Abstract
HIV-1 viral protein U (Vpu) is involved in ubiquitination and degradation of BM stromal cell Ag 2 and surface receptor CD4 through their recruitment to SCFβ-TrcP (Skp1/Cul1/F-box) ubiquitin ligase (SCF) complex. Here, we show that specific interaction of wild-type Vpu protein with SCF complex leads to inhibition of ubiquitination and proteasomal degradation of p53 protein in a β-TrcP–dependent manner. Successful interaction of SCFβ-TrcP complex with β-TrcP binding motif (DS52GNES56) present in Vpu is essential because mutant Vpu possessing specific alanine substitutions (DA52GNEA56) in the β-TrcP binding motif not only failed to stabilize p53 protein but was also unable to inhibit ubiquitination of p53 protein. Furthermore, Vpu competes efficiently with the interaction of p53 protein with the β-TrcP subunit of the SCF complex and inhibits subsequent ubiquitination of p53 proteins in a dose-dependent manner. We also observed potent apoptotic activity in a p53 null cell line (H-1299) that was cotransfected with p53 and Vpu-expressing plasmids. Furthermore, MOLT-3 (human T-lymphoblast) cells when infected with vesicular stomatitis virus glycoprotein–pseudotypic HIV-1 possessing wild-type vpu gene exhibited maximum activation of p53/Bax proteins and p53-mediated cell death. These findings establish a novel function of Vpu in modulating the stability of p53 protein that correlates positively with apoptosis during late stages of HIV-1 infection.
Introduction
HIV type 1 (HIV-1) Vpu (viral protein U) is an accessory gene exclusive to HIV-1 but not present in HIV-2 and in most SIVs.1-3 Studies in a macaque model have shown that Vpu from subtype B plays a crucial role in massive loss of circulating CD4+ T lymphocytes,4-6 which can be modulated by replacing it with Vpu from subtype C.7 The exact mechanism(s) underlying how Vpu makes HIV-1 more pathogenic is only partially understood.8,9 Vpu is involved in ubiquitination and degradation of antiretroviral restriction factor BM stromal cell Ag 2 (BST-2; also known as tetherin) and surface receptor CD4 through their recruitment to SCFβ-TrcP (Skp1/Cul1/F-box) ubiquitin ligase (SCF) complex.10-13 The key to biologic function of Vpu is the presence of a highly conserved 6–amino acid (DS52GNES56) sequence that constitutes the β-TrcP (β-transducin repeat-containing proteins) binding motif.14 This motif is constitutively phosphorylated and mediates interaction of Vpu with β-TrcP, the substrate recognition subunit of SCFβ-TrcP complex. Hence, Vpu acts as an adaptor linking its target proteins (CD4 and BST-2) to a host E3 ubiquitin ligase SCFβ-TrcP complex, which are otherwise not the natural substrates of this complex. However, unlike natural substrates of β-TrcP, which are targeted for degradation, Vpu itself is resistant to degradation and can form stable complexes with β-TrcP.15 Thus, expression of constitutively phosphorylated Vpu in infected cells leads to competitive inhibition of ubiquitination and subsequent proteosomal degradation of many natural substrates of SCFβ-TrcP complex (β-catenin, activating transcription factor 4, and Iκβ-α) and results in changes in the profile of cellular proteins that may contribute to cytopathic effects during HIV-1 infection.9,15,16 The inability to degrade Iκβ-α and to activate NF-κβ on stimulation with TNF-α explains the TNF-α–sensitive phenotype of Vpu-expressing cells.9 Interestingly, more recent data suggest that Vpu is also degraded by both β-TrcP–dependent17 and –independent pathways,18 which in turn may have a role in modulating biologic activities of Vpu.
The SCFβ-TrcP ubiquitin ligase complex controls the functions of a wide spectrum of cellular proteins16,19-22 ; many of them are involved in pathways crucial to HIV-1 pathology. Tumor suppressor protein p53 is reported to be a major player involved in HIV-1–induced apoptosis.23-25 p53 protein has been shown to be an important substrate of β-TrcP–dependent ubiquitination. Small interfering RNA (siRNA)–mediated depletion of components of SCFβ-TrcP complex or the expression of a dominant-negative form of β-TrcP was shown to enhance the stability of p53 protein and also to activate p53 downstream signaling events.22 Like most of the natural β-TrcP substrates (Iκβ-α, β-catenin, p65, and steroid receptor coactivator 3), the Iκβ kinase 2 (IKK2/IKKβ) also phosphorylates p53 at serine 362/366 (Ser362/366) position directing it for β-TrcP–mediated ubiquitination in an Mdm-2 independent manner,22 which is a well-studied negative regulator (E3 ubiquitin ligase) of p53.26 Interestingly, multiple viral proteins (Env and Vif) are known to trigger activation of p53 by different mechanisms with possible involvement of Mdm-2 ubiquitin ligase.27-30 Sequestration of β-TrcP by Vpu however is speculated to result in alteration of β-TrcP–dependent p53 ubiquitination that may further potentiate p53 activation during HIV-1 infection. In the present study, we demonstrate a novel effect of Vpu expression on p53 metabolism. We show that Vpu stabilizes p53 and inhibits its ubiquitination. This leads to enhancement of p53-mediated apoptosis during HIV-1 infection. Our findings suggest that Vpu (a late-stage protein unique to the HIV-1 genome) potently inhibits the β-TrcP–mediated pathway responsible for p53 degradation during HIV-1 infection that results in higher cell death observed during viral infection.
Methods
Plasmid constructs, proviral DNAs, and transient siRNA-mediated knockdown
Vpu from subtype B (pNL4-3) and C (Indian Isolate 93IN905) HIV-1 (obtained from Acquired Immunodeficiency Syndrome Research and Reference Reagent Program, Division of Acquired Immunodeficiency Syndrome, National Institute of Allergy and Infectious Diseases [NIAID], National Institutes of Health [NIH]) were amplified by PCR and cloned in the mammalian expression vector pCMV-HA (Clontech) to generate Vpu B-HA and Vpu C-HA constructs. The S52A and S56A mutations were introduced by site-directed mutagenesis with Pfu Turbo polymerase in pCMV Vpu B-HA to generate pCMV M-Vpu-HA (mutant Vpu) construct with the use of overlapping primers with the following sequences: motif forward, 5′-GGCAGAAGACGCAGGCAATGAGGCAGA-3′, and motif reverse, 5′-GGCTCGCACTCATTGCCGCAGTCTTCT-3′.
The pNL4-3 HIV-1 clone as well as mutant pNL4-3 (Mt; having Ser52/56 substituted with alanine 52/56 in Vpu open reading frame) or pNL4-3ΔVpu (having deletion in Vpu initiation codon) variants were kind gifts from K. Strebel (NIH).9 We used the infectious molecular clone of HIV-1 subtype C, pIndie as originally described by Mochizuki et al.31 Reporter virus pNLΔGFP (obtained from NIH) was used to ensure uniform infection in different cell lines. Viral stocks of pNL4-3 and its variants were prepared by cotransfecting different proviral constructs (pNL4-3, Mt pNL4-3, pNL4-3ΔVpu, and pIndie) and plasmid-encoding vesicular stomatitis virus glycoprotein (VSV-G) into HEK 293T cells, followed by collection of virion particles from culture supernatant fluid at 48 and 72 hours.9 The shRNA sequence against β-TrcP that was reported previously22 was cloned in RetroQ-Zs Green Vector. HA-p53 and GST-p53 expression plasmids were a kind gift from Yukiko Gotoh (Institute of Molecular and Cellular Biosciences, University of Tokyo)32 and Sanjeev Das (National Institute of Immunology)33 respectively. p53ΔI-expressing plasmid was provided by Karen Vousden (Beatson Institute of Cancer Research).34
Cell culture, transfections, and immunoblot analysis
HEK 293T (human embryonic kidney 293 cells), MCF-7 (breast cancer cell line, p53 Wt), and H-1299 (nonsmall cell lung carcinoma, p53 null) cells were maintained in DMEM (Gibco, Invitrogen) supplemented with glutamine, 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) at 37°C with 5% CO2. MOLT-3 T cells (T-lymphoblastoid cell line, p53 Wt) and K-562 cells (human erythroleukemia cell line, p53 null) were maintained in RPMI (Gibco, Invitrogen) media supplemented with glutamine, 10% FCS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen) at 37°C with 5% CO2. Plasmid transfections were performed with Lipofectamine 2000 (Invitrogen). Relative levels of different proteins were compared by immunoblot analysis. Cells were lysed with RIPA Lysis buffer (20mM Tris [pH 7.5], 150mM NaCl, 1mM Na2ethylenediaminetetraacetic acid, 1mM EGTA, 1% NP-40, 1% sodium deoxycholate, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, and 1 μg/mL leupeptin). Protein estimation was done with the use of BCA Protein Estimation Kit (Pierce Biotechnology Inc). Proteins were resolved with PAGE and transferred to Immobilon membrane (Millipore). The membranes were blocked with 5% nonfat dry milk (Sigma-Aldrich) in PBS, washed with PBS containing 0.1% Tween 20 (Merck) and incubated in the same buffer overnight at 4°C in the presence of primary Ab (1:2000 dilution). The primary Abs used were anti-p53, anti-GST, anti-His, anti-HA, anti-GAPDH, anti–β-TrcP, anti–Iκβ-α, anti-Bax (Santa Cruz Biotechnology), anti–Ser362/366 phosphorylated p53 (P-362/366 p53; Abcam) and anti-Ser15 phosphorylated p53 (P-15 p53; Cell Signaling Technology). The membranes were washed with PBS containing 0.1% Tween 20 and then incubated with secondary Ab of either anti–mouse or anti–rabbit conjugated with HRP (1:10 000 dilution; Jackson ImmunoResearch Laboratories Inc) in 5% nonfat dry milk in PBS with 0.1% Tween 20 at room temperature. The proteins of interest were detected with EZ Western HRP substrate (Biologic Industries). GAPDH was used as a loading control in all cases.
Cycloheximide chase assay
Untransfected (1.2 × 106) or transfected (2 μg of Wt or mutant Vpu) MCF-7 cells in a 35-mm dish were incubated for 36 hours. Subsequently, cycloheximide (Sigma-Aldrich) was added to a final concentration of 100 μg/mL, and cells were harvested after the indicated time points. The lysate was prepared, and immunoblotting was performed as described in the preceding paragraph.
GST pull-down assay
To study the effect of Vpu on the interaction between p53 and β-TrcP, GST pull-down assay was performed. In 100-mm dishes HEK 293T cells were transfected with 5 μg of GST-p53–expressing plasmid pEBG-p53 in the presence or absence of Vpu-expressing plasmid in a 1:1 ratio. GST-expressing plasmid pEBG was used as control. Cells were harvested 48 hours after transfection and suspended in 1 mL of GST lysis buffer (50mM Tris [pH 7.5], 0.1M NaCl, 50mM β-glycerophosphate, 50mM NaF, 1mM Na3VO4, 50mM sodium pyrophosphate, 2mM EDTA, 1mM EGTA, 1% NP-40, 2 μg/μL aprotinin, 1 μg/μL leupeptin, 0.7 μg/mL pepstatin, and 1mM phenylmethylsulfonyl fluoride). The samples were sonicated on ice and centrifuged, and 60 μL of 50% Glutathione Agarose beads (Sigma-Aldrich) were added. The samples were incubated at 4°C for 4 hours while rotating; subsequently, the beads were washed twice with lysis buffer and boiled in SDS loading buffer. The proteins were separated on 12% SDS-PAGE and immunoblotted with anti-GST and anti–β-TrcP Abs.
In vivo ubiquitination assay
For detection of ubiquitinated p53 protein, HEK 293T cells were grown in 100-mm dishes and transfected with 5 μg of 6× His-ubiquitin expression plasmid35 along with equal amounts of various Vpu-expressing plasmids. To equalize the DNA amount pCMV-HA vector was used. After 36 hours of transfections, 25 μM of MG-132 (Sigma-Aldrich) was added, and cells were further incubated for 8 hours. Thereafter, cells were collected in PBS and were resuspended in 1 mL of lysis buffer (6M guanidinium-HCl, 0.1M Na2HPO4/NaH2PO4, 0.01M Tris, pH 8.0, 5mM imidazole, and 10mM 2-ME), sonicated, and centrifuged. Ni-NTA beads (50 μL) were added to the supernatant fluid, and the mixture was incubated at room temperature for 4 hours while rotating. Subsequently, the beads were washed for 5 minutes at room temperature with 750 μL of each of the following buffers: 6M guanidinium-HCl, 0.1M Na2HPO4/NaH2PO4, 0.01M Tris, pH 8.0, and 10mM 2-ME; 8M urea, 0.1M Na2HPO4/NaH2PO4, 0.01M Tris, pH 8.0, 10mM 2-ME; 8M urea, 0.1M Na2HPO4/NaH2PO4, 0.01M Tris, pH 6.3, 10mM 2-ME (buffer A) plus 0.2% Triton X-100; buffer A and then buffer A plus 0.1% Triton X-100. After the last wash ubiquitinated proteins were eluted by incubating the beads in 75 μL of buffer containing 200mM imidazole, 5% SDS, 0.15M Tris, pH 6.7, 30% glycerol, 0.72M 2-ME for 20 minutes at room temperature. The eluates were mixed in a 1:1 ratio with 2× Laemmli buffer and separated the proteins on 8% SDS-PAGE followed by immunoblotting with anti-p53 Ab.
SubG1 DNA content analysis and cell death assay
SubG1 DNA content analysis was performed for determination of apoptotic population.36 Cells were harvested, fixed in ethanol, and stained with propidium iodide (PI) staining solution (50 μg/mL PI, 50 μg/mL ribonuclease A, 0.1% saponin, and 5mM EDTA in PBS) for 30 minutes at 37°C and analyzed on a BD FACSCaliber. To study the effect of p53 in Vpu-mediated apoptosis, we transfected H-1299 cells (1.2 × 106 cells in 35-mm culture plates) with 2 μg of p53-expressing plasmid alone or in combination with Vpu variants in a 1:1 ratio. We used the empty control vector pCMV-HA to normalize equal amounts of DNA in each transfection. Two to 3 days after transfection cells were harvested, fixed in ethanol, and stained with PI for SubG1 DNA content analysis. Cell death was measured according to the earlier published procedures.37,38 The FACS data were analyzed by WinMDI 2.8 software (Joseph Trotter, Scripps Research Institute).
Infection by HIV-1 pNL4-3 or HIV-1 mutants
MOLT-3 T cells were infected with pNL4-3, Mt pNL4-3, or pNL4-3ΔVpu or mock infected (as a control) in the presence of 4 μg/mL Polybrene (Sigma-Aldrich) for 4 hours at 37°C. Infection was accomplished by incubating the cells for 4 hours with equal amounts of infectious virus (1 MOI) assessed by β-galactosidase staining with the use of HIV-1 indicator Tzmbl cells.39 The infected cells were harvested 48 hours after infection and subjected to immunoblotting with indicated Abs. Another set of cells was stained with PI (10 μg/mL) for determining the total cell death. To determine whether Vpu potentiated p53-mediated apoptosis in HIV-infected cells, we treated a parallel set of infected cells with 30μM Pifithrin-α (Santa Cruz Biotechnology), which is a small molecule inhibitor of p53 transcriptional activity.29,40 Briefly, after 24 hours of infection, the samples were divided into 2 equal sets, one set was treated with Pifithrin-α and the other set served as an untreated control. The cell death was determined by PI staining.
Results
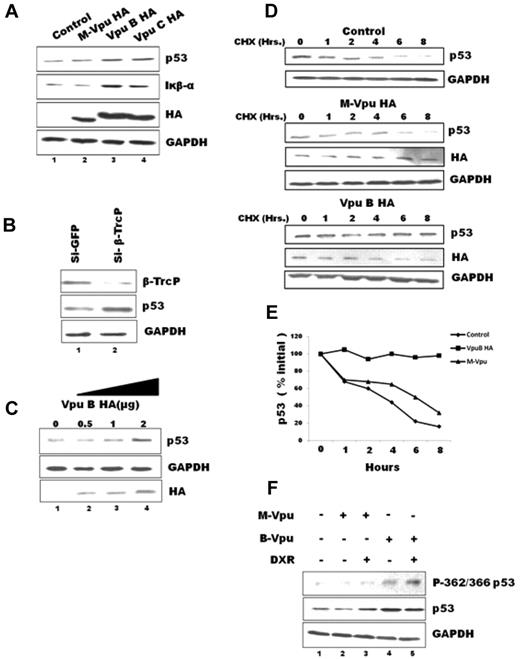
Vpu expression leads to cellular accumulation and stabilization of p53 and P-362/366 p53
Vpu is a competitive inhibitor of β-TrcP function and is known to stabilize natural β-TrcP substrates. To test whether Vpu expression can inhibit β-TrcP–dependent degradation of p53 protein, we compared relative p53 protein levels in MCF-7 cells on transfecting Vpu variants. This cell line expresses Wt p53 protein constitutively. Expression of Wt subtype B and C Vpu led to cellular accumulation of p53 (Figure 1A lanes 3-4). Iκβ-α, which is another β-TrcP substrate known to be stabilized on Vpu expression,15 was also elevated in Wt Vpu-expressing cells (Figure 1A lanes 3-4). The observed effect of Wt Vpu on p53 level was β-TrcP dependent, because the mutant Vpu B (M-Vpu), in which the 2 serine residues were mutated to alanine in the β-TrcP binding motif, failed to show any stabilization of p53 protein (Figure 1A lane 2). All the 3 Vpu constructs directed synthesis of comparable levels of intracellular Vpu protein that was detected by immunoblotting with the use of anti-HA Ab (Figure 1A lanes 2-4). The GAPDH protein was used as a loading control for each lane that remained unchanged. We further confirmed this finding by knocking down β-TrcP with specific siRNA (Figure 1B lane 2, explained in the legend), which also led to accumulation or stabilization of p53 protein (Figure 1B lane 2). The cellular expression of Wt Vpu B also up-regulated p53 protein in a dose-dependent manner (Figure 1C lanes 2-4). The cycloheximide (CHX) chase assay was performed to study the effect of Vpu expression on the turnover of p53 protein (Figure 1D). After 8 hours of chase period, the level of p53 was reduced by ∼ 80% in untransfected MCF-7 cells as well as in M-Vpu–expressing cells (Figure 1D and quantitation shown in Figure 1E). In Wt Vpu B–expressing cells, the p53 levels remained almost unchanged after 8 hours of CHX chase (Figure 1E), confirming that Vpu protein increases half-life of p53 protein by decreasing its rate of degradation. We conclude that Wt Vpu expression led to stabilization of p53 protein via inhibition of β-TrcP function. We next examined the cellular levels of P-362/366 p53, which is the substrate form of p53 protein recognized by β-TrcP, using specific phospho-Ab (Abcam) by immunoblot analysis (Figure 1F). As expected, control untransfected cells and M Vpu–expressing cells showed barely detectable levels of phosphorylated p53 (Figure 1F lanes 1-2). However, expression of Wt Vpu resulted in significant up-regulation of phosphorylated p53 (Figure 1F lane 4). Up-regulation of phosphorylated p53 was also observed when cells were treated with IKK2 activator doxorubicin (Figure 1F lane 3). Interestingly, highest levels of phosphorylated p53 were observed when Vpu-expressing MCF-7 cells were treated with doxorubicin, showing an additive effect of Vpu and doxorubicin on the levels of phosphorylated p53 (Figure 1F lane 5). These data suggest that phosphorylated p53 gets accumulated because of inactive SCFβ-TrcP complex rendered by Vpu interaction. An internal control (GFP) was always included to normalize transfection efficiency, and equal loading was confirmed by GAPDH levels for all transfection-based experiments.
Expression of Vpu B-HA or Vpu C-HA in MCF-7 cells results in the accumulation of p53 and P-362/366 p53. (A) Equal amounts (2 μg) of Vpu B, Vpu C, or M Vpu-HA were transfected into MCF-7 cells by Lipofectamine 2000 for 48 hours. Cell lysates were subjected to immunoblotting with indicated Abs. (B) MCF-7 cells were transfected with siRNA against GFP or β-TrcP, and immunoblot analysis was done. (C) Increasing amounts (0.5, 1, and 2 μg) of Vpu B-HA was transfected into MCF-7 cells. (D) Cycloheximide (CHX) chase to check half-life of endogenous p53, MCF-7 cells either untransfected or transfected with M-Vpu HA or Vpu B-HA, were treated with 100 μg/mL CHX and harvested at the indicated time points for immunoblotting. (E) After quantification, the signals obtained in panel D were used to calculate the p53/GAPDH ratios that were plotted with respect to treatment period. (F) Equal amounts of Vpu B, Vpu C, or M Vpu-HA were transfected into MCF-7 followed by treatment with 1 μg/mL doxorubicin for 12 hours. Immunoblotting analysis was performed with anti-p53, anti–Iκβ-α, anti–β-TrcP, anti-HA, anti–P-362/366 p53, and anti-GAPDH Abs. GAPDH was used as a loading control. This is a representative result obtained from 3 independent experiments.
Expression of Vpu B-HA or Vpu C-HA in MCF-7 cells results in the accumulation of p53 and P-362/366 p53. (A) Equal amounts (2 μg) of Vpu B, Vpu C, or M Vpu-HA were transfected into MCF-7 cells by Lipofectamine 2000 for 48 hours. Cell lysates were subjected to immunoblotting with indicated Abs. (B) MCF-7 cells were transfected with siRNA against GFP or β-TrcP, and immunoblot analysis was done. (C) Increasing amounts (0.5, 1, and 2 μg) of Vpu B-HA was transfected into MCF-7 cells. (D) Cycloheximide (CHX) chase to check half-life of endogenous p53, MCF-7 cells either untransfected or transfected with M-Vpu HA or Vpu B-HA, were treated with 100 μg/mL CHX and harvested at the indicated time points for immunoblotting. (E) After quantification, the signals obtained in panel D were used to calculate the p53/GAPDH ratios that were plotted with respect to treatment period. (F) Equal amounts of Vpu B, Vpu C, or M Vpu-HA were transfected into MCF-7 followed by treatment with 1 μg/mL doxorubicin for 12 hours. Immunoblotting analysis was performed with anti-p53, anti–Iκβ-α, anti–β-TrcP, anti-HA, anti–P-362/366 p53, and anti-GAPDH Abs. GAPDH was used as a loading control. This is a representative result obtained from 3 independent experiments.
Wt Vpu inhibits association of p53 with β-TrcP and its subsequent ubiquitination
Having established that Vpu inhibits β-TrcP–mediated degradation of p53, we next investigated the molecular mechanism underlying this phenomenon. Degradation of cellular proteins by SCFβ-TrcP complex involves binding of natural substrates with WD40 repeat domain of β-TrcP, and Vpu is known to compete with natural substrates for binding with β-TrcP.16 We therefore evaluated whether Vpu affects intracellular interaction of β-TrcP with p53 protein by pull-down assay. As shown (Figure 2A) expression of M Vpu had little or no effect on β-TrcP binding with p53 (Figure 2A lane 2) and is the same as in control cells (Figure 2A lane 1). However, expression of both Wt Vpu B and C proteins inhibited β-TrcP binding with p53 (Figure 2A lanes 3-4). The total β-TrcP levels were not altered by Vpu expression. Because binding of natural substrate with β-TrcP leads to its ubiquitination, we next evaluated whether Vpu expression inhibited p53 ubiquitination. Both Wt Vpu B and Vpu C inhibited ubiquitination of p53 (Figure 2B lanes 3-4). However, cells transfected with M Vpu showed p53 ubiquitination profile identical to that of untransfected cells (Figure 2B lanes 1-2). Furthermore, we performed a dose-response study of inhibition of p53 ubiquitination with Wt Vpu B and M Vpu. Wt Vpu B transfections (Figure 2C lanes 5-7) resulted in significant inhibition of p53 ubiquitination in a dose-dependent manner, confirming the competitive nature of inhibition of SCFβ-TrcP function by Vpu. In contrast M Vpu (Figure 2C lanes 2-4) did not inhibit p53 ubiquitination in all 3 doses tested. These results show that Vpu competitively inhibits association of β-TrcP with p53 protein and its subsequent ubiquitination in a dose-dependent manner.
Effect of Vpu expression on β-TrcP interaction with and ubiquitination of p53. (A) HEK 293T cells were cotransfected with plasmids encoding GST-p53 and either Vpu B, Vpu C, or M Vpu-HA for 48 hours as described in “Cell culture, transfections, and immunoblot analysis.” Cell lysates were used to pull down GST-p53–associated proteins with the use of Glutathione beads, and the resulting eluent was used to measure GST-p53–bound β-TrcP by immunoblotting with anti–beta]-TrcP Ab. Total cellular β-TrcP levels were not altered on Vpu expression. (B) HEK 293T cells were cotransfected with His-ubiquitin (His-Ub) and either Vpu B, Vpu C, or M Vpu-HA. After 36 hours cells were treated with MG132 for 8 hours followed by lysis in denaturation buffer, and then total ubiquitinated proteins were pulled down with the use of Ni-NTA beads, and p53 ubiquitination was checked by immunoblotting with anti-p53 Ab. (C) HEK 293T cells were cotransfected with His-Ub, and an increasing dose (0.5, 1, and 2 μg) of either M-Vpu or Wt Vpu B-HA and p53 ubiquitination was checked as described in panel B.
Effect of Vpu expression on β-TrcP interaction with and ubiquitination of p53. (A) HEK 293T cells were cotransfected with plasmids encoding GST-p53 and either Vpu B, Vpu C, or M Vpu-HA for 48 hours as described in “Cell culture, transfections, and immunoblot analysis.” Cell lysates were used to pull down GST-p53–associated proteins with the use of Glutathione beads, and the resulting eluent was used to measure GST-p53–bound β-TrcP by immunoblotting with anti–beta]-TrcP Ab. Total cellular β-TrcP levels were not altered on Vpu expression. (B) HEK 293T cells were cotransfected with His-ubiquitin (His-Ub) and either Vpu B, Vpu C, or M Vpu-HA. After 36 hours cells were treated with MG132 for 8 hours followed by lysis in denaturation buffer, and then total ubiquitinated proteins were pulled down with the use of Ni-NTA beads, and p53 ubiquitination was checked by immunoblotting with anti-p53 Ab. (C) HEK 293T cells were cotransfected with His-Ub, and an increasing dose (0.5, 1, and 2 μg) of either M-Vpu or Wt Vpu B-HA and p53 ubiquitination was checked as described in panel B.
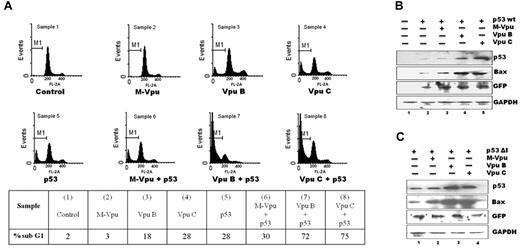
Vpu potentiates apoptotic activity of exogenous p53 in p53 null (H-1299) cells
We further analyzed the functional effect of Vpu-mediated inhibition of p53 ubiquitination on biologic activity of both Vpu and p53. Expression of p53 leads to apoptosis because of activation of proapoptotic protein Bax,41 whereas Vpu sensitizes cells for apoptosis by inhibiting NF-κβ activation.9,15 To address the question of whether Vpu induces apoptosis by the activation of p53 protein, we used a p53-null cell line (H-1299) and exogenously expressed both proteins followed by measurement of subG1 DNA content containing apoptotic population by PI staining (Figure 3A) according to previously reported protocol.36 The proportion of apoptotic cells was < 5% in M Vpu–transfected as well as untransfected cells (Figure 3A samples 1-2). Cells transfected either with p53 or Wt Vpu B or Vpu C constructs showed moderate levels of apoptosis (Figure 3A samples 3-5, between 18% and 30%). However, when both p53 and Wt Vpu B or C were expressed, there was a synergistic increase in the apoptotic population (Figure 3A samples 7-8, between 72% and 75%). In contrast, cells transfected with p53 and M Vpu showed apoptotic population close to what was observed in cells transfected with p53 construct alone (Figure 3A sample 6, 30%). These results clearly establish the physiologic consequences that result from inhibitory influence of Vpu on p53 degradation. Bax is an immediate downstream target gene known to get up-regulated in p53-mediated apoptotic pathway, cellular levels of which is directly controlled by p53. We therefore checked cellular levels of Bax in H-1299 cells after cotransfection of p53- and Vpu-expressing constructs. As shown (Figure 3B), we failed to detect any expression of Bax protein in untransfected H-1299 cells (Figure 3B lane 1), but its expression was induced in cells transfected with p53 (Figure 3B lane 2). Wt Vpu B and C constructs were able to significantly up-regulate Bax protein (Figure 3B lanes 4-5). Furthermore, to rule out the possibility of involvement of Mdm-2 ubiquitin ligase for p53 degradation, we performed a parallel set of transfections for 48 hours with plasmid encoding Mdm-2–binding mutant form of p53 protein (p53ΔI).34 As shown (Figure 3C) the Bax levels increased substantially in cells coexpressing p53ΔI and Wt Vpu B or C (Figure 3C lanes 3 and 4) in comparison to cells expressing M Vpu and p53ΔI (Figure 3C lane 2). Hence, we conclude that Vpu augments apoptotic activity of p53 in a synergistic and β-TrcP–dependent but Mdm-2–independent manner.
The functional effect of Vpu expression on p53-mediated apoptosis. (A) H-1299 (p53 null) cells were cotransfected with plasmids encoding p53 and either Vpu B, Vpu C, or M Vpu-HA. Then after 72 hours, cells were harvested, fixed, and stained with PI, and subG1 DNA content was determined by FACS to see relative proportion of apoptotic population that is summarized below the histograms. (B.) H-1299 cells were cotransfected with p53wt and Vpu B, Vpu C, or M Vpu-HA, and endogenous Bax levels were checked by immunoblotting analysis with the indicated Abs. (C.) H-1299 cells were cotransfected with indicated plasmids, and cell lysates were subjected to immunoblot analysis. This is a representative result obtained from 3 independent experiments.
The functional effect of Vpu expression on p53-mediated apoptosis. (A) H-1299 (p53 null) cells were cotransfected with plasmids encoding p53 and either Vpu B, Vpu C, or M Vpu-HA. Then after 72 hours, cells were harvested, fixed, and stained with PI, and subG1 DNA content was determined by FACS to see relative proportion of apoptotic population that is summarized below the histograms. (B.) H-1299 cells were cotransfected with p53wt and Vpu B, Vpu C, or M Vpu-HA, and endogenous Bax levels were checked by immunoblotting analysis with the indicated Abs. (C.) H-1299 cells were cotransfected with indicated plasmids, and cell lysates were subjected to immunoblot analysis. This is a representative result obtained from 3 independent experiments.
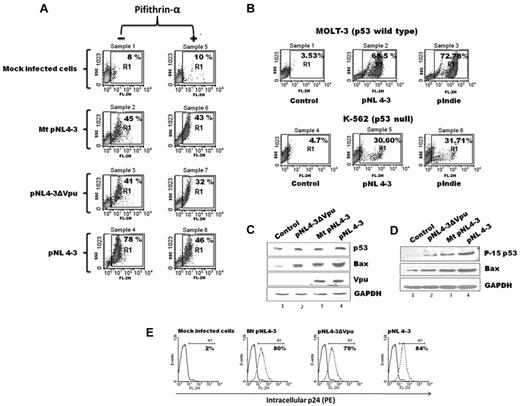
Vpu contributes to p53-dependent apoptosis in HIV-1–infected MOLT-3 cells
Next, we investigated whether the observed phenomenon of p53 induction and p53-dependent apoptosis by exogenously expressed Vpu contributes to apoptotic potential of HIV-1 during infection in a biologically relevant T-cell line (MOLT-3). To address this question, MOLT-3 T cells expressing Wt p53 were infected with an equal MOI (1) of the following VSV-G–pseudotyped viruses: pNL4-3 (Wt-HIV), pNL4-3ΔVpu, and Mt pNL4-3 for 48 hours. Thereafter, the cells were stained with PI and subjected to FACS analysis to determine the extent of cell death, and the results are shown in Figure 4A left panels. As expected the control cells showed only background activity (8%). The Mt pNL4-3 and ΔVpu-HIV constructs exhibited 45% and 41% cell death, respectively. In comparison, HIV harboring the Wt Vpu B (pNL4-3) exhibited 78% cell death under exactly similar conditions. A parallel set of experiments was performed with the use of the same viral constructs in the presence of p53 inhibitor Pifithrin-α to determine the cytotoxic contribution of p53 alone in these cells,29,40 and the results are shown in Figure 4A right panels. Interestingly, the extent of inhibition of cell death in the presence of Pifithrin-α was also highest in Wt HIV–infected cells (Figure 4A sample 8, 46% from 78% in sample 4) than in cells infected with either Mt HIV (Figure 4A sample 6, 43% from 45% in sample 2) or ΔVpu HIV (Figure 4A sample 7, 32% from 41% in sample 3). These data confirm that Vpu independently contributes substantially to p53-dependent apoptosis during HIV-1 infection. Next, we wanted to compare the ability of HIV-1 subtypes B and C in mediating p53-mediated apoptosis. For this purpose MOLT-3 (p53 Wt) and K-562 (p53 null) cells were infected with an equal MOI (1) of pNL 4-3 (subtype B) and pIndie (subtype C). We initially ensured that pseudotyped viruses were equally infectious for both the cell lines with the use of the pNLΔGFP reporter virus. The extent of cell death was determined by PI staining shown in Figure 4B, and the results are shown in Figure 4B. We always observed more cell death (almost double) in MOLT-3 (Figure 4 B samples 2 and 3, 68% and 72%) than in K-562 cells (Figure 4 B samples 5 and 6, 30% and 31%). Thus, HIV-1 subtypes B and C were equally potent in causing cell death, and p53 contributed substantially to the cell death.
Vpu contributes to p53-mediated apoptosis during infection. MOLT-3 T cells or K-562 cells were infected with an MOI of 1 with indicated VSV-G–pseudotyped HIV-1 virus. (A) Total cell death was measured after 48 hours of infection of MOLT-3 cells by PI staining by FACS: PI (FL-2H), (left) live cells (PI−), (right; region R1): dead cells (PI+). p53-mediated apoptosis was inhibited by treating cells with 30μM Pifithrin-α for 24 hours (samples 5-8) as described in “Infection by HIV-1 pNL4-3 or HIV-1 mutants.” Percentage of cell death is indicated for each sample. (B) Total cell death (region R1) was measured 48 hours after infection of MOLT-3 and K-562 cells with indicated viruses. After 48 hours of infection cell lysates were prepared, and immunoblotting was done with indicated Abs (C-D). Cells were evaluated for the extent of HIV-1 infection by intracellular p24 staining with primary p24 rabbit Ab (NIH) followed by secondary anti–rabbit Ab (phycoerythrin conjugated). (E) Representative pictures showing comparable infection efficiency of the various subtype B viruses in MOLT-3 cells by intracellular p24 staining.
Vpu contributes to p53-mediated apoptosis during infection. MOLT-3 T cells or K-562 cells were infected with an MOI of 1 with indicated VSV-G–pseudotyped HIV-1 virus. (A) Total cell death was measured after 48 hours of infection of MOLT-3 cells by PI staining by FACS: PI (FL-2H), (left) live cells (PI−), (right; region R1): dead cells (PI+). p53-mediated apoptosis was inhibited by treating cells with 30μM Pifithrin-α for 24 hours (samples 5-8) as described in “Infection by HIV-1 pNL4-3 or HIV-1 mutants.” Percentage of cell death is indicated for each sample. (B) Total cell death (region R1) was measured 48 hours after infection of MOLT-3 and K-562 cells with indicated viruses. After 48 hours of infection cell lysates were prepared, and immunoblotting was done with indicated Abs (C-D). Cells were evaluated for the extent of HIV-1 infection by intracellular p24 staining with primary p24 rabbit Ab (NIH) followed by secondary anti–rabbit Ab (phycoerythrin conjugated). (E) Representative pictures showing comparable infection efficiency of the various subtype B viruses in MOLT-3 cells by intracellular p24 staining.
Finally, we examined the cellular levels of p53 and Bax protein in MOLT-3 cells infected with the 3 subtype B viruses mentioned in the previous paragraph. Moderate elevation in the levels of total cellular p53 protein was observed after Wt HIV infection, but a significant up-regulation was observed for Bax protein compared with the other 2 viruses (Figure 4C lane 4). Intracellular expression of Vpu protein was monitored in each case, and as expected only Wt and Mt HIV virus directed the synthesis of authentic Vpu proteins (Figure 4C lanes 3-4). GAPDH protein was monitored as internal control, which remained unchanged. We conclude that HIV-1 infection in T cells results in moderate elevation of total intracellular p53 but a significant elevation in the Bax protein levels, both of which were mediated by Vpu. We then wanted to determine whether P-15 p53 levels correlated with Bax levels, because it is known to get specifically up-regulated under stress conditions, including viral infection.23 Remarkably, the intracellular levels of biologically more active P5 p5342 correlated with the Bax expression levels (Figure 4D).
Discussion
Exploitation of cellular pathways that govern apoptosis plays a critical role in pathophysiologic outcomes of viral infection and is achieved by direct or indirect interaction of viral proteins with key components of highly regulated cell survival and death pathways. The outcome of this interaction depends on the viral life cycle where the viral genes are expressed in a temporal manner during the course of virus replication.43 Early suppression of apoptotic pathways in an infected mammalian cell is desired because it will result in more virus production and subsequently the release at a later stage because of apoptosis and cell lysis.
HIV-1 has evolved several mechanisms to modulate apoptotic pathways differentially during viral life cycle. Most of the HIV-1–encoded proteins are known to have either proapoptotic or antiapoptotic properties or both, for example, Gp120 as well as protease are known to be proapoptotic, whereas Tat, Nef, and Vpr proteins have been shown to possess both antiapoptotic and proapoptotic activities.44,45 Because some of these proteins (Tat, Nef) are expressed early in HIV-1 infection and some are expressed late (Gp120, Vpu, Vpr), the ultimate outcome with respect to apoptosis will vary at different stages of virus life cycle. Clearly, HIV-1 uses multiple genes to modulate apoptotic machinery to its advantage.
The tumor suppressor protein p53 is one of the dominant proapoptotic transcriptional factors elicited specifically in HIV-1–infected cells and not in uninfected lymphocytes.24,25 The exact contribution of p53 protein in HIV-1 replication has been controversial because it has been shown to play a positive as well as a negative role in HIV-1 replication.46,47 Besides, multiple viral proteins are known to alter cellular p53 levels and its phosphorylation status to manipulate cell survival and death pathways.27-29,48 Cellular expression of Vif and Tat, as well as Env is known to trigger p53 activation and associated downstream events. Nef, however, is a potent inhibitor of p53 functions, binds to p53 protein, and targets it for degradation.48 In summary, overall p53 activity is modulated differentially by viral proteins. Although Vpu is known to influence a number of key cellular events, its possible involvement with p53 function was not reported earlier.
A recent report identified β-TrcP as a novel regulator of p53 protein stability and biologic activity.22 Because Vpu is a general inhibitor of β-TrcP function,16 we investigated a potential role of Vpu on the stability of p53 protein and its ensuing physiologic consequences. We clearly show that Vpu up-regulates p53 in a cell line that constitutively expresses Wt p53 (MCF-7). The same was observed when Vpu and p53 were cotransfected in a p53-null cell line (H-1299). The results are in agreement with the known functions of Vpu. It exploits SCFβ-TrcP complex for degradation of CD4 and BST-2 and simultaneously causes the accumulation of natural β-TrcP substrates. The increase in the stability of p53 protein in the presence of Vpu suggests that the accumulation is because of inhibition of p53 degradation. In addition, inhibitory influence of Vpu on interaction between p53 and β-TrcP further supports the hypothesis that Vpu inhibits p53 degradation by exploitation of SCF complex needed for β-TrcP–mediated ubiquitination. The ubiquitination profile of p53 protein in the presence of Wt and Mt Vpu clearly establish the inhibitory role of Vpu in p53 ubiquitination. Having shown the effect of Vpu expression on p53 metabolism, we further investigated the physiologic consequences of p53 accumulation during virus infection. Vpu is a late-stage viral protein, presence of which has been shown to correlate with massive loss of CD4+ T lymphocytes in macaque models. The p53 protein was also found to be elevated in the late stage of viral infection that is associated with increased apoptosis.23,25 On the basis of our findings it can be safely argued that Vpu is an important contributor to this phenomenon. To study additive or synergistic influence of Vpu on p53-mediated apoptosis, we used a p53-null cell line. The total apoptotic cells (as measured quantitatively by subG1 DNA-containing cells) in Mt Vpu- and p53-transfected cells were found to be additive. In contrast, cells expressing Wt Vpu and p53 protein exhibited synergistic effect with respect to apoptosis. These data functionally complement our earlier results of Vpu-mediated p53 accumulation. Because p53-mediated apoptosis involves Bax up-regulation, we measured the Bax and p53 protein levels in Wt and Mt Vpu-expressing cells. The Bax level was highly elevated in Wt Vpu-expressing cells that further confirmed that Vpu mediated accumulated p53 protein has an important functional consequence in promoting Bax-mediated apoptosis. A similar pattern was observed with Mdm-2 binding mutant of p53, indicating the Mdm-2–independent nature of this phenomenon. The higher Bax activation in samples containing Mdm-2 binding p53 mutant is most probably because of its high transactivation potential as reported previously.34 Finally, we demonstrate the role of Vpu in up-regulating p53, which, in turn, results in augmenting p53-mediated cell death in HIV-1–infected cells. It is important to note that we observed maximum cell death in Wt HIV-1 (pNL 4-3) infected MOLT-3 cells and on Pifithrin-α treatment, the same cells exhibited maximum reduction with respect to cell death. The importance of p53 in causing cell death was evaluated by infecting MOLT-3 cells (p53 Wt) and K-562 cells (p53 null) with HIV-1 subtypes B or C. As expected, under identical conditions higher cell death was observed in MOLT-3 cells than in K-562 cells, establishing the role of p53 in augmenting HIV-1–mediated apoptosis. We observed that the proapoptotic Bax levels correlated with P-15 p53 and not total cellular p53 in infected cells. It is predominantly this phosphorylated p53 form that gets up-regulated on stress conditions or on viral infection.23 These findings further established the important contribution of Vpu in up-regulating p53 levels during HIV-1 infection.
Taken together, in this study we demonstrate that Vpu inhibits p53 ubiquitination and degradation by inhibiting β-TrcP function, thereby leading to its enhanced stability, cellular accumulation with concurrent increase in apoptosis. Earlier studies9,15 clearly have shown that Vpu causes apoptosis by inhibiting activation of NF-κβ–induced antiapoptotic gene expression. The sub-G1 DNA content analysis presented in Figure 3A (samples 3 and 4) verified that this pathway is indeed active and that stabilization of p53 by Vpu further enhances apoptosis (Figure 3A samples 7 and 8). Recently HIV-1 Vif was shown to promote p53 activation by inhibition of Mdm-2–mediated pathway,29 but our work establishes that another late accessory protein, Vpu, competitively blocks the β-TrcP–mediated pathway for p53 degradation. Therefore, by exploiting SCFβ-TrcP complex, Vpu enables HIV-1 to completely hijack β-TrcP/p53/Mdm-2 axis to achieve higher p53-mediated apoptotic potential. These results show an important implication of inhibition of p53 ubiquitination by Wt Vpu that correlates positively toward promoting apoptosis.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Sanjeev Das (NII, India) for GST-p53 expression plasmid, Yukiko Gotoh (Institute of Molecular and Cellular Biosciences, The University of Tokyo) for HA-p53 expression plasmid, Karen Vousden (The Beatson Institute of Cancer Research) for p53ΔI expression plasmid, Dimitris Xirodimas (University of Dundee) for His-Ubiquitin expression plasmid. We thank Klaus Strebel (NIH) for generously providing HIV-1 clone pNL4-3 and its Vpu variants and Udaykumar Ranga (JNCASR, India) for subtype C pIndie HIV-1 plasmid. We also thank Aalia Shahr Bano for technical help.
This work was supported by the Department of Biotechnology, Government of India (A.C.B. and NII, New Delhi). Several reagents were obtained from the Acquired Immunodeficiency Syndrome Research and Reference Reagent Program, Division of Acquired Immunodeficiency Syndrome, NIAID, NIH.
Authorship
Contribution: S.V. and A.C.B. designed the research; S.V. and A.A. performed the research; S.V. analyzed and interpreted the data; and S.V., A.A., S.A., and A.C.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Akhil C. Banerjea, Laboratory of Virology, National Institute of Immunology, JNU Campus, New Delhi-110067, India; e-mail: akhil@nii.res.in or akhil_banerjea@yahoo.com.

![Figure 2. Effect of Vpu expression on β-TrcP interaction with and ubiquitination of p53. (A) HEK 293T cells were cotransfected with plasmids encoding GST-p53 and either Vpu B, Vpu C, or M Vpu-HA for 48 hours as described in “Cell culture, transfections, and immunoblot analysis.” Cell lysates were used to pull down GST-p53–associated proteins with the use of Glutathione beads, and the resulting eluent was used to measure GST-p53–bound β-TrcP by immunoblotting with anti–beta]-TrcP Ab. Total cellular β-TrcP levels were not altered on Vpu expression. (B) HEK 293T cells were cotransfected with His-ubiquitin (His-Ub) and either Vpu B, Vpu C, or M Vpu-HA. After 36 hours cells were treated with MG132 for 8 hours followed by lysis in denaturation buffer, and then total ubiquitinated proteins were pulled down with the use of Ni-NTA beads, and p53 ubiquitination was checked by immunoblotting with anti-p53 Ab. (C) HEK 293T cells were cotransfected with His-Ub, and an increasing dose (0.5, 1, and 2 μg) of either M-Vpu or Wt Vpu B-HA and p53 ubiquitination was checked as described in panel B.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/117/24/10.1182_blood-2011-01-333427/4/m_zh89991173070002.jpeg?Expires=1767708735&Signature=xChaifWRVSB6qC6DdY491k1geB7DnZikHwZZK1Kod-HkoqkLAuvZMBNP67U7zPMarYGidBsCUL8nNA6GS08mAGPdDRLEEO19q1v2641tiTXa81fP-cI-pA8t-9Ss3xbDAgHo-mGwsBbUUFyePDDU4VvnGls3thbrwYi0Od0Ym4GU07CNhA3SatmtBzDZEzhvrdQgGV7q-szN8p0K5btuHQEQnu57lVJwt4W0ind0Z7DAOvtQQo6R2WFZjZBhHbec98F5rTkbPtHdoe7Qx8jTKVTwKu79vybQAnXrXY6rw9z3gw5RKjppqIVUBYJolN6mGyDJ20XbN2DE0kYpTV373Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)