In this issue of Blood, Agar and colleagues provide evidence that the plasma protein β2-glycoprotein I (β2GPI) changes conformation to a fish hook structure to bind to bacterial LPS via the protein's carboxyterminal domain, and that this binding promotes the clearance of LPS from the blood circulation.1
Beta2GPI is a plasma protein that is familiar to hematologists as the primary antigenic target of autoantibodies in the antiphospholipid syndrome (APS). Although a range of properties and affinities have been described, some of which are reviewed in the article by Agar et al,1 the protein's biologic functions have not been established. The mystery is compounded by the observation that deficiency of β2GPI in humans has not been associated with any obvious disease or alterations.2 Furthermore, investigation of transgenic β2GPI-null mice reported evidence for impaired in vitro thrombin generation but the absence of any obvious phenotypes other than the interesting finding of the births of fewer than expected homozygous null mice to heterozygous parents.3
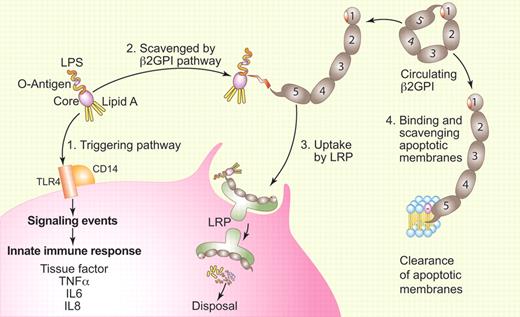
Model for LPS scavenging by β2GPI. (1) Free unbound LPS triggers the innate immune response by binding to a receptor complex that includes TLR4 and CD14 via its Lipid A domain. (2) LPS can be captured by the carboxyterminal 5th domain of β2GPI after a change in the protein's conformation. Domain 5 binds to the non-Lipid A portion of LPS and does not directly neutralize its activity. The opening of β2GPI exposes epitopes that are hypothesized to trigger an autoimmune response leading to antiphospholipid syndrome in susceptible patients. (3) The β2GPI-LPS complex is taken up by LRP, endocytosed, and degraded within the cell. (4) As previously described, β2GPI can also change conformation to bind to membranes that display anionic phospholipids. This binding similarly exposes the epitope on domain 1 that may also trigger an autoimmune response leading to antiphospholipid syndrome. Author's adaptation of Figure 7 of the article by Agar et al1 executed by Paulette Dennis.
Model for LPS scavenging by β2GPI. (1) Free unbound LPS triggers the innate immune response by binding to a receptor complex that includes TLR4 and CD14 via its Lipid A domain. (2) LPS can be captured by the carboxyterminal 5th domain of β2GPI after a change in the protein's conformation. Domain 5 binds to the non-Lipid A portion of LPS and does not directly neutralize its activity. The opening of β2GPI exposes epitopes that are hypothesized to trigger an autoimmune response leading to antiphospholipid syndrome in susceptible patients. (3) The β2GPI-LPS complex is taken up by LRP, endocytosed, and degraded within the cell. (4) As previously described, β2GPI can also change conformation to bind to membranes that display anionic phospholipids. This binding similarly exposes the epitope on domain 1 that may also trigger an autoimmune response leading to antiphospholipid syndrome. Author's adaptation of Figure 7 of the article by Agar et al1 executed by Paulette Dennis.
Last year, Agar et al reported that plasma β2GPI has a circular conformation that is maintained by affinity of its carboxyterminal domain V for aminoterminal domain I and that the protein opens into a fish hook conformation when it becomes bound to phospholipid via its hydrophobic “barb” on domain V.4 They showed that this alteration exposes an epitope on domain I that is cryptic in the circular conformation and proposed that immunologic recognition of this epitope was part of the APS disease process. In the current paper, Agar et al demonstrate that β2GPI also binds LPS through domain V and that this binding is associated with a similar conformational change (see figure).1
An interesting aspect of their results was the finding that β2GPI binds to LPS at sites other than the lipid A core, where the molecule's endotoxin activity resides. In addition, β2GPI does not neutralize the endotoxin activity of LPS directly. Rather, the β2GPI-LPS complexes are bound and endocytosed via low density lipoprotein-related protein receptors on cell surfaces—monocyte-macrophages in their experiments—but they propose that vascular endothelial cells can have a similar function. They also show that this uptake occurs via affinity of receptors for the open, but not the circular, conformation of β2GPI.
Agar and colleagues provide convincing evidence for the biologic significance of this β2GPI-LPS affinity in humans.1 Under a protocol approved by their institutional scientific and ethics committees, healthy human volunteers were intravenously infused with low doses of LPS. A rapid decline of plasma β2GPI levels was observed. In addition, febrile responses and serum levels of inflammatory cytokines correlated inversely with the subjects' baseline β2GPI levels. Also, plasma β2GPI levels of hospital patients with Gram-negative sepsis were reduced during the acute event and increased after recovery.
In addition to shedding new light on the biologic function of β2GPI and extending knowledge about host responses to infection, these results by Agar et al also fuel consideration of potential clinical implications. For example, might β2GPI levels be helpful in defining prognostic factors in Gram-negative sepsis? Agar and colleagues also suggest the possibility of novel biopharmaceutical approaches for this often fatal disorder.
To “close the circle,” these results regarding LPS also return our attention to the APS disease process because clinical studies indicate that infections may be one of the triggers for APS.5 This is particularly true for a severe form of the disorder, catastrophic APS, that is marked by disseminated small vessel occlusions and multiorgan failure.6 It is possible, as Agar and colleagues suggest, that in susceptible patients, the binding of β2GPI to Gram-negative bacteria leads to a conformational change in β2GPI with exposure of former cryptic epitopes that in turn lead to the immunologic response and the devastating thromboembolic sequelae.1
This article by Agar et al represents a significant advance in the biology of β2GPI and in the elucidation of innate host defenses against microorganisms. We can hope for even more solid confirmation of the hypothesis with additional clinical studies of the β2GPI-deficient patients to learn whether they might be more susceptible to sepsis and when investigators return and to the β2GPI-null animal model to determine whether these mice might have an altered response to LPS challenge.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal