HIT is caused by antibodies specific to PF4/heparin complexes. In this issue of Blood, Krauel et al report novel findings supporting the hypothesis that primary synthesis of these antibodies results from bacterial infections. HIT, therefore, appears to be a misdirected antibacterial host defense response.1
The immune response to heparin that leads to heparin-induced thrombocytopenia (HIT), a severe drug-induced thrombocytopenia observed in 1% to 3% of heparin-treated patients, is considered to be atypical because IgG and IgM antibodies are both synthesized shortly after heparin administration.2 HIT IgG antibodies bind to epitopes that are exposed by modified platelet factor 4 (PF4, or CXCL4), a tetrameric member of the C-X-C subfamily of chemokines, after its interaction with heparin. However, a variety of sulfated polysaccharides other than heparin can also induce antigenic changes in the PF4 molecule similar to those implicated in HIT.
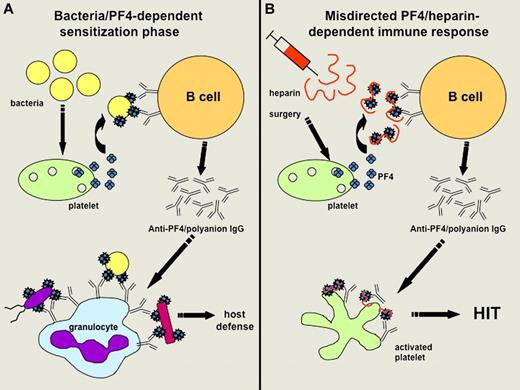
Hypothetical representation. The image shows the hypothetical representation of the mechanisms involved (A) in sensitizing infected patients to PF4 and (B) favoring further development of HIT when treated by heparin (adapted from Krauel et al's Figure 6). (A) Bacteria can induce the release of PF4, which then binds polyanions present on the bacterial surface. This interaction triggers the synthesis of antibodies specific to modified PF4, which can bind to variable bacterial strains and favor their phagocytosis by granulocytes. (B) After initiation of heparin treatment (particularly in surgical patients), PF4/heparin complexes are formed, mimic PF4 bound to bacteria and thus trigger the synthesis of modified PF4-specific antibodies in sensitized patients. Anti-PF4/polyanion IgG antibodies strongly activate platelets in some cases and promote the development of HIT.
Hypothetical representation. The image shows the hypothetical representation of the mechanisms involved (A) in sensitizing infected patients to PF4 and (B) favoring further development of HIT when treated by heparin (adapted from Krauel et al's Figure 6). (A) Bacteria can induce the release of PF4, which then binds polyanions present on the bacterial surface. This interaction triggers the synthesis of antibodies specific to modified PF4, which can bind to variable bacterial strains and favor their phagocytosis by granulocytes. (B) After initiation of heparin treatment (particularly in surgical patients), PF4/heparin complexes are formed, mimic PF4 bound to bacteria and thus trigger the synthesis of modified PF4-specific antibodies in sensitized patients. Anti-PF4/polyanion IgG antibodies strongly activate platelets in some cases and promote the development of HIT.
In this context, Krauel et al hypothesized that PF4 can also bind to negatively charged polysaccharides present on the surface of bacteria, this interaction leading to the formation of complexes triggering an immune response to modified PF4 that contributes to defense against infections. The results obtained by Krauel et al support this original concept because they showed that human and murine PF4 bound to different strains of Gram-negative and Gram-positive bacteria in a dose-dependent and saturable manner, and this binding was inhibited by high concentrations of heparin. In addition, PF4-coated bacteria could be used to adsorb anti-PF4/heparin antibodies from the sera of patients with HIT, the IgG fractions eluted exhibiting a significant reactivity against PF4/heparin complexes and the ability to activate platelets in the presence of heparin. Using a mouse polymicrobial sepsis model, the authors also demonstrated that bacterial infection was associated with the development of anti-PF4/heparin antibodies, although the mice did not receive any heparin injection. Importantly, the kinetics of the response observed in infected mice resembled a primary immune response with early IgM synthesis followed by relatively low titers of IgG antibodies, which bound to murine PF4 alone. In humans, the specificity of HIT antibodies is mainly directed against PF4/heparin complexes, but IgG may also bind to PF4 alone in some cases. If such a close connection between bacterial infection, PF4, and HIT does exist in humans, we should expect a relatively high prevalence of seroconversion with the development of antibodies to PF4 in patients with sepsis, and this possibility has to be investigated.
Finally, Krauel et al concluded that HIT can be considered as a misdirected host-defense immune response that occurs in some patients exposed to heparin who have been previously immunized with PF4-coated bacteria. This new concept is also supported by the relatively high prevalence of IgM and IgG anti-PF4/heparin antibodies evidenced in a large population of German individuals who had not received any heparin treatment in the 12 months preceding blood sampling. However, it is noteworthy that the same team failed to detect memory B cells in cardiac surgery patients who frequently develop anti-PF4/heparin antibodies postoperatively.3 To explain this apparent discrepancy, Krauel et al suggest that the immune response associated with HIT could mainly involve marginal zone (MZ) B cells that are major players at the interface between the initial innate immune response and the delayed adaptive response. Indeed, the ability of MZ B cells to respond rapidly to encapsulated bacteria by differentiating into antigen-specific plasma cells helps keep such infections under control. MZ B cells might thus also contribute to the rapid synthesis of IgG, IgM, and IgA PF4-specific antibodies in HIT and explain why there is a rapid decline in antibody titers, which usually disappear within 100 days, whereas a normal memory B-cell response maintains these antibodies for a longer time. The role of T cells in the HIT immune response remains uncertain in this context, although it was supported by a study based on a murine immunization model.4
As illustrated in their article (see figure), Krauel et al also deduced from their experiments that antibodies specific to modified PF4 and synthesized in infected patients could contribute to host defenses by allowing the binding of PF4-coated bacteria to granulocytes and their subsequent phagocytosis. Their findings thus support the concept that PF4 has a significant role in bacterial defense. This process could be viewed as a “generic” host-defense mechanism because PF4 was shown to bind to several different strains of bacteria and anti-PF4/polyanion antibodies might therefore react with bacteria not previously encountered by the host-immune system. Whether it is an “ancient” host-defense mechanism, as suggested by Krauel et al, remains to be established. Their findings are also reminiscent of what was observed more than 20 years ago when PF4 was shown to modulate the antibody response to pneumococcal polysaccharides.5 Moreover, the binding of PF4 to bacteria could also result in direct bactericidal activity dependent on peptides derived from PF4 and containing heparin-binding motifs.6
Whether the phenomenon observed by Krauel et al can also occur with other endogenous proteins warrants further study. Intriguingly, peptides derived from another autoantigen, beta-2 glycoprotein I (β2GPI), were recently shown to exhibit antibacterial activities against Gram-positive and Gram-negative bacteria,7 but it is not known whether the binding of β2GPI to bacteria elicits conformational changes and subsequent anti-β2GPI immune response. However, this protein is the main target of antibodies associated with the “antiphospholipid syndrome” which exhibits several similarities to HIT8 and can also occur in the context of bacterial infections.
In conclusion, this study is an important contribution that significantly adds to our understanding of the mechanisms potentially responsible for HIT, and opens new areas of research concerning host-defense mechanisms and their links with autoimmunity.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal