadsorbed virus, cell-associated virus was quantified by measuring the p24 content. Data shown correspond to the means ± SD of triplicate samples from 3 combined independent experiments. The statistical significance of differences between untreated and treated Raji-CD4-DCIR is denoted by asterisks: *P < .05; **P < .01. (B) Cells were either left untransfected or transfected with sense or antisense oligonucleotides specific for the signaling protein of interest. Next, cells were pulsed with NL4-3 for 60 minutes. After 3 washes with PBS to eliminate unbound virus, cell-associated virus was quantified by measuring the p24 content. Data shown correspond to the means ± SD of triplicate samples from 3 combined independent experiments. The statistical significance of differences between nontransfected and Raji-CD4-DCIR transfected with antisense oligonucleotides is denoted by an asterisk: *P < .05. After gene silencing, the diminution of the targeted protein was verified by Western blotting and actin was used as a loading control marker (provided as inserts for each graph).
DCIR-mediated enhancing effect on HIV-1 binding/entry involves SHP-1 and SHP-2. (A) Raji-CD4 and Raji-CD4-DCIR cells were either left untreated or treated with SSG (100 μg/mL) for 10 minutes at 37°C. Thereafter, cells were pulsed with NL4-3 for 60 minutes. After 3 washes with PBS to remove nonadsorbed virus, cell-associated virus was quantified by measuring the p24 content. Data shown correspond to the means ± SD of triplicate samples from 3 combined independent experiments. The statistical significance of differences between untreated and treated Raji-CD4-DCIR is denoted by asterisks: *P < .05; **P < .01. (B) Cells were either left untransfected or transfected with sense or antisense oligonucleotides specific for the signaling protein of interest. Next, cells were pulsed with NL4-3 for 60 minutes. After 3 washes with PBS to eliminate unbound virus, cell-associated virus was quantified by measuring the p24 content. Data shown correspond to the means ± SD of triplicate samples from 3 combined independent experiments. The statistical significance of differences between nontransfected and Raji-CD4-DCIR transfected with antisense oligonucleotides is denoted by an asterisk: *P < .05. After gene silencing, the diminution of the targeted protein was verified by Western blotting and actin was used as a loading control marker (provided as inserts for each graph).
Src and Syk family members are involved in the HIV-1–mediated DCIR signalosome
PTPs such as SHP-1 and SHP-2 are recruited to the phosphorylated ITIM domain of DCIR, but nothing is known about TKs involved in the phosphorylation of this region.16 We thus next assessed whether Src, Tec, and Syk TKs were responsible for this process. The possible involvement of Src TKs was assessed using the specific inhibitor PP2. Data shown in Figure 3A indicate that HIV-1 binding/entry is decreased in presence of the inhibitor. To specify which Src(s) is involved in the DCIR signalosome, cells were transfected with antisense oligonucleotides against Src, Fyn, Fgr, Lyn, and Hck. The results indicate that Src, Fyn, and Hck, but neither Fgr nor Lyn, participate in the DCIR-mediated binding/entry of HIV-1 (Figure 3B and data not shown).
DCIR-mediated enhancing effect on HIV-1 binding/entry requires Src and Syk family members. Experimental procedures used here are similar to those described for Figure 2 except that the following inhibitors and oligonucleotides were tested: (A) Src inhibitor PP2 (10μM), (B) oligonucleotides specific for Src, (C) BTK inhibitor LFM-A13 (2.5μM), (D) Syk inhibitor piceatannol (10μM), (E) oligonucleotides specific for Syk, and (F) PI3K inhibitor wortmannin (50nM). Data shown correspond to the means ± SD of triplicate samples from 3 combined independent experiments. The statistical significance of differences between untreated and treated cells or nontransfected and Raji-CD4-DCIR transfected with antisense oligonucleotides is denoted by asterisks: *P < .05; **P < .01; ***P < .001. After gene silencing, the diminution of the targeted protein was verified by Western blotting and actin was used as a loading control marker (provided as inserts for each graph). Vertical lines have been inserted to indicate repositioned gel lanes.
DCIR-mediated enhancing effect on HIV-1 binding/entry requires Src and Syk family members. Experimental procedures used here are similar to those described for Figure 2 except that the following inhibitors and oligonucleotides were tested: (A) Src inhibitor PP2 (10μM), (B) oligonucleotides specific for Src, (C) BTK inhibitor LFM-A13 (2.5μM), (D) Syk inhibitor piceatannol (10μM), (E) oligonucleotides specific for Syk, and (F) PI3K inhibitor wortmannin (50nM). Data shown correspond to the means ± SD of triplicate samples from 3 combined independent experiments. The statistical significance of differences between untreated and treated cells or nontransfected and Raji-CD4-DCIR transfected with antisense oligonucleotides is denoted by asterisks: *P < .05; **P < .01; ***P < .001. After gene silencing, the diminution of the targeted protein was verified by Western blotting and actin was used as a loading control marker (provided as inserts for each graph). Vertical lines have been inserted to indicate repositioned gel lanes.
The Tec family comprises NRTKs, among which the Bruton TK (BTK) is crucial for the maturation of B lineage cells. Moreover, BTK shares a feature with the Src family kinase, namely a Src homology 3 (SH3) domain.23 In BTK, this domain may be involved in the regulation of kinase activity in a manner different from that of Src. This kinase is fully activated on tyrosine phosphorylation of its catalytic domain by TKs of the Src family and recruitment to the cellular membrane by PI3K.24 We tested the possible implication of BTK by using the LFM-A13 inhibitor, and our data suggest that this enzyme is not involved in the DCIR signalosome induced by HIV-1 (Figure 3C).
The third family of NRTKs possibly involved in DCIR signal transduction is the Syk family. It is known that Syk acts in several endocytosis/phagocytosis signaling pathways, especially after DC-SIGN stimulation.25,26 Moreover, it has been shown that Syk can be recruited by tyrosine phosphorylation of the ITAM-like motif of dectin-1 and CLEC-2.27 We thus investigated whether Syk is implicated in the signaling cascade induced upon ligation of DCIR by HIV-1. Cells were first treated with the Syk inhibitor piceatannol before virus exposure. Pretreatment of Raji-CD4-DCIR cells with piceatannol significantly reduces HIV-1 binding/entry (Figure 3D). The specificity of the piceatannol-dependent effect was confirmed by the inability of the inactive analog trans-stilbene to induce a comparable decrease in HIV-1 binding/entry (data not shown). The importance of Syk family members was further confirmed by using a specific antisense oligonucleotide (Figure 3E).
Activation of Syk can regulate the activity of PI3K.28 To investigate the role of this important cellular sensor, 2 selective pharmacologic inhibitors of PI3K, namely wortmannin and LY294002, were used before pulsing cells with HIV-1. No modulation of virus binding/entry was observed when using both compounds (Figure 3F for wortmannin and data not shown for LY294002). Based on these observations, we concluded that DCIR stimulation by HIV-1 is independent of PI3K activation.
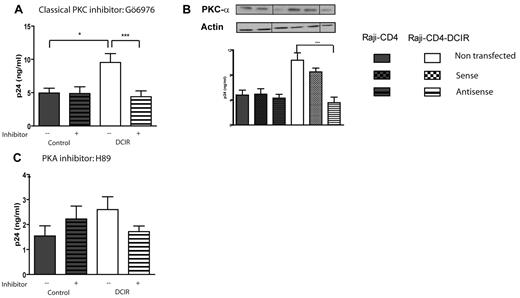
PKC-α, but not PKA, is involved in DCIR-dependent increase in HIV-1 binding/entry
The serine and threonine kinases also participating in several signaling cascades and in regulating protein activity. For example, SHP-1 turnoff is regulated by Ser591 phosphorylation, which is achieved by PKCs.29 Moreover, Syk has been recently identified as a substrate for PKC.30 The role of classic PKCs was monitored with the Gö6976 inhibitor and our data indicate that these serine–threonine protein kinase isoenzymes are implicated (Figure 4A). Classic PKCs comprise at least 4 distinct isoforms (ie, α, BI, BII, and γ) and our next series of investigations was aimed at assessing the contribution of each of them. To this end, Raji-CD4 and Raji-CD4-DCIR cells were transfected with sense or antisense oligonucleotides. Our findings indicate that PKC-α is the only classic PKC isoform that plays a role in the signaling induced by DCIR after virus attachment (Figure 4B and data not shown for the 3 other isoforms).
DCIR-mediated enhancing effect on HIV-1 binding/entry involves PKC-α. Experimental procedures used here are similar to those described for Figure 2 except that the following inhibitors and oligonucleotides were tested: (A) classic PKC inhibitor Gö6976 (1μM), (B) oligonucleotides specific for PKC-α, and (C) PKA inhibitor H89 (10μM). Data shown correspond to the means ± SD of triplicate samples from 3 combined independent experiments. The statistical significance of differences between untreated and treated cells or nontransfected and Raji-CD4-DCIR transfected with antisense oligonucleotides is denoted by asterisks: *P < .05; ***P < .001. After gene silencing, the diminution of the targeted protein was verified by Western blotting and actin was used as a loading control marker (provided as inserts for each graph). Vertical lines have been inserted to indicate repositioned gel lanes.
DCIR-mediated enhancing effect on HIV-1 binding/entry involves PKC-α. Experimental procedures used here are similar to those described for Figure 2 except that the following inhibitors and oligonucleotides were tested: (A) classic PKC inhibitor Gö6976 (1μM), (B) oligonucleotides specific for PKC-α, and (C) PKA inhibitor H89 (10μM). Data shown correspond to the means ± SD of triplicate samples from 3 combined independent experiments. The statistical significance of differences between untreated and treated cells or nontransfected and Raji-CD4-DCIR transfected with antisense oligonucleotides is denoted by asterisks: *P < .05; ***P < .001. After gene silencing, the diminution of the targeted protein was verified by Western blotting and actin was used as a loading control marker (provided as inserts for each graph). Vertical lines have been inserted to indicate repositioned gel lanes.
PKA regulates a number of cellular processes important for immune activation. For example, PKA activation resulting from an increase of the intracellular level of cAMP represents one mechanism for regulating antigen receptor signaling.31 The possible implication of this kinase in DCIR signaling was tested by using the specific inhibitor H89 and our observations suggest that PKA does not contribute to the DCIR-mediated augmentation in virus binding/entry (Figure 4C).
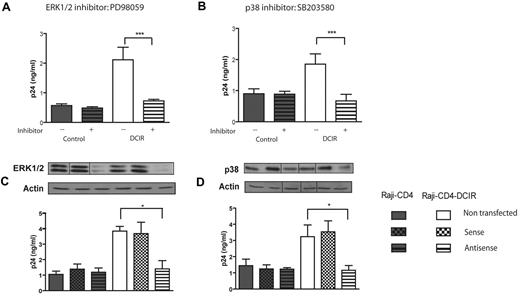
ERK1/2 and p38 are involved in DCIR signaling induced by HIV-1
Next, we focused on kinases intervening more downstream in the signaling cascade such as the classic mitogen-activated protein (MAP) kinases ERK1/2 and p38. To this end, cells were pretreated either with PD98059, an inhibitor of ERK1/2,32 or SB203580, an inhibitor of p38,33 before virus exposure. The results depicted in Figure 5A and B show that both inhibitors induce a significant decrease in HIV-1 DCIR binding/entry in Raji-CD4-DCIR cells but not in parental Raji-CD4 cells. The implication of these kinases in the DCIR signalosome was confirmed using specific antisense oligonucleotides (Figure 5C and D, respectively). Altogether these results suggest that the virus-directed DCIR signaling pathway involved activation of ERK1/2 and p38.
DCIR-mediated enhancing effect on HIV-1 binding/entry involves ERK1/2 and p38. Experimental procedures used here are similar to the ones described for Figure 2 except that the following inhibitors and oligonucleotides were tested: (A) ERK1/2 inhibitor PD98059 (20nM), (B) p38 inhibitor SB203580 (2μM), and oligonucleotides specific for (C) ERK1/2 and (D) p38. Data shown correspond to the means ± SD of triplicate samples from 3 combined independent experiments. The statistical significance of differences between untreated and treated cells or nontransfected and Raji-CD4-DCIR transfected with antisense oligonucleotides is denoted by asterisks: *P < .05; ***P < .001. After gene silencing, the diminution of the targeted protein was verified by Western blotting and actin was used as a loading control marker (provided as inserts for each graph). Vertical lines have been inserted to indicate repositioned gel lanes.
DCIR-mediated enhancing effect on HIV-1 binding/entry involves ERK1/2 and p38. Experimental procedures used here are similar to the ones described for Figure 2 except that the following inhibitors and oligonucleotides were tested: (A) ERK1/2 inhibitor PD98059 (20nM), (B) p38 inhibitor SB203580 (2μM), and oligonucleotides specific for (C) ERK1/2 and (D) p38. Data shown correspond to the means ± SD of triplicate samples from 3 combined independent experiments. The statistical significance of differences between untreated and treated cells or nontransfected and Raji-CD4-DCIR transfected with antisense oligonucleotides is denoted by asterisks: *P < .05; ***P < .001. After gene silencing, the diminution of the targeted protein was verified by Western blotting and actin was used as a loading control marker (provided as inserts for each graph). Vertical lines have been inserted to indicate repositioned gel lanes.
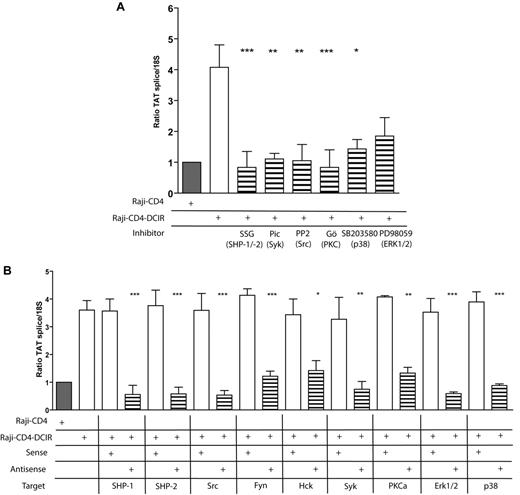
HIV-1 replication is increased by the virus-mediated engagement of DCIR and subsequent signaling through SHP-1, SHP-2, Src, Fyn, Hck, Syk, PKC-α, ERK1/2, and p38
We next verified the contribution of the signal transducers identified here in the process of virus replication. Results displayed in Figure 6A demonstrate that signal transducers involved in DCIR-mediated HIV-1 entry/binding are also important for productive virus infection. These observations are corroborated when using antisense oligonucleotides against each protein of interest (Figure 6B). It can be concluded that the DCIR-mediated enhancement in virus production requires SHP-1, SHP-2, Src, Fyn, Hck, Syk, PKC-α, ERK1/2, and p38.
Signaling proteins responsible for the DCIR-mediated enhancing effect on HIV-1 binding/entry are also required to achieve a superior virus infection. (A) Raji-CD4 and Raji-CD4-DCIR were either left untreated or preincubated for 10 minutes with the tyrosine phosphatase inhibitor SSG (100μg/mL), Syk inhibitor piceatannol (10μM), Src inhibitor PP2 (10μM), classic PKC inhibitor Gö6976 (1μM), MAPK p38 inhibitor SB203580 (2 μM), or MAP kinase inhibitor PD98059 (20nM). (B) Raji-CD4 and Raji-CD4-DCIR were treated with Oligofectamine and then either left untreated or treated for 5 hours with sense or antisense oligonucleotides specific for the listed signaling proteins. Next, cells were exposed to NL4-3 for 24 hours. Virus infection was determined by real-time PCR of spliced Tat mRNA. Data shown correspond to the means ± SD of triplicate samples from 3 independent experiments. The statistical significance of differences between untreated and treated cells or nontransfected and Raji-CD4-DCIR transfected with antisense oligonucleotides is denoted by asterisks: *P < .05; **P < .01; ***P < .001.
Signaling proteins responsible for the DCIR-mediated enhancing effect on HIV-1 binding/entry are also required to achieve a superior virus infection. (A) Raji-CD4 and Raji-CD4-DCIR were either left untreated or preincubated for 10 minutes with the tyrosine phosphatase inhibitor SSG (100μg/mL), Syk inhibitor piceatannol (10μM), Src inhibitor PP2 (10μM), classic PKC inhibitor Gö6976 (1μM), MAPK p38 inhibitor SB203580 (2 μM), or MAP kinase inhibitor PD98059 (20nM). (B) Raji-CD4 and Raji-CD4-DCIR were treated with Oligofectamine and then either left untreated or treated for 5 hours with sense or antisense oligonucleotides specific for the listed signaling proteins. Next, cells were exposed to NL4-3 for 24 hours. Virus infection was determined by real-time PCR of spliced Tat mRNA. Data shown correspond to the means ± SD of triplicate samples from 3 independent experiments. The statistical significance of differences between untreated and treated cells or nontransfected and Raji-CD4-DCIR transfected with antisense oligonucleotides is denoted by asterisks: *P < .05; **P < .01; ***P < .001.
DCIR-dependent increase in virus replication necessitates phosphorylation of both tyrosine and threonine residues in the ITIM of DCIR
The ITIM of DCIR (ie, ITYAEV) contains 2 contiguous potential sites of phosphorylation, a tyrosine and a threonine residue. However, there is no information about the importance of these amino acids in virus-mediated signal transduction events through DCIR. Therefore, we constructed 2 mutants of DCIR, namely T6F, in which the threonine was replaced by a phenylalanine, and Y7F, in which the tyrosine was replaced by a phenylalanine. Thereafter, using the same experimental procedure that we used for Raji-CD4-DCIR, we constructed stable cell lines expressing these 2 DCIR mutants. The surface expression of both forms of DCIR was evaluated by flow cytometry, and we found that wild-type DCIR, DCIR-T6F, and DCIR-Y7F were expressed at roughly similar levels (Figure 7A). An HIV-1 binding/entry assay was performed and the results show a statistically significant diminution in HIV-1 binding/entry in cells bearing threonine and tyrosine mutants (Figure 7B left). Moreover, the decrease in virus binding/entry is reflected in HIV-1 replication (Figure 7B right). To confirm the importance of phosphorylation of the 2 amino acids in a more physiologically relevant model, an ITIM peptide, phosphorylated or not on the threonine or the tyrosine, was introduced in IM-MDDCs. This approach relies on competition of the introduced peptide with the ITIM domain of DCIR for signaling molecules to be recruited. The peptides were inserted in such primary human cells with the Pro-Ject lipidic delivery system and, 15 minutes later, HIV-1 binding/entry and infection assays were performed. The presence of the peptides inside the cells was confirmed by flow cytometry (Figure 7C). A decrease in virus binding/entry and HIV-1 production was seen in the presence of the synthetic peptides (Figure 7D left and right). Therefore, it can be proposed that recruitment of signaling protein(s) to the ITIM domain of DCIR is important for its activity with regard to HIV-1 binding/entry and replication.
DCIR-mediated enhancing effect on HIV-1 replication requires phosphorylation of the ITIM domain. (A) Raji-CD4 cells were transduced with a retroviral vector expressing a wild-type form of DCIR, a T6F mutant of DCIR, or a Y7F mutant of DCIR. Surface expression levels of DCIR was assessed by flow cytometry using a combination of PE-labeled anti-DCIR Ab (dotted lines) and a control isotype-matched Ab (continuous lines). Data shown correspond to a single experiment representative of 3 independent experiments. (B) For the virus binding/entry assay shown in the left panel, cells were exposed to NL4-3. For the infection assay shown in the right panel, the same cells were exposed to NL4-3 for 2 hours at 37°C, and then maintained in culture for 9 days. Data shown correspond to the means of triplicate samples from 3 independent experiments. The statistical significance of differences between Raji-CD4-DCIR, Raji-CD4-DCIR T6F, and Raji-CD4-DCIR Y7F is denoted by asterisks: *P < .05; **P < .01. (C) IM-MDDCs were treated with Pro-Ject only, with a control peptide or an ITIM peptide, either not phosphorylated or phosphorylated on the tyrosine or threonine residue, during 5 minutes at 37°C. All peptides used in this study were labeled with the fluorescent dye TAMRA. The total cell uptake of peptides was determined by flow cytometry. (D) Cells were next pulsed with NL4-3balenv for 60 minutes at 37°C and washed extensively before measuring the p24 content (left panel). In some experiments, similarly treated IM-MDDCs were pulsed with NL4-3balenv for 2 hours at 37°C, washed extensively, and maintained in complete culture medium supplemented with GM-CSF and IL-4. Cell-free culture supernatants were quantified by measuring the p24 content. Data shown correspond to the means of triplicate samples from 3 independent experiments. The statistical significance of differences between cells treated with nonphosphorylated ITIM peptide, ITIM peptide phosphorylated on the tyrosine residue, and ITIM peptide phosphorylated on the tyrosine residue is denoted by asterisks: ***P < .001.
DCIR-mediated enhancing effect on HIV-1 replication requires phosphorylation of the ITIM domain. (A) Raji-CD4 cells were transduced with a retroviral vector expressing a wild-type form of DCIR, a T6F mutant of DCIR, or a Y7F mutant of DCIR. Surface expression levels of DCIR was assessed by flow cytometry using a combination of PE-labeled anti-DCIR Ab (dotted lines) and a control isotype-matched Ab (continuous lines). Data shown correspond to a single experiment representative of 3 independent experiments. (B) For the virus binding/entry assay shown in the left panel, cells were exposed to NL4-3. For the infection assay shown in the right panel, the same cells were exposed to NL4-3 for 2 hours at 37°C, and then maintained in culture for 9 days. Data shown correspond to the means of triplicate samples from 3 independent experiments. The statistical significance of differences between Raji-CD4-DCIR, Raji-CD4-DCIR T6F, and Raji-CD4-DCIR Y7F is denoted by asterisks: *P < .05; **P < .01. (C) IM-MDDCs were treated with Pro-Ject only, with a control peptide or an ITIM peptide, either not phosphorylated or phosphorylated on the tyrosine or threonine residue, during 5 minutes at 37°C. All peptides used in this study were labeled with the fluorescent dye TAMRA. The total cell uptake of peptides was determined by flow cytometry. (D) Cells were next pulsed with NL4-3balenv for 60 minutes at 37°C and washed extensively before measuring the p24 content (left panel). In some experiments, similarly treated IM-MDDCs were pulsed with NL4-3balenv for 2 hours at 37°C, washed extensively, and maintained in complete culture medium supplemented with GM-CSF and IL-4. Cell-free culture supernatants were quantified by measuring the p24 content. Data shown correspond to the means of triplicate samples from 3 independent experiments. The statistical significance of differences between cells treated with nonphosphorylated ITIM peptide, ITIM peptide phosphorylated on the tyrosine residue, and ITIM peptide phosphorylated on the tyrosine residue is denoted by asterisks: ***P < .001.
Discussion
Despite intensive efforts to improve our understanding of HIV-1 pathogenesis and correlates of immune protection, the pandemic continues to expand and no effective vaccine is available or appears likely to become available in the near future. Our study provides novel and cogent insight into salient aspects of HIV-1 transmission by DCs and more particularly into the role of the virus-mediated DCIR signaling events in this process. The molecular and functional characterization of intracellular signal transducers engaged by DCIR after HIV-1 ligation is important because it provides fundamental information useful for the development of novel approaches to control HIV-1 propagation. Intracellular protein phosphorylation represents one of the most critical and dynamic mechanisms by which cellular functions are regulated. The present study examined the signaling cascade induced by DCIR on its engagement by HIV-1. We provide novel and relevant insight into significant aspects of DCIR signaling, more particularly into the nature of intracellular biochemical events involved in HIV-1–mediated DCIR signalosome. This study also identified, using different experimental approaches, several components of the signal transduction pathway involved in DCIR stimulation. Briefly, the importance of PTPs such as SHP-1 and SHP-2, some Src and Syk family members, PKC-α, and MAP kinases (ERK1/2 and p38) was shown, as well as the pivotal role of the tyrosine and threonine residues located in the intracellular ITIM domain of DCIR.
CLRs are known to play a dominant role in pathogen recognition within the immune system. To develop better means of controlling infection, it is crucial to understand signaling events that are induced by the interaction between a pathogen and its cognate receptor. CLRs are known to recruit signaling adaptor molecules like DAP12 or contain various intracellular signaling sequences such as ITAM or ITIM in their cytoplasmic regions.34 These signaling motifs are involved in regulation of effector-related functions of the receptors. For example, it has been established that binding of ligands to CLRs such as BDCA-2, DCAL-1, or dectin-1 induces tyrosine phosphorylation of ITAM and that this event promotes recruitment of the tyrosine kinase Syk, subsequently inducing adaptive immune responses.35 On the other hand, DCAL-2, another CLR carrying ITIM domain and present on DCs, induces activation of ERK1/2 and p38, allowing programming of DC activities. Moreover, DCAL-2 signaling modulates the ability of DCs to direct downstream T-cell polarization.36 In general, receptors possessing an ITAM domain acts as activators, whereas receptors bearing an ITIM region act as inhibitors in signal transduction pathways.37 For example, PECAM-1, which promotes migration of endothelial cells through a process relying on PTP activity, can reduce B-cell receptor–mediated calcium and tyrosine phosphorylation events because of its ITIM region.37 Interestingly, it was reported that chimeric molecules consisting of the extracellular domain of FcγRIIB and the cytoplasmic portion of murine DCIR display an ITIM-mediated inhibitory role.38 Nevertheless, in the context of our study, we did not observe any inhibitory signal mediated by DCIR at least with respect to HIV-1 biology. This is reminiscent of previous findings where the ITIM domain can sometimes propagate positive signals.37
Ligation of some CLRs can result in phosphorylation of ITIM domain by Src TKs.37 However, nothing is known about the identity of the phosphorylated amino acid(s) on this motif and the precise nature of the Src involved in this process. Some studies postulated that tyrosine phosphorylation is important for signaling induced by an ITIM domain,37,39 but for DCIR it remains to be established. Site-directed mutagenesis of the tyrosine residue present in the ITIM domain revealed the important function of this amino acid in induction of DCIR signaling. For another CLR, namely CLEC-2, a tyrosine residue is important for signal transduction but it was also demonstrated that an upstream serine residue is also needed to achieve CLEC-2–mediated biochemical events.27 We thus performed site-directed mutagenesis on the threonine preceding the tyrosine and showed the importance of this residue in DCIR-dependent increase in HIV-1 replication. As a complementary experiment to site-directed mutagenesis, we introduced ITIM peptides bearing a phosphorylated tyrosine or threonine residue in primary human cells known to express high levels of DCIR (ie, DCs). The use of such specific peptides that can compete with the natural intracellular partners of the ITIM region of DCIR confirmed that ligation of DCIR by HIV-1 leads to phosphorylation of both residues.
Knowing that engagement of DCIR by HIV-1 leads to phosphorylation of the 2 amino acids located in its ITIM domain, the obvious next question is the identity of the phosphatase(s) and/or kinase(s) involved in this process. This goal was reached by using pharmacologic inhibitors and well as antisense oligonucleotides. We provide evidence that the Syk and Src family kinase members are involved in the HIV-1–mediated DCIR signalosome. The Src family comprises 8 members of NRTKs such as Src, Fyn, Yes, Lyn, Fgr, Hck, Lck, Blk, and Csk. Three of them (ie, Src, Fyn, and Yes) are ubiquitously expressed, whereas the other 5 display a tissue-specific expression. For example, Hck is expressed only in hematopoietic cells. Among the Src family members tested, only3 of them seem to be involved in DCIR signaling (ie, Scr, Fyn, and Hck).
Likewise, our results indicate that PKC-α participates in DCIR signaling. The PKC family constitutes a large group of Ser/Thr kinases but nothing is known about the implication of PKC-α in DCIR signaling. We hypothesize that, among other possible roles discussed below, PKC-α phosphorylates the threonine of the ITIM to induce signaling. Additional studies are warranted to confirm this postulate.
Subtle differences in ITIM sequences can result in the differential recruitment of phosphatases by various receptor systems, presumably because of differences in the binding specificity of the phosphatase SH2 domains. Therefore, some ITIMs recruit the tyrosine phosphatases SHP-1 and SHP-2, whereas others bind the inositol polyphosphate 5′-phosphatase, SHIP, and some bind all 3 of them.15,40 Previous observations in neutrophils had shown the tyrosine-phosphorylated ITIM of DCIR to bind to SHP-1 and SHP-2.16 Our results suggest that ligation of DCIR by HIV-1 also leads to SHP-1 and SHP-2 activation, because blocking them either with an inhibitor or with antisense oligonucleotides causes a significant diminution in HIV-1 binding/entry and virus infection. It is noteworthy that SHP-1 can be a substrate of PKC-α.30 Interestingly, it has recently been demonstrated that PKC-α mediates serine phosphorylation of Syk and this event is necessary for tyrosine phosphorylation and activity of SHP-1.41 Thus, PKC-α is likely to be involved in different signal transduction pathways engaged by DCIR.
Altogether, the data presented herein suggest that the interaction between cell surface DCIR and HIV-1 leads to phosphorylation of tyrosine and threonine residues located in the ITIM domain of DCIR and activation of several second messengers. The association between this CLR and HIV-1 engages signal transduction events that positively affect the process of virus infection in cells proposed to play a determinant role in the pathogenesis of the disease (ie, DCs). The present work bears a clinical relevance, because blocking HIV-1 attachment to DCIR may represent a useful new strategy for fighting this retrovirus. Indeed, preventing the interaction between DCIR and HIV-1 can lead to a decrease of virus transmission during primo-infection, a period during which the virus is disseminated by mucosal DCs that can express DCIR toward CD4+ T cells residing in draining lymphoid organs. The importance of DCIR in HIV-1 pathogenesis is supported further by the recent demonstration that this CLR is promoted in CD4+ T cells upon virus infection (ie, under in vitro and in vivo situations) and this phenomenon results in more efficient HIV-1 attachment/entry, replication, and transfer processes.42
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Sylvie Méthot for her excellent technical assistance in writing this manuscript.
This work was supported by an operating grant to M.J.T. from the Canadian Institutes of Health Research (CIHR; MOP-79542). A.A.L. holds a CIHR Doctoral Award. C.G. is the recipient of a Scholarship Award Junior 1 level from the Fonds de la Recherche en Santé du Québec and a New Investigator Award from CIHR. M.J.T. holds the Canada Research Chair in Human Immuno-Retrovirology (Tier 1 level).
Authorship
Contribution: A.A.L. performed research, analyzed data, and wrote the paper; F.B. contributed plasmids used for derived stable cell lines expressing DCIR and wrote the paper; and C.G. and M.J.T. designed research, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Michel J. Tremblay, Centre de Recherche en Infectiologie, Centre Hospitalier Universitaire de Québec-CHUL, 2705 Boulevard Laurier, Québec (QC), Canada, G1V 4G2; e-mail: michel.j.tremblay@crchul.ulaval.ca; and Dr Caroline Gilbert, Centre de Recherche en Rhumatologie et Immunologie, Centre Hospitalier Universitaire de Québec-CHUL, 2705 Boulevard Laurier, Québec (QC), Canada, G1V 4G2; e-mail: caroline.gilbert@crchul.ulaval.ca.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal